Abstract
Alzheimer’s disease is a complex neurodegenerative disorder leading to a decline in cognitive function and mental health. Recent research has positioned the gut microbiota as an important susceptibility factor in Alzheimer’s disease by showing specific alterations in the gut microbiome composition of Alzheimer’s patients and in rodent models. However, it is unknown whether gut microbiota alterations are causal in the manifestation of Alzheimer’s symptoms.
To understand the involvement of Alzheimer’s patient gut microbiota in host physiology and behaviour, we transplanted faecal microbiota from Alzheimer’s patients and age-matched healthy controls into microbiota-depleted young adult rats.
We found impairments in behaviours reliant on adult hippocampal neurogenesis, an essential process for certain memory functions and mood, resulting from Alzheimer’s patient transplants. Notably, the severity of impairments correlated with clinical cognitive scores in donor patients. Discrete changes in the rat caecal and hippocampal metabolome were also evident. As hippocampal neurogenesis cannot be measured in living humans but is modulated by the circulatory systemic environment, we assessed the impact of the Alzheimer’s systemic environment on proxy neurogenesis readouts. Serum from Alzheimer’s patients decreased neurogenesis in human cells in vitro and were associated with cognitive scores and key microbial genera.
Our findings reveal for the first time, that Alzheimer’s symptoms can be transferred to a healthy young organism via the gut microbiota, confirming a causal role of gut microbiota in Alzheimer’s disease, and highlight hippocampal neurogenesis as a converging central cellular process regulating systemic circulatory and gut-mediated factors in Alzheimer’s.
Keywords: Alzheimer’s disease, faecal microbiota transplantation, memory, adult hippocampal neurogenesis
Grabrucker et al. show that young adult rats that received transplants of gut microbiota from patients with Alzheimer’s disease performed poorly in memory tests, and produced fewer new neurons, compared to rats that received microbiota from age-matched controls. Serum from patients also decreased neurogenesis in human cells in vitro.
See Gubert and Hannan (https://doi.org/10.1093/brain/awad368) for a scientific commentary on this article.
Introduction
Alzheimer’s disease, the leading cause of dementia, is a complex neurodegenerative disorder characterized by progressive impairments in cognitive function, which are often comorbid with neuropsychiatric symptoms.1-3 Neuropathological hallmarks of Alzheimer’s disease include extracellular deposition of amyloid-β (Aβ) plaques, intraneuronal accumulation of neurofibrillary tangles composed of hyperphosphorylated tau, neuroinflammation and neuronal death.4-6 The hippocampus plays a critical role in learning and memory7 and is particularly vulnerable to Alzheimer’s pathology, being one of the earliest brain areas to be affected.8 The hippocampus is also host to a population of neural stem cells, which generate new neurons throughout the lifespan in a process called adult hippocampal neurogenesis (AHN).9,10 This unique form of cellular plasticity is a key mediator of cognitive functions such as spatial learning, pattern separation (an ability to distinguish between highly similar events or environments),11-13 as well as the regulation of emotion.14 Notably, these behaviours are impaired in Alzheimer’s disease.15-17 Crucially, AHN is altered in early Braak stages, preceding neurofibrillary tangles and Aβ plaque formation in the hippocampus of humans,18 suggesting that AHN dysfunction is an early feature of Alzheimer’s pathogenesis.19
It is increasingly recognized that Alzheimer’s is a multifactorial disease, substantially influenced by genetic, lifestyle and environmental factors.20-22 While it is well established that changes in the systemic circulatory environment may accelerate the development of Alzheimer’s pathology,23 the gut microbiome is now emerging as a key target for investigation in Alzheimer’s disease due to its particular susceptibility to lifestyle and environmental influences.24-26 Moreover, the microbiota–gut–brain axis is now recognized as a significant mediator of behaviour throughout the lifespan.27,28 Altered microbial composition, microbial metabolites and bacterial endotoxins have been reported in Alzheimer’s patients.29-36 Recently, a significant genetic overlap and correlation between Alzheimer’s disease and gastrointestinal tract disorders has been suggested.37
Faecal microbiota transplantation (FMT) studies using mouse models of Alzheimer’s disease have implicated the gut microbiota in pathological features of Alzheimer’s disease; endoplasmic reticulum stress in recipient mice is enhanced after FMT from APP/PS1 mice and Alzheimer’s patients,38 and transplantation of gut microbiota derived from a 5xFAD mouse model of Alzheimer’s disease impairs spatial memory and AHN in C57BL/6 mice.39 Notably, the microbiota–gut–brain axis has been shown to regulate AHN.40-42 However, it is as yet unexplored if cognitive symptoms in human Alzheimer’s patients coupled with underlying cellular changes such as AHN can be transmitted to a healthy organism via the gut microbiota to thus position the gut microbiota as a critical mediator of Alzheimer’s symptomology.
We addressed this using FMT from Alzheimer’s participants to young adult rats to determine behavioural, neurogenic and metabolic features mediated through gut microbiota. The influence of the systemic milieu of Alzheimer’s participants on hippocampal neurogenesis was assessed in parallel in human hippocampal progenitor cells in vitro to establish AHN as a converging central cellular process from systemic circulatory and gut-mediated factors in Alzheimer’s disease.
Materials and methods
Human participants
Patients with Alzheimer’s disease (n = 69) and cognitively healthy control subjects (n = 64) were recruited at the IRCCS Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy. All participants underwent clinical assessment for cognitive function and a physical examination. See Supplementary material for details.
Human biological samples
Venous blood was sampled from an antecubital vein between 8–10 a.m. and collected in EDTA tubes (BD 186 Vacutainer Systems) for preparation of plasma. Stool samples were collected from a subset of participants (n = 54 for Alzheimer’s disease and n = 41 for controls) at their own home in a sterile plastic cup, stored at −20°C and subsequently delivered to IRCCS Centro San Giovanni di Dio Fatebenefratelli, for storage at −20°C until processing. For stool used in FMT to rats, frozen samples were transferred into an anaerobic chamber, thawed and homogenized at a 100 mg/ml concentration in reduced sterile PBS containing 20% glycerol (w/v) and filtered through a stomacher bag. The filtered slurry was aliquoted and frozen at −80°C. Samples were defrosted immediately before inoculating recipient rats.
Human immune profile
For cytokine expression analysis in plasma, total RNA was isolated using the PAXgene blood miRNA kit according to the manufacturer’s protocol (PreAnalytiX) and gene expression analyses was performed using real-time PCR (Supplementary material).
Animals
To avoid effects of ageing, adult male Sprague-Dawley rats (310–350 g) aged 11 weeks, (Envigo) were used and maintained in environmentally controlled conditions at the ambient temperature of 21°C ± 2°C, 55 ± 10% humidity, and under a 12-h light-dark cycle. Animals were group-housed (four per cage) in autoclaved cages with autoclaved bedding (Eco-Pure Aspen chips) and had access to autoclaved water and chow (Teklad Global Diet 2018S; Envigo) ad libitum.
Faecal microbiota transplantation
Following 2 weeks acclimatization, rats were administered an antibiotic cocktail of ampicillin (1 g/l), vancomycin (500 mg/l), ciprofloxacin HCL (200 mg/l) and imipenem (250 mg/l) for seven consecutive days in autoclaved drinking water, which has previously been shown to ensure sufficient microbiota depletion.43,44 Rats were colonized 72 h later via daily oral gavage of donor microbiota (300 μl of 100 mg/ml homogenized faecal slurry) for 3 days.45 See Supplementary material for details.
Study design and experimental timeline for animal experiments
After 7 days of antibiotics, rats were randomly allocated into one of the two groups, balanced by weight (using a random number table): rats receiving FMT from control subjects (control FMT, n = 16) and rats receiving FMT from Alzheimer’s patients (AD-FMT, n = 16). See Supplementary material for details.
Faecal sample collection and faecal water content in rats
Faecal samples were collected and faecal water content determined as previously described.46 (Supplementary material).
16S microbiota and bioinformatic analysis of human and rat samples
DNA was extracted from 180–200 mg of frozen stool using the QIAamp DNA Stool Mini Kit (Qiagen Retsch GmbH) according to the manufacturer’s instructions. For details and bioinformatic analysis see Supplementary material.
Histological analysis of ileal and colonic sections from rats
Colon and ileum sections were prepared using the Swiss-roll technique. After fixation, Swiss-rolls were embedded in paraffin and stained with haematoxylin and eosin. Alcian Blue/Periodic acid-Schiff (PAS) staining was performed to identify goblet cells (Supplementary material).
Behavioural testing in rats
Behavioural testing started 10 days after the first inoculation and behaviours were conducted between 9 a.m. and 7 p.m. during the light phase of the light cycle with a minimum of 36 h between each behavioural test. Rats underwent a battery of behavioural tests conducted in the following order: Open Field, Elevated Plus Maze, Modified Spontaneous Location Recognition Test, Novel Object Recognition, Novel Location Recognition, Morris Water Maze and Forced Swim Test. A detailed description of the behavioural tests can be found in the Supplementary material.
Immunohistochemistry of rat brain tissue
BrdU/NeuN, DCX, Ki67 and Iba-1 immunohistochemistry was performed in the hippocampus, as previously described.47,48 See Supplementary material for details of immunohistochemistry, analysis of cell density and 3D reconstruction of DCX + cells.
Thioflavin S staining
For visualization of amyloid plaques, Thioflavin S staining was carried out on hippocampal and cortex sections (Supplementary material).
Cell culture and serum treatment
All experiments were performed using the multipotent human hippocampal progenitor/stem cell line HPC0A07/03C (HPC, ReNeuron) derived from the first trimester female foetal hippocampal tissue following medical termination (in accordance with the UK and USA ethical and legal guidelines, and obtained from Advanced Bioscience Resources). See Supplementary material for details on cell culture, serum treatments, immunocytochemistry, high-content imaging and neurite outgrowth analysis.
Rat hippocampal and caecal metabolomics
Metabolomic analysis was carried out on caecum and hippocampus by Ms-OMICS. See Supplementary material for details on the preparation of hippocampal tissue and caecal content, mass spectrometry and bioinformatic analysis.
Quantitative real-time PCR for immune profile in rat colon
Isolation of total RNA was performed using the GenElute kit (Sigma) as described by the manufacturer (Supplementary material).
Cytokine assay in rat plasma
Tail blood (10 days after FMT) and trunk blood (at the end of the study) was collected in tubes containing heparin and immediately centrifuged at 1000g for 15 min at 4°C. The resulting supernatant (plasma) was collected, aliquoted and stored at −80°C. Plasma levels of IL-6, IL-4, IL-10 IFN-γ, TNF-α and IL-1β were assayed in duplicate using high sensitivity commercially available electrochemiluminescence MULTI-SPOT® Meso Scale Discovery kits (MSD) as per the manufacturer’s instructions. Reading and analysis were conducted using the MESO QuickPlex SQ 120, Sector Imager 2400. Concentration is expressed in pg/ml.
Ethics statement
The human study was approved by the Ethics Committee of ‘Comitato Etico dell’IRCCS San Giovanni di Dio—Fatebenefratelli’ (Brescia, Italy) under registration number 92/2017. Informed consent to participate in this study was given by all subjects or their caregivers. All animal experiments were performed in compliance with the guidelines for the welfare of experimental animals issued by the Health Products Regulatory Authority (HPRA, Ireland), and the European Communities Council Directive (2010/63/EU) and approved by the Animal Experimentation Ethics Committee (#AE19130/P108) of University College Cork.
