Abstract
Three genes coding for the endonuclease, methylase, and specificity subunits of a type I restriction-modification (R-M) system in the Lactococcus lactis plasmid pIL2614 have been characterized. Plasmid location, sequence homologies, and inactivation studies indicated that this R-M system is most probably of type IC.
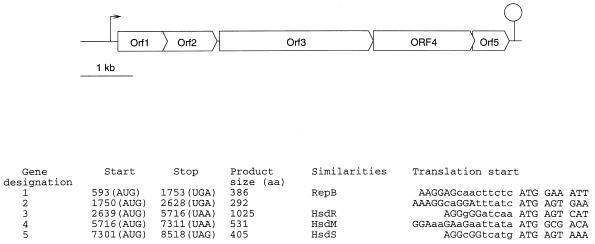
Restriction-modification systems (R-M) are an effective barrier to protect strains from phage infection. As expected for bacteria which are under strong selective pressure in the dairy environment, due to the presence of bacteriophages, a number of R-M systems have been found in Lactococcus lactis strains (18). However, only four have been studied in detail. Three are of type II (5, 9, 31, 33, 34, 41) and one, composed of three genes associated with restriction activity and a type IIs methylase, is unclassified (35). We previously established that plasmid pIL2614 codes for both the Abi420 phage abortive infection and for an R-M activity (efficiency of 10−4) (36). To characterize the R-M system, plasmid pIL2614, extracted from strain IL1403 (4), was sequenced by chromosome walking in cycle extension reactions, using appropriate primers, Taq polymerase, fluorescent dye-coupled dideoxynucleotides and an Applied Biosystems sequencer ABI-373. The DNA and protein sequences were analyzed with the Genetics Computer Group software (6), Genmark (3), and Blast (1) programs. A sequence of 8.6 kb (accession no. U90222), localized upstream of the abi420 genes, revealed five open reading frames (ORFs). The organization of this sequence and the general features of the ORFs are summarized in Fig. 1.
FIG. 1.
(Top) Organization of the pIL2614 sequenced fragment. A putative promoter sequence is indicated by an arrow, and a transcription terminator is indicated by a circle atop a vertical line. (Bottom) Features of the ORFs. Start and stop numbers refer to positions in the sequence, the corresponding codons are shown in parentheses. The putative ribosome binding site and the beginning of the ORFs are shown in capital letters.
The orf1-specified protein has 66 to 78% identity with replication proteins designated RepB from lactococcal plasmids pSK11 (19), pSL2 (20), pCI528 (29), pUCL22 (11), pSV40 (44), pCI305 (17), and pWV02 (22). All these plasmids belong to a family replicating via theta intermediates. They have structural and DNA sequence similarities at the replication origins (11). This origin is composed of two 10-bp repeats ([T/A]TATATATTT) spaced by 3 bp and followed by an AT-rich core containing CG clusters. This core is followed by three 22-bp repeats (TATAn7AAAAAnCn2TG [where n stands for any base pair]) and one that is truncated (11). The −35 box of the promoter is located immediately downstream of this origin. All these features are present upstream of orf1. There are two 10-bp repeats (ATTATTATTTn3 TTATATATTT), an AT-rich core, three 22-bp repeats (CTTATACCTAGAAAAAACAATG), one truncated repeat (CTTATACCTAGAAA), and a putative promoter sequence (TTGTATn17TATAAT). Therefore, orf1 and the upstream DNA sequence are most probably involved in pIL2614 replication that proceeds as described previously for plasmid pUCL22 (11). RepB initiates replication by binding to the origin. This replication is bidirectional and would be under the control of RepB itself (11).
The orf2-specified protein has 53 and 57% identity with proteins encoded by orfx genes present downstream of repB on plasmids pUCL22 (11) and pJW563 (15), respectively. OrfX does not participate in plasmid replication, and its function remains unknown (11). The homology lies in two domains localized at the N and C terminal parts of the orf2-encoded protein. Between these two domains, this protein comprises a long helical domain that contains 10 repeats of 11 amino acids (aa) ([N/D]SLEDKQEKA). These repeats are hydrophilic, and the protein is acidic (Pi 4.5). Therefore, Orf2 is probably neither involved in DNA binding nor membrane anchored as expected for proteins with such repeats.
