Abstract
In the field of neurodegeneration, speech and language assessments are useful for diagnosing aphasic syndromes and for characterizing other disorders. As a complement to classic tests, scalable and low-cost digital tools can capture relevant anomalies automatically, potentially supporting the quest for globally equitable markers of brain health. However, this promise remains unfulfilled due to limited linguistic diversity in scientific works and clinical instruments.
Here we argue for cross-linguistic research as a core strategy to counter this problem.
First, we survey the contributions of linguistic assessments in the study of primary progressive aphasia and the three most prevalent neurodegenerative disorders worldwide—Alzheimer’s disease, Parkinson’s disease, and behavioural variant frontotemporal dementia. Second, we address two forms of linguistic unfairness in the literature: the neglect of most of the world’s 7000 languages and the preponderance of English-speaking cohorts. Third, we review studies showing that linguistic dysfunctions in a given disorder may vary depending on the patient’s language and that English speakers offer a suboptimal benchmark for other language groups. Finally, we highlight different approaches, tools and initiatives for cross-linguistic research, identifying core challenges for their deployment.
Overall, we seek to inspire timely actions to counter a looming source of inequity in behavioural neurology.
Keywords: neurodegenerative diseases, linguistic testing, language diversity, cross-linguistic research
García et al. argue that speech and language research on neurodegeneration is undermined by limited linguistic diversity and by the predominance of English-speaking cohorts. They maintain that this can create new forms of global inequity, and outline cross-linguistic approaches, tools, and initiatives to counter the problem.
Introduction
Speech and language assessments are a pillar of neurodegeneration research. They are vital for diagnosing syndromes involving perisylvian damage, such as the non-fluent, semantic and logopenic variants of primary progressive aphasia.1 Moreover, they are useful for characterizing, phenotyping and monitoring more prevalent conditions with distinct anatomical vulnerabilities, including Alzheimer’s disease,2,3 Parkinson’s disease4,5 and behavioural variant frontotemporal dementia.6 Importantly, predominant speech and language deficits diverge among these disorders and correlate with their distinct atrophy patterns (Table 1). Thus, speech and language assessments can inform translational neurolinguistic models26,27 and contribute to clinical diagnosis.9
Table 1.
Main neurolinguistic patterns reported in neurodegenerative disorders
| Disorder | Main speech and/or language deficits | Neural correlates of main deficits | Key references |
|---|---|---|---|
| Non-fluent/agrammatic variant primary progressive aphasia | Impaired motor speech and/or agrammatism | Inferior frontal and motor regions | García et al.,7 Gorno-Tempini et al.,1 Montembeault et al.,8 Tee and Gorno-Tempini,9 Wilson et al.10 |
| Semantic variant primary progressive aphasia | Multimodal semantic deficits | Anterior temporal lobe | |
| Logopenic variant primary progressive aphasia | Word-finding and phonological deficits | Parieto-temporal regions | |
| Alzheimer’s disease | Lexico-semantic deficits, poor figurative language comprehension, simplified grammar | Hippocampal, temporal and temporo-parietal regions | Birba et al.,11 Domoto-Reilly et al.,12 Fraser et al.,13 Grossman et al.,14 Hirni et al.,15 Rapp and Wild.16 |
| Parkinson’s disease | Hypokinetic dysarthria, morphosyntactic and action-verb deficits | Basal ganglia, thalamus, motor cortex, temporal lobe | Abrevaya et al.,17 Alm,18 Birba et al.,4,11 Eyigoz et al.,19 García et al.,20 Grossman et al.21 |
| Behavioural variant frontotemporal dementia | Deficits in naming prosody, reading and social concept processing | Fronto-insulo- temporal regions | Birba et al.,11 Geraudie et al.,6 Hardy et al.,22 Hughes et al.,23 Nevler et al.,24 Saxon et al.25 |
Specific disturbances, such as those listed in Table 1, are common and fast in occurrence. Per current diagnostic criteria, speech and language impairments are the most salient feature in all persons with primary progressive aphasia.1 Notably, they are also prevalent in the early stages of Alzheimer’s disease, Parkinson’s disease and behavioural variant frontotemporal dementia—often appearing alongside core memory, motoric and sociobehavioural symptoms, respectively.28 Distinct deficits have been observed in Alzheimer’s disease (lexico-semantic impairment,2,29 simplified syntax,13 altered figurative language processing16), Parkinson’s disease (dysarthria,30,31 difficulties with specific word patterns4 and action concepts4) and behavioural variant frontotemporal dementia (picture naming deficits,6,32 atypical speech rhythm, poor reading skills6). Some of these deficits may actually occur preclinically in each of these disorders.3,33–36 Specific linguistic domains, then, emerge as important targets in early clinical testing of numerous populations.37
More particularly, language tests may be relevant for a pressing challenge of neurology: the quest for globally equitable markers of brain health.38–40 Gold standard methods for detecting and monitoring neurodegenerative diseases are not equally available worldwide. For instance, CSF and imaging biomarkers have been deemed critical in a recent consensus for Alzheimer’s disease diagnosis,41,42 but they are scant, unevenly distributed and often unaffordable across developing countries,43,44 which face the greatest burden of dementia.45 In Latin America, for example, the number of cases is rapidly increasing but there is a lack of biospecimen and neuroimaging facilities, culturally valid tests and specialized staff.46 Similar scenarios are found in other under-represented and underserved world regions, such as Africa and India.43,44