Statistics
Sample size for experiments were determined based on prior publication and power analysis using the G*Power software. Normal distribution was determined by the Shapiro-Wilk test. For normally distributed data, single comparisons were tested using independent Student’s t-test. Non-parametric data were examined using the Mann-Whitney U-test. Experiments with more than two groups were subjected to a one-way ANOVA if normally distributed and compared using Bonferroni’s post hoc test. Multiple comparisons of non-parametric data were made by a Kruskal-Wallis analysis followed by Dunn’s post hoc test. Experiments with two ‘between factors’ (treatment, donors) were analysed using a two-way ANOVA to determine genotype and treatment effect or interaction between both factors. Experiments with one ‘between-subjects’ factor (treatment group) and one ‘within-subjects’ factor (trial) were analysed using a two-way mixed ANOVA. Pearson or Spearman’s rank correlation were performed when data were normally or not normally distributed, respectively. Statistical analyses were performed using GraphPad Prism 8.0 or in R (v.3.6.3) with the Rstudio GUI (v.1.2.5033) for bioinformatic analysis of 16S microbiota sequencing and R (v.4.1.1) for metabolomics analysis. See Supplementary material for details. Statistical significance was set at P ≤ 0.05 and differences are indicated in the figures by *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
Results
Alterations in circulatory system and gut microbiota are associated with cognitive status in Alzheimer’s patients
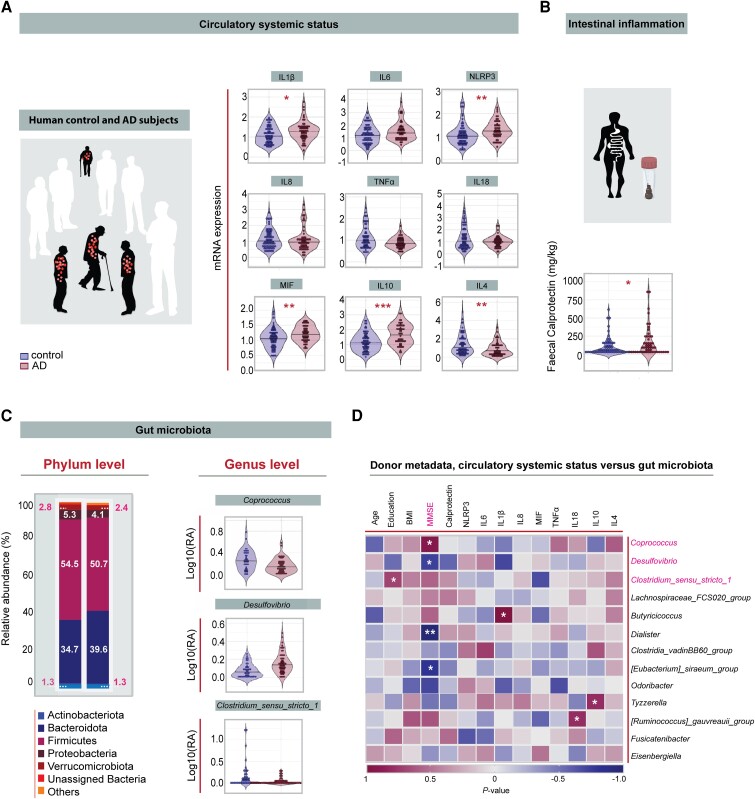
Recent studies have described a potential link between the development of Alzheimer’s disease and alterations in the intestinal microbial community.49 Dysregulation in systemic inflammatory status has long been associated with the aetiology and progression of Alzheimer’s disease50 but there is little evidence showing that alterations in gut microbiota composition are associated with circulatory systemic factors and cognitive status in Alzheimer’s patients. Assessment of plasma in a cohort of 69 healthy control subjects and 64 Alzheimer’s patients (Table 1) show that IL-1β, the inflammasome marker NLRP3 and the macrophage migration inhibitory factor (MIF) were significantly upregulated in Alzheimer’s patients, indicating increased systemic inflammation in Alzheimer’s patients (Fig. 1A). These changes were irrespective of biological sex (Supplementary material, Extended Table 6). Lipopolysaccharide (LPS), which has previously been reported to be elevated in the serum of Alzheimer’s disease patients, and can influence Aβ homeostasis, tau pathology and neuroinflammation is not significantly changed in plasma of Alzheimer’s disease participants compared to healthy control participants in our study (Supplementary Fig. 10) but this does not exclude the possibility that LPS may indirectly influence the brain, neurogenesis and cognition in our cohort through the release of pro-inflammatory cytokines from circulating immune cells in the plasma, as we have reported here (Fig. 1A), or through other routes, such as the brain–gut axis.51 In addition, faecal calprotectin, which has been shown to correlate with the presence and severity of intestinal inflammation,52 was significantly increased in Alzheimer’s patients compared to control subjects (Fig. 1B). Analysis of gut microbiota composition by bacterial 16S rRNA gene sequencing in a subset of Alzheimer’s patients (Supplementary Fig. 1A–E) revealed no significant change in alpha and beta diversities between control subjects and Alzheimer’s patients (Supplementary Fig. 1B–D). However, at phylum level, Alzheimer’s patients had a higher abundance of Bacteroidetes (Fig. 1C) reported to comprise many pro-inflammatory species,53 and a lower abundance of the phyla Firmicutes and Verruocomicrobiota, reported to produce beneficial metabolites.54 At the genus level, Alzheimer’s patients had on average a significant reduction in the relative abundance of Clostridium sensu stricto 1 and the short chain fatty acid (SCFA) butyrate-producing genera Coprococcus, which is associated with healthy ageing in general55 (Fig. 1C). In addition, similar to a previous report using a transgenic mouse model of Alzheimer’s disease,56 the relative abundance of the pathobiont genera Desulfovibrio was significantly increased in Alzheimer’s patients compared to cognitively healthy control subjects (Fig. 1C). There was no variability in gut microbiota composition between males and females (Supplementary material, Extended Table 7 and Supplementary Fig. 9).
Table 1.
Demographic and clinical features of control subjects and Alzheimer’s patients
| Control subjects | Alzheimer’s disease subjects | P-value | |
|---|---|---|---|
| n | 69 | 64 | – |
| Age, years | 71.4 (7.9) | 74.8 (7.3) | 0.007** |
| Female, n (%) | 42 (61) | 34 (53) | 0.367 |
| ApoE4, n (%) | 16 (24) | 33 (58) | <0.001*** |
| Education, years | 11.3 (5.4) | 7.6 (3.9) | <0.001*** |
| BMI | 25.2 (3.7) | 24.7 (3.7) | 0.750 |
| Dementia score | |||
| MMSE | 29.0 (1.2) | 19.8 (5.8) | <0.001*** |
Demographic and clinical features of control subjects (n = 69) and Alzheimer’s patients (n = 64), P-values were calculated using unpaired, two-tailed Student’s t-test for continuous Gaussian variables (or Mann-Whitney test for non-Gaussian variables) and chi-square test for categorical data. There were eight missing values for ApoE4, six missing values for MMSE and BMI. Alzheimer’s patients were significantly older (**P = 0.007), showed a significant increase in the prevalence of ApoE4 carriers (***P < 0.001), were less educated (***P < 0.001) and had lower MMSE score (***P < 0.001) than cognitively healthy controls. Except for values on females and ApoE4, data are presented as means + SEM. A greater proportion of patients with Alzheimer’s disease presented with higher incidence of hypercholesterolemia and vascular diseases and were medicated with antidepressant/anxiolytic drugs than healthy controls (Supplementary material, Extended Table 8). BMI = body mass index; MMSE = Mini-Mental State Examination.
Figure 1.
Associations between circulatory systemic factors, gut microbiota and cognitive status of Alzheimer’s disease patients. (A) There was increased expression of the cytokines IL-1β (*P = 0.018), NLRP3 (**P = 0.003), MIF (**P = 0.008), IL-10 (***P < 0.001) and decreased expression of IL-4 (**P = 0.006) in Alzheimer’s disease patients (n = 52) compared to control subjects (n = 68), unpaired, two-tailed Student’s t-test for continuous Gaussian variables (or Mann-Whitney test for non-Gaussian variables). (B) Faecal calprotectin was significantly increased in Alzheimer’s patients (n = 64) compared to control subjects (n = 69), Mann-Whitney test. *P = 0.022. (C) Gut microbiota composition at phylum level from control (n = 41) and Alzheimer’s patients (n = 54). Alzheimer’s patients had higher abundance of phyla Bacteroides, and lower abundance of phyla Firmicutes and Verrucomicrobiota. Relative abundance of genera that differed significantly between controls and Alzheimer’s patients after batch correction using percentile-normalization. Mann-Whitney tests, multiple testing corrections using the Benjamini–Hochberg method, and FDR ≤ 0.1 was considered significant (Coprococcus, P = 0.099; Clostridium in sensu stricto 1, P = 0.099; Desulfovibrio, P = 0.006). (D) Correlation between human gut microbiota, human donor metadata, faecal calprotectin and serum markers. Heat map shows Spearman rank coefficients, with red indicating strong positive correlation, and blue indicating strong negative correlation. P-values for significant correlations (α < 0.05) are noted (*P < 0.05, **P < 0.01, ***P < 0.001). Black horizontal lines in violin plots indicate medians. *P < 0.05, **P < 0.01, ***P < 0.001. NS = not significant; AD = Alzheimer’s disease.
To further understand whether these peripheral alterations are associated with the clinical status of Alzheimer’s patients, we correlated Mini-Mental State Examination (MMSE) scores with the circulatory systemic profile and with the gut microbiota alterations. Notably, we observed significant associations between the MMSE score and the gut microbiota signature (Fig. 1D). Importantly, a positive correlation was observed between the abundance of the health-associated SCFA producer Coprococcus and the MMSE score, and inverse correlations were detected between the abundance of the disease-associated pathobionts Desulfovibrio, Dialister and the MMSE score, supporting a microbiome signature for cognitive performance in Alzheimer’s disease.
Gut microbiota from Alzheimer’s patients altered gut microbiota and intestinal epithelium in rats
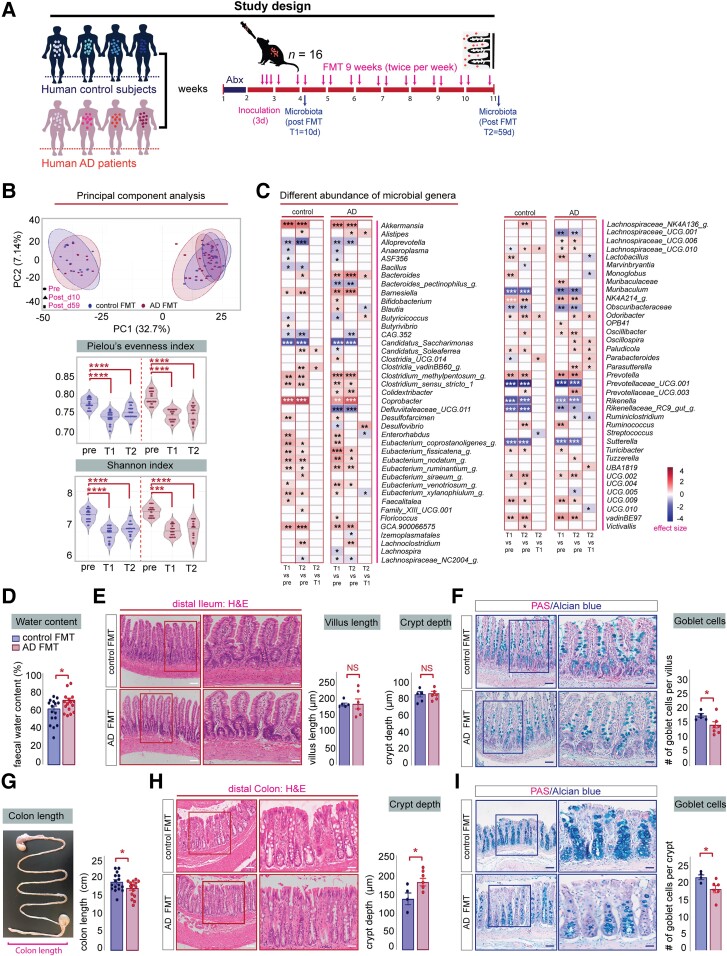
To elucidate the functional contribution of human gut microbiota to Alzheimer’s aetiology, we transplanted faecal samples from cognitively healthy subjects and Alzheimer’s patients into microbiota-depleted young adult rat (Fig. 2A). For this purpose, cognitively healthy and Alzheimer’s disease human donors were selected based on clinical features, using the MMSE and Clinical Dementia Rating (CDR) (Table 2). To validate donor engraftment and the temporal colonization dynamics of gut microbiota transplantation, we analysed baseline faecal samples using 16S rRNA gene sequencing, reassessed composition and diversity at time point 1 (T1) 10 days after human donor colonization (10d) and again at time point 2 (T2), which was the end of the study at Day 59 (59d; Fig. 2A).
Figure 2.