The orf3-specified protein has 29.8, 28.4, and 24% identity with the Escherichia coli EcoR124II (8), Mycoplasma pulmonis (7), and Haemophilus influenzae (10) endonuclease (R) subunits of type IC R-M systems, respectively. This homology is particularly high at the level of seven helicase-like domains (14, 32) (Fig. 2). Conservation of these domains suggested that the R subunits of type I R-M enzymes may possess helicase activity, playing a role in local unwinding of DNA at the cleavage sites and in DNA translocation (14). Recently, Titheradge et al. (40) identified two additional domains (X and Y) which were well conserved among the four enzymes presented in Fig. 2. Moreover, a 10th domain, localized between domains IA and II, designated Z, is well conserved in R subunits of type IC (Fig. 2). Conserved domains and sequence homologies suggest that orf3 codes for a type IC endonuclease subunit.
FIG. 2.
Alignment of the predicted amino acids of the pIL2614 HsdR peptide with the R subunits of EcoR124II (8), M. pulmonis (7), and H. influenzae (10). Sequence accession numbers are U90222, X13145, L25415, and L45919, respectively. Helicase-like domains I to VI (14) and X and Y domains (40) as well as the additional conserved domain Z are shown in boldface letters. Conserved amino acids and conservative or semiconservative substitutions are indicated by an asterisk and a period, respectively.
The orf4-specified protein has 36.9, 36.9, and 35.5% identity with the E. coli EcoR124II (21) and prr (42) and M. pulmonis (7) methylase (M) subunits respectively. This identity is in agreement with those (32% [38]) usually found for M polypeptides, thus indicating that Orf4 could be part of a type IC R-M system. An alignment of the sequences is shown in Fig. 3 (because of the identity between EcoR124II- and prr-encoded M subunits, only that from EcoR124II is shown). Two sequence motifs conserved in the adenine methyltransferases (MTases), motif CMI ([D/E/S]X[F/A]XGXG) and motif CMII ([L/I/V/M/A/C]X[D/N]PP[Y/F]) (24, 28, 45) are present in Orf4 (Fig. 3). Motif CMI, found in both N and C MTases is the binding site for the cofactor S-adenosylmethionine. This has been shown by mutational analysis of EcoKI N6-adenine MTase (45) and by crystallographic studies of C5-cytosine HhaI MTase (23). Motif II probably plays a role in catalysis (45). The aromatic residue has been shown to be essential for methyl group transfer (45). Moreover, the nature of the conserved amino acid residue preceding PP(Y/F) is characteristic for different classes of MTases and correlates with the base methylation specificity of the enzymes (39, 45). Therefore, depending on the nature of this amino acid (D, N, or S) and on consensus sequences at two additional conserved motifs (CMIs and CMIII), the subdivision of the N4-cytosine and N6-adenine MTases into five classes has been proposed (39). The presence of the NPPY motif together with sequence conservation at the two other domains (Fig. 3) suggests that Orf4 may be an N6-adenine MTase (N12 class). Moreover, for the three enzymes compared in Fig. 3, the CMIs motif defined by Timinkas et al. (39) can be extended to the 22 upstream amino acids.
FIG. 3.
Alignment of the predicted amino acids of the pIL2614 HsdM peptide with the M subunits of EcoR124II (21) and M. pulmonis (7) (GenBank accession no. U90222, X13145, and L25415, respectively). Conserved amino acids and conservative or semiconservative substitutions are indicated by an asterisk and a period, respectively. Conserved motifs (CMIs, CMI, CMII, and CMIII) are shown in boldface letters, and the proposed consensus for the N12 class and all MTases (39) are indicated below. Different groups of amino acids are indicated as follows: p, polar (D, E, N, H, K, R, S, Q, and G); h, hydrophobic (W, F, I, L, M, V, A, P, Y, C, and T); n, negatively charged (D and E); f, aromatic (F, W, Y, and H); a, aliphatic (I, L, V, and M); c, charged (D, E, K, R, and H); and s, small, nonbulky (G, A, S, T, D, N, P, and V).
Four additional domains, conserved among DNA methylases of type II R-M systems, have been identified by structure-guided analysis (30). These domains, not clearly apparent in the MTases compared in Fig. 3, could be absent from type I MTases. Two other motifs are well conserved among the three MTases aligned on Fig. 3: GQEX4TXNLARMNX2L located downstream of CMI and LAPKSKADFAF located just upstream of CMIII. They could be significant in relation to special properties of MTases of the type IC R-M systems.