Given their non-invasive, cost-effective nature, speech and language tests could reveal widely applicable markers, especially via automated speech and language analysis (ASLA). ASLA offers objective, examiner-independent, multidimensional results via brief oral production tasks, through measures of the acoustic speech signal (e.g. speech timing, pitch variability) and/or its transcription (e.g. syntactic complexity, semantic specificity).47,48 Across primary progressive aphasia variants, ASLA markers capture syndrome-specific patterns49–53 that predict underlying neuropathology years before death7 and correlate with variant-specific atrophy,53–56 even longitudinally.57 In Alzheimer’s disease, they differentiate patients from healthy persons13,58–62 and other patient groups,63 predict overall cognitive status,64 outperform certain cognitive tests in predicting dementia onset,65 and correlate with volume of the hippocampus and other core atrophy regions.66,67 In Parkinson’s disease, ASLA features identify early-stage patients,19,68 discriminate between cognitive phenotypes,20,69,70 correlate with motor symptom severity19,68 and track medication status.71 In behavioural variant frontotemporal dementia, they capture prosodic24 and linguistic72 alterations as well as their worsening in the course of disease.57 As a corollary, speech and language assessments and ASLA in particular, emerge as powerful tools in the pursuit of globally fair and scalable markers of neurodegeneration.47,63,73,74
Worryingly, however, this potential is undermined by widespread lack of linguistic diversity. Like other disciplines,75 research on neurodegenerative conditions has neglected most of the world’s 7000 languages.76–79 Batteries for primary progressive aphasia diagnosis are validated for only a few linguistic communities, many of which use verbatim translations from West European languages.80 Also, as shown by systematic reviews, speech and language studies across neurodegenerative diseases span fewer than 20 languages, most of them tested in only a handful of papers.4,5,47,81–83 Of note, most languages in this literature are mainly spoken in high-income regions, which already concentrate ≈90% of dementia research.84,85
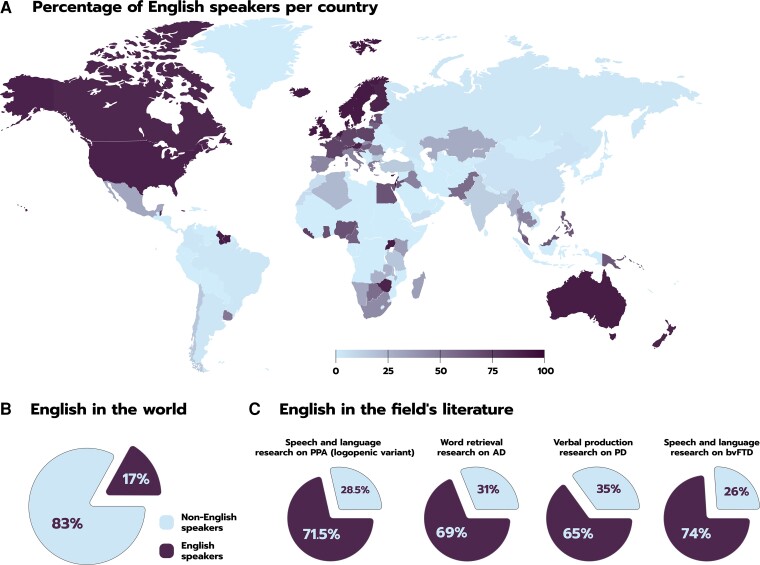
Compounding these issues is the field’s Anglocentrism. English is a minority language in most of the globe (Fig. 1A), being spoken to some proficiency by only 17% of the world’s population (Fig. 1B).79 Nevertheless, it dominates research on neurocognition, in general,75 and on neurodegeneration, in particular (Fig. 1C). Diagnostic criteria for primary progressive aphasia syndromes are based on English-speaking cohorts, and tests for other populations are typically translated (though rarely adapted) from English.80 General overviews of such syndromes exhibit the same bias. In a systematic review of logopenic variant primary progressive aphasia, for instance, 71.5% of findings came from speakers of English.81 This language is also predominant in Alzheimer’s disease research, accounting for 69% of word retrieval studies83 and over 40% of ASLA reports47 (with recent findings coming increasingly from the same dataset).86 Furthermore, English has been targeted by 65% of verbal production studies on Parkinson’s disease82 and by 74% of speech and language studies on behavioural variant frontotemporal dementia6—few of which come from low-income (e.g. Latin American) countries.32 Briefly, we know less about neurodegenerative disorders of language than we do about neurodegenerative disorders of one language.
Figure 1.
Anglocentrism in speech and language research on neurodegenerative disorders. (A) English speakers are proportionally few in most countries. (B) Most of the world’s population speaks languages other than English. (C) Yet, most reports of speech and language difficulties in neurodegenerative diseases target English speakers, outnumbering studies on non-English speakers. Data were obtained from Wikipedia (https://en.wikipedia.org/wiki/List_of_countries_by_English-speaking_population) for A; Eberhard and Simmons79 for B; and relevant reviews and/or meta-analyses for the insets of C: from left to right: Conca et al.,81 Kavé and Goral,83 Camerino et al.82 and Geraudie et al.6 AD = Alzheimer’s disease; bvFTD = behavioural variant frontotemporal dementia; PD = Parkinson’s disease; PPA = primary progressive aphasia.
Speech and language dysfunctions across languages
The above scenario would not be problematic if relations between brain and language were universal or if English were an apt model to understand every other language. Prima facie, this might seem the case. Typologically different languages may engage similar perisylvian regions during receptive tasks87 and many of them share key properties with English (e.g. subject-verb agreement). Moreover, specific acoustic and discourse markers of Alzheimer’s disease in English speakers may generalize onto Spanish speakers,88 and dysarthric aspects that typify Parkinson’s disease89 and its phenotypes20,69,90 seem similar across the languages studied so far.
Nevertheless, more fine-grained phenomena differ widely across languages and often deviate from findings in English.75,91–93 For example, noun-verb dissociations and predominant left-hemisphere activations for pitch processing are typical in Germanic and Romance languages, but such patterns are not commonly found in Mandarin Chinese and other tonal languages.94,95 Similarly, different fronto-posterior regions are engaged during reading depending on the script (alphabetic, in English and ideographic, in Chinese).96 Also, while subordination (grammatical dependencies between sentence components) manifests at the syntactic level in English, it operates mainly at the morphological level in Turkish—influencing the assessment of standard tasks, such as picture description.97 More generally, myriad phonological, orthographic, morphological, syntactic and lexico-semantic systems, as well as their interfaces with non-linguistic mechanisms, differ radically between English and most of the world’s languages.75,92 Naturally, these and other cross-linguistic differences impinge on neurolinguistic breakdown (Table 2).
Table 2.