Adult rats colonized with faecal material from Alzheimer’s patients harbour different bacterial genera and display alterations in intestinal epithelial structure. (A) Schematic representation of the experimental procedure: microbiota-depleted young adult rats were colonized with human faecal samples from cognitively healthy control or Alzheimer’s donors (n = 4). One faecal sample from each human donor was used to colonize four microbiota-depleted rats (n = 16). Faecal pellets were collected at Day 0 (pre), 10 days after human donor colonization (10d; T1) and at the end of the study (T2 = 59 days). Vertical bars represent weekly intervals. (B) Top: Principal coordinate analysis showing the effects of FMT on faecal microbiome in rats in terms of β-diversity as measured by Bray-Curtis distance. Ellipses indicate 95% confidence intervals per group. Linear mixed-effects model, significant Time × FMT interaction effect (*P = 0.047). Bottom: Violin plots displaying the effects of FMT on rats in terms of α-diversity. Black horizontal lines in violin plots indicate medians. The Pielou’s evenness and Shannon index decreased following FMT, regardless of donor group (ANOVA with repeated measures *P < 0.05, ***P < 0.001, ****P < 0.0001). (C) Left: Heat map showing genera differentially altered by FMT. Colour depicts effect size, with blue (negative) indicating higher abundances pre-treatment and red (positive) indicating higher abundances post-treatment, Wilcoxon signed-rank test followed by Benjamini–Hochberg correction as per the ALDEx2 library, *q < 0.1, **q < 0.01, ***q < 0.001. (D) Alzheimer’s-FMT rats (n = 16) display a significant increase in faecal water content compared to control FMT rats (n = 16), unpaired, two-tailed Student’s t-test, *P = 0.0309. (E) Left: Representative images of haematoxylin and eosin stained sections of the distal ileum from control and Alzheimer’s-FMT rats. Scale bars = 200 µm (left magnification), 100 µm (right magnification). Right: Alzheimer’s colonized rats show no significant change in ileal villus length, unpaired, two-tailed Student’s t-test, P = 0.7749 and crypt depth, unpaired, two-tailed Student’s t-test, P = 0.9060. (F) Left: Representative PAS/Alcian blue staining of ileal sections. Scale bars = 200 µm (left magnification), 100 µm (right magnification). Right: Alzheimer’s-FMT rats display significant goblet cell loss in the ileum, unpaired, two-tailed Student’s t-test, *P = 0.0426. (G) Average colon length was significantly reduced in Alzheimer’s-FMT rats compared to control FMT rats, two-tailed Student’s t-test, *P = 0.0500. (H) Left: Representative images of haematoxylin and eosin stained sections of the proximal colon from control and Alzheimer’s-FMT rats. Scale bars = 200 µm (left magnification), 100 µm (right magnification). Right: Alzheimer’s-FMT rats displayed a significant increase in colonic crypt depth, two-tailed Student’s t-test, *P = 0.0356. (I) Left: Representative PAS/Alcian blue staining of colon sections. Right: Alzheimer’s-FMT rats display a significant goblet cell loss in the proximal colon, unpaired, two-tailed Student’s t-test, *P = 0.0403. Unless otherwise indicated, data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001. AD = Alzheimer’s disease; FMT= faecal microbiota transplantation; NS = not significant; PAS = periodic acid-Schiff.
Table 2.
Demographic information of human donors
| Donor | Sex | APOE | Age | E | BMI | MMSE | CDR |
|---|---|---|---|---|---|---|---|
| Control_1 | M | ε3ε3 | 80 | 5 | 24.6 | 26 | 0 |
| Control_2 | F | ε3ε4 | 71 | 5 | 25.6 | 29 | 0 |
| Control_3 | M | ε3ε3 | 67 | 13 | 24.7 | 27 | 0 |
| Control_4 | M | ε3ε3 | 68 | 19 | 25.1 | 30 | 0 |
| AD_1 | M | ε3ε4 | 74 | 8 | 24.9 | 12 | 2 |
| AD_2 | F | ε3ε3 | 78 | 8 | 23.1 | 24 | 1 |
| AD_3 | F | ε3ε4 | 77 | 5 | 34.2 | 20 | 3 |
| AD_4 | F | ε3ε3 | 80 | 5 | 23.8 | 19 | 1 |
Donor metadata: demographic and clinical characteristics of human donors (n = 4) used for rat colonization. MMSE and CDR scores were used to determine cognitive function applicable to dementia of human donors. Cognitive function was significantly impaired in human Alzheimer’s donors compared to control donors as measured by MMSE, unpaired, two-tailed Student’s t-test, *P = 0.0131 and CDR scores, Mann-Whitney test, *P = 0.0286. Human donors in both groups displayed no significant difference in age, unpaired, two-tailed Student’s t-test, P = 0.8326; education level, Mann-Whitney test, P = 0.6571 and BMI, Mann-Whitney test, P = 0.6857. BMI = body mass index; CDR = Clinical Dementia Rating; E = Education; MMSE = Mini-Mental State Examination.
Principal coordinate analysis (PCoA) of the Bray-Curtis distance matrix revealed that the temporal microbial changes were not influenced by the clinical status of the donors (Fig. 2B, top). A decrease in alpha diversity was observed in both groups of rats at both time points after human donor colonization, indicating a loss of bacterial species (Fig. 2B, bottom), which may be due to sample processing or host incompatibility. These differences were independent from the clinical status of the donors. At the end of the study (59 days after FMT), a decrease in gained taxa was observed in rats that received faecal material from Alzheimer’s patients, suggesting that their microbiota tended to return to its original state (Supplementary Fig. 2C). On average, 40% of the taxa from human donors engrafted into recipient rats (Supplementary Fig. 2D). Transplantation efficiency was lowest for taxa belonging to the phyla Bacteroidota and Proteobacteria (Supplementary Fig. 2F). Post-FMT, 78 genera were significantly altered in recipient rats (Fig. 2C). Compared with the original microbiota, human Alzheimer’s colonized rats displayed greater alterations in microbial genera than rats receiving control FMT at 10d (51 genera versus 41) but not at 59d (44 versus 45) (Fig. 2C). Notably, we detected specific differences in the abundance of the genera Desulfovibrio in human Alzheimer’s colonized rats compared to control colonized rats (Fig. 2C), which reflects our findings in Alzheimer’s patients (Fig. 1C) and correlates with the MMSE score (Fig. 1D).
To elucidate whether human Alzheimer’s gut microbiota induce pathophysiological processes in the gastrointestinal (GI) tract of healthy young rats, we evaluated the general health of the rat intestine after FMT. Human Alzheimer’s-FMT had no significant impact on faecal pellet output, the caecum weight of recipient rats (Supplementary Fig. 3A and B), body weight composition or food intake (Supplementary Fig. 3C and D). Additionally, no significant differences were detected in the expression of genes encoding pro-inflammatory cytokines in colonic tissue of rats after FMT (Supplementary Fig. 4A–I) and the presence of cytokines in the systemic circulation was unaltered (Supplementary Fig. 5A and B), suggesting that no local inflammatory event occurred in the GI tract due to humanization with Alzheimer’s-FMT. However, human Alzheimer’s disease colonized rats displayed a significant increase in faecal water content (Fig. 2D) and intake (Supplementary Fig. 3E) along with a reduction in colon length (Fig. 2G), suggesting specific effects of the human Alzheimer’s gut microbiota on colonic function. These findings were further supported by structural changes in the depth of the colonic but not ileal crypts as determined by haematoxylin and eosin staining (Fig. 2E and H). Compared to control colonized rats, Alzheimer’s recipient rats displayed crypt hyperplasia in the proximal colon, with the depths of the crypt significantly elongated (Fig. 2H). Additionally, we found a reduction in the number of goblet cells in the colon and ileum, suggesting altered mucin production after transplantation with human Alzheimer’s gut microbiota (Fig. 2F and I).
Alzheimer’s FMT induced cognitive deficits in hippocampal-neurogenesis dependent behaviours in rats
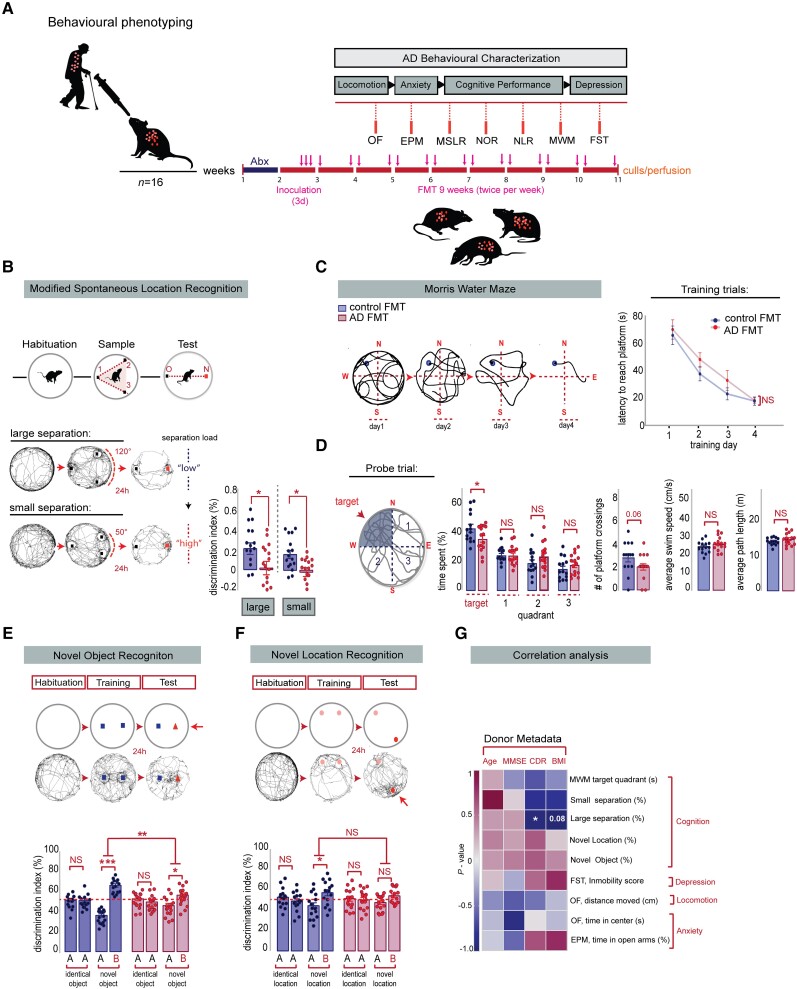
To investigate whether alterations of the human gut microbiota contribute to the cause of the Alzheimer’s behavioural phenotype, we evaluated behavioural performance of young adult rats colonized with faecal material from human Alzheimer’s patients and age-matched control subjects. Rats were tested 2 weeks after initial human donor colonization in a behavioural test battery to assess task-specific memory performance and other Alzheimer’s-related co-morbiditie (Fig. 3A). Rats colonized with faecal material from Alzheimer’s donors exhibited no change in locomotor parameters in the Open Field Test (Supplementary Fig. 6A), no change in anxiety-related behaviours in the Elevated Plus Maze (EPM) (Supplementary Fig. 6B), or in antidepressant-like behaviour in the Forced Swim test (FST) (Supplementary Fig. 6C), indicating no specific effects of the Alzheimer’s human gut microbiota on comorbid features of Alzheimer’s disease in rats.
Figure 3.
