Abstract
The dorsomedial prefrontal cortex/dorsal anterior cingulate cortex (dmPFC/dACC) is a brain area subject to many theories and debates over its function(s). Even its precise anatomical borders are subject to much controversy. In the past decades, the dmPFC/dACC has been associated with more than 15 different cognitive processes, which sometimes appear quite unrelated (e.g. body perception, cognitive conflict). As a result, understanding what the dmPFC/dACC does has become a real challenge for many neuroscientists. Several theories of this brain area's function(s) have been developed, leading to successive and competitive publications bearing different models, which sometimes contradict each other. During the last two decades, the lively scientific exchanges around the dmPFC/dACC have promoted fruitful research in cognitive neuroscience.
In this review, we provide an overview of the anatomy of the dmPFC/dACC, summarize the state of the art of functions that have been associated with this brain area and present the main theories aiming at explaining the dmPFC/dACC function(s). We explore the commonalities and the arguments between the different theories.
Finally, we explain what can be learned from these debates for future investigations of the dmPFC/dACC and other brain regions' functions.
Keywords: dACC, dmPFC, cognitive control, foraging value, hierarchical error representation
The precise anatomy and functions of the dorsomedial prefrontal cortex/dorsal anterior cingulate cortex have been subject to debate for decades. Clairis and Lopez-Persem summarize the main arguments of this debate and offer suggestions for future research.
Introduction
In their impossible quests for the ‘philosophers’ stone’ and the quinta essentia, alchemists made many discoveries that are still widely in use. For instance, in the 14th century, the French Franciscan Jean de Roquetaillade, while searching for the quinta essential, an ‘incorruptible’ substance that would not depend upon water, air, fire or earth, discovered the aqua vitae, a highly concentrated solution of almost pure ethanol, that he thought would keep people in good health. Unravelling the function(s) supported by some brain regions can sometimes appear a quest as impossible as the alchemists’ quest. Still, the discoveries it may lead to can be just as fruitful. A brain region located between the dorsomedial prefrontal cortex (dmPFC) and the dorsal anterior cingulate cortex (dACC) has been the focus of many studies in the past two decades. This brain area roughly corresponds to a cluster of activity commonly observed in functional neuroimaging studies (Fig. 1C). It reflects the average brain activity of a group of subjects and lacks precise anatomical boundaries. Depending on studies, it has been labelled with at least 10 different names, which either refer to the cingulate cortex,1–16 to the prefrontal cortex,4,16–23 or to motor actions.1,2,24–26 Because this brain area pertains to a functional cluster that overlaps both the cingulate gyrus and frontal lobe, and lacks clear anatomical boundaries, we chose to designate it as the dmPFC/dACC area. This label denotes its location in a general sense, encompassing both the dmPFC and dACC regions. The dmPFC/dACC is involved in a wide range of cognitive functions, such as time estimation,27–29 body perception,6,30 computing foraging value,2,31 processing aversive events32 or processing conflict,33 which partially overlap. Many scientists have attempted to unify these functions into a single theory, resulting in numerous theories and models over the past three decades. In the present review, the main unifying theories of the dmPFC/dACC will be explored with particular emphasis on three major models (see Vassena et al.34 for a more exhaustive list). These include the error likelihood model,35 which has since developed into the predicted response-outcome (PRO) model20,36,37 and the hierarchical error representation (HER) model,3,38 the conflict monitoring theory,33,39 which was further developed into the expected value of control (EVC) theory9 and the foraging value theory.40 However, very early on, some researchers argued that it would be impossible to identify one single theory that would be able to summarize all the functions of the dmPFC.1,7 We will refer to this fourth view as the multiple signals view (MSV), which differs from the others as it is not a unifying theory per se.
Figure 1.
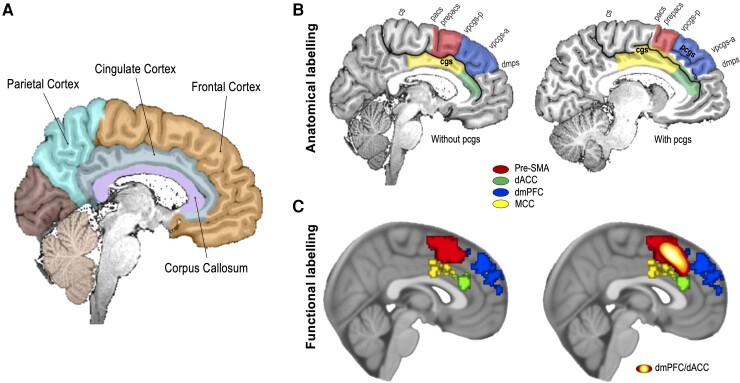
Functional and anatomical labelling of the dmPFC/dACC surrounding brain regions. (A) Brain segmentation from the USCLobes atlas42 in which the frontal cortex, parietal cortex, cingulate cortex and corpus callosum are highlighted. (B) Anatomical delineations in a brain hemisphere with (left) or without (right) paracingulate sulcus. Main sulci [in black: cingulate sulcus (cgs) and paracingulate sulcus (pcgs)] are used to delineate the pre-SMA and dmPFC from the MCC and dACC. Secondary sulci (dark grey) are used to delineate the rostral and caudal boundaries of the pre-SMA and dmPFC. The pre-SMA and the dmPFC lie within the frontal cortex and are ventrally bordered by the cingulate sulcus. The pre-SMA is immediately anterior to the SMA. Its posterior boundary appears to lie between the paracentral sulcus (pacs) or the pre-paracentral sulcus (prepacs), but this boundary is somewhat uncertain.43–45 The posterior vertical paracingulate sulcus (vpcgs-p) seems to constitute an anatomical landmark for the anterior frontier of the pre-SMA and the posterior frontier of the dmPFC.30 We propose that the anterior boundary of the dmPFC can be delineated by the dorsomedial polar sulcus (dmps), which appears to limit Brodmann area 10 dorsally.46 The dACC and MCC are subdivisions of the cingulate cortex, which are ventrally bordered by the corpus callosum, and dorsally by the pcgs, when present, or the cgs, when there is no pcgs. The frontier between the dACC and the MCC is mostly based on neuroanatomical criteria such as cytoarchitectural differences across the different cortical layers,47,48 but it is roughly located above the genu of the corpus callosum48,49 and below the anterior vertical paracingulate sulcus (vpcgs). We acknowledge that the dACC label is controversial among neuroanatomists.8 Our use of this term in this review corresponds to the dorsal part of the ACC, which is anterior to the MCC.8,49 cs = central sulcus; pacs = paracentral sulcus; prepacs = pre-paracentral sulcus; vpcgs-p = posterior vertical paracingulate sulcus; vpcgs-a = anterior vertical paracingulate sulcus.46 Note that some discrepancies exist in the literature about the labels. (C) Functional labels of the pre-SMA, dACC, dmPFC and MCC. Left: Brain activations associated with each label extracted from Neurosynth (association tests). Right: Same as left but with the functional cluster corresponding to the dmPFC/dACC depicted on top, generated with data from Lopez-Persem et al.50 for negative decision value during value-based forced choice, with permission. Note that these functional associations are displayed on the MNI152 template, as it reflects an averaged brain, without clear sulcal delineation in the prefrontal and cingulate areas. dACC = dorsal anterior cingulate cortex; dmPFC = dorsomedial prefrontal cortex; MCC = mid-cingulate cortex; pre-SMA = pre-supplementary motor area.
The various theories (HER/foraging value/EVC) and views (one versus multiple signals) regarding the role of the dmPFC/dACC have sparked a series of antagonist publications, which will be reviewed here. First, we will provide an overview of the diverse signals observed in the dmPFC/dACC and briefly introduce the theories that attempt to explain these findings. Next, we will present the key points of agreement and of conflict between these different theories.