Examples of cross-linguistic differences
| Disorder | Languages | Structural contrast | Distinct marker | Key references |
|---|---|---|---|---|
| Non-fluent/agrammatic variant primary progressive aphasia | English | Greater phonetic and lesser morphosyntactic complexity | Phonetic distortions as most salient symptom | Canu et al.98 |
| Italian | Lesser phonetic and greater morphosyntactic complexity | Distinct syntactic alterations | ||
| Semantic variant primary progressive aphasia | English | Alphabetic script (letters represent phonemes) | High prevalence of surface dysgraphia | Graham,99 Sepelyak et al.,100 Tee et al.101 |
| Chinese | Logographic script (logograms convey semantic or phonological information) | Low prevalence of surface dysgraphia | ||
| Logopenic variant primary progressive aphasia | English | Less diverse morphosyntactic patterns | Frequent sentence repetition deficits | Mesulam et al.,102 Hohlbaum et al.103 |
| German | More diverse morphosyntactic patterns | Infrequent sentence repetition deficits | ||
| Alzheimer’s disease | English | Simpler pronominal system | Overuse of pronouns | Ahmed et al.,104 Fraser et al.,13 Bose et al.105 |
| Bengali | More complex pronominal system | Underuse of pronouns | ||
| Parkinson’s disease | Spanish | Verb-framed language with rich verb vocabulary | Selective action-verb deficits | Birba et al.,11 García et al.,106 Møller et al.107 |
| Dutch | Satellite-framed language with fewer verbs | Non-selective action-verb deficits | ||
| Behavioural variant frontotemporal dementia | No clear crosslinguistic contrast reported yet. | |||
As long acknowledged in stroke aphasia,108,109 the same primary progressive aphasia syndrome may present different symptoms depending on the patient’s language. For example, a picture description study on English and Italian speakers with the non-fluent/agrammatic variant revealed significantly more speech distortions in the former and distinct syntactic alterations in the latter. According to the authors, this might reflect the greater motor speech complexities of English and the elevated morphosyntactic demands of Italian’s synthetic grammar (which, unlike English grammar, indicates syntactic relations through multiple word inflections for gender, person, tense and number).98 Also, in semantic variant primary progressive aphasia, writing tests consistently reveal surface dysgraphia (spelling words via letter-sound correspondences) in English-speaking patients99,100 but not in Chinese-speaking patients—whose writing errors, instead, abound in homophones (similar-sounding words).101 By the same token, sentence repetition deficits in logopenic variant primary progressive aphasia may be more frequent across German speakers103 than across English speakers,102 arguably because German requires storing more diverse morpho-phonological patterns across stimuli. Importantly, translations of tests developed for English may overlook language-specific markers of these syndromes, compromising diagnosis.80
Cross-linguistic differences have also been reported in Alzheimer’s disease. As shown in a machine learning study, the contribution of semantic, syntactic and paralinguistic features for disease identification differs between speakers of English and French.110 Also, a study on error patterns111 showed that subject omissions were recurrent in Italian-speaking patients, but absent in their English-speaking counterparts. Suggestively, note that subjects can be inferred from verbs’ conjugations in Italian, but not in English (e.g. the Italian verb ‘camminiamo’, on its own, entails a first person plural subject, but the English verb ‘walk’ can only entail first person plural if preceded by ‘we’). More notably, while pronouns are often overused by Anglophone Alzheimer’s disease cohorts,13,104 their proportion is abnormally low in Bengali-speaking patients.105 Reading dysfunctions may also depend on language (or, more particularly, on its script type), as suggested by assessments of English and Chinese-speaking persons with atypical forms of Alzheimer’s disease, such as posterior cortical atrophy.112,113 In Alzheimer’s dementia, then, linguistic disruptions may be different, absent or reversed depending on the language at hand.
Linguistic idiosyncrasies are also found in Parkinson’s disease research. An analysis of acoustic features114 showed that reduced speech rhythm variability in Parkinson’s disease was more marked in patients who spoke Korean than in those who spoke English, a pattern that could reflect prosodic differences—e.g. each language uses different pause and tone patterns to mark phrase boundaries, and only English uses word accents to signal new information.115,116 Furthermore, while morphosyntactic patterns differentiated Parkinson’s disease patients from healthy persons in German, Spanish and Czech, the most discriminatory features diverged across these languages (e.g. classification was mainly driven by verb-related features in Spanish and by pronoun-related features in German), arguably due to their typological grammatical differences.19 By the same token, whereas a text comprehension paradigm revealed selective action-verb deficits in speakers of Spanish,11,106 no such distinct impairment was observed in speakers of Danish.107 This might be so because Spanish possesses multiple verbs that encode motion direction (resembling the English verb ‘exit’, which directly implies ‘outwards’), while Danish features fewer, more context-sensitive verbs that require other words to encode direction (resembling the English phrase ‘go out’, where outwardness is conveyed by ‘out’).117 In short, cross-linguistic differences also influence the utility of language markers of Parkinson’s disease.
Finally, to our best knowledge, no cross-linguistic studies have been performed on behavioural variant frontotemporal dementia. Yet, some evidence suggests that lexico-semantic skills are more frequently impaired in English than in Spanish-speaking cohorts.6 That being said, the evidence is altogether mixed,6 calling for harmonized protocols that enable robust comparisons across languages while accounting for socio-cultural factors in this syndrome.32
In short, speech and language markers of neurodegeneration prove sensitive across speech communities, but they vary greatly among them. Individuals with the same diagnosis may present different verbal dysfunctions depending on their primary language and evidence from English speakers offers a suboptimal benchmark for other populations. Moreover, validated tools are unavailable for most languages and the powerful field of ASLA, based mainly on English-specific methods, is quickly reproducing these disparities. The resulting scenario is paradoxical, as potentially equitable tools seem to be generating new forms of inequity.
Ways forward and main challenges
This situation calls for a cross-linguistic and cross-cultural framework. The field requires broader representation of languages to identify their shared and distinguishing properties, leading to enhanced testing and treatment. Though still limited, existing efforts reveal fruitful ways forward.
Different approaches can be exploited to further cross-linguistic research. For example, Lindsay et al.110 and Pérez-Toro et al.88 performed cross-linguistic experiments by combining public data from the Pitt corpus (comprising English-speaking Alzheimer’s disease patients and control subjects) with proprietary data from French and Spanish-speaking cohorts, respectively. This could be expanded onto different language pairs and replicated with public data from other conditions, including speech recordings from persons with primary progressive aphasia and Parkinson’s disease in the DementiaBank. Progress can also be made through multicentric collaborations, as shown by the works of Canu et al.98 on primary progressive aphasia or Eyigoz et al.19 on Parkinson’s disease. This can be achieved by identifying similarities among primary or secondary outcome measures in each centre’s existing datasets. Even more directly, harmonized, hypothesis-driven protocols can be designed for new data collection across countries and languages.
Future efforts can benefit from existing cross-linguistic tools. For example, the Comprehensive Aphasia Test, which spans over 20 subtests of receptive and productive skills, is available in Basque, Catalan, Croatian, Cypriot Greek, English, French, Greek, Hungarian, Norwegian, Serbian, Spanish, Swedish and Turkish.80,118 Likewise, the Quick Aphasia Battery119 is available in English, Arabic, Danish, French, Spanish and Korean. Also, the more recent Mini Linguistic State Examination was first developed in English and has been validated in Spanish and Italian for cross-cohort comparisons.120 Note, however, that versions of these tests vary in the parameters used for adapting the original stimuli’s spelling-to-sound patterns, word properties and sentence characteristics—for details of key challenges and solutions, see Fyndanis et al.118 In particular, tests may present low construct validity if based on direct translations or validated only via back-translations.121
Standardized tests can be complemented with experiments targeting more fine-grained hypotheses. To this end, cross-linguistically comparable stimuli can be built with multilingual resources on word frequency (e.g. Worldlex, with estimations for 66 languages derived from big data sources), phonological and lexical properties (e.g. Lexibank, offering descriptions, transcriptions and semantic glosses for over 1000 languages),122 picture-word pairs (e.g. the MULTIMAP test, providing 218 word-image pairs matched across Spanish, Basque, Catalan, Italian, French, English, German, Mandarin Chinese and Arabic),123 and grammar (e.g. the World Atlas of Language Structures, covering over 2600 languages).124 Cross-linguistic resources are also available for ASLA, as seen, for example, in FreeLing, an open-source library providing diverse functionalities (e.g. part-of-speech tagging, morphological tagging, parsing, semantic role labelling) in typologically different languages (e.g. Croatian, English, Italian, Russian, Spanish, Slovene).125 Promising avenues for cross-linguistic research also come from novel speech perception paradigms, which capture syndrome-differential deficits by manipulating temporal and spectral properties of recorded speech.126–128 Although these resources do not cover all of the world’s most spoken languages, they enable rich comparisons among patients from different speech communities.