The orf5-specified protein has 26% identity (45% homology) with the putative specificity (S) subunit of a Spiroplasma citri type I R-M system (27). Moreover, Orf5 presents structural organization characteristics of S subunits. Two repeats of 38 aa (designated A and A′), present in the central and the C-terminal part of the protein, respectively, have 87% identity. Parts of these repeats are homologous to the repeats (24 aa) identified in all S subunits from type I restriction enzymes (21) (Fig. 4). Two split repeats (designated D and D′), characteristic of type IC S subunits (25), are present in the N-terminal and the central parts of Orf5. Homologies between the central conserved domain and sequences near the N and C termini were proposed to favor a circular organization of the domains of the S subunit, which provides the required symmetry for interactivity with the M subunits and the target DNA sequence (40).
FIG. 4.
Predicted amino acid sequence of the pIL2614 HsdS peptide. Thirty-nine-amino-acid repeats (A and A′) and split repeats (D and D′) (25) are shown in boldface letters. The consensus for the 24-aa repeats present in all S peptides (21) is indicated below, together with sequences from EcoR124II (21), M. pulmonis (7), and S. citri (27).
These sequence homologies and gene structure suggest that orf5 codes for an S subunit which is part of an R-M system including the R (Orf3) and M (Orf4) subunits described above. Based on amino acid identities observed for both R and M, this system must be of type IC. However, the L. lactis HsdS protein lacks the TAEL direct repeats characteristic of S subunits of type IC enzymes (37), the number of which has been shown to determine the length of the nonspecific spacer between the specific domains of the recognition sequence (2). Nevertheless, these repeats are absent in the S subunit of the type IC R-M system of M. pulmonis (7) as well as in S subunits of other type I enzymes.
In order to confirm that the region from Orf3 to Orf5 confers the R-M phenotype, a 5,358-bp EcoRV-SacI segment, from position 2968 (309 bp downstream of the start codon of hsdR) to position 8326 (192 bp upstream of the stop codon of hsdS), was deleted from plasmid pIL2614. This segment was replaced by a chloramphenicol resistance cassette recovered from plasmid pGKV259 (43) and previously cloned in pBluescript plasmid (pIL1388) (1a). The construct was designated pIL1032. Phage bIL170 propagated on strain IL1403 showed efficiencies of plating of 3 × 10−3 and 1 when plated on strains IL1403(pIL2614) and IL1403(pIL1032), respectively. Phages picked up from plaques formed on the pIL2614-harboring strain were no longer restricted by this strain. In contrast, phages picked up on the pIL1032-harboring strain were still restricted by IL1403(pIL2614) with an efficiency of plating of 5 × 10−3. The loss by pIL1032 of the aptitude to restrict and/or modify the growth of phage bIL170 indicated that the region from Orf3 to Orf5 confers the R-M phenotype. In contrast, pIL1032 still conferred the Abi420 phenotype active on the phage bIL41 (35).
Genes of type I R-M systems of enterobacteria are arranged into two contiguous transcription units, with hsdM and hsdS forming an operon and hsdR being transcribed from its own promoter. The order of the two transcriptional units is different for different families (46), and this has been proposed as an additional evidence for a horizontal transfer of the hsd genes (40). This organization differs in M. pulmonis, in which the gene order is hsdS hsdR hsdM, with only one promoter upstream of hsdS and the expression of the genes being controlled by inversion of a DNA element (7). In L. lactis, the absence of consensus sequences for a promoter upstream of hsdR and hsdM together with gene organization and the presence of a putative terminator structure downstream of hsdS (GCCCCTAAGATCTAACCTTTATATCTTAGGGGCTATTT TTTT) suggests that the five genes identified could be transcribed from the promoter located upstream of repB. However, as weak promoters transcribing type I genes are difficult to spot in DNA sequences, functional analysis will be needed to identify transcriptional units. It has been proposed that autoregulation of the RepB protein could be under the control of heat-shock proteins (11). If this were true, hsd genes would be activated under stress conditions and therefore perhaps after phage infection.