Multiple signals for an anatomically ill-defined brain region
Anatomical discrepancies in what is the dmPFC/dACC
One difficulty in solving the different conflicts over the dmPFC/dACC function(s) is its anatomical definition. Anatomical borders of clusters of activity in this brain area are ill-defined and vary from one study to another. Furthermore, there is considerable inconsistency in how this area is labelled, both between laboratories and sometimes even within the same laboratory across different publications. Therefore, to develop a comprehensive theory of the dmPFC/dACC function, it is essential to establish a consensus on the anatomical description of this cluster. Otherwise, there is a possibility of referring to different brain areas using the same label or using different labels for the same brain area. This becomes critical when researchers make reverse inferences based on the assumption that the activation of a brain region associated with a particular function implies the involvement of that cognitive process.41
For the sake of clarity in brain region labelling, in the current review, we first define four main brain regions that surround the dmPFC/dACC (Fig. 1): the pre-supplementary motor area (pre-SMA) and the dorsomedial prefrontal cortex (dmPFC), which both belong to the frontal cortex (Fig. 1A) and the dorsal anterior cingulate cortex (dACC) and mid-cingulate cortex (MCC), which belong to the cingulate cortex. These four brain regions can be defined anatomically (Fig. 1B) or functionally (i.e. according to how activity peaks have been labelled by researchers in functional neuroimaging studies) (Fig. 1C).
The functional dmPFC/dACC, as we observe it in the literature, seems to partially overlap these four areas, along the cingulate sulcus (Fig. 1C). It roughly corresponds to the junction between Brodmann areas 4, 6, 24 and 32.51 As with many other brain regions, its functional definition implies that its name and location can vary between studies. This area has, for example, been called the ACC1–4 or dACC,9–16 referring to its location above the corpus callosum and close to the anterior part of the cingulate cortex. Similarly, others have called it the MCC5,6,8,47 or dorsal anterior mid-cingulate cortex (daMCC),7 referring to the fact that the neuronal morphology differs between the anterior and the middle areas of the cingulate cortex (with a transition of laminar thickness located dorsally to the genu of the corpus callosum).8,47,48 Other studies have labelled it the posterior fronto-medial cortex (pFMC),22,23 medial prefrontal cortex (mPFC)20 or dmPFC,4,16–19 referring broadly to its spatial location within the PFC. Finally, others have labelled it with a functional name as pre-SMA referring to its proximity (anatomically and functionally) with the SMA,2,24 or even as SMA.25,26
These labelling discrepancies are problematic because some of the aforementioned names refer to areas with a specific profile regarding their anatomy,8,47,52 function,1,2,5,53,54 neurometabolism55–57 and anatomical58 and functional connectivity.59–63 These discrepancies cause even more trouble when attempting to investigate homologous brain regions in animal studies.8 Furthermore, inconsistencies in anatomical labelling can cause great confusion, especially when coordinates and figures of the cluster location are not displayed, leading to uncertainty regarding whether one refers to the same brain area or not. Therefore, for simplicity's sake, we adopted the term dmPFC/dACC. Although, we acknowledge that this label is debatable, there is still no ideal label to mention this brain area, when observed as a group-level activity cluster.
Moreover, note that the presence or absence of a paracingulate sulcus (pcgs) could greatly impact the exact location of the functional clusters related to the dmPFC/dACC activity.5 While all healthy subjects possess a cingulate sulcus in both hemispheres, only 60% of people have a pcgs in the left hemisphere and 40% in the right hemisphere64 (Fig. 1B). Although the impact of pcgs presence on exact anatomical location of the dmPFC/dACC cluster has not been extensively studied, more studies are now considering it.65 Better consideration of individual anatomy in functional MRI (fMRI) preprocessing software could help reconcile studies with differences in the MNI coordinates of observed dmPFC/dACC clusters.
A diversity of signals in the dmPFC/dACC
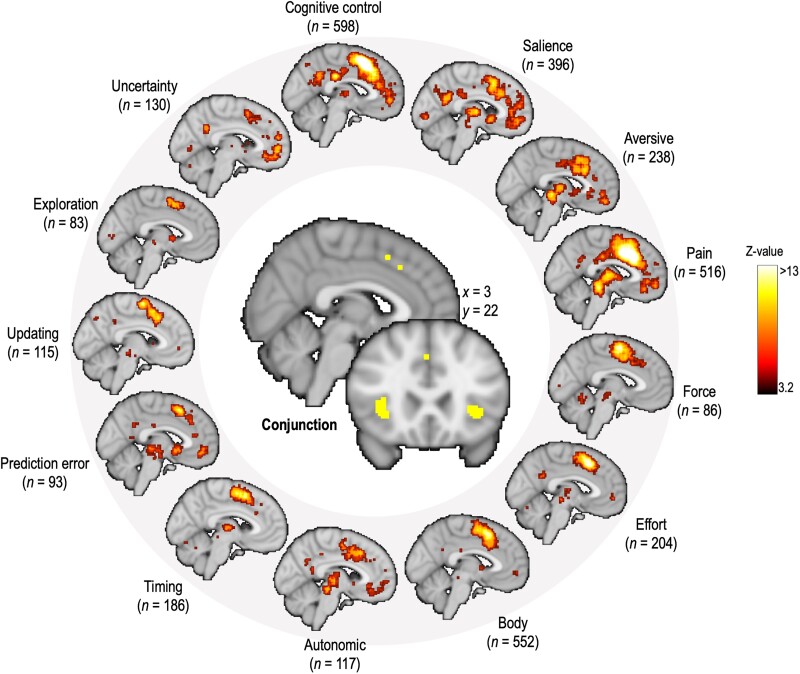
Understanding what the dmPFC/dACC does is a challenge. Indeed, the dmPFC/dACC has been linked to a tremendous number of functions.66,67 It is one of the brain areas more commonly activated across brain imaging studies,68 which has even led some researchers to state—with humour—that ‘the cingulate cortex does everything’.69 Given the number of functions associated with the dmPFC/dACC, one could hardly pretend not to have missed one in the literature. As stated by other researchers, ‘we all see something different in it, and what we see may tell us more about ourselves-and our research priorities-than about the function of the region’.70 Without pretending to provide an exhaustive list, we provide here a list of some of the functions that have been related to the activity of this cluster (Fig. 2).
Figure 2.
Non-exhaustive list of multiple signals related to the dmPFC/dACC. All maps have been extracted through a uniformity test in Neurosynth (see https://www.neurosynth.org/faq/#q18 for more details). All maps are displayed in Montreal Neurological Institute (MNI) coordinates and centred at x = −4. Each meta-analysis is based on a number n (displayed below each keyword) of neuroimaging studies based on Neurosynth automatic word extraction. For the conjunction, made with the SPM12 toolbox (Wellcome Trust Center for NeuroImaging) ImCalc function running in MATLAB 2021b, all maps have been binarized to keep only clusters surviving a significant threshold of P < 0.01 after false discovery rate (FDR) correction for multiple comparisons and they have then been multiplied with each other to only keep the voxels that are shared across all these maps. The anatomical image used for the background is the anatomical template used by Neurosynth.