Global language investigations can also be bolstered through formal alliances among numerous sites. ASLA research has been incorporated by the Alzheimer’s Disease Neuroimaging Initiative, a long-standing multicentric effort to capture neuroanatomical, biochemical and cognitive changes in the course of Alzheimer’s disease.129 Another relevant effort can be found in the International Network for Cross-Linguistic Research on Brain Health, better known as Include (https://include-network.com/). Spanning over 60 sites in roughly 20 countries, Include fosters the discovery of language markers in under-represented languages (e.g. Hebrew, Hindi, Turkish), together with comparisons between these and more widely studied ones (e.g. English, Italian, Spanish). Collaborations are promoted among neurologists, linguists, neuroscientists, speech pathologists and engineers to jointly analyse linguistic, cognitive and imaging data via statistical and machine learning tools. New members are welcome from any world region, especially if they provide data from or access to cohorts who speak underexamined languages. With its transdisciplinary, multi-methodological ethos, Include seeks to align cross-linguistic research with current trends in behavioural and translational neurology at large.
Although extensive research of all living languages is likely unachievable, specific strategies could foster sustainable progress. For instance, primary progressive aphasia symptoms could be examined and validated in cohorts spanning diverse language families. Likewise, when investigating reading and writing deficits, users of different scripts (e.g. logographic, alphabetic, abjad abugida) should be evenly represented. Furthermore, researchers should avoid over-generalizing their findings with universalistic claims unless adequate replications have been made on different languages. In addition, statistical harmonization methods could facilitate cross-linguistic research when tools targeting the same cognitive process in different speech communities are structured differently due to linguistic variations.130–133 In this sense, it might be strategic to focus on under-represented languages with the largest numbers of speakers, such as those spoken in India (e.g. Hindi, Bengali, Marathi, Telugu), Indonesia (Urdu), Vietnam (Vietnamese), Africa (e.g. Swahili, Arabic, Hausa) and Latin America (Spanish, Portuguese). These efforts would be vital to bridge not only the lack of language diversity in the literature but also the need for increased neurodegeneration research in underserved regions at large.
Cross-linguistic approaches should also be pursued in the therapeutic domain. Language or typology-specific frameworks could be crucial to develop more effective treatments, beyond the importation of mainstream (often English-based) procedures. In fact, speech assessments from trained English-speaking experts prove inaccurate when they are faced with an unknown language.134 Rehabilitation practices might also benefit from a focus on pragmatic or broad communicative skills that may cut across language-specific differences.135 In addition, these efforts should contemplate cross-cultural differences in attitudes towards speech disorders, which are attributed to different factors (emotional alterations, lack of effort) depending on the country.136
Optimal leveraging of these strategies, tools and initiatives faces numerous challenges. First, while language is widely recognized as centrally affected in primary progressive aphasia and Alzheimer’s disease, it has long been described as broadly spared in Parkinson’s disease and behavioural variant frontotemporal dementia.137–139 However, recent works underscore the broad clinical utility of speech and language testing in these4,6 and other140 neurodegenerative disorders, even if many deficits are secondary to broader motoric or cognitive dysfunction. Wider recognition of language changes across diagnoses would be critical for cross-linguistic findings to be incorporated in clinical toolkits. Second, typological and neurocognitive differences among languages can be easily confounded with broader cultural idiosyncrasies across cohorts. New cross-linguistic studies would benefit from incorporating relevant cross-cultural measures (e.g. surveys on social determinants of health) to disentangle linguistic and non-linguistic sources of commonality and differentiation across language groups.75 Third, financial resources are unevenly available for language research across world regions. Trans-regional funding schemes should be systematically pursued to boost research on sub-represented languages and align it with world-leading initiatives. Current and future efforts in these directions will be critical to the success of the cross-linguistic framework advocated here.
Conclusion
Speech and language assessments can reveal cognitive markers of several brain disorders in an equitable fashion. However, a global approach is necessary for these tools to be useful across languages and cultures. Incipient evidence indicates that the linguistic symptomatology of a given disease may manifest differently depending on the patients’ language, calling for wider empirical diversity and comparative efforts. Increased awareness of the transdiagnostic utility of speech and language measures, their limited availability across the world’s languages, and existing resources to counter this imbalance are critical to prevent the emergence of a new source of global inequity in behavioural neurology.
Acknowledgements
We are thankful for the thought-provoking discussions around this article’s topic with members of the International Network for Cross-Linguistic Research on Brain Health (Include).
Contributor Information
Adolfo M García, Global Brain Health Institute, University of California, San Francisco, CA 94143, USA; Cognitive Neuroscience Center, Universidad de San Andrés, Buenos Aires B1644BID, Argentina; Departamento de Lingüística y Literatura, Facultad de Humanidades, Universidad de Santiago de Chile, Santiago 9160000, Chile; Latin American Brain Health (BrainLat) Institute, Universidad Adolfo Ibáñez, Avenida Diagonal Las Torres 2640 (7941169), Santiago, Peñalolén, Región Metropolitana, Chile.
Jessica de Leon, Memory and Aging Center, Department of Neurology, University of California, San Francisco, CA 94143, USA.
Boon Lead Tee, Global Brain Health Institute, University of California, San Francisco, CA 94143, USA; Memory and Aging Center, Department of Neurology, University of California, San Francisco, CA 94143, USA.
Damián E Blasi, Data Science Initiative, Harvard University, Cambridge, MA 02138, USA; Department of Human Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA; Department of Linguistic and Cultural Evolution, Max Planck Institute for the Science of Human History, Jena 07745, Germany.
Maria Luisa Gorno-Tempini, Memory and Aging Center, Department of Neurology, University of California, San Francisco, CA 94143, USA.
Funding
A.M.G. is an Atlantic Fellow at the Global Brain Health Institute (GBHI) and is supported with funding from National Institute on Aging of the National Institutes of Health (R01AG075775); GBHI, Alzheimer’s Association, and Alzheimer’s Society (Alzheimer’s Association GBHI ALZ UK-22-865742); ANID (FONDECYT Regular 1210176, 1210195); Latin American Brain Health Institute (BrainLat), Universidad Adolfo Ibáñez, Santiago, Chile (#BL-SRGP2021-01); Universidad de Santiago de Chile (DICYT 032351GA_DAS); the Network of European Institutes for Advanced Study; and Programa Interdisciplinario de Investigación Experimental en Comunicación y Cognición (PIIECC), Facultad de Humanidades, USACH. J.d.L. is supported by funding from the Alzheimer’s Association (AARGD-22-923915) and National Institutes of Health (NIDCD K23 DC018021, R01AG080396, P01AG019724, P30AG062422). B.L.T. is supported by the Global Brain Health Institute (GBHI ALZ UK-19-589585); Alzheimer’s Association (AACSFD-22-972143); National Institutes of Health (NIA R21AG068757, NIA R56-AG069130, U01 NS128913); and Alzheimer’s Disease Research Center of California (P30 AG062422). M.L.G-T. is supported by grants from the National Institutes of Health (NIA R21AG068757, NINDS R01 NS050915, NIDCD K24 DC015544, NIA P01 AG019724, NIA U01 AG052943, UTA 17-000879, UG3 NS105557, R01 AG038791, R01 NS100440-01, R01AG058233, U01 AG045390, U54 NS092089).