Alzheimer’s-FMT induced cognitive deficits in hippocampal-neurogenesis dependent behaviours in young adult rats. (A) Overview of the Alzheimer’s-related behaviours. Vertical bars represent weekly intervals. Behaviours include: Open Field (OF), Elevated Plus Maze (EPM), Modified Spontaneous Location Recognition (MSLR), Novel Object Recognition (NOR), Novel Location Recognition (NLR), Morris Water Maze (MWM) and Forced Swim test (FST). (B) Top: Illustration of the MSLR task. Bottom: Alzheimer’s-FMT rats displayed a significant reduction in the discrimination index in the large separation task, two-tailed Student’s t-test, *P = 0.0353 and small separation task, Mann-Whitney U-test, *P = 0.0108. (C) Left: Representative tracings in platform trials in the MWM. Right: Escape latency during platform trials in MWM. No significant differences in learning were detected during the acquisition training days in the MWM test, two-way mixed ANOVA, effect of FMT: P = 0.2148, effect of time: ***P < 0.0001, FMT × Time interaction: P = 0.6672. (D) When challenged in the probe trial, Alzheimer’s-FMT rats spent significantly less time in the target quadrant, unpaired, two-tailed Student’s t-test, *P = 0.0498. Average number of platform crossings was reduced in Alzheimer’s-FMT rats compared to control FMT rats, Mann-Whitney test, P = 0.0670. Alzheimer’s-FMT colonized rats displayed no significant change in the locomotory parameters of the probe trial, as determined by average swim speed, unpaired, two-tailed Student’s t-test, P = 0.1582 and path length, unpaired, two-tailed Student’s t-test, P = 0.2246. (E) Top: Illustration of the NOR test. Bottom: In the training session, no significant difference was detected between the differentiation index of the two identical objects in the control FMT rats, two tailed paired Student’s t-test, P = 0.9859 and Alzheimer’s-FMT rats, P = 0.4149. In the test session, Alzheimer’s-FMT rats show a significant reduction in the discrimination index of the novel objects compared to control FMT rats, unpaired, two-tailed Student’s t-test, **P = 0.0019. (F) Top: Illustration of the NOL test. Bottom: In the training sessions, no significant difference was detected between the differentiation index of the two identical locations in the control FMT rats, two tailed paired Student’s t-test, P = 0.4244 and Alzheimer’s-FMT rats, P = 0.8267. In the test session, Alzheimer’s-FMT rats show no significant reduction in the discrimination index of the novel location compared to control FMT rats, unpaired, two-tailed Student’s t-test, P = 0.4032. (G) Spearman’s rank and Pearson correlation between rat behaviour and human donor metadata. Heat map showing Spearman p (rho) or Pearsons R correlation coefficients, with red indicating strong positive correlation, and blue indicating strong negative correlation. P-values for significant correlations (α<0.05) are noted. All data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001. AD = Alzheimer’s disease; BMI = body mass index; CDR = Clinical Dementia Rating; FMT= faecal microbiota transplantation; MMSE = Mini-Mental State Examination; NS = not significant.
Given previous reports in Alzheimer’s patients and transgenic animal models of Alzheimer’s disease that impairments of AHN contribute to memory deficits in Alzheimer’s disease,18 we employed the modified spontaneous location recognition (MSLR) task to assess pattern separation (Fig. 3B), which has been shown to be reliant on AHN.57 We found that rats harbouring human Alzheimer’s microbiota were significantly impaired in discriminating between the familiar and novel locations in the ‘large separation condition’ (low cognitive load on pattern separation) and ‘small separation condition’ (high cognitive load on pattern separation) (Fig. 3B). Memory performance was consistently affected in a number of other cognitive tasks (Fig. 3C–F), which have also been shown to be dependent on AHN.58-60 In the Morris Water Maze (MWM) task (Fig. 3C), humanized Alzheimer’s colonized rats spent significantly less time in the target quadrant during the probe trial than control FMT-treated rats, indicating impairments in long-term spatial memory (Fig. 3D). Consistent with Alzheimer’s-relevant cognitive deficits, we also found that rats colonized with material from Alzheimer’s donors performed significantly worse in a novel recognition memory task (Fig. 3E) and failed to discriminate the novel location in the novel location recognition test (Fig. 3F).
These observations were further corroborated by correlating the clinical human donor profile to the Alzheimer’s behavioural readouts of the recipient rats. We found an inverse correlation between the discrimination index of the MSLR (large separation) and the CDR of the human donors (Fig. 3G), supporting the hypothesis that dementia donor-specific microbiota changes impact on cognitive function. Overall, our results demonstrate that the presence of human gut microbiota from Alzheimer’s patients promotes cognitive deficits related to the clinical symptoms of Alzheimer’s disease and impairs, in particular, AHN-dependent memory performance in recipient rats.
Transplantation of gut microbiota from Alzheimer’s patients decreased neurogenesis in adult rats
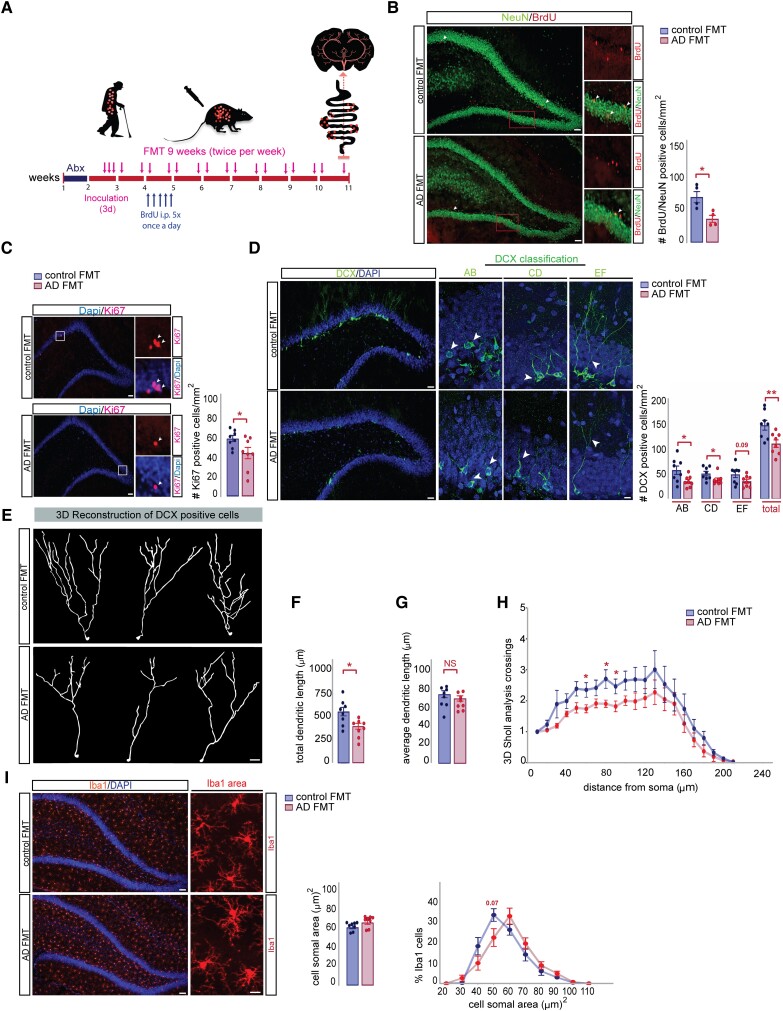
Based on these results and given the well documented role of the dentate gyrus (DG) in supporting pattern separation, we assessed AHN in the rat DG after human FMT (Fig. 4A). We measured the survival of new neurons in the DG by performing BrdU labelling and co-localization with the neuronal marker NeuN 6 weeks after injection of BrdU (Fig. 4B). A significant decrease in the number of BrdU/NeuN positive cells in the DG of rats colonized with Alzheimer’s faecal material compared to control recipient rats was evident, indicating reduced survival of new neurons in the DG (Fig. 4B). In line with these findings, we observed that the number of Ki67 (Fig. 4C) and DCX-positive cells was significantly lower in Alzheimer’s colonized rats (Fig. 4D). This density reduction was specific to AB-type (proliferative, short or no dendrites) and CD-type (intermediate, dendrites reaching molecular layer) cells but not EF-type (postmitotic, multi-branched dendrites) cells.61
Figure 4.
Transplantation of gut microbiota from Alzheimer’s patients decreased neurogenesis and dendritogenesis of adult-born hippocampal neurons in rats. (A) Experimental timeline showing the effect of FMT on the survival of newborn neurons and microglia activation: rats were injected with BrdU (150 mg/kg) once per day for 5 days, 10 days post donor colonization and sacrificed 6 weeks later at Day 59. (B) Left: Representative images of BrdU/NeuN positive cells in DG of control and Alzheimer’s-FMT rats. Scale bars = 200 µm (left magnification), 50 µm (right magnification). Newborn neurons were analysed using double labelling for BrdU (red) and the neuronal marker NeuN (green). Arrowheads indicate double-positive cells (orange). Right: Alzheimer’s-FMT rats show a significant reduction in the number of BrdU/NeuN cells, two-tailed Student’s t-test, *P = 0.0159. (C) Left: Representative images of Ki67 positive cells in DG of control and Alzheimer’s-FMT rats. Scale bars = 200 µm (left magnification), 50 µm (right magnification). Right: A significant reduction in the number of Ki67 positive cells in rats after FMT from Alzheimer’s disease, two-tailed Student’s t-test, *P = 0.0411. (D) Left: Representative images of AB, CD and EF types of DCX-positive cells classified based on their dendritic tree morphology. Scale bars = 200 µm (left magnification), 40 µm (right magnification). Right: Alzheimer’s-FMT rats show a significant reduction in the number of AB type, two-tailed Student’s t-test, *P = 0.0290; CD type, Mann-Whitney test, *P = 0.0281 and total number of DCX-positive cells in the DG, two-tailed Student’s t-test, **P = 0.0099. (E) Representative 3D reconstructions of DCX-positive cells from the DG of young adult rats after control and Alzheimer’s-FMT. Scale bar = 20 μm. (F) Morphological analysis revealed a significant decrease in the total dendritic length of DCX-positive cells in Alzheimer’s-FMT treated rats, two-tailed Student’s t-test, *P = 0.0124 (G) The average dendritic length of DCX-positive cells was unaltered between control and Alzheimer’s-FMT rats, two-tailed Student’s t-test, P = 0.3533. (H) Sholl analysis revealed a reduction in dendritic complexity of DCX-positive cells in Alzheimer’s-FMT rats compared to control FMT rats, two-way mixed ANOVA, effect of FMT: *P = 0.0452, effect of distance from soma: ***P < 0.0001, FMT × Distance interaction: P = 0.9358. (I) Left: Representative images of Iba1 positive cells in the DG of control and Alzheimer’s-FMT rats. Scale bars = 200 µm (left magnification), 40 µm (right magnification). Right: Quantification of cell soma area of Iba1-positive cells in the DG of control and Alzheimer’s-FMT rats, Mann-Whitney test, P = 0.1605. Distribution analysis of soma area shows a shift from small to larger cell body sizes in Alzheimer’s-FMT colonized rats in comparison to control FMT colonized rats. Two-way mixed ANOVA, effect of FMT: P = 0.3154, effect of soma area: ***P < 0.0001, FMT × Soma area interaction: *P = 0.0240. All data are presented as mean ± SEM, *P < 0.05, **P < 0.01. AD = Alzheimer’s disease; DG = dentate gyrus; FMT = faecal microbiota transplantation; NS = not significant.
New neurons produced in the hippocampus of the DG have been shown to functionally integrate into mature circuits and contribute to the ability to recall memories. One requirement, however, for the successful synaptic integration and subsequent functionality of these neurons is the dendritic sprouting and proper arborization into the molecular layer of the DG.62 To gain insight into the mechanism that may contribute to the observed memory deficits in Alzheimer’s colonized rats, we carried out 3D-reconstruction of DCX-positive cells using Sholl analysis to evaluate the complexity of their dendritic trees (Fig. 4E). Compared to control colonized rats, DCX labelled cells in Alzheimer’s-FMT treated rats displayed a significant reduction in the total dendritic length, whereas the average dendritic length per neuron was unaltered (Fig. 4F and G). When we quantified the number of dendritic intersections, we observed a significant reduction of dendritic complexity in Alzheimer’s colonized rats compared to control recipient rats (Fig. 4H). These data indicate that gut microbiota transferred from Alzheimer’s disease negatively affect the survival and dendritic arborization of adult-born neurons into the molecular layer of the DG of the rat.