Type I R-M systems are able to evolve rapidly. A single subunit that concomitantly confers sequence specificity to both restriction and modification facilitates the acquisition of new specificities. Moreover, an S polypeptide has two recognition domains, each specifying one component of the bipartite target sequence (2). In a given family of S polypeptides, the two variable recognition domains are separated by a conserved core sequence. It has been established in vivo (12) and in vitro (13, 16) that the hsdS genes can recombine at the level of the conserved domain, creating a functional R-M system with an entirely new specificity.
In conclusion, our report describes the second functionally characterized (7) and the third (47) type I R-M system described for gram-positive bacteria. Its location on a plasmid and probably under the control of its replication machinery could both increase plasmid stability by postsegregational killing of plasmid-free cells (26) and possibly allow activation of the R-M system by the stress due to phage infection. This, in addition to the facility to acquire new specificities, confers an obvious selective advantage. Therefore, plasmid-encoded type I R-M systems are likely to be widespread in the L. lactis species and possibly other bacteria exposed to phage-abundant environments.
Acknowledgments
We thank C. Anagnostopoulos for critically reading the manuscript and J. Anba for the gift of pIL1388.
Alda Luisa Lerayer was supported by the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (Brasilia, Brazil).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 1a.Anba, J. Unpublished data.
- 2.Bickle T A, Krüger D H. Biology of DNA restriction. Microbiol Rev. 1993;57:434–450. doi: 10.1128/mr.57.2.434-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodorovsky M, Initch J M. Genmark: parallel gene recognition for both DNA strands. Comput Chem. 1993;17:123–133. [Google Scholar]
- 4.Chopin A, Chopin M C, Moillo-Batt A, Langella P. Two plasmid determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984;11:260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- 5.Davis R, van der Lelie D, Mercenier A, Daly C, Fitzgerald G F. ScrFI restriction-modification system of L. lactis subsp. cremoris UC503: cloning and characterization of two ScrFI methylase genes. Appl Environ Microbiol. 1993;59:777–785. doi: 10.1128/aem.59.3.777-785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the vax. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dybvig K, Huilan Y. Regulation of a restriction and modification system via DNA inversion in Mycoplasma pulmonis. Mol Microbiol. 1994;12:547–560. doi: 10.1111/j.1365-2958.1994.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 8.Firman K, Glover W. Basis for changes in DNA recognition by the EcoR124 and EcoR124/3 type I DNA restriction and modification enzymes. J Mol Biol. 1989;205:115–125. doi: 10.1016/0022-2836(89)90369-0. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald G F, Daly C, Brown L R, Gingeras T R. ScrFI: a new sequence-specific endonuclease from Streptococcus cremoris. Nucleic Acids Res. 1982;10:8171–8179. doi: 10.1093/nar/10.24.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleishmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Suton G, FitzHugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodeck A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Uterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J-L, Fuhrmann J-L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Frère J, Novel M, Novel G. Molecular analysis of the Lactococcus lactis subspecies lactis CNRZ270 bidirectional theta replicating lactose plasmid pUCL22. Mol Microbiol. 1993;10:1113–1124. doi: 10.1111/j.1365-2958.1993.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 12.Fuller-Pace F V, Bullas L R, Delius H, Murray N E. Genetic recombination can generate altered restriction specificity. Proc Natl Acad Sci USA. 1984;81:6095–6099. doi: 10.1073/pnas.81.19.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gann A A F, Campbell A J B, Collins F F, Coulson A F W, Murray N E. Reassortment of DNA recognition domains and the evolution of new specificities. Mol Microbiol. 1987;1:13–22. doi: 10.1111/j.1365-2958.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 14.Gorbalenya A E, Koonin E V. Endonuclease (R) subunits of type-I and type-III restriction-modification enzymes contain a helicase-like domain. FEBS Lett. 1991;291:277–281. doi: 10.1016/0014-5793(91)81301-n. [DOI] [PubMed] [Google Scholar]