Competing interests
The authors report no competing interests.
References
- 1. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams E, McAuliffe M, Theys C. Language changes in Alzheimer’s disease: A systematic review of verb processing. Brain Lang. 2021;223:105041. [DOI] [PubMed] [Google Scholar]
- 3. Taler V, Phillips NA. Language performance in Alzheimer's disease and mild cognitive impairment: A comparative review. J Clin Exp Neuropsychol. 2008;30:501–556. [DOI] [PubMed] [Google Scholar]
- 4. Birba A, García-Cordero I, Kozono G, et al. Losing ground: Frontostriatal atrophy disrupts language embodiment in Parkinson's and huntington's disease. Neuroscience & Biobehavioral Reviews. 2017;80:673–687. [DOI] [PubMed] [Google Scholar]
- 5. García AM, Bocanegra Y, Birba A, Orozco-Arroyave JR, Sedeño L, Ibáñez A. Disruptions of frontostriatal language functions in Parkinson’s disease. In: Martin C, Preedy VR, eds. The neuroscience of Parkinson’s disease: Genetics, neurology, behavior, and diet. Elsevier Academic Press; 2020:413–430. [Google Scholar]
- 6. Geraudie A, Battista P, García AM, et al. Speech and language impairments in behavioral variant frontotemporal dementia: A systematic review. Neurosci Biobehav Rev. 2021;131:1076–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. García AM, Welch AE, Mandelli ML, et al. Automated detection of speech timing alterations in autopsy-confirmed nonfluent/agrammatic variant primary progressive aphasia. Neurology. 2022;99:e500–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R. Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: A review. Front Neurol. 2018;9:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tee BL, Gorno-Tempini ML. Primary progressive aphasia: A model for neurodegenerative disease. Curr Opin Neurol. 2019;32:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson SM, Dronkers NF, Ogar JMet al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive phasia. J Neurosci. 2010;30:16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birba A, Fittipaldi S, Cediel Escobar JC, et al. Multimodal neurocognitive markers of naturalistic discourse typify diverse neurodegenerative diseases. Cerebral Cortex. 2022;32:3377–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domoto-Reilly K, Sapolsky D, Brickhouse M, Dickerson BC. Naming impairment in Alzheimer's disease is associated with left anterior temporal lobe atrophy. NeuroImage. 2012;63:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fraser KC, Meltzer JA, Rudzicz F. Linguistic features identify Alzheimer's disease in narrative speech. J Alzheimers Dis. 2015;49:407–422. [DOI] [PubMed] [Google Scholar]
- 14. Grossman M, Koenig P, Glosser G. Neural basis for semantic memory difficulty in Alzheimer's disease: an fMRI study. Brain. 2003;126:292–311. [DOI] [PubMed] [Google Scholar]
- 15. Hirni DI, Kivisaari SL, Monsch AU, Taylor KI. Distinct neuroanatomical bases of episodic and semantic memory performance in Alzheimer’s disease. Neuropsychologia. 2013;51:930–937. [DOI] [PubMed] [Google Scholar]
- 16. Rapp AM, Wild B. Nonliteral language in Alzheimer dementia: A review. J Int Neuropsychol Soc. 2011;17:207–218. [DOI] [PubMed] [Google Scholar]
- 17. Abrevaya S, Sedeño L, Fitipaldi S, et al. The road less traveled: Alternative pathways for action-verb processing in Parkinson’s disease. J Alzheimer's Dis. 2016;55:1429–1435. [DOI] [PubMed] [Google Scholar]
- 18. Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. J Commun Dis. 2004;37:325–369. [DOI] [PubMed] [Google Scholar]
- 19. Eyigoz E, Courson M, Sedeño L, et al. From discourse to pathology: Automatic identification of Parkinson's disease patients via morphological measures across three languages. Cortex. 2020;132:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. García AM, Arias-Vergara T, C Vasquez-Correa J, et al. Cognitive determinants of dysarthria in Parkinson's disease: An automated machine learning approach. Mov Disord. 2021;36:2862–2873. [DOI] [PubMed] [Google Scholar]
- 21. Grossman M, Cooke A, DeVita Cet al. Grammatical and resource components of sentence processing in Parkinson's disease: An fMRI study. Neurology. 2003;60:775–781. [DOI] [PubMed] [Google Scholar]
- 22. Hardy CJ, Buckley AH, Downey LEet al. The language profile of behavioral variant frontotemporal dementia. J Alzheimers Dis. 2016;50:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hughes LE, Nestor PJ, Hodges JR, Rowe JB. Magnetoencephalography of frontotemporal dementia: spatiotemporally localized changes during semantic decisions. Brain. 2011;134:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nevler N, Ash S, Jester C, Irwin DJ, Liberman M, Grossman M. Automatic measurement of prosody in behavioral variant FTD. Neurology. 2017;89:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saxon JA, Thompson JC, Jones Met al. Examining the language and behavioural profile in FTD and ALS-FTD. J Neurol Neurosurg Psychiatry. 2017;88:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tremblay P, Dick AS. Broca and wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 2016;162:60–71. [DOI] [PubMed] [Google Scholar]
- 27. Ullman MT. The declarative/procedural model: A neurobiological model of language learning, knowledge, and use. In: Hickok G , Small SL, eds. Neurobiology of language. Academic Press; 2016:953–968. [Google Scholar]
- 28. García AM, DeLeon J, Tee BL. Neurodegenerative disorders of speech and language: Non-language-dominant diseases. In: Della Sala S, ed. Encyclopedia of behavioral neuroscience. 2nd ed. Elsevier; 2022:66–80. [Google Scholar]
- 29. Feldman HH, Woodward M. The staging and assessment of moderate to severe Alzheimer disease. Neurology. 2005;65(6 suppl 3):S10–S17. [Google Scholar]
- 30. Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson's disease. Behav Neurol. 1998;11:131–137. [PubMed] [Google Scholar]
- 31. Ramig LO, Fox C, Sapir S. Speech treatment for Parkinson's disease. Expert Rev Neurother. 2008;8:297–309. [DOI] [PubMed] [Google Scholar]
- 32. Geraudie A, Díaz Rivera M, Montembeault M, García AM. Language in behavioral variant frontotemporal dementia: Another stone to be turned in Latin America. Front Neurol. 2021;12:702770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cuetos F, Arango-Lasprilla JC, Uribe C, Valencia C, Lopera F. Linguistic changes in verbal expression: A preclinical marker of Alzheimer's disease. J Int Neuropsychol Soc. 2007;13:433–439. [DOI] [PubMed] [Google Scholar]
- 34. Rusz J, Hlavnička J, Tykalová T, et al. Quantitative assessment of motor speech abnormalities in idiopathic rapid eye movement sleep behaviour disorder. Sleep Med. 2016;19:141–147. [DOI] [PubMed] [Google Scholar]