Rats harbouring gut microbiota from Alzheimer’s patients did not show significant differences in microglia density in the DG (Supplementary Fig. 7A) and showed only minor differences in Iba1 somal size (Fig. 4I and Supplementary Fig. 7B) compared to control FMT colonized rats, suggesting that neuroinflammatory processes in this brain region may have a minimal role in the cognitive impairments observed in recipient rats colonized with microbiota from Alzheimer’s patients. To examine whether the impaired cognitive function in Alzheimer’s colonized rats might be related to plaque formation, we performed Thioflavin-S fluorescent microscopy of coronal brain sections, which revealed the absence of plaque deposition in the hippocampus and cortex in both groups (Supplementary Fig. 7C and D). Overall, these data reinforce previous findings in post-mortem human brains that cognition and AHN is already altered before extracellular amyloid deposition.18
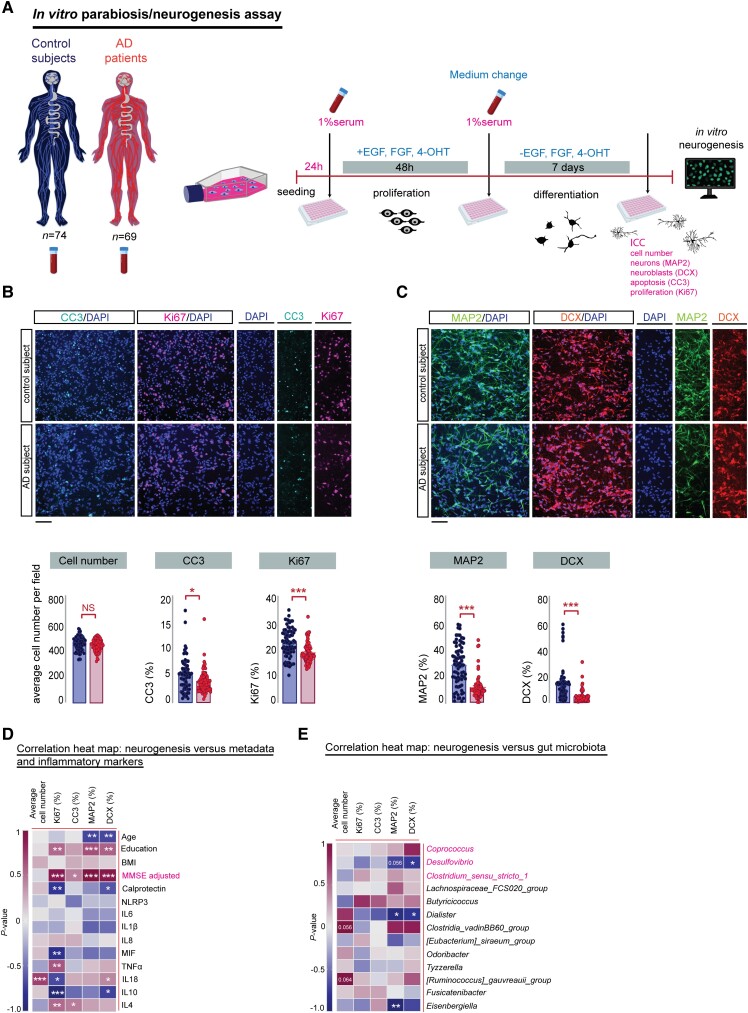
Serum from Alzheimer’s patients decreased neurogenesis in human hippocampal progenitor cells in vitro
Since the hippocampal neurogenic niche is highly vascularized63-65 and gut microbiota composition explains up to 58% of the variance of individual plasma metabolites,66 Alzheimer’s gut microbiota-induced changes in the systemic environment, mediated by blood, have the potential to modulate AHN. Given the inaccessibility of the DG and the absence of validated biomarkers and neuroimaging tools to quantify hippocampal neurogenesis in living humans, we used our in vitro neurogenesis assay to study how the Alzheimer’s systemic environment modulates hippocampal neurogenesis67-70 (Fig. 5). To that end, embryonic human hippocampal progenitor cells (HPCs) were exposed to serum from control subjects and Alzheimer’s patients (Fig. 5A) and the percentage of cells expressing markers for neural stem cell proliferation (Ki67), differentiation (MAP2, DCX), and programmed cell death (CC3) was determined.71 Our results show that, while the average cell density of control and Alzheimer’s serum exposed HPCs cells remained unaffected (Fig. 5B), a reduction in the expression of Ki67 positive cells (Fig. 5B) in response to Alzheimer’s serum occurred, indicating that the serum of Alzheimer’s patients decreases the proliferative capacity of differentiating HPCs. Furthermore, following 7 days of neuronal differentiation, we observed a decrease in the percentage of MAP2 and DCX positive neuroblasts in Alzheimer’s serum-treated cells (Fig. 5C), suggesting that the serum of Alzheimer’s patients impaired neuronal differentiation. Similar to previous reports using an Alzheimer’s disease transgenic mouse model,72 we found that neuronal HPCs treated with Alzheimer’s serum displayed a substantially different morphology, characterized by an increase in the total neurite length and highly increased branching points in both MAP2 and DCX-positive cells (Supplementary Fig. 8B and C) compared to HPCs exposed to control serum. Overall, these data highlight that the systemic, circulatory environment of Alzheimer’s patients is capable of affecting neuronal proliferation, differentiation and dendritic morphology.
Figure 5.
Serum from Alzheimer’s patients decreased neurogenesis in human hippocampal progenitor cells. (A) Experimental timeline of the in vitro parabiosis/neurogenesis assay: human hippocampal progenitor cells (HPCs) were cultured with 1% human serum from age-matched cognitively healthy and Alzheimer’s patients. Cellular readouts are expressed as percentage relative to neural cell number. Fifteen fields were analysed per well, n = 3 biological replicates. (B) Top: Representative confocal micrographs showing CC3-positive HPCs cells (green: CC3-positive cells, blue: DAPI) and Ki67-positive HPCs cells (red: DCX-positive cells, blue: DAPI) during the differentiation phase of the assay. Scale bar = 100 μm. Bottom: No significant change was detected in the average number of HPCs per field between control and Alzheimer’s patients during the differentiation phase of the human serum assay, unpaired, two-tailed Student’s t-test, P = 0.1855. Staining for the apoptotic marker CC3 revealed a significant decrease in cell death in HPCs cells receiving serum from Alzheimer’s patients compared to controls, Mann-Whitney test, *P = 0.0100. Serum from Alzheimer’s patients induced a significant reduction in the expression level of the proliferation cell marker Ki67, Mann-Whitney test, ***P < 0.0001. (C) Top: Immunofluorescence staining of MAP2-positive HPCs cells (green: MAP2-positive cells, blue: DAPI) and DCX-positive HPCs cells (red: DCX-positive cells, blue: DAPI) during the differentiation phase of the assay. Scale bar = 100 μm. Bottom: Serum from Alzheimer’s patients caused a significant reduction in the expression levels of neurons (MAP2), Mann-Whitney test, ***P < 0.0001 and neuroblasts (DCX), Mann-Whitney test, ***P < 0.0001. (D) Spearman’s rank correlation between in vitro cellular neurogenesis readouts, human donor metadata and serum inflammatory markers. Correlation heat maps showing Spearman’s rank coefficients, with red indicating strong positive correlation and blue indicating strong negative correlation. (E) Spearman correlation between in vitro cellular neurogenesis readouts and human donor gut microbiota. P-values for significant correlations (α < 0.05) are noted. Control serum n = 74, Alzheimer’s serum n = 69. All data are presented as mean ± SEM. Panel (A) was created with BioRender.com. *P < 0.05, **P < 0.01, ***P < 0.001. AD = Alzheimer’s disease; ICC = immunocytochemistry.
Notably, we could again link these observations to the clinical phenotype of Alzheimer’s patients. We found a significant positive association between the MMSE score (Fig. 5D) and the prevalence of DCX, Ki67 and MAP2 cells. Additionally, in line with our previous findings in rats (Fig. 2E), the abundance of the parabiont genera Desulfovibrio and Dialister inversely correlated with the in vitro expression of the neuronal marker DCX (Fig. 5E). The association of specific bacterial genera with markers for neurogenesis, which are also highly correlated with the MMSE score (Fig. 2E), further support the hypothesis that impaired neurogenesis may be the converging link between the observed altered gut microbiota composition and cognitive impairment in Alzheimer’s disease.
Recipient rats transplanted with human Alzheimer’s gut microbiota show discrete metabolomic profiles
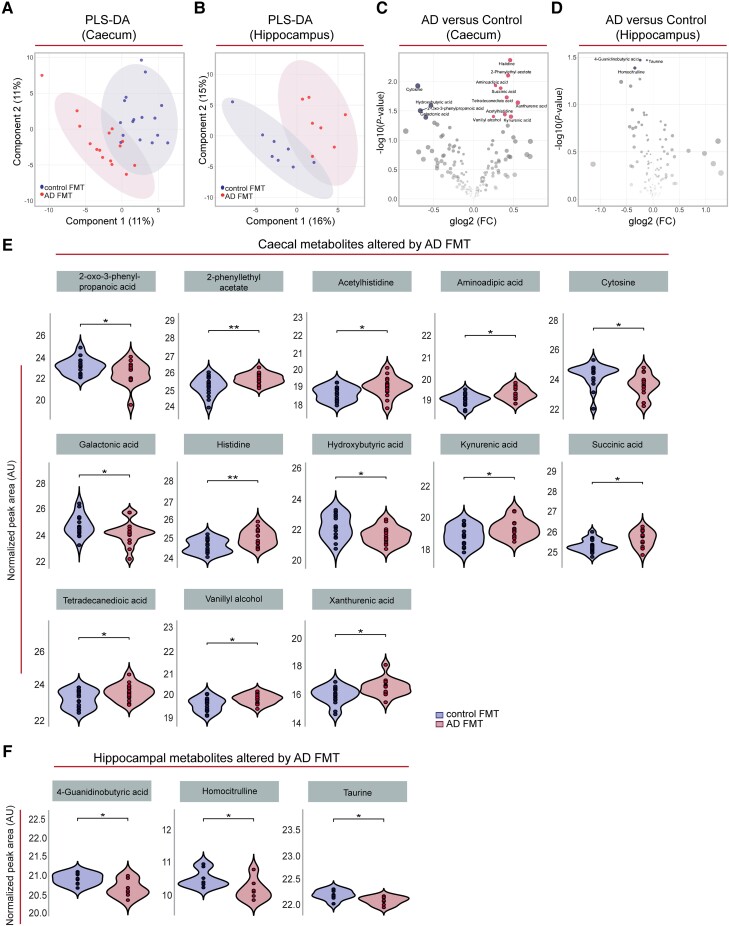
To better understand which factors may trigger the observed effects of gut microbiota transferred from Alzheimer’s patients on AHN and associated behaviours, we performed untargeted metabolomic analyses of caecal content and hippocampal tissue from control and Alzheimer’s colonized rats. Partial least squares discriminant analyses suggested an effect of donor status on the caecal (Fig. 6A) and hippocampal (Fig. 6B) metabolome. Indeed, differential expression analyses revealed that 13 (out of 184) metabolites in the caecal content (Fig. 6C and E) and three (out of 123) metabolites in the hippocampus (Fig. 6D and F) were nominally (P < 0.05) differentially abundant in Alzheimer’s-FMT compared to control FMT-colonized rats (for a full list of quantified features, see Supplementary material, Extended Table 4). It should be noted however that metabolic features in either dataset did not pass the 5% false discovery rate (FDR)-based correction for multiple testing.
Figure 6.
FMT from Alzheimer’s patients induced alterations in the caecal and hippocampal metabolomes of young adult rats. (A and B) Partial least squares discriminant analysis (PLS-DA) plot depicting the effect of control and Alzheimer’s-FMT on the caecal content (A) and hippocampal (B) metabolomes of young adult rats (with 95% concentration ellipses). Both models were non-significant (pQ2 > 0.05) by n = 1000 permutation tests. (C and D) Volcano plots of metabolites quantified in caecal content (n = 184 features) (C) and hippocampal tissue (n = 123 features) (D) of rats colonized with control and Alzheimer’s-FMT. Red dots represent metabolites nominally (P < 0.05) upregulated in Alzheimer’s-FMT rats, and blue dots represent metabolites nominally (P < 0.05) upregulated in control FMT rats. (E and F) Violin and box-and-whisker plot (median represented by horizontal line) of normalized peak area of the caecal (n = 15–16) and hippocampal (n = 7–8) metabolites differentially regulated between rats receiving human FMT from control subjects and Alzheimer’s patients, as depicted in C and D. *P < 0.05, **P < 0.01 (unadjusted P-values from moderated t-tests, Limma). Histidine, P = 0.0043; 2-phenylethyl acetate, P = 0.0079; aminoadipic acid, P = 0.012; cytosine, P = 0.012; succinic acid, P = 0.013; tetradecanedioic acid, P = 0.019; xanthurenic acid, P = 0.023; hydroxybutyric acid, P = 0.026; 2-oxo-3-phenylpropanoic acid, P = 0.031; acetylhistidine, P = 0.036; vanillyl alcohol, P = 0.039; kynurenic acid, P = 0.040; galactonic acid, P = 0.041. 4-guanidinobutyric acid, P = 0.034; taurine, P = 0.034; homocitrulline, P = 0.041. AD = Alzheimer’s disease; FC = fold change; FMT = faecal microbiota transplantation; glog2 = generalized logarithm base 2.