Time perception
The dmPFC/dACC activity is associated with time perception.27,29,71 Its different subparts are tuned to different durations in chronotopic maps.28
Bodily representation
Different parts of the dmPFC/dACC seem tuned to different parts of the body in motor maps.6,30
Uncertainty
The dmPFC/dACC activity correlates with the volatility of the environment,72 with choice uncertainty, reflected in choice difficulty23,73,74 and also in encoding different learning rates according to the volatility of the environment, with different subparts of the dmPFC/dACC tuned to different learning rates.75
Goal-directed behaviour variables
Many studies have tried to explain the role of the dmPFC/dACC in goal-directed behaviour. Some of these results contradict each other, while others suggest that the dmPFC/dACC could encode several variables independently during value-based decision-making. For example, the dmPFC/dACC activity has been associated with negative subjective value76,77 and more generally in response to any type of aversive stimulus, including both non-painful and physically painful aversive stimuli,32 or even social rejection.78 It is also associated with the integrated net value,14,18,79 saliency,80 physical effort anticipation and exertion,25,81–83 physical fatigue,84 cognitive control exertion,33 the expected value of exerting cognitive control,9 the difference between the value of exploring the environment and the value of keeping with the ongoing behaviour,31 choice difficulty23,73,74 and also prediction errors and surprise.85–89
Model updating
To navigate our environments, we build internal models of the world. It has been shown that the dmPFC/dACC gets more active when these internal models need to be updated based on external events.2,90
Autonomic sympathetic activity
The dmPFC/dACC blood oxygen level-dependant (BOLD) activity has been consistently associated with heart-rate variability91–94 and pupil diameter size17,81,95–99 (see Amiez and Procyk100 for a more exhaustive review).
Anatomical overlap, convergences and divergences of the previous results
Interestingly, when looking at the common voxels activated by all these concepts through a meta-analytic approach based on Neurosynth, we found clusters located in the dmPFC/dACC, the bilateral anterior insula and in the right dorsolateral PFC (Fig. 2). Note that the identified cluster in this meta-analysis is somewhat posterior and does not cover the whole cluster usually observed in fMRI studies, which is displayed in Fig. 1C. Nevertheless, this result confirms that all these different processes recruit the dmPFC/dACC. Some of these functions sometimes overlap or even contradict each other.
Overlaps
It has been suggested that the mere correlation between the dmPFC/dACC activity and uncertainty can be explained by the exertion of cognitive control by the dmPFC/dACC.13 Similarly, it has been suggested that the correlation between the dmPFC/dACC activity and time reflects cognitive control processes.101 Another striking example is the case of pain. The dmPFC and the ventral ACC are often activated in situations that trigger pain.102–105 Neurons in the cingulate cortex respond to physical pain,106 making it part of the ‘pain matrix’.107 However, cingulotomy, a treatment for chronic pain syndrome,108 was abandoned, due to inconsistent results and personality changes.108 Neuroimaging studies are mostly correlational and not causal. Because a given brain area is recruited when a specific cognitive function is operating does not necessarily mean that the brain area is performing that cognitive process. Stimulating neurons in the human dmPFC and the adjacent ACC did not cause pain,106,109 suggesting that this area activity is triggered by painful stimuli rather than causing the subjective sensation of pain. It was therefore proposed that the dmPFC/ACC activity is elicited by any salient stimulus that requires a reaction.107
Contradictions
The dmPFC/dACC has been related to negative subjective value,76,77 to the integrated net value14,18,79 and to saliency.80 These three claims are not compatible with each other. One states that the dmPFC/dACC activity should increase when anticipating more aversive events, the second that it should increase with the anticipation of more positive events, therefore promoting the execution of a motor action when the net value is appetitive110 and the last that it should increase with the exposure to both positive and negative events.
In summary, given the number of findings related to the dmPFC/dACC, gathering all the literature into one single theoretical framework of the dmPFC/dACC activity thus appears as an unsolvable issue. However, this multiplicity of results has called for the development of theories, each aiming at reducing the number of dimensions associated to the dmPFC/dACC, either by explaining all or at least part of the functions associated to it.
A multiplicity of theories of the dmPFC/dACC
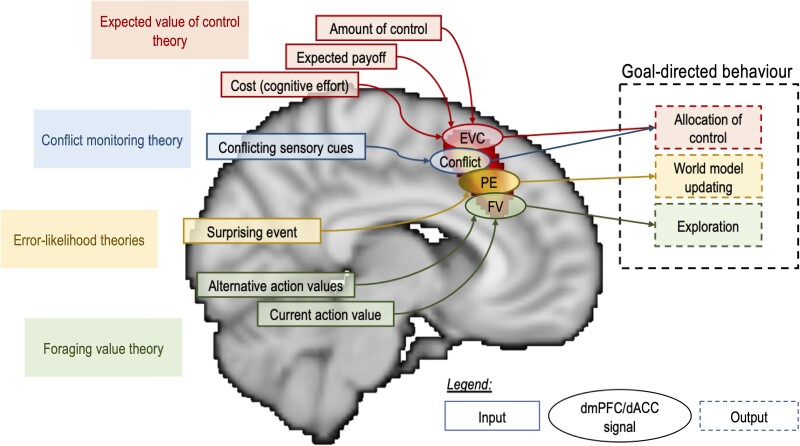
Studying the brain at a finer scale could reveal specific anatomical areas that have different connectivity and activity despite their proximity, therefore explaining the wide range of functions correlated with the dmPFC/dACC. Alternatively, one tempting approach is to unite them under a single theory of the dmPFC/dACC's function (Fig. 3). The functional overlaps between some of the functions related to the dmPFC/dACC confirm the validity of this ‘one theory to rule them all’ approach, however, please also note that, as proposed by the MSV, some of these functions are independent and could be encoded in parallel by the dmPFC/dACC. Some of the more influential theories of the dmPFC/dACC will be briefly exposed below.
Figure 3.
Schematic summary of the main dmPFC/dACC theories (without the multiple signal view). Note that the dorso-ventral or rostro-caudal orientation depicted in the figure is for illustration purposes only and we do not intend to suggest that those theories are distributed specifically along these axes in the dmPFC/dACC. dACC = dorsal anterior cingulate cortex; dmPFC = dorsomedial prefrontal cortex; EVC = expected value of control (red); FV = foraging value (green); PE = prediction error (yellow).
Cognitive control theories
Conflict detection and information theory
Only a few years after the invention of fMRI in the 1990s, Cohen's team started gathering evidence that the dmPFC/dACC was involved in conflict detection and conflict monitoring by using fMRI. A series of publications revealed that the BOLD activity of the dmPFC/dACC increased in situations involving higher levels of difficulty and conflict11,12,111–115 leading them to build up the conflict-monitoring theory of the dmPFC/dACC. They also showed that the dmPFC/dACC BOLD activity increased when errors were detected.112,116 As Botvinick and colleagues39 mentioned, ‘The occurrence of pain and feedback indicating error commission fall into the same class of signals as conflict, all of which indicate that the current distribution of attention is failing to prevent negative outcomes’.
In other terms, the dmPFC/dACC becomes more active in situations requiring behavioural adjustment, due to an increase of cognitive conflict or aversive outcomes. This increase in dmPFC/dACC activity would then trigger increased activity in the dorsolateral prefrontal cortex (dlPFC), which is assumed to implement cognitive control to adjust behaviour.114
Grounded on the conflict monitoring theory, Fan later built the information theory of the dmPFC/dACC. In this theory, the main role of recruiting cognitive control is to deal with uncertainty by trying to reduce it to a manageable level allowing to react appropriately.117 The role of the dmPFC/dACC is to detect situations where uncertainty is high and could be reduced by the application of cognitive control by the dlPFC. Within this theory, conflict processing consists in a subcase of an increase in uncertainty, which drives the recruitment of the dmPFC/dACC.118 This would explain why the dmPFC/dACC has been associated with both cognitive control and uncertainty. Thus, it was suggested that the dmPFC/dACC was broadly recruited by situations related to more uncertainty.118
Expected value of control
Later, Shenhav, Botvinick and Cohen developed a new theory called the EVC theory.9 Applying more cognitive control is subjectively costly as cognitive control goes along with a sensation of mental effort. While the conflict theory does not take the cost of cognitive control into account, the EVC theory states that one will spend cognitive control only when the EVC computation suggests that doing so is worth the effort. The EVC theory posits that the dmPFC/dACC detects situations where the implementation of more cognitive control would be beneficial, despite its cost. Based on the result of the EVC computation, the dmPFC/dACC will then eventually recruit the dlPFC to implement cognitive control. Situations involving conflict between multiple responses can induce a change in EVC (due to potential changes in control demands) and therefore the potential allocation of cognitive control.