- 35. Hlavnička J, Čmejla R, Tykalová T, Šonka K, Růžička E, Rusz J. Automated analysis of connected speech reveals early biomarkers of Parkinson's disease in patients with rapid eye movement sleep behaviour disorder. Sci Rep. 2017;7:12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheran G, Wu L, Lee S, et al. Cognitive indicators of preclinical behavioral variant frontotemporal dementia in MAPT carriers. J Int Neuropsychol Soc. 2019;25:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. García AM, Ibáñez A, Miller B, Gorno Tempini ML. Editorial: The unusual suspects: Linguistic deficits in non-language-dominant neurodegenerative diseases. Front Aging Neurosci. 2022;14:861041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sexton C, Snyder HM, Chandrasekaran L, Worley S, Carrillo MC. Expanding representation of low and middle income countries in global dementia research: Commentary from the Alzheimer's association. Front Neurol. 2021;12:633777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kivipelto M, Mangialasche F, Snyder HM, et al. World-wide FINGERS network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16:1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu L, Feigin V, Sacco RL, Koroshetz WJ. Promoting global collaboration for brain health research. BMJ. 2020;371:m3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parra M, Orellana P, Leon T, et al. Biomarkers for dementia in Latin American countries: Gaps and opportunities. Alzheimers Dement. 2023;19:721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chávez-Fumagalli MA, Shrivastava P, Aguilar-Pineda JA, et al. Diagnosis of Alzheimer's disease in developed and developing countries: Systematic review and meta-analysis of diagnostic test accuracy. J Alzheimer's Dis Rep. 2021;5:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parra MA, Baez S, Allegri R, et al. Dementia in Latin America: Assessing the present and envisioning the future. Neurology. 2018;90:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de la Fuente Garcia S, Ritchie CW, Luz S. Artificial intelligence, speech, and language processing approaches to monitoring Alzheimer’s disease: A systematic review. J Alzheimers Dis. 2020;78:1547–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boschi V, Catricalà E, Consonni M, Chesi C, Moro A, Cappa SF. Connected speech in neurodegenerative language disorders: A review. Front Psychol. 2017;8:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Themistocleous C, Webster K, Afthinos A, Tsapkini K. Part of speech production in patients with primary progressive aphasia: An analysis based on natural language processing. Am J Speech Lang Pathol. 2021;30(1s):466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cho S, Shellikeri S, Ash S, et al. Automatic classification of AD versus FTLD pathology using speech analysis in a biologically confirmed cohort. Alzheimers Dement. 2021;17(S5):e052270. [Google Scholar]
- 51. Faroqi-Shah Y, Treanor A, Ratner NB, Ficek B, Webster K, Tsapkini K. Using narratives in differential diagnosis of neurodegenerative syndromes. J Commun Disord. 2020;85:105994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fraser KC, Meltzer JA, Graham NL, et al. Automated classification of primary progressive aphasia subtypes from narrative speech transcripts. Cortex. 2014;55:43–60. [DOI] [PubMed] [Google Scholar]
- 53. Nevler N, Ash S, Irwin DJ, Liberman M, Grossman M. Validated automatic speech biomarkers in primary progressive aphasia. Ann Clin Transl Neurol. 2019;6:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cordella C, Quimby M, Touroutoglou A, Brickhouse M, Dickerson BC, Green JR. Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology. 2019;92:e1992–e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ballard KJ, Savage S, Leyton CE, Vogel AP, Hornberger M, Hodges JR. Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLoS One. 2014;9:e89864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ash S, Evans E, O'Shea J, et al. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology. 2013;81:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ash S, Nevler N, Phillips J, et al. A longitudinal study of speech production in primary progressive aphasia and behavioral variant frontotemporal dementia. Brain Lang. 2019;194:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Orimaye SO, Wong JS-M, Wong CP. Deep language space neural network for classifying mild cognitive impairment and Alzheimer-type dementia. PLoS One. 2018;13:e0205636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. König A, Satt A, Sorin A, et al. Automatic speech analysis for the assessment of patients with predementia and Alzheimer's disease. Alzheimers Dement. 2015;1:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hernández-Domínguez L, Ratté S, Sierra-Martínez G, Roche-Bergua A. Computer-based evaluation of Alzheimer's disease and mild cognitive impairment patients during a picture description task. Alzheimers Dement. 2018;10:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. López-de-Ipiña K, Alonso J-B, Travieso CM, et al. On the selection of non-invasive methods based on speech analysis oriented to automatic Alzheimer disease diagnosis. Sensors. 2013;13:6730–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Orimaye SO, Wong JSM, Golden KJ, Wong CP, Soyiri IN. Predicting probable Alzheimer's disease using linguistic deficits and biomarkers. BMC Bioinform. 2017;18:34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanz C, Carrillo F, Slachevsky A, et al. Automated text-level semantic markers of Alzheimer's disease. Alzheimers Dement. 2022;14:e12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Al-Hameed S, Benaissa M, Christensen H, Mirheidari B, Blackburn D, Reuber M. A new diagnostic approach for the identification of patients with neurodegenerative cognitive complaints. PLoS One. 2019;14:e0217388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eyigoz E, Mathur S, Santamaria M, Cecchi G, Naylor M. Linguistic markers predict onset of Alzheimer's disease. EClinicalMedicine. 2020;28:100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jonell P, Moëll B, Håkansson K, et al. Multimodal capture of patient behaviour for improved detection of early dementia: Clinical feasibility and preliminary results. Front Comput Sci. 2021;3:642633. [Google Scholar]
- 67. Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR. Early life linguistic ability, late life cognitive function, and neuropathology: Findings from the nun study. Neurobiol Aging. 2005;26:341–347. [DOI] [PubMed] [Google Scholar]
- 68. García AM, Carrillo F, Orozco-Arroyave JR, et al. How language flows when movements don’t: An automated analysis of spontaneous discourse in Parkinson’s disease. Brain Lang. 2016;162:19–28. [DOI] [PubMed] [Google Scholar]
- 69. Rusz J, Tykalová T. Does cognitive impairment influence motor speech performance in de novo Parkinson's disease? Mov Disord. 2021;36:2980–2982. [DOI] [PubMed] [Google Scholar]
- 70. García AM, Escobar-Grisales D, Vásquez Correa JC, et al. Detecting Parkinson’s disease and its cognitive phenotypes via automated semantic analyses of action stories. NPJ Parkinsons Dis. 2022;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Norel R, Agurto C, Heisig S, et al. Speech-based characterization of dopamine replacement therapy in people with Parkinson’s disease. NPJ Parkinsons Dis. 2020;6:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cho S, Nevler N, Ash S, et al. Automated analysis of lexical features in frontotemporal degeneration. Cortex. 2021;137:215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Laske C, Sohrabi HR, Frost SM, et al. Innovative diagnostic tools for early detection of Alzheimer's disease. Alzheimers Dement. 2015;11:561–578. [DOI] [PubMed] [Google Scholar]
- 74. Petti U, Baker S, Korhonen A. A systematic literature review of automatic Alzheimer’s disease detection from speech and language. J Am Med Inform Assoc. 2020;27:1784–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Blasi DE, Henrich J, Adamou E, Kemmerer D, Majid A. Over-reliance on English hinders cognitive science. Trends Cogn Sci (Regul Ed). 2022;26:1153–1170. [DOI] [PubMed] [Google Scholar]
- 76. Blasi D, Anastasopoulos A, Neubig G. Systematic inequalities in language technology performance across the world’s languages. In: Proceedings of the 60th Annual Meeting of the Association for Computational Linguistics. Association for Computational Linguistics. 2022:5486–5505.