In caecal content, the amino acid histidine and its derivative acetylhistidine were nominally altered, in line with previous reports on peripheral metabolic profiles of both Alzheimer’s patients and transgenic Alzheimer’s disease mouse models.73-77 Increased levels of histidine may indicate perturbed homeostasis of histamine,73 which has been shown to influence AHN as well blood–brain barrier integrity.78 Additionally, histidine plays a critical role in the membrane binding and neurotoxicity of Aβ fibrils.79,80 Aminoadipic acid was found to be upregulated, and this has also been found in the plasma metabolome of people with Alzheimer’s and mild cognitive impairment compared to age- and sex-matched controls.81 Galactonic acid and succinic acid (nominally decreased and increased, respectively, in Alzheimer’s-FMT rats) were found in one study to be increased in the urine metabolome of presenilin double knockout mice, and this was correlated with gut microbiota composition changes.82 The tryptophan metabolites kynurenic acid and xanthurenic acid were upregulated in Alzheimer’s recipient rats, which have been found to be altered in Alzheimer’s serum, urine and CSF.83 Increases in faecal concentration of the carboxylate tetradecanedioic acid, found upregulated here, have been closely linked to ageing and the prediction of neurocognitive disorders.84,85 We also detected decreased levels of the metabolite hydroxybutyrate. Beta-hydroxybutyrate mitigates against the pathogenesis of Alzheimer’s disease through various mechanisms, including through mitochondrial metabolism, metabolism of Aβ and tau proteins, inhibition of inflammation, promotion of AHN and through the regulation of cognitive function.38,86
While many of the above metabolites can cross the blood–brain barrier, they were not differentially abundant in the hippocampus. In the hippocampus, 4-guanidinobutyric acid and taurine were nominally downregulated. These metabolites were similarly found to be decreased in an age-dependent manner in the hippocampi of Alzheimer’s disease (3xTg) model mice.87 Critically, taurine has been found to regulate adult88 and developmental89 AHN, as evidenced both in vivo and in vitro. Homocitrulline was also found to be downregulated. Interestingly, (homo)citrullination of Alzheimer’s-related proteins in the human medial temporal lobe proteome has been found to be altered in early Alzheimer’s disease compared to healthy age-matched controls.90 Additionally, citrulline is increased in the plasma of people with Alzheimer’s91 and the hippocampus of Alzheimer’s disease model (3xTg) mice.92
Discussion
In this study, we demonstrate that the transplantation of human gut microbiota from Alzheimer’s patients is sufficient to produce core cognitive symptoms of Alzheimer’s disease coupled with an impairment in AHN, in healthy young adult rats. Moreover, application of human Alzheimer’s disease serum provoked an impairment in AHN in human cells in vitro, supporting AHN as a converging cellular process regulating systemic circulatory and gut-mediated factors in Alzheimer’s disease.
The results of the present study confirm that Alzheimer’s disease is characterized by systemic and gut inflammation.29,93 Moreover, the decrease in abundance of the phyla Firmicutes and the increase in Bacteroidetes observed in Alzheimer’s patients compared with age-matched cognitively healthy subjects is in line with several findings in US35 and Chinese populations.34,36 At genera level and in agreement with our findings, reduced abundance of Clostridium sensu stricto 1 is associated with adverse outcomes in Alzheimer’s disease.33,35 The parabiont Desulfovibrio has also been found to be enriched in other Alzheimer’s cohorts33,94 and is associated with reduced caecal levels of SCFAs and with inflammation in mice.95 As previously reported, we found a lower proportion of bacteria with the potential to synthetize butyrate,33,94 a microbial metabolite negatively associated with cortical amyloid accumulation.96 Furthermore, we confirmed reduced abundance of the genus Coprococcus in Alzheimer’s disease,33 which is associated with amyloid accumulation.97 When interpreting microbiota data however, it is important to keep in mind that results may be influenced by geography,98 the inclusion criteria of study participants and methodological difference (i.e. sample collection and processing, freeze-thaw, storage methods, different bioinformatics pipelines).99,100 Not surprisingly, human faeces from different donors engrafted in rats at different rates. Notwithstanding, transfer of faecal microbiota from cognitively healthy subjects to rats resulted in the taxa diversity remaining relatively stable over time, whereas after transfer from Alzheimer’s donors, there was a greater alteration in taxa between 10 and 59 days post-FMT. Notably, one of the genera that was increased at Day 59 post Alzheimer’s-FMT compared to Day 10 was Desulfovibrio, a genus that was also significantly enriched in Alzheimer’s participants. It should be noted that healthy and Alzheimer’s disease FMT donors were not balanced by biological sex, a factor that may influence the gut microbiota.101 Although no biological sex-associated differences emerged in the gut microbiota analysis (Supplementary Fig. 9), future research investigating the role of biological sex in gut microbiota diversity in the context of Alzheimer’s disease is necessary. Factors such as diet, physical activity, stress and other environmental influences, such as medication use, can also influence the composition of the gut microbiota. Thus, comprehensive assessments in future studies of health status, lifestyle factors, medication history and other factors, which specifically shape the gut microbiota, will inform potential implications for disease progression, management and the development of strategies to prevent or delay cognitive decline through modulation of the gut microbiota.
To determine whether the changes in the gut microbiota composition in Alzheimer’s patients precipitate symptoms associated with the disease, we performed a series of behavioural tests on young adult rats colonized with faecal material from cognitively healthy or Alzheimer’s subjects. We found a reduction in pattern separation, a hippocampal neurogenesis-reliant memory process, which is frequently reported to be impaired in Alzheimer’s disease15,17 and is correlated with indicators of pathology, such as increased cortical Aβ burden,102 medial temporal lobe tau,103 CSF levels of phosphorylated tau,104 and apolipoprotein E genotype.105 In addition, we demonstrated that Alzheimer’s-FMT induced impairment in long-term spatial memory in the MWM, which is also associated with AHN levels.58 This is in line with a recent intra-species report using transfer of a faecal sample from the 5xFAD mouse model of Alzheimer’s disease into wild-type recipient mice.39 We further demonstrate that recognition memory was impaired in Alzheimer’s-FMT rats, which agrees with previous work showing an impairment in germ-free mice that received FMT from one Alzheimer’s patient.106 While alterations in the gut microbiome have been associated with altered behaviour, such as hippocampal-dependent cognition during ageing,33 there is no evidence to date of transfer of Alzheimer’s-associated cognitive behaviour to a healthy young organism via gut microbiota to confirm a causal role of gut microbiota in Alzheimer’s disease, such as we have shown here.
Interestingly, the memory impairments we observed in young adult rats induced by gut microbiota from Alzheimer’s patients are reliant on AHN. Our analysis of AHN in recipient rats revealed that the survival of new neurons and the number of newly born neurons was decreased after Alzheimer’s-FMT, which supports our behavioural observations. We also found that rats humanized with Alzheimer’s-FMT displayed an impairment in the morphological development of these newborn neurons, rendering a delay or a defect in this critical process of enabling functional integration into hippocampal circuits.107,108 In line with our findings, the dendritic complexity of immature neurons was deficient in a familial Alzheimer’s mouse model109 and intra-species FMT (5xFAD to wild-type mice) reduced the survival and proliferation of neurons.39 It has recently been reported that reconstitution of gut microbiota in antibiotic-treated newborn mice via FMT prevented the decrease in AHN and cognitive deficits in later life in these mice,110 suggesting that AHN is a cellular target modifiable by gut microbiota for potential therapeutic gain. Coupled with mounting evidence of a role for the microbiota–gut–brain axis in regulating AHN, and that impairments in AHN contribute to Alzheimer’s symptoms,19 our results highlight AHN as a critical process mediating cognitive changes impacted by gut microbiota in Alzheimer’s disease.
In line with our findings in rats in vivo, we observed decreased neurogenesis in human-derived HPCs treated with blood serum from Alzheimer’s patients; we found a lower level of DCX-positive neuroblasts, Map2-positive immature neurons and proliferating HPCs. These findings are in agreement with studies that report fewer immature neurons in Alzheimer’s patients compared to cognitively healthy controls.18,111-113 Intriguingly, we previously found that an increased baseline cell death during differentiation predicts cognitive decline over the subsequent 12 years in cognitively healthy subjects,68 whereas, here, we observed a decrease in cell death after treatment with Alzheimer’s serum, suggesting that there is a differential effect of Alzheimer’s serum on cell death during the disease course. Neurite outgrowth is a critical step in the formation of new neurons and integration into the hippocampal circuitry.114 When we examined how the Alzheimer’s systemic environment influences neurite outgrowth in vitro, complexity measures were increased after Alzheimer’s serum treatment. Our findings are in line with a study that showed enhanced neurite outgrowth and arborization in a primary neuronal culture from transgenic Drosophila melanogaster larvae expressing human Aβ42 compared to control,115 which occurred prior to the Alzheimer’s pathology. Surprisingly, our findings of longer and more complex neurites under Alzheimer’s serum conditions in vitro contrasts with our observations in the rat DG. One of the reasons for the opposing finding in the Alzheimer’s-FMT adult rat HPCs and the Alzheimer’s-serum treated embryonic human HPCs in vitro could be the difference in maturation rates of HPCs between immortalized foetal human HPCs and adult rat HPCs. It has been demonstrated that adult HPCs exhibit various molecular and cellular properties that differ from those present in developmental HPCs including more restricted multipotency, acquisition of a quiescence state and different transcriptomic programmes underlying those cellular processes.116 When we investigated the association between in vitro neurogenic readouts and the gut microbiota composition of the serum donors, we found that the percentage of DCX-positive neuroblasts inversely correlated with parabiont genera Desulfovibrio and Dialister, and the percentage of Map2-positive cells inversely correlated with genera Desulfovibrio, Dialister and Eisenbergiella. This is interesting considering a previously published observation that genus Eisenbergiella increases with severity of cognitive impairment117 and that genus Desulfovibrio was negatively correlated with MMSE scores.118,119 Furthermore, Desulfovibrio has been found to be increased by a Western style diet and by a high fat diet but decreased by endurance exercise,120,121 in mice suggesting susceptibility to lifestyle factors on its relative abundance in the gut. Conversely, the relative abundance of the parabiont genus Eisenbergiella has been reported to be decreased by a Western style diet122 and increased in a model of stress in rats123 as well as in adult male athletes124 indicating a complex relationship between lifestyle factors and the host microbiome, which may influence cognitive function and the risk of cognitive decline, possibly through changes in hippocampal neurogenesis. In a previous study involving cognitively healthy individuals, we demonstrated the association between altered apoptosis, reduced HPC integrity, and lifestyle factors such as exercise and diet. These findings were further linked to future cognitive decline and dementia.68 Additionally, we recently demonstrated the predictive value of neurogenesis assay readouts, combined with years of education, in identifying individuals at risk of developing Alzheimer’s disease before clinical diagnosis.125 Future studies will aim to validate and expand upon these observed correlations between neurogenesis assay readouts and specific microbiota genera, by investigating the underlying mechanisms by which these genera influence neurogenesis and their potential implications for Alzheimer’s disease pathogenesis. Longitudinal studies are also warranted to assess whether these correlations persist over time and to determine if they have any predictive value for Alzheimer’s disease development or progression. Finally, intervention studies targeting specific microbiota genera could help elucidate the causal relationship between the microbiota, neurogenesis and Alzheimer’s disease, potentially opening avenues for novel therapeutic approaches.