Error-likelihood theories
A wide range of evidence shows that the dmPFC/dACC activity increases in response to unsigned prediction errors (also referred to as surprise)65,86,88,119–121 and to error detection.99,122–124 This led to the development of the error likelihood theories of the dmPFC/dACC.
Error likelihood model
Brown and Braver35 developed the error likelihood model in 2005. This model posits that the dmPFC/dACC is involved in computing the likelihood of committing an error, even in cases with no error or response conflict. This theory states that the dmPFC/dACC activity level would serve as an early warning signal for other brain areas to detect when cognitive control needs to be implemented based on the predicted level of errors associated with a given context.
Predicted response-outcome model and hierarchical error representation model
Later on, in 2010–11, Alexander and Brown evolved this model into the PRO model.36,37,125 The central aspect of the PRO model is that the dmPFC/dACC computes the various possible outcomes related to a given set of actions to allow for action selection. Then, at the time of the feedback, the dmPFC/dACC would compute the difference between the prediction and the actual outcome (prediction error) to update its internal models of the world.
A few years later, in 2015, Alexander and Brown38 updated their PRO model to the HER model. The HER model shares the same principles as the PRO model but it specifies its anatomo-functional organization by including hierarchic prediction errors organized in a rostrocaudal gradient depending on the level of the prediction error. Sensory and concrete prediction errors would be encoded rostrally, while more abstract and theoretical prediction errors, for example, at the level of rules, would be encoded caudally.89 The HER model also assumes that the dmPFC/dACC and dlPFC interact bilaterally. The dmPFC/dACC prediction error signals would drive learning by the dlPFC, while the dlPFC would modulate specific predictions generated by the dmPFC/dACC. The role of the dlPFC would be mostly to maintain in working memory a representation of stimuli that reliably co-occur with prediction errors, while the dmPFC/dACC generates these prediction errors.38
Brown and Alexander126 also developed another modified version of the PRO model named the PRO-control model. This variant incorporates both a proactive control signal and a reactive control signal. The proactive control signal inhibits actions that lead to aversive outcomes because they entail a high risk. While this proactive signal was originally present in the PRO model, the authors extended its functionality by including the capacity to stimulate actions leading to desirable outcomes through excitatory projections to the dorsolateral prefrontal cortex. On the other hand, the reactive control signal is derived from the computation of negative prediction errors, allowing it to rapidly and temporarily inhibit the future selection of actions that previously led to undesirable outcomes.
Foraging value theory
Rushworth, Kolling and colleagues, aiming to apply optimal foraging models of ecology to humans, demonstrated that the dmPFC/dACC was involved in the value of foraging the environment instead of exploiting the current patch.31,40 The foraging value theory (FVT) is inspired from behavioural ecology127 and considers that many naturalistic situations do not involve two well defined options as is often the case with binary choice tasks conducted in laboratory settings. In this vein, the FVT considers that individuals constantly weigh the option of exploiting an ongoing option (such as a default option) against the possibility of switching to explore other alternatives, when making decisions about which action to take. This theory has received some support in non-human primates' electrophysiological recordings of the dmPFC/dACC,128,129 and other similar accounts by neuroimaging studies in humans showing that the dmPFC/dACC activity increases to signal the need to switch from exploitation to exploration of the environment.19,130,131 In the framework of this theory, the dmPFC/dACC would monitor the value of alternative actions and compare them to the current action to indicate when going back to foraging is more valuable than keeping with the ongoing action. In addition, research has shown that the dmPFC/dACC is also involved in processing physical fatigue.84 While traditional views of fatigue solely focused on muscular exhaustion, recent studies propose that fatigue may also involve the computation of opportunity cost.132–135 This account is compatible with the FVT, as it states that dmPFC/dACC activity should increase with opportunity cost, i.e. when switching from the current behaviours to alternative ones is more rewarding. However, Rushworth and colleagues do not claim that this theory can account for all dmPFC/dACC activity. They propose that foraging value encoding is just one of the multiple functions performed by the dmPFC/dACC.1
Multiple signals view
As mentioned, foraging value encoding is only one of the functions attributed to the dmPFC/dACC by the upholders of the FVT. They, as well as other researchers,7,136 propose that not all dmPFC/dACC-related activity can be summarized by a single theory. This view states that the dmPFC/dACC neurons may have distinct roles depending on the ongoing task and brain networks at work. The MSV could also be understood as a multiple functions view. Indeed, it proposes that the dmPFC/dACC not only represents multiple signals, but also that it implements different functions depending on the context and task at hand. While a unifying theory implies that multiple signals can be conveyed to the dmPFC/dACC area and integrated according to its main single function, the MSV proposes that this brain region can compute several independent functions simultaneously (either in parallel or based on the current task requirements). This view is supported by considerable evidence about a vast range of distinct functions that are related to ACC and dmPFC activity in humans,2,31,72,137 in non-human primates138 and rodents,139–141 which have been summarized in several reviews.15,60,142–145 The MSV suggests that rather than searching for a single theory to explain all dmPFC/dACC activity across all paradigms and situations, it is better to document the independent functions of the dmPFC/dACC depending on the situation.
Agreements and conflicts around the role(s) of the dmPFC/dACC
As seen in previous sections, the dmPFC/dACC is associated with multiple cognitive functions, with some overlap, suggesting that different theories may explain some of these functions. Many teams have tried to demonstrate how these theories explain the observed results in the literature (Fig. 3). Some researchers have even compared the different theories to determine which one is better. The next section explores the commonalities and criticisms/conflicts between these theories.
Agreements
The dmPFC/dACC has a key role in goal-directed behaviour
Selecting optimal actions to increase reward rate
One striking aspect of all the theories outlined in the previous section is their agreement that the dmPFC/dACC plays a key role in goal-directed behaviour. Indeed, they concur that the dmPFC/dACC activity is stimulated by behaviours involving pursuing or achieving goals. In the case of the FVT, the dmPFC/dACC signals when it is more beneficial to return to foraging instead of continuing with the current behaviour, to improve the utility of the current behaviour. The conflict theory posits that the dmPFC/dACC activity indicates when an ongoing task induces cognitive conflict (such as determining the correct answer in a Stroop task) that must be dealt with to sustain a good reward rate. The EVC theory proposes that the dmPFC/dACC calculates the value of spending more cognitive control based on the integration of various signals, including the cost of cognitive control and the expected reward from increasing cognitive control. The information theory also contends that the dmPFC/dACC activity identifies situations with high uncertainty that can be reduced by applying more cognitive control. Increasing cognitive control decreases uncertainty and increases reward rate by providing a better understanding of the world, which is corroborated by previous findings in which the dmPFC/dACC activity is triggered when internal models of the world need updating.2 The error likelihood, the PRO and the HER models also all assert that the dmPFC/dACC enables the updating of internal models of the world by computing prediction errors at different levels, thereby increasing the likelihood of selecting optimal actions over time.
Integration of multiple signals
Furthermore, as would be expected by a brain region related to goal-directed behaviour, all models indicate that the dmPFC/dACC integrates multiple signals. The HER model proposes that the dmPFC/dACC integrates prediction errors across a broad spectrum of tasks, as evidenced by several paradigms involving pain, cognitive control or visual perception.65,89 This finding was also supported by a meta-analysis on prediction error.86 The FVT and the EVC theories also propose that the dmPFC/dACC integrates costs (i.e. the cost of foraging in the case of FVT, the cost of performing cognitive control in the case of EVC) and benefits (i.e. the expected mean reward rate if one starts foraging for the FVT, the expected reward from increasing cognitive control for the EVC) allowing to increase one's utility by adapting behaviour (i.e. either through switching from exploitation to exploration in FVT, or by triggering cognitive control in EVC).