- 77. Kidd E, Garcia R. How diverse is child language acquisition research? First Lang. 2022;42:703–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Christiansen MH, Contreras Kallens P, Trecca F. Toward a comparative approach to language acquisition. Curr Dir Psychol Sci. 2022;31:131–138. [Google Scholar]
- 79. CDF. In: Eberhard DM, Simons GF, eds. Ethnologue: Languages of the world. 23rd ed. SIL International; 2020. [Google Scholar]
- 80. Weekes BSH. Aphasia in Alzheimer's disease and other dementias (ADOD): Evidence from Chinese. Am J Alzheimers Dis Other Demen. 2020;35:153331752094970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Conca F, Esposito V, Giusto G, Cappa SF, Catricalà E. Characterization of the logopenic variant of primary progressive aphasia: A systematic review and meta-analysis. Ageing Res Rev. 2022;82:101760. [DOI] [PubMed] [Google Scholar]
- 82. Camerino I, Ferreira J, Vonk JM, et al. Systematic review and meta-analyses of word production abilities in dysfunction of the basal ganglia: Stroke, small vessel disease, Parkinson’s disease, and Huntington’s disease. Neuropsychol Rev. Published online 24 December 2022. doi: 10.1007/s11065-022-09570-3 [DOI] [PubMed] [Google Scholar]
- 83. Kavé G, Goral M. Word retrieval in connected speech in Alzheimer’s disease: A review with meta-analyses. Aphasiology. 2018;32:4–26. [Google Scholar]
- 84. Mattap SM, Mohan D. The economic burden of dementia in low- and middle-income countries (LMICs): A systematic review. BMJ Glob Health. 2022;7:e007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Prince MJ. World Alzheimer report 2015: The global impact of dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer's Disease International. 2015. [Google Scholar]
- 86. Luz S, Haider F, de la Fuente Garcia S, Fromm D, MacWhinney B. Editorial: Alzheimer's dementia recognition through spontaneous speech. Front Comput Sci. 2021;3:780169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Malik-Moraleda S, Ayyash D, Gallée J, et al. An investigation across 45 languages and 12 language families reveals a universal language network. Nat Neurosci. 2022;25:1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pérez-Toro PA, Klumpp P, Hernández A, et al. Alzheimer’s detection from English to Spanish using acoustic and linguistic embeddings. Proc. Interspeech; 2022:2483–2487. [Google Scholar]
- 89. Pinto S, Chan A, Guimarães I, Rothe-Neves R, Sadat J. A cross-linguistic perspective to the study of dysarthria in Parkinson’s disease. J Phon. 2017;64:156–167. [Google Scholar]
- 90. García AM, Orozco-Arroyave JR. Reply to: “does cognitive impairment influence motor speech performance in De Novo Parkinson's Disease”. Mov Disord. 2021;36:2982–2983. [DOI] [PubMed] [Google Scholar]
- 91. Malt BC, Sloman SA, Gennari SP. Universality and language specificity in object naming. J Mem Lang. 2003;49:20–42. [Google Scholar]
- 92. Kemmerer D. Messages must be tuned to the target language: Some implications of crosslinguistic semantic diversity for neurolinguistic research on speech production. J Neurolinguistics. 2019;52:100861. [Google Scholar]
- 93. Kemmerer D. Concepts in the brain: The view from cross-linguistic diversity. Oxford University Press; 2019. [Google Scholar]
- 94. Li P, Jin Z, Tan LH. Neural representations of nouns and verbs in Chinese: An fMRI study. NeuroImage. 2004;21:1533–1541. [DOI] [PubMed] [Google Scholar]
- 95. Qi Z, Han M, Garel K, San Chen E, Gabrieli JDE. White-matter structure in the right hemisphere predicts mandarin Chinese learning success. J Neurolinguistics. 2015;33:14–28. [Google Scholar]
- 96. Zhang J, Chen J, Ding G. Universality and language specificity of brain Reading networks: A developmental perspective. Dev Sci. Published online 10 March 2023. doi: 10.1111/desc.13379 [DOI] [PubMed] [Google Scholar]
- 97. Seçkin M, Savaş M. Picnic, accident or cookies? A systematic approach to guide the selection of the picture definition tasks in linguistic assessment. Arch Clin Neuropsychol. 2023;38:236–246. [DOI] [PubMed] [Google Scholar]
- 98. Canu E, Agosta F, Battistella G, et al. Speech production differences in English and Italian speakers with nonfluent variant PPA. Neurology. 2020;94:e1062–e1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Graham NL. Dysgraphia in primary progressive aphasia: Characterisation of impairments and therapy options. Aphasiology. 2014;28(8–9):1092–1111. [Google Scholar]
- 100. Sepelyak K, Crinion J, Molitoris J, et al. Patterns of breakdown in spelling in primary progressive aphasia. Cortex. 2011;47:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tee BL, Lorinda Kwan-Chen LY. Dysgraphia phenotypes in native Chinese speakers with primary progressive aphasia. Neurology. 2022;98:e2245–e2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hohlbaum K, Dressel K, Lange I, et al. Sentence repetition deficits in the logopenic variant of PPA: Linguistic analysis of longitudinal and cross-sectional data. Aphasiology. 2018;32:1445–1467. [Google Scholar]
- 104. Ahmed S, Haigh AM, de Jager CA, Garrard P. Connected speech as a marker of disease progression in autopsy-proven Alzheimer's disease. Brain. 2013;136(Pt 12):3727–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bose A, Dash NS, Ahmed S, et al. Connected speech characteristics of bengali speakers with Alzheimer's disease: Evidence for language-specific diagnostic markers. Front Aging Neurosci. 2021;13:707628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. García AM, Bocanegra Y, Herrera E, et al. Parkinson's disease compromises the appraisal of action meanings evoked by naturalistic texts. Cortex. 2018;100:111–126. [DOI] [PubMed] [Google Scholar]
- 107. Møller MLH, Høj SH, Østergaard K, Wallentin M, Højlund A. No selective action verb impairment in patients with Parkinson's disease: Evidence from Danish patients reading naturalistic texts, a commentary on García et al., 2018. Cortex. 2023;158:176–180. [DOI] [PubMed] [Google Scholar]
- 108. Bates E, Wulfeck B, MacWhinney B. Cross-linguistic research in aphasia: An overview. Brain Lang. 1991;41:123–148. [DOI] [PubMed] [Google Scholar]
- 109. Paradis M. The need for awareness of aphasia symptoms in different languages. J Neurolinguistics. 2001;14:85–91. [Google Scholar]