The composition of the human gut microbiota has been found to account for a significant amount of variation in individual circulating metabolite levels.66 We found alterations in the caecal and hippocampal metabolomes of young adult rats colonized with Alzheimer’s-FMT compared to control FMT. The majority occurred in the caecum, but these were not reflected in the hippocampus, suggesting they may not directly influence AHN. The limited amount of differential expression and low effect sizes may be due to the variable levels of engraftment (i.e. by donor and genera) and reversion of microbial signatures with time, and thus did not allow for pathway analyses. Notably, however, the free amino acid taurine was decreased in the hippocampus of Alzheimer’s-FMT rats. Taurine administration has repeatedly been shown to increase hippocampal neural stem and progenitor cell proliferation, survival and neurogenesis both in vivo and in vitro.88 Given its blood–brain barrier permeability,126 it is conceivable that some of the observed effects of Alzheimer’s-FMT on AHN could partly be mediated via taurine. The current data suggest that microbial metabolites could impact both cognitive function and Alzheimer’s pathology, possibly linking specific microbial signatures to the observed changes in hippocampal neurogenesis and associated cognitive behaviours. Future studies could incorporate metabolomic analyses of both donor and recipient sera to investigate gut microbiota-metabolite associations moderated by case-control status.
In conclusion, our results demonstrate that colonization of healthy young adult rats with gut microbiota from Alzheimer’s patients induced behavioural and neurogenic alterations typical of Alzheimer’s disease. We show that the expression of caecal metabolites involved in the neurogenic and cognitive function are altered after FMT from Alzheimer’s patients, and report a direct and negative impact of serum from Alzheimer’s patients on neurogenesis in vitro. Overall, our findings reveal that Alzheimer’s symptoms can be transferred to a healthy young organism via the gut microbiota, confirming a causal role of gut microbiota in Alzheimer’s disease. Furthermore, AHN is established as a converging central cellular process for cognitive changes influenced by both systemic circulatory and gut-mediated factors in Alzheimer’s disease.
Supplementary Material
Acknowledgements
We thank Tara Foley, Gerry Moloney, Patrick Fitzgerald, Suzanne Crotty and Anna Golubeva for technical assistance. We thank Samantha Saleri for assistance with analysis of microbiota.
Contributor Information
Stefanie Grabrucker, Department of Anatomy and Neuroscience, University College Cork, Ireland; APC Microbiome Ireland, University College Cork, Ireland.
Moira Marizzoni, Biological Psychiatry Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy; Laboratory of Neuroimaging and Alzheimer’s Epidemiology, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy.
Edina Silajdžić, Department of Basic and Clinical Neuroscience, Institute of Psychiatry, King’s College London, SE5 9NU London, UK.
Nicola Lopizzo, Biological Psychiatry Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy; Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy.
Elisa Mombelli, Biological Psychiatry Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy.
Sarah Nicolas, Department of Anatomy and Neuroscience, University College Cork, Ireland; APC Microbiome Ireland, University College Cork, Ireland.
Sebastian Dohm-Hansen, Department of Anatomy and Neuroscience, University College Cork, Ireland; APC Microbiome Ireland, University College Cork, Ireland; INFANT Research Centre, University College Cork, T12 DC4A Cork, Ireland.
Catia Scassellati, Biological Psychiatry Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy.
Davide Vito Moretti, Alzheimer Unit, IRCCS Fatebenefratelli, Brescia, Italy.
Melissa Rosa, Biological Psychiatry Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy.
Karina Hoffmann, Department of Basic and Clinical Neuroscience, Institute of Psychiatry, King’s College London, SE5 9NU London, UK.
John F Cryan, Department of Anatomy and Neuroscience, University College Cork, Ireland; APC Microbiome Ireland, University College Cork, Ireland.
Olivia F O’Leary, Department of Anatomy and Neuroscience, University College Cork, Ireland; APC Microbiome Ireland, University College Cork, Ireland.
Jane A English, Department of Anatomy and Neuroscience, University College Cork, Ireland; INFANT Research Centre, University College Cork, T12 DC4A Cork, Ireland.
Aonghus Lavelle, Department of Anatomy and Neuroscience, University College Cork, Ireland; APC Microbiome Ireland, University College Cork, Ireland.
Cora O’Neill, APC Microbiome Ireland, University College Cork, Ireland; School of Biochemistry and Cell Biology, BioSciences Institute, University College Cork, T12 YT20 Cork, Ireland.
Sandrine Thuret, Department of Basic and Clinical Neuroscience, Institute of Psychiatry, King’s College London, SE5 9NU London, UK.
Annamaria Cattaneo, Biological Psychiatry Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy; Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy.
Yvonne M Nolan, Department of Anatomy and Neuroscience, University College Cork, Ireland; APC Microbiome Ireland, University College Cork, Ireland.
Data availability
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Funding
This project was funded by Science Foundation Ireland (SFI) for S.G., S.N., S.D.-H., J.E., A.L., C.O’N. and Y.N. (SFI 17/COEN/3475 and SFI 19/FFP/6820), the Italian Ministry of Health for M.M., N.L., E.M., C.S., D.V.M., M.R. and A.C., and the Medical Research Council (MRC) UK for E.S., K.H. and S.T. (MR/S00484X/1) under the Network of Centres of Excellence in Neurodegeneration initiative.
Competing interests
Y.M.N. and O.F.O. have received funding from Marigot Limited. O.F.O. has received funding for unrelated research from Alkermes plc. J.F.C. has received research funding from Mead Johnson, Cremo, Nutricia, Pharmavite, Reckitt, and DuPont; and has served as a consultant for Nestle. This support neither influenced nor constrained the contents of this article. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. [DOI] [PubMed] [Google Scholar]
- 2. Gaugler J, James B, Johnson T, Scholz K, Weuve J. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509.27570871 [Google Scholar]
- 3. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iqbal K, del Alonso AC, Chen S, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. [DOI] [PubMed] [Google Scholar]
- 6. Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306–318. [DOI] [PubMed] [Google Scholar]
- 7. Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. [DOI] [PubMed] [Google Scholar]
- 8. Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. [DOI] [PubMed] [Google Scholar]
- 9. Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7:a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kozareva DA, Cryan JF, Nolan YM. Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell. 2019;18:e13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garthe A, Kempermann G. An old test for new neurons: Refining the morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci. 2013;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahay A, Scobie KN, Hill AS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levone BR, Cryan JF, O’Leary OF. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol Stress. 2015;1:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern separation and pattern completion in Alzheimer’s disease: Evidence of rapid forgetting in amnestic mild cognitive impairment. Hippocampus. 2013;23:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herbert J, Lucassen PJ. Depression as a risk factor for Alzheimer’s disease: Genes, steroids, cytokines and neurogenesis—What do we need to know? Front Neuroendocrinol. 2016;41:153–171. [DOI] [PubMed] [Google Scholar]
- 17. Parizkova M, Lerch O, Andel R, et al. Spatial pattern separation in early Alzheimer’s disease. J Alzheimers Dis. 2020;76:121–138. [DOI] [PubMed] [Google Scholar]
- 18. Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–560. [DOI] [PubMed] [Google Scholar]
- 19. Salta E, Lazarov O, Fitzsimons CP, Tanzi R, Lucassen PJ, Choi SH. Adult hippocampal neurogenesis in Alzheimer’s disease: A roadmap to clinical relevance. Cell Stem Cell. 2023;30:120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellenguez C, Küçükali F, Jansen IE, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54:412–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eiser AR, Fulop T. Alzheimer’s disease is a multi-organ disorder: It may already be preventable. J Alzheimers Dis JAD. 2023;91:1277–1281 . [DOI] [PubMed] [Google Scholar]
- 22. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–666. [DOI] [PubMed] [Google Scholar]
- 23. Ganz T, Fainstein N, Ben-Hur T. When the infectious environment meets the AD brain. Mol Neurodegener. 2022;17:53–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. [DOI] [PubMed] [Google Scholar]
- 25. Donoso F, Cryan JF, Olavarría-Ramírez L, Nolan YM, Inflammation CG. Lifestyle factors, and the microbiome-gut-brain axis: Relevance to depression and antidepressant action. Clin Pharmacol Ther. 2023;113:246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gubert C, Kong G, Renoir T, Hannan AJ. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol Dis. 2020;134:104621. [DOI] [PubMed] [Google Scholar]
- 27. Cowan CSM, Cryan JF. The microbiome-gut-brain axis in neurocognitive development and decline. Mod Trends Psychiatry. 2021;32:12–25. [DOI] [PubMed] [Google Scholar]
- 28. Heijtz RD, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. [DOI] [PubMed] [Google Scholar]
- 30. Connell E, Le Gall G, Pontifex MG, et al. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol Neurodegener. 2022;17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo M, Peng J, Huang X, Xiao L, Huang F, Zuo Z. Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis. 2021;80:299–310. [DOI] [PubMed] [Google Scholar]
- 32. Li B, He Y, Ma J, et al. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement J Alzheimers Assoc. 2019;15:1357–1366. [DOI] [PubMed] [Google Scholar]
- 33. Ling Z, Zhu M, Yan X, et al. Structural and functional dysbiosis of fecal Microbiota in Chinese patients with Alzheimer’s disease. Front Cell Dev Biol. 2020;8:634069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu P, Wu L, Peng G, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019;80:633–643. [DOI] [PubMed] [Google Scholar]
- 35. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhuang ZQ, Shen LL, Li WW, et al. Gut Microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63:1337–1346. [DOI] [PubMed] [Google Scholar]
- 37. Adewuyi EO, O’Brien EK, Nyholt DR, Porter T, Laws SM. A large-scale genome-wide cross-trait analysis reveals shared genetic architecture between Alzheimer’s disease and gastrointestinal tract disorders. Commun Biol. 2022;5:691–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang F, Gu Y, Xu C, et al. Transplantation of fecal microbiota from APP/PS1 mice and Alzheimer’s disease patients enhanced endoplasmic reticulum stress in the cerebral cortex of wild-type mice. Front Aging Neurosci. 2022;14:858130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim N, Jeon SH, Ju IG, et al. Transplantation of gut microbiota derived from Alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav Immun. 2021;98:357–365. [DOI] [PubMed] [Google Scholar]
- 40. Dohm-Hansen S, Donoso F, Lucassen PJ, Clarke G, Nolan YM. The gut microbiome and adult hippocampal neurogenesis: A new focal point for epilepsy? Neurobiol Dis. 2022;170:105746. [DOI] [PubMed] [Google Scholar]
- 41. Guzzetta KE, Cryan JF, O’Leary OF. Microbiota-Gut-Brain axis regulation of adult hippocampal neurogenesis. Brain Plast Amst Neth. 2022;8:97–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sarubbo F, Cavallucci V, Pani G. The influence of gut Microbiota on neurogenesis: Evidence and hopes. Cells. 2022;11:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gheorghe CE, Ritz NL, Martin JA, Wardill HR, Cryan JF, Clarke G. Investigating causality with fecal microbiota transplantation in rodents: Applications, recommendations and pitfalls. Gut Microbes. 2021;13:1941711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Staley C, Kaiser T, Beura LK, et al. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome. 2017;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Golubeva AV, Joyce SA, Moloney G, et al. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine. 2017;24:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kozareva DA, Hueston CM, Ó’Léime CS, et al. Absence of the neurogenesis-dependent nuclear receptor TLX induces inflammation in the hippocampus. J Neuroimmunol. 2019;331:87–96. [DOI] [PubMed] [Google Scholar]
- 48. Nicolas S, McGovern AJ, Hueston CM, et al. Prior maternal separation stress alters the dendritic complexity of new hippocampal neurons and neuroinflammation in response to an inflammatory stressor in juvenile female rats. Brain Behav Immun. 2022;99:327–338. [DOI] [PubMed] [Google Scholar]
- 49. Liu S, Gao J, Zhu M, Liu K, Zhang HL. Gut Microbiota and dysbiosis in Alzheimer’s disease: Implications for pathogenesis and treatment. Mol Neurobiol. 2020;57:5026–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bettcher BM, Tansey MG, Dorothée G, Heneka MT. Peripheral and central immune system crosstalk in Alzheimer disease—A research prospectus. Nat Rev Neurol. 2021;17:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marizzoni M, Mirabelli P, Mombelli E, et al. A peripheral signature of Alzheimer’s disease featuring microbiota-gut-brain axis markers. Alzheimers Res Ther. 2023;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bjarnason I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol Hepatol. 2017;13:53–56. [PMC free article] [PubMed] [Google Scholar]