In addition to the consensus among the different theories regarding the link between the dmPFC/dACC and goal-directed behaviour, several other lines of research provide further evidence supporting the predominant role of the dmPFC/dACC in goal-directed behaviour.
Task variables correlated with dmPFC/dACC activity relate to goal-directed behaviour
It is remarkable that the majority of variables that have been related to the dmPFC/dACC activity, as discussed in the ‘Multiple signals for an anatomically ill-defined brain region’ section, are directly or indirectly related to goal-directed behaviour. While a few of these variables, such as chronotopic maps, may not have an immediate and apparent connection to goal-directed behaviour, most other functions, including model updating (for efficient goal achievement), body maps (enhancing locomotor activity towards goals) and triggering autonomic nervous system (facilitating effort expenditure) can be easily linked to goal-directed behaviour.
Other theoretical accounts of the dmPFC/dACC function(s) relate to goal-directed behaviour
Other models of the dmPFC/dACC that we did not develop in this review also propose a direct link between the dmPFC/dACC and goal-directed behaviour. For example, the hierarchical reinforcement learning (HRL) model posits that the dmPFC/dACC is an essential node for initiating, maintaining and organizing a sequence of goal-directed actions based on a hierarchical reinforcement learning146; the volatility model proposes that the dmPFC/dACC adapts learning rate based on the detected volatility of the environment72; and the reward value and prediction model (RVPM) suggests that the dmPFC/dACC predicts the value of future outcomes when reward is at stake.147
Lesions to dmPFC/dACC alter goal-directed behaviours
Studies of human brain lesions have revealed that unilateral148 or bilateral149,150 anterior cerebral artery occlusion, which typically affects the dmPFC/dACC and the ACC, can result in akinetic mutism, a phenomenon characterized by a loss of motivation to speak or to move, despite the patients retaining full consciousness.151 Although reflexes and physical capacity to exert actions remain relatively intact in these patients, lesions affecting the dmPFC/dACC generally engender a decrease in their desire to act (volition) and their sense of responsibility (agency).152 Recently, another study has also found that lesions in the dmPFC/dACC regions of patients with frontotemporal dementia can lead to an increased aversion to perform efforts compared to healthy participants.153
Stimulations of the dmPFC/dACC induce an ‘urge’ to act
Electrical stimulation of dmPFC/dACC intracranial electrodes in implanted epileptic patients provokes an ‘urge’ to act in a goal-directed manner, either to protect oneself or to move towards a goal,53,154,155 again confirming the involvement of the dmPFC/dACC in goal-directed behaviour. Nevertheless, quite surprisingly, many patients under stimulation were not necessarily capable of explaining towards which goal they were acting or why they were acting the way they were acting,53 suggesting that the dmPFC/dACC can trigger a chain of actions, based on goal values defined in other parts of the brain.
In summary, all of these theories attribute a role to the dmPFC/dACC in goal-directed behaviour and adaptive fitness, and this is supported by numerous findings in the literature, including studies of lesions and electrical stimulation in humans. However, the means by which the dmPFC/dACC achieves this function and the variables it computes to do so vary greatly among theories.
The dmPFC/dACC activity reflects the need for a change
Another clear agreement is that when the dmPFC/dACC is more active, adaptation seems necessary.2,100,156,157 In the case of the FVT, adaptation corresponds to a switch from exploitative to explorative behaviour when the foraging value encoded by the dmPFC/dACC is high. In the case of the conflict theory and of the EVC theory, adaptation consists in applying more cognitive control when it allows to better deal with the current situation. In most of these theories, cognitive control is applied by the dlPFC,9,114 which is known to be functionally tightly connected to the dmPFC/dACC.59,61,62 Finally, the error likelihood models propose that the dmPFC/dACC activity calls for updating internal models of the world. All theories highlight that the dmPFC/dACC activity relates to adaptation in behaviour (explore/exploit, cognitive control/habitual behaviour) or updating internal models.
Additional convergences
On top of the convergence of most theories towards the role of the dmPFC/dACC in goal-directed behaviour, all the teams involved in the debate also agree on three additional conceptual key aspects: (i) the dmPFC/dACC is one of the most interesting areas of the brain, as it has been suggested previously68; (ii) computational modelling can be used as a tool to test and support theories on the brain; and (iii) the activity of the dmPFC/dACC seems to drive the activity of the dlPFC.2,9,38,117
Unresolved debates
Several antagonistic publications have revealed disagreements between the different teams involved in these debates. One major issue comes from the lack of convergence between data coming from multiple experiments over which theory is best at explaining dmPFC/dACC activity in a foraging task in humans. In the following section, we highlight these disagreements and propose that there are also different scientific approaches behind the arguments around the dmPFC/dACC function(s) that can explain, at least in part, the reasons for the debate.
Which theory/theories better account for the dmPFC/dACC activity: a matter of debate
Importantly, throughout the past two decades, the authors of the different theories presented in this review have actively engaged with the other theories surrounding the dmPFC/dACC. Rather than ignoring alternative perspectives, they have confronted their own theories to rigorous evaluation through a wide series of experiments that incorporate empirical data and simulations. In the subsequent section, we provide a brief summary of these exchanges. However, it is important to note that this summary offers only an overview and does not delve into the specifics of the experimental designs used in the referenced studies. Therefore, to gain a comprehensive understanding, we encourage readers to refer to these studies in the order suggested in Tables 1 –3.
Table 1.
Foraging value and difficulty (conflict monitoring theory/EVC)
| Recommended order of reading | Reference | Type of experiment | Compared variables and associated theories (defended versus confronted) |
|---|---|---|---|
| 1 | Kolling et al.31 | One fMRI experiment (n = 20, 12 females) | Foraging value (FVT) versus conflict (conflict monitoring theory/EVC) |
| 2 | Shenhav et al.162 | Two fMRI experiments (n = 15 for Experiment 1, 9 females; n = 14 for Experiment 2, 8 females) | Difficulty (conflict monitoring theory/EVC) versus foraging value (FVT) |
| 3 | Kolling et al.1 | Reanalysis of the Kolling et al.31 experiment and of the O’Reilly et al.90 experiment | Foraging value (FVT) versus difficulty (conflict monitoring theory/EVC) |
| 4 | Shenhav et al.163 | One fMRI experiment (n = 34, 30 females) | Difficulty (conflict monitoring theory/EVC) versus Foraging value (FVT) |
| 5 | Zacharopoulos et al.156 | One fMRI experiment (n = 30, 21 females) | Difficulty (conflict monitoring theory/EVC) versus Foraging value (FVT) |
| 6 | Kolling et al.164 | One fMRI experiment (n = 25, 11 females) | Foraging value (FVT) versus difficulty (conflict monitoring theory/EVC) |
This table lists the main research articles that have been at the core of the debate between difficulty encoding (compatible with conflict monitoring theory, EVC and the reactive control signal in the PRO model) and foraging value (compatible with FVT and the proactive signal in the PRO-control model) encoding in the dmPFC/dACC. The reader is kindly invited to delve into these papers to understand in more detail the arguments of the controversy. References are indicated in chronological order but the column on the left provides a suggested order of reading for the naïve reader.
Table 3.
PRO models, conflict monitoring theory, FVT and EVC
| Recommended order of reading | Reference | Type of experiment | Compared theories (defended versus confronted) |
|---|---|---|---|
| 1 | Brown125 | Perspective | PRO model versus conflict monitoring theory |
| 2 | Brown and Alexander126 | Simulations | PRO-control versus FVT and difficulty |
| 3 | Vassena et al.88 | One fMRI experiment (n = 23, 13 females) | PRO versus difficulty and EVC |
| 4 | Shenhav et al.174 | Commentary on Vassena et al.88 | EVC versus PRO |
| 5 | Bush et al.175 | One fMRI experiment (n = 97, 61 females) | EVC versus PRO, error likelihood model, conflict monitoring theory and error detection |
This table lists the main research articles that have been at the core of the debate between PRO models (original PRO model and other variants) and the other theories of the dmPFC/dACC. The reader is kindly invited to delve into these papers to understand in more details the arguments of the controversy. References are indicated in chronological order but the column on the left provides a suggested order of reading for the naïve reader.