- 15.Gravesen A, Josephsen J, von Wright A, Vogensen F K. Characterization of the replicon from the lactococcal theta-replicating plasmid pJW563. Plasmid. 1995;34:105–118. doi: 10.1006/plas.1995.9996. [DOI] [PubMed] [Google Scholar]
- 16.Gubler M, Braguglia D, Meyer J, Piekarowicz A, Bickle T A. Recombination of constant and variable modules alters DNA sequence recognition by type IC restriction-modification enzymes. EMBO J. 1992;11:233–240. doi: 10.1002/j.1460-2075.1992.tb05046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes F, Daly C, Fitzgerald G F. Identification of the minimal replicon of Lactococcus lactis subsp. lactis UC317 plasmid pCI305. Appl Environ Microbiol. 1990;56:202–209. doi: 10.1128/aem.56.1.202-209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill C. Bacteriophage and bacteriophage resistance in lactic acid bacteria. FEMS Microbiol Lett. 1993;12:87–108. [Google Scholar]
- 19.Horng J S, Polzin K M, McKay L L. Replication and temperature-sensitive maintenance functions of lactose plasmid pSK11L from Lactococcus lactis subsp. cremoris. J Bacteriol. 1991;173:7573–7581. doi: 10.1128/jb.173.23.7573-7581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahns A, Schafer A, Geis A, Teuber M. Identification, cloning and sequencing of the replication region of Lactococcus lactis subsp. lactis biovar diacetylactis Bu2 citrate plasmid pSL2. FEMS Microbiol Lett. 1991;80:253–258. doi: 10.1016/0378-1097(91)90605-a. [DOI] [PubMed] [Google Scholar]
- 21.Kannan P, Cowan G M, Daniel A S, Gann A A F, Murray N E. Conservation of organization in the specificity polypeptides of two families of type I restriction enzymes. J Mol Biol. 1989;209:335–344. doi: 10.1016/0022-2836(89)90001-6. [DOI] [PubMed] [Google Scholar]
- 22.Kiewet R, Bron S, de Jonge K, Venema G, Seegers J F M L. Theta replication of the lactococcal plasmid pWV02. Mol Microbiol. 1993;10:319–327. [PubMed] [Google Scholar]
- 23.Klimasauskas S, Kumar S, Roberts R J, Cheng X. HhaI methyltransferase flips its target base out of the DNA helix. Cell. 1994;7:357–369. doi: 10.1016/0092-8674(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 24.Klimasauskas S, Timinkas A, Menkevicius S, Butkiene D, Butkus V, Janulaitis A. Sequence motifs characteristic of DNA [cytosine-N′] methyltransferases: the two domains of global similarity within DNA-methylases. Exp Biol. 1990;1:4–12. [Google Scholar]
- 25.Kneale G G. A symmetrical model for the domain structure of type I DNA methyltransferases. J Mol Biol. 1994;243:1–5. doi: 10.1006/jmbi.1994.1624. [DOI] [PubMed] [Google Scholar]
- 26.Kulakauskas S, Lubys A, Ehrlich S D. DNA restriction-modification systems mediate plasmid maintenance. J Bacteriol. 1995;177:3451–3454. doi: 10.1128/jb.177.12.3451-3454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laigret F, Gaurivaud P, Bove J-M. The unique organization of the rpoB region of Spiroplasma citri: a restriction and modification system gene is adjacent to rpoB. Gene. 1996;171:95–98. doi: 10.1016/0378-1119(96)00071-6. [DOI] [PubMed] [Google Scholar]
- 28.Lauster R. Evolution of type II DNA methyltransferases. A gene duplication model. J Mol Biol. 1989;206:313–321. doi: 10.1016/0022-2836(89)90481-6. [DOI] [PubMed] [Google Scholar]
- 29.Lucey M, Daly C, Fitzgerald G F. Identification and sequence analysis of the replication region of the phage resistance plasmid pCI528 from Lactococcus lactis ssp. cremoris UC503. FEMS Microbiol Lett. 1993;110:249–256. doi: 10.1111/j.1574-6968.1993.tb06330.x. [DOI] [PubMed] [Google Scholar]
- 30.Malone T, Blumenthal R M, Cheng X. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyl-transferases, and suggests a catalytic mechanism for these enzymes. J Mol Biol. 1995;253:618–632. doi: 10.1006/jmbi.1995.0577. [DOI] [PubMed] [Google Scholar]