- 110. Lindsay H, Tröger J, König A. Language impairment in Alzheimer’s disease—Robust and explainable evidence for AD-related deterioration of spontaneous speech through multilingual machine learning. Front Aging Neurosci. 2021;13:642033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bencini GML, Pozzan L, Biundo R, et al. Language-specific effects in Alzheimer’s disease: Subject omission in Italian and English. J Neurolinguistics. 2011;24:25–40. [Google Scholar]
- 112. Li J, Wu L, Tang Y, et al. Differentiation of neuropsychological features between posterior cortical atrophy and early onset Alzheimer’s disease. BMC Neurol. 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yong KX, Shakespeare TJ, Cash D, Henley SM, Warren JD, Crutch SJ. (Con)text-specific effects of visual dysfunction on Reading in posterior cortical atrophy. Cortex. 2014;57:92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kim Y, Choi Y. A cross-language study of acoustic predictors of speech intelligibility in individuals with Parkinson's disease. J Speech Lang Hear Res. 2017;60:2506–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pierrehumbert JB, Hirschberg J. The meaning of intonational contours in the interpretation of discourse. In: Cohen PR, Morgan J, Pollack ME, eds. Intentions in communication. MIT Press; 1990:271–311. [Google Scholar]
- 116. Jun SA. Korean Intonational phonology and prosodic transcription. In: Jun SA, ed. Prosodic typology: The phonology of intonation and phrasing. Oxford University Press; 2005:9–54. [Google Scholar]
- 117. García AM, Ibáñez A. On the replicability of action-verb deficits in Parkinson's disease. Cortex. 2023;158:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fyndanis V, Lind M, Varlokosta S, et al. Cross-linguistic adaptations of The Comprehensive Aphasia Test: Challenges and solutions. Clin Linguist Phon. 2017;31(7–9):697–710. [DOI] [PubMed] [Google Scholar]
- 119. Wilson SM, Eriksson DK, Schneck SM, Lucanie JM. A quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. PLoS One. 2018;13:e0192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Patel N, Peterson KA, Ingram RU, et al. A ‘Mini linguistic state examination’ to classify primary progressive aphasia. Brain Commun. 2022;4(2):fcab299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bender HA, Martín García A, Barr WB. An interdisciplinary approach to neuropsychological test construction: Perspectives from translation studies. J Int Neuropsychol Soc. 2010;16:227–232. [DOI] [PubMed] [Google Scholar]
- 122. List J-M, Forkel R, Greenhill SJ, Rzymski C, Englisch J, Gray RD. Lexibank, a public repository of standardized wordlists with computed phonological and lexical features. Sci Data. 2022;9:316. [Google Scholar]
- 123. Gisbert-Muñoz S, Quiñones I, Amoruso L, et al. MULTIMAP: Multilingual picture naming test for mapping eloquent areas during awake surgeries. Behav Res Methods. 2021;53:918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dryer MS, Haspelmath M. The World Atlas of Language Structures Online. Accessed 2023–01-03. http://wals.info.
- 125. Padró L, Stanilovsky E. Freeling 3.0: Towards wider multilinguality. European Language Resources Association; 2012. [Google Scholar]
- 126. Hardy CJD, Agustus JL, Marshall CR, et al. Behavioural and neuroanatomical correlates of auditory speech analysis in primary progressive aphasias. Alzheimers Res Ther. 2017;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hardy CJD, Agustus JL, Marshall CR, et al. Functional neuroanatomy of speech signal decoding in primary progressive aphasias. Neurobiol Aging. 2017;56:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jiang J, Johnson JCS, Requena-Komuro M-C, et al. Comprehension of acoustically degraded speech in Alzheimer’s disease and primary progressive aphasia. Brain. 2023;146:4065-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Veitch DP, Weiner MW, Aisen PS, et al. Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2019;15:106–152. [DOI] [PubMed] [Google Scholar]
- 130. Vonk JMJ, Gross AL, Zammit AR, et al. Cross-national harmonization of cognitive measures across HRS HCAP (USA) and LASI-DAD (India). PLoS One. 2022;17:e0264166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Briceño EM, Gross AL, Giordani BJ, et al. Pre-Statistical considerations for harmonization of cognitive instruments: Harmonization of ARIC, CARDIA, CHS, FHS, MESA, and NOMAS. J Alzheimers Dis. 2021;83:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chan KS, Gross AL, Pezzin LE, Brandt J, Kasper JD. Harmonizing measures of cognitive performance across international surveys of aging using item response theory. J Aging Health. 2015;27:1392–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kobayashi LC, Gross AL, Gibbons LE, et al. You say tomato, I say radish: Can brief cognitive assessments in the U.S. Health retirement study be harmonized with its international partner studies? J Gerontol B Psychol Sci Soc Sci. 2021;76:1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Stoehr JR, Park E, Reddy NK, Rychlik K, Raj B, Gosain AK. The feasibility of cross-linguistic speech evaluation in the care of international cleft palate patients. J Craniofac Surg. 2022;33:1413–1417. [DOI] [PubMed] [Google Scholar]
- 135. Volkmer A, Walton H, Swinburn K, Spector A, Warren JD, Beeke S. Results from a randomised controlled pilot study of the better conversations with primary progressive aphasia (BCPPA) communication partner training program for people with PPA and their communication partners. Pilot Feasibility Stud. 2023;9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bebout L, Arthur B. Cross-cultural attitudes toward speech disorders. J Speech Lang Hear Res. 1992;35:45–52. [DOI] [PubMed] [Google Scholar]
- 137. Rodriguez-Oroz MC, Jahanshahi M, Krack P, et al. Initial clinical manifestations of Parkinson's disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. [DOI] [PubMed] [Google Scholar]
- 138. Piguet O, Hodges JR. Behavioural-variant frontotemporal dementia: An update. Dement Neuropsychol. 2013;7:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bott NT, Radke A, Stephens ML, Kramer JH. Frontotemporal dementia: Diagnosis, deficits and management. Neurodegener Dis Manag. 2014;4:439–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Suárez-González A, Cassani A, Gopalan R, Stott J, Savage S. When it is not primary progressive aphasia: A scoping review of spoken language impairment in other neurodegenerative dementias. Alzheimers Dement. 2021;7:e12205. [DOI] [PMC free article] [PubMed] [Google Scholar]