Foraging value or difficulty?
One of the main debates surrounding the dmPFC/dACC function concerns its role in foraging choice. Six publications illustrate this debate (Table 1). Following the 2012 study31 that proposed the FVT theory and showed that the dmPFC/dACC reflected search value in the context of foraging rather than difficulty or conflict, a 2014 study162 challenged this view. The authors of the latter study argued that a potential confound between foraging value and choice difficulty could exist, depending on the value range used.162 Next, the two research teams involved in these studies engaged in a series of publications1,2,156,163,164 aiming (but not only) at disentangling which of the two variables (difficulty or foraging value) better reflected the dmPFC/dACC activity by using several variants of the initial task. Despite tremendous efforts to address criticisms raised by the other team, a consensus over whether the dmPFC/dACC better reflects difficulty or foraging value remains elusive until now (but see the following sections and our discussion for potential leads out of this conundrum). Moreover, it is important to note that this debate has been centred on one experiment and its variants. Also, this discrepancy is not circumscribed to these two research teams, as a large and growing body of evidence in humans, non-human primates and rodents supports the idea that the dmPFC/dACC encodes foraging value on one side128,165–168 and difficulty on the other side.12,111,114,169–173 This suggests that both functions could actually be supported by the dmPFC/dACC either in different anatomical subdivisions of the dmPFC/dACC1,126 or with different timings.1,126,164 Overall and until now, it seems that the debate around whether the dmPFC/dACC encodes difficulty or foraging value is one of the hardest to resolve.
Error likelihood model or conflict?
The supporters of the error likelihood models also confronted their own dmPFC/dACC model to the others. A series of six antagonistic publications (Table 2) centred around whether the dmPFC/dACC predicts error likelihood in a given context, as predicted by the error likelihood model, or whether it encodes conflict, as predicted by the conflict monitoring theory. Initially, the error likelihood model posited that the dmPFC/dACC predicts error likelihood in a given context, and not conflict or error detection.35 However, subsequent criticisms emerged when other researchers defending the conflict monitoring theory identified conflict, error detection and negative feedback signals in the dmPFC/dACC, while finding no significant correlation between dmPFC/dACC activity and error likelihood in both fMRI and EEG studies.158,160 In response to those criticisms, the authors of the error likelihood model updated their model to take into account these criticisms by positing that the dmPFC/dACC does not only predict the error likelihood in a given context, but also the ‘predicted error consequence magnitude’ (the product of those two variables can be understood as the expected risk of a given behaviour). They showed that, in line with this modified version of the error likelihood model, the dmPFC/dACC activity increases in situations when the expected risk (classically defined as the subjective probability of not being correct) is high,159 even in situations with no response conflict.161 Furthermore, they proposed that interindividual variability in risk-attitude could potentially explain why previous research did not replicate the error likelihood encoding in the dmPFC/dACC.159 Nevertheless, they also later demonstrated that both signals (conflict and error likelihood) seemed to be encoded by the dmPFC/dACC in a task-dependent manner.136
Table 2.
Error-likelihood model and conflict monitoring theory
| Recommended order of reading | Reference | Type of experiment | Compared theories (defended versus confronted) |
|---|---|---|---|
| 1 | Brown and Braver35 | One fMRI experiment (n = 16, gender not reported) | Error-likelihood model versus conflict monitoring theory |
| 2 | Nieuwenhuis et al.158 | Two fMRI experiments (n = 14, 10 females for Experiment 1; n = 14, 8 females for Experiment 2) and one EEG experiment (n = 8, 7 females for Experiment 3) | Conflict monitoring theory versus error likelihood model |
| 4 | Brown and Braver159 | One fMRI experiment (n = 21, 9 females) | Updated error likelihood model versus conflict monitoring theory |
| 6 | Brown136 | One fMRI experiment (n = 20, 11 females) | Updated error likelihood model versus conflict monitoring theory |
| 3 | Yeung and Nieuwenhuis160 | Simulations and one EEG experiment (n = 16, 10 females) | Conflict monitoring theory versus error likelihood model |
| 5 | Jahn et al.161 | One fMRI experiment (n = 22, 11 females) | Updated error likelihood model versus conflict monitoring theory |
This table lists the main research articles that have been at the core of the debate between the error likelihood model and the conflict monitoring theory of the dmPFC/dACC. The reader is kindly invited to delve into these papers to understand in more detail the arguments of the controversy. References are indicated in chronological order but the column on the left provides a suggested order of reading for the naïve reader.
PRO model(s) versus FVT, difficulty, conflict and EVC
More recently, the PRO model and its variant known as the PRO-control model have been subjected to comparisons with other theories (Table 3).
Simulations of the PRO-control model126 on the foraging task used to develop the FVT31 yielded results similar to the behavioural and neural findings reported previously.31,162 In particular, the model exhibited human-like behaviour in terms of foraging choices. Also, the proactive control signal predicted by the model showed similarity to the changes of activity of the dmPFC/dACC in response to variations in relative foraging value, as expected by the FVT. Additionally, the reactive control signal aligned with the changes of dmPFC/dACC activity in response to choice difficulty (negative surprise), as predicted by conflict monitoring theory. Interestingly, these two signals displayed distinct temporal dynamics, with the model activation being correlated early in the trial with relative foraging value and later with difficulty.
Subsequently, the predictions of the original PRO model were applied to fMRI data and compared to the predictions of the EVC theory.88 The study found that the neural responses observed in the dmPFC/dACC were better explained by the PRO model than by the EVC. Nevertheless, a commentary authored by proponents of the EVC theory criticized this result, claiming that the EVC was misunderstood and misinterpreted as an ‘Expected Value of Vigor’ model, which failed to better explain the fMRI data compared to the PRO model.174 Furthermore, a recent independent study comparing the EVC, the error likelihood model and the original PRO model during an emotion regulation task favoured the EVC theory in explaining the dmPFC/dACC activity.175
In summary, while many studies have attempted to disentangle which of the different theories could better reflect the dmPFC/dACC activity across different situations, none has consistently outperformed the others. It is worth mentioning that to date, there has not been a formal comparison of the PRO model, the PRO-control model, the HER model, the EVC theory and the FVT predictions. Moreover, as suggested in some of the studies discussed above, this debate raises questions (i) about the anatomical location of the cluster related to cognitive control versus foraging value versus prediction error1,2,65,176; (ii) about the number of functions assumed by the dmPFC/dACC since, as suggested by the MSV,1,15 the dmPFC/dACC could be involved in computing several independent functions, including both difficulty, FVT, conflict and prediction error1,136; and (iii) about the timing when each function is encoded in the dmPFC/dACC since difficulty-related signals are often observed to appear later than foraging value.1,126,164
On top of these direct conflicts between theories, most researchers have realized that the dmPFC/dACC correlates with time-on-task and have tried to explain it in the frame of their own theory, while also ruling out that the link between dmPFC/dACC activity and their own theory could be just a byproduct of this correlation (Box 1). The debate over the function(s) of the dmPFC/dACC is not solved yet, but there are many interesting points to be taken from the scientific discussions that took place, and we will try to summarize them in the two following sections.
Box 1. dmPFC/dACC and time-on-task.
In addition to the main theories presented here, other researchers have argued that the dmPFC/dACC activity reflects time-on-task rather than response conflict or error likelihood.177,178 This is evidenced by its correlation with time perception27–29 and prolonged reaction times.17,178–182
According to cognitive control theories, this phenomenon has been interpreted as reflecting higher levels of mental effort,17,29,101 because higher levels of conflict require more deliberation and are thus related to slower reaction times.183 According to this view, the dmPFC/dACC activity should not increase with reaction time in situations where it does not reflect mental effort or conflict, but only when longer reaction times are necessary to increase confidence in a decision where initial confidence is low.184,185 For instance, in tasks where the goal is to reach a target as fast as possible, dmPFC/dACC activity should not be related to longer reaction times. Consistent with this hypothesis, studies have shown that the dmPFC/dACC activity correlates with faster reaction times in a task where the goal is to answer as quickly as possible when a target appears.186 Conversely, in a task where participants were asked to click on a button when a stimulus disappears, the dmPFC/dACC activity was found to correlate with longer durations despite the absence of any conflict.177 The information theory also accounts for the correlation between dmPFC/dACC activity and reaction times by explaining that it computes information uncertainty and generates a behavioural response to it according to Hick-Hyman law. Hick-Hyman law posits a linear link between information uncertainty and reaction times.182
The PRO model also links the dmPFC/dACC activity to time-on-task, suggesting that the dmPFC/dACC activity ramps up over time until an expected outcome occurs and then shuts off once the predicted response occurs.37 If the outcome is unexpectedly delayed, either due to internal factors such as slower reaction times187 or to external factors,188 the dmPFC/dACC signal continues to ramp up and, if the outcome does not occur at all when it was expected, the dmPFC/dACC will increase its activity due to the prediction error. In agreement with the PRO model, the increase in dmPFC/dACC activity during task performance and its immediate cessation afterwards157,177,178 could partially explain why the dmPFC/dACC activity correlates with a wide range of task variables in a rather unspecific manner.67 However, understanding why the dmPFC/dACC activity correlates with time-on-task and whether this is related to one of the dmPFC/dACC theories is still a matter to be solved. Furthermore, this would not explain why there is a linear correlation between the dmPFC/dACC activity and the level of conflict in the environment, foraging value or prediction error, as this would only predict binary activation during mental or physical effort (as opposed to rest).
Importantly, it is worth noting that the authors of the different theories have also demonstrated that their variable of interest, namely foraging value for FVT, difficulty for cognitive control theories and prediction error for HER, was still significantly correlated with the dmPFC/dACC activity after controlling for reaction time.2,10,65,126 These findings rule out the possibility that the dmPFC/dACC only reflects time-on-task and does not correlate with the variables related to the main theories presented here.
In summary, many of the theories described above can account for why the dmPFC/dACC correlates with time-on-task. Conflict and information theories propose that longer reaction times reflect the exertion of cognitive control in response to situation of uncertainty and/or conflict, while error models suggest that the relation between dmPFC/dACC and longer reaction times is due to prediction errors about internal or external events that are unexpectedly delayed. Others argue that this correlation cannot be explained by these theories and that the dmPFC/dACC is merely encoding time per se.27,177,178,189,190
One versus multiple brain regions
Differences in cluster location have been suggested as a partial explanation for disparate findings among teams studying the dmPFC/dACC.1,2 It has been proposed that the cluster associated with foraging value would be located in the dACC (inside the cingulate cortex, at the level of the frontier between the ACC and the MCC in Fig. 1), while the cluster associated with choice difficulty and conflict monitoring appears to be more dorsal and closer to the pre-SMA.2,191,192 Similarly, it has been argued that the antagonism between the FVT and the EVC theories may be related to the distinct spatial gradients followed by the dmPFC/dACC and the dlPFC.3 One rostro-caudal gradient is associated with abstract prediction errors, computed in the rostral regions and concrete prediction errors located in the caudal regions. Additionally, a dorso-ventral gradient dissociates pain, control and foraging value signals in the ventral parts of the dmPFC/dACC, from the computation of prediction error in dorsal regions recruited by situations where the EVC would be higher.3 Similarly, while both cognitive control theories103 and error likelihood models193 of the dmPFC/dACC are compatible with its correlation with pain and negative affect, a recent study65 showed that pain and conflict are encoded in different locations, with pain being encoded more ventrally (in the MCC) than conflict (in the dmPFC/dACC).
While interindividual anatomical differences in the brain have often been disregarded in neuroimaging studies, future studies may consider the precise location of functional clusters. Indeed, several factors of non-interest (fMRI sequence used, the size of the smoothing kernel used during the preprocessing, the software used for fMRI analysis, etc.) can alter the anatomical location of clusters. Those factors could prevent the generalization of results over multiple studies depending on the preprocessing techniques used,194 at least in terms of precise anatomical coordinates. In the case of the dmPFC/dACC, considering the proportion of subjects with or without a pcgs in each hemisphere could allow for better disentangling where precisely the functional clusters are located, since functional activities related to the dmPFC/dACC depend on its presence.5,65 Such consideration might affect conclusions related to the dmPFC/dACC theories, by dissociating subregions implementing each theory for instance. Moreover, improvements in the anatomical frontiers of the different brain areas and of the software programs used for delimitating these borders at the individual and group level will prove of great assistance to make the field progress. Knowing whether all the signals that have been related to this cluster in the brain actually relate to one single brain area or to multiple substructures, as suggested previously,65 will be essential to build better maps of how the brain works. Moreover, variations in subject neuroanatomy or the specific anatomical localization of the cluster of activity may contribute to the disparities observed among the different studies. A comparative analysis of the neuroanatomy of individuals across the datasets could potentially help in resolving the conflicts surrounding the role(s) attributed to the dmPFC/dACC. By investigating the subject-specific neuroanatomical differences, a deeper understanding of the underlying mechanisms of dmPFC/dACC function may be gained, potentially shedding light on the discrepancies in theoretical perspectives.
One versus multiple functions
While the idea of ‘one brain area = one cognitive function’ seems relatively valid for sensory or motor areas, many suggest that we should completely abandon the assumption that ‘brain regions are both unifunctional and domain dedicated’.195,196
The overall brain activity pattern must be considered when looking at the function of a single brain region. Indeed, cortical networks can reconfigure their functional connectivity according to the task at stake,197,198 and the role of a given brain area can thus differ depending on the cortical network that is currently active.195 Strikingly, the dmPFC/dACC belongs to both the salience network and the executive control network.199 Altogether, this suggests that the dmPFC/dACC could bear different roles depending on its co-activated partners (anterior insula for salience and dlPFC for executive control, for instance).
Taken together, the controversy over whether brain regions have multiple or single functions raises a fundamental question about brain functioning. It dissociates two views. The first view suggests that each brain region is specialized for a specific transformation of input information (a cognitive working as proposed by Bergeron196), without being specialized into a single cognitive function (a cognitive role196). The MSV supports this first view, where each brain area can be recruited by different networks and cognitive functions. The second view suggests that each brain area implements a specific cognitive function (e.g. visual cortex and vision, motor cortex and locomotor action, etc.), which is more consistent with dmPFC/dACC unifying theories. This view allows for reverse inferences, such as ‘brain area X1 is active, therefore the cognitive process Y1 is currently active’, but requires great caution in its use.41,200,201
This conceptual difference has also reached its peak in the debates over the dmPFC/dACC function(s), given its association with multiple cognitive functions. Some teams aim to identify the primary function of the dmPFC/dACC to account for all the related data in a parsimonious way,34,162 while others argue that it is impossible to isolate a single function that would summarize all the others,1,2,7 as the MSV.
Further studies will allow us to better understand whether we should consider each brain area as a functional node involved in many different cognitive functions or whether each brain area is associated with a particular cognitive state and process. It is essential to bear these concepts in mind when discussing the functional roles of different brain areas.
Discussion
Understanding what is/are the cognitive function(s) supported by the dmPFC/dACC is a real challenge. Nevertheless, like the alchemists’ quests, even if it never gives rise to one single and unifying theory, the research it has promoted has greatly advanced our knowledge of the human brain. The vast amount of theoretical and practical work performed in the last decades has already allowed us to narrow down the possibilities about what the dmPFC/dACC does. For instance, it has become clear that some functions often associated with its activity can be explained because they are indirectly related to other functions, such as pain or uncertainty, which are both better explained by a relationship with cognitive control13,103,118 or with saliency encoding for pain.107 Moreover, careful examinations, in the same participants, of the correlates of both pain and cognitive control have revealed that pain was related to a more ventral cluster than cognitive control in the brain.65 Thanks to the different theories surrounding the dmPFC/dACC, great advances have been achieved in disentangling what is provoking a rise in the dmPFC/dACC activity and what is causally provoked by a rise in the dmPFC/dACC activity in terms of behavioural output. Moreover, many authors have consistently put the different theories into competition when trying to interpret their data, which has also helped to significantly advance our knowledge on the dmPFC/dACC. While all theories identify the dmPFC/dACC as a key component of goal-directed behaviour, indicating the need for an internal and/or external adaptation, the exact computation performed by the dmPFC/dACC is still a matter of debate.
Nevertheless, further studies are needed to better understand what the dmPFC/dACC is doing. We foresee several main lines of research that could be followed and address them later.
Electrophysiological recordings in the dmPFC/dACC
Most of the theories reviewed here have been developed based on fMRI studies, which lack precise time resolution and do not provide a quantification of the proportion of neurons in a given area for which activity correlates with a specific variable. As previously suggested,1,164 it is possible that the dmPFC/dACC encodes different signals at different timings of a task with foraging value encoded first and difficulty encoded later, which is also compatible with the PRO-control model.126 However, fMRI is not the best tool to test this assumption. Although several of the theories have received support from electrophysiological recordings in animal models, research on which theory best accounts for electrophysiological recordings of the dmPFC/dACC remains subject to debate.2,34,37,202,203 Future studies could therefore explore multi-unit and local field potential recordings in rodents, non-human primates or humans using intra-electroencephalography (iEEG) to gain a better insight into the proportion of neurons related to each of the theories within the dmPFC/dACC with precise anatomical locations.
Developing new artificial intelligence-inspired approaches to the dmPFC/dACC
Future studies could draw inspiration from recent advances in artificial intelligence (AI). Artificial neural networks, which were initially inspired by biological neural networks204 have paved the way for the development of intelligent robots that are based on the latest research in neuroscience.205 After 80 years of research on artificial neural networks, the field of neuroscience is now drawing inspiration back from AI research. For instance, a recent architecture has been proposed206 to construct autonomous intelligent agents, based on deep neural networks. In this architecture, there is a configurator module that resembles the dmPFC/dACC in the way it integrates multiple inputs to facilitate goal-directed behaviour by identifying a sequence of subgoals required to reach a global goal. Future studies could compare the artificial neural activity of the configurator to the neural activity in humans and possibly propose a new AI-inspired theory about the function of the dmPFC/dACC (e.g. other research in which the dmPFC/dACC has been associated with a monitoring module in a computational approach205,207). A similar approach has been performed with the development of a multi-task learning model.208 To behave optimally across a wide range of tasks and contexts, this model relies on habits as much as possible but, when it has no other choice, it relies on a set of controlled behaviours that correspond to task-specific policies that could be perceived as more costly because they are less generalizable. They propose that such a model would be compatible with the EVC model, therefore confirming the potential role of the dmPFC/dACC in computing the EVC. However, to our knowledge, their model remains to be tested at the neural level. Interestingly, the goal-oriented learning and selection of action (GOLSA) model, which is an algorithm that incorporates neurobiological Hebbian constraints,209 has allowed us to identify other brain areas than the dmPFC/dACC in relationship to goal-directed behaviour, such as the hippocampus, basal ganglia and ventral PFC.210 These AI-based approaches therefore suggest that research on goal-directed behaviour by AI could open unexpected new avenues for better understanding the exact role of the dmPFC/dACC.
Taking dmPFC/dACC interindividual anatomical differences into account
Future studies could benefit from considering interindividual sulcal morphology variability in the brain. As explained above, the presence or absence of a paracingulate sulcus in the dmPFC/dACC can impact the location of functional clusters. This approach has also shed light on other brain areas, such as the ventromedial prefrontal cortex (vmPFC), where different morphological patterns211–213 can affect the localization of functional clusters related to experienced value214 and the default mode network.215 By using large datasets and classifying participants according to sulcal morphology, future studies could clarify the exact location of activity in response to different tasks and potentially dissociate as many distinct brain regions as there are theories, as some authors have suggested previously.1–3,54
Considering dmPFC/dACC connectivity
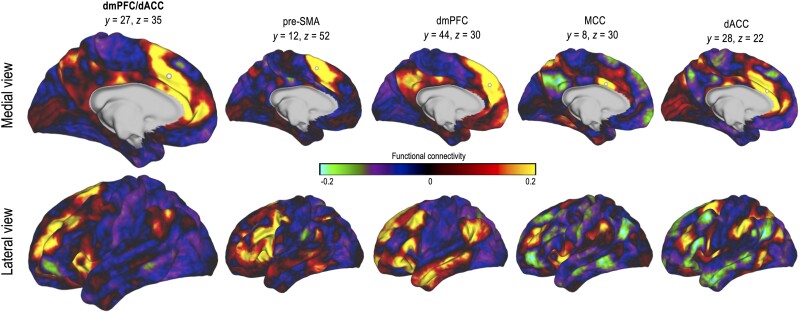
Instead of focusing on precise anatomical boundaries within the dmPFC/dACC, it may be useful to consider anatomical and functional connectivity. The anatomical58 and functional connectivity59–63 of the dmPFC/dACC and its neighbours can vary greatly. Recent advances in mapping the connectivity of the human brain, such as with the Human Brain Connectome project216 have been essential in refining our understanding of the brain organization, at both individual and group levels. Comparative neuroscience can also benefit from such investigations. For instance, Sallet and colleagues217 demonstrated that functional and anatomical connectivity could serve at finding similarities between frontal regions in human and non-human primates. These approaches challenge assumptions in brain region labelling and uncover correspondences that were not previously known. Although neurons in different subparts of the dmPFC/dACC may be physiologically and neuroanatomically equivalent, they may connect to different parts of the brain and serve different functions. To illustrate this argument, we used data released as part of the Human Connectome Project218 to compute the functional connectivity of the dmPFC/dACC and its four neighbouring regions (Fig. 4). All connectivity maps are qualitatively different, despite the anatomical closeness of the seeds. Current dmPFC/dACC theories can also benefit from integrating functional connectivity. For instance, the EVC theory posits that the dmPFC/dACC is functionally connected to other brain regions involved in decision-making and cognitive control (such as the dlPFC and the vmPFC).9 This integration of connectivity provides a mechanistic account of how the brain processes and integrates information to guide decision-making and cognitive control. Overall, future studies assessing each theory could benefit from considering both anatomical boundaries and functional connectivity within the dmPFC/dACC, for instance by coupling functional connectivity analyses with individual-level anatomical boundaries, to better specify the brain regions of interest.
Figure 4.
Connectivity of the dmPFC/dACC area. Functional connectivity maps in medial (top) and lateral (bottom) view for seeds (grey dots) in the dmPFC/dACC, pre-supplementary motor area (pre-SMA), dmPFC, mid-cingulate cortex (MCC) and dACC. Seeds were defined according to the functional labelling provided in Fig. 1C. Data are from the Human Connectome Project216 (HCP; Washington University-University of Minnesota Consortium of the Human Connectome Project; RRID: SCR_008749; http://db.humanconnectome.org; S900 subjects release