- 31.Moineau S, Walker S A, Vedamuthu E R, Vandenbergh P A. Cloning and sequencing of LlaII restriction/modification genes from Lactococcus lactis and relatedness of this system to the Streptococcus pneumoniae DpnII system. Appl Environ Microbiol. 1995;61:2193–2202. doi: 10.1128/aem.61.6.2193-2202.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray N E, Daniel A S, Cowan G M, Sharp P M. Conservation of motifs within the unusually variable polypeptide sequences of type I restriction and modification enzymes. Mol Microbiol. 1993;9:133–143. doi: 10.1111/j.1365-2958.1993.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 33.Nyengaard N, Vogensen F K, Josephsen J. LlaAI and LlaBI, two type II restriction endonucleases from Lactococcus lactis subsp. cremoris W9 and W56 recognizing, respectively, 5′-/GATC-3′ and 5′-C/TRYAG-3′. Gene. 1992;136:371–372. doi: 10.1016/0378-1119(93)90499-s. [DOI] [PubMed] [Google Scholar]
- 34.Nyengaard N, Vogensen F K, Josephsen J. Restriction-modification systems in Lactococcus lactis. Gene. 1995;157:13–18. doi: 10.1016/0378-1119(95)91235-r. [DOI] [PubMed] [Google Scholar]
- 35.O’Sullivan D J, Zagula K, Klaenhammer T R. In vivo restriction by LlaI is encoded by three genes, arranged in an operon with llaIM, on the conjugative Lactococcus plasmid pTR2030. J Bacteriol. 1995;177:134–143. doi: 10.1128/jb.177.1.134-143.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portugal, C., F. Clier, R. Valyasevi, S. D. Ehrlich, and M.-C. Chopin. Characterization of the lactococcal Abi420 determinant for abortive infection. Unpublished data.
- 37.Price C, Lingner J, Bickle T A, Firman K, Glover S W. Basis for changes in DNA recognition by the EcoR124 and EcoR124/3 type I DNA restriction and modification enzymes. J Mol Biol. 1989;205:115–125. doi: 10.1016/0022-2836(89)90369-0. [DOI] [PubMed] [Google Scholar]
- 38.Sharp P M, Kelleher J E, Daniel A S, Cowan G M, Murray N E. Roles of selection and recombination in the evolution of type I restriction-modification systems in enterobacteria. Proc Natl Acad Sci USA. 1992;89:9836–9840. doi: 10.1073/pnas.89.20.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timinkas A, Butkus V, Janulaitis A. Sequence motifs characteristic for DNA [cytosine-N4] and DNA [adenine-N6] methyltransferases. Classification of all DNA methyltransferases. Gene. 1995;157:3–11. doi: 10.1016/0378-1119(94)00783-o. [DOI] [PubMed] [Google Scholar]
- 40.Titheradge A J B, Ternent D, Murray N E. A third family of allelic hsd genes in Salmonella enterica: sequence comparisons with regions implicated in restriction of DNA. Mol Microbiol. 1996;22:437–447. [PubMed] [Google Scholar]
- 41.Towney D P, Davis R, Daly C, Fitzgerald G F. Sequence of the gene encoding a second ScrFI m5 C methyltransferase of Lactococcus lactis. Gene. 1993;136:205–209. [Google Scholar]
- 42.Tyndall C, Meister J, Bickle T A. The Escherichia coli prr region encodes a functional type IC DNA restriction system closely integrated with an anticodon nuclease gene. J Mol Biol. 1994;237:266–274. doi: 10.1006/jmbi.1994.1230. [DOI] [PubMed] [Google Scholar]
- 43.Van der Vossen J, Van der Lelie D, Venema G. Isolation and characterization of Lactococcus lactis subsp. cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987;53:2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Wright A, Wessels S, Tynkkynen S, Saarela M. Isolation of a replication region of a large lactococcal plasmid and use in cloning of a nisin resistance determinant. Appl Environ Microbiol. 1990;56:2029–2035. doi: 10.1128/aem.56.7.2029-2035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willcock D F, Dryden D T F, Murray N E. A mutational analysis of the two motifs common to adenine methyltransferases. EMBO J. 1994;13:3902–3908. doi: 10.1002/j.1460-2075.1994.tb06701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson G G. Organization of restriction-modification systems. Nucleic Acids Res. 1991;19:2539–2566. doi: 10.1093/nar/19.10.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu G, Willert J, Kapfer W, Trautner T A. BsuCI, a type I restriction-modification system in Bacillus subtilis. Gene. 1995;157:59. doi: 10.1016/0378-1119(95)00724-k. [DOI] [PubMed] [Google Scholar]