- 53. Nomura K, Ishikawa D, Okahara K, et al. Bacteroidetes Species are correlated with disease activity in ulcerative colitis. J Clin Med. 2021;10:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Murray ER, Kemp M, Nguyen TT. The microbiota-gut-brain axis in Alzheimer’s disease: A review of taxonomic alterations and potential avenues for interventions. Arch Clin Neuropsychol. 2022;37:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ghosh TS, Shanahan F, O’Toole PW. The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol. 2022;19:565–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen Y, Fang L, Chen S, et al. Gut microbiome alterations precede cerebral amyloidosis and microglial pathology in a mouse model of Alzheimer’s disease. BioMed Res Int. 2020;2020:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bekinschtein P, Kent BA, Oomen CA, et al. BDNF In the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 2013;5:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goodman T, Trouche S, Massou I, et al. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–778. [DOI] [PubMed] [Google Scholar]
- 60. Jessberger S, Clark RE, Broadbent NJ, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Plümpe T, Ehninger D, Steiner B, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rosenzweig S, Wojtowicz JM. Analyzing dendritic growth in a population of immature neurons in the adult dentate gyrus using laminar quantification of disjointed dendrites. Front Neurosci. 2011;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Licht T, Keshet E. The vascular niche in adult neurogenesis. Mech Dev. 2015;138:56–62. [DOI] [PubMed] [Google Scholar]
- 64. Pluvinage JV, Wyss-Coray T. Author correction: Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. 2020;21:298. [DOI] [PubMed] [Google Scholar]
- 65. Smith LK, White CW, Villeda SA. The systemic environment: At the interface of aging and adult neurogenesis. Cell Tissue Res. 2018;371:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dekkers KF, Sayols-Baixeras S, Baldanzi G, et al. An online atlas of human plasma metabolite signatures of gut microbiome composition. Nat Commun. 2022;13:5370–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De Lucia C, Murphy T, Maruszak A, et al. Serum from older adults increases apoptosis and molecular aging markers in human hippocampal progenitor cells. Aging Dis. 2021;12:2151–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Du Preez A, Lefèvre-Arbogast S, Houghton V, et al. The serum metabolome mediates the concert of diet, exercise, and neurogenesis, determining the risk for cognitive decline and dementia. Alzheimers Dement J Alzheimers Assoc. 2022;18:654–675. [DOI] [PubMed] [Google Scholar]
- 69. Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Lucia C, Murphy T, Steves CJ, Dobson RJB, Proitsi P, Thuret S. Lifestyle mediates the role of nutrient-sensing pathways in cognitive aging: Cellular and epidemiological evidence. Commun Biol. 2020;3:157–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ferreiro E, Lanzillo M, Canhoto D, et al. Chronic hyperglycemia impairs hippocampal neurogenesis and memory in an Alzheimer’s disease mouse model. Neurobiol Aging. 2020;92:98–113. [DOI] [PubMed] [Google Scholar]
- 73. González-Domínguez R, García-Barrera T, Vitorica J, Gómez-Ariza JL. High throughput multiorgan metabolomics in the APP/PS1 mouse model of Alzheimer’s disease. Electrophoresis. 2015;36:2237–2249. [DOI] [PubMed] [Google Scholar]
- 74. Hunsberger HC, Greenwood BP, Tolstikov V, Narain NR, Kiebish MA, Denny CA. Divergence in the metabolome between natural aging and Alzheimer’s disease. Sci Rep. 2020;10:12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mazurkiewicz-Kwilecki IM, Nsonwah S. Changes in the regional brain histamine and histidine levels in postmortem brains of Alzheimer patients. Can J Physiol Pharmacol. 1989;67:75–78. [DOI] [PubMed] [Google Scholar]
- 76. Nielsen JE, Maltesen RG, Havelund JF, et al. Characterising Alzheimer’s disease through integrative NMR- and LC-MS-based metabolomics. Metab Open. 2021;12:100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xi J, Ding D, Zhu H, et al. Disturbed microbial ecology in Alzheimer’s disease: Evidence from the gut microbiota and fecal metabolome. BMC Microbiol. 2021;21:226–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Song J, Yang L, Nan D, He Q, Wan Y, Guo H. Histidine alleviates impairments induced by chronic cerebral hypoperfusion in mice. Front Physiol. 2018;9:662–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Smith DG, Ciccotosto GD, Tew DJ, et al. Histidine 14 modulates membrane binding and neurotoxicity of the Alzheimer’s disease amyloid-beta peptide. J Alzheimers Dis. 2010;19:1387–1400. [DOI] [PubMed] [Google Scholar]
- 80. Brännström K, Islam T, Sandblad L, Olofsson A. The role of histidines in amyloid β fibril assembly. FEBS Lett. 2017;591:1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang G, Zhou Y, Huang FJ, et al. Plasma metabolite profiles of Alzheimer’s disease and mild cognitive impairment. J Proteome Res. 2014;13:2649–2658. [DOI] [PubMed] [Google Scholar]
- 82. Gao J, Zhou N, Wu Y, et al. Urinary metabolomic changes and microbiotic alterations in presenilin1/2 conditional double knockout mice. J Transl Med. 2021;19:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Whiley L, Chappell KE, D’Hondt E, et al. Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimers Res Ther. 2021;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Han Y, Quan X, Chuang Y, et al. A multi-omics analysis for the prediction of neurocognitive disorders risk among the elderly in macao. Clin Transl Med. 2022;12:e909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xie K, Qin Q, Long Z, et al. High-Throughput metabolomics for discovering potential biomarkers and identifying metabolic mechanisms in aging and Alzheimer’s disease. Front Cell Dev Biol. 2021;9:602887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Benjamina JS, Pilarowskia GO, Carossoa GA, et al. A ketogenic diet rescues hippocampal memory defects in a mouse model of kabuki syndrome. Proc Natl Acad Sci U S A. 2017;114:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhao Y, Chen H, Iqbal J, et al. Targeted metabolomics study of early pathological features in hippocampus of triple transgenic Alzheimer’s disease male mice. J Neurosci Res. 2021;99:927–946. [DOI] [PubMed] [Google Scholar]
- 88. Gebara E, Udry F, Sultan S, Toni N. Taurine increases hippocampal neurogenesis in aging mice. Stem Cell Res. 2015;14:369–379. [DOI] [PubMed] [Google Scholar]
- 89. Hernández-Benítez R, Ramos-Mandujano G, Pasantes-Morales H. Taurine stimulates proliferation and promotes neurogenesis of mouse adult cultured neural stem/progenitor cells. Stem Cell Res. 2012;9:24–34. [DOI] [PubMed] [Google Scholar]
- 90. Gallart-Palau X, Serra A, Lee BST, Guo X, Sze SK. Brain ureido degenerative protein modifications are associated with neuroinflammation and proteinopathy in Alzheimer’s disease with cerebrovascular disease. J Neuroinflammation. 2017;14:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Corso G, Cristofano A, Sapere N, et al. Serum amino acid profiles in normal subjects and in patients with or at risk of Alzheimer dementia. Dement Geriatr Cogn Disord Extra. 2017;7:143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhao Y, Jaber V, Lukiw WJ. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol. 2017;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Leblhuber F, Geisler S, Steiner K, Fuchs D, Schütz B. Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J Neural Transm. 2015;122:1319–1322. [DOI] [PubMed] [Google Scholar]
- 94. Haran JP, Bhattarai SK, Foley SE, et al. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio. 2019;10:e00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sawin EA, De Wolfe TJ, Aktas B, et al. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G590–G601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Marizzoni M, Cattaneo A, Mirabelli P, et al. Short-Chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis. 2020;78:683–697. [DOI] [PubMed] [Google Scholar]
- 97. Verhaar BJH, Hendriksen HMA, de Leeuw FA, et al. Gut Microbiota composition is related to AD pathology. Front Immunol. 2022;12:794519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. He Y, Wu W, Zheng HM, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24:1532–1535. [DOI] [PubMed] [Google Scholar]
- 99. Choo JM, Leong LEX, Rogers GB. Sample storage conditions significantly influence faecal microbiome profiles. Sci Rep. 2015;5:16350–16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Marizzoni M, Gurry T, Provasi S, et al. Comparison of bioinformatics pipelines and operating systems for the analyses of 16S rRNA gene amplicon sequences in human fecal samples. Front Microbiol. 2020;11:1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shobeiri P, Kalantari A, Teixeira AL, Rezaei N. Shedding light on biological sex differences and microbiota-gut-brain axis: A comprehensive review of its roles in neuropsychiatric disorders. Biol Sex Differ. 2022;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Webb CE, Foster CM, Horn MM, Kennedy KM, Rodrigue KM. Beta-amyloid burden predicts poorer mnemonic discrimination in cognitively normal older adults. NeuroImage. 2020;221:117199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Leal SL, Ferguson LA, Harrison TM, Jagust WJ. Development of a mnemonic discrimination task using naturalistic stimuli with applications to aging and preclinical Alzheimer’s disease. Learn Mem. 2019;26:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Berron D, Cardenas-Blanco A, Bittner D, et al. Higher CSF tau levels are related to hippocampal hyperactivity and object mnemonic discrimination in older adults. J Neurosci Off J Soc Neurosci. 2019;39:8788–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sinha N, Berg CN, Tustison NJ, et al. APOE ε4 status in healthy older African Americans is associated with deficits in pattern separation and hippocampal hyperactivation. Neurobiol Aging. 2018;69:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fujii Y, Nguyen TTT, Fujimura Y, et al. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci Biotechnol Biochem. 2019;83:2144–2152. [DOI] [PubMed] [Google Scholar]
- 107. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. [DOI] [PubMed] [Google Scholar]
- 108. Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mishra R, Phan T, Kumar P, et al. Augmenting neurogenesis rescues memory impairments in Alzheimer’s disease by restoring the engram. SSRN Electron J. 2021;219:e20220391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu G, Yu Q, Tan B, et al. Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome. Gut Microbes. 2022;14:2104089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhou Y, Su Y, Li S, et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature. 2022;607:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li B, Yamamori H, Tatebayashi Y, et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Walgrave H, Balusu S, Snoeck S, et al. Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer’s disease. Cell Stem Cell. 2021;28:1805–1821.e8. [DOI] [PubMed] [Google Scholar]
- 114. Rodríguez-Iglesias N, Sierra A, Valero J. Rewiring of memory circuits: Connecting adult newborn neurons with the help of microglia. Front Cell Dev Biol. 2019;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Saad Y, Segal D, Ayali A. Enhanced neurite outgrowth and branching precede increased amyloid-β-induced neuronal apoptosis in a novel Alzheimer’s disease model. J Alzheimers Dis. 2015;43:993–1006. [DOI] [PubMed] [Google Scholar]
- 116. García-Corzo L, Calatayud-Baselga I, Casares-Crespo L, et al. The transcription factor LEF1 interacts with NFIX and switches isoforms during adult hippocampal neural stem cell quiescence. Front Cell Dev Biol. 2022;10:912319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stadlbauer V, Engertsberger L, Komarova I, et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020;20:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Palmas V, Pisanu S, Madau V, et al. Gut Microbiota markers and dietary habits associated with extreme longevity in healthy sardinian centenarians. Nutrients. 2022;14:2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ren T, Gao Y, Qiu Y, et al. Gut Microbiota altered in mild cognitive impairment compared with normal cognition in sporadic Parkinson’s disease. Front Neurol. 2020;11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yun EJ, Imdad S, Jang J, et al. Diet is a stronger covariate than exercise in determining gut microbial richness and diversity. Nutrients. 2022;14:2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Huang WC, Tung CL, Yang YCSH, Lin IH, Ng XE, Tung YT. Endurance exercise ameliorates Western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci Rep. 2022;12:3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pessoa J, Belew GD, Barroso C, Egas C, Jones JG. The gut microbiome responds progressively to fat and/or sugar-rich diets and is differentially modified by dietary fat and sugar. Nutrients. 2023;15:2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang X, Eguchi A, Fujita Y, et al. Abnormal compositions of gut microbiota and metabolites are associated with susceptibility versus resilience in rats to inescapable electric stress. J Affect Disord. 2023;331:369–379. [DOI] [PubMed] [Google Scholar]
- 124. Jang LG, Choi G, Kim SW, Kim BY, Lee S, Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J Int Soc Sports Nutr. 2019;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Maruszak A, Silajdžić E, Lee H, et al. Predicting progression to Alzheimer’s disease with human hippocampal progenitors exposed to serum. Brain J Neurol. 2023;146:2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tachikawa M, Hosoya KI. Transport characteristics of guanidino compounds at the blood-brain barrier and blood-cerebrospinal fluid barrier: Relevance to neural disorders. Fluids Barriers CNS. 2011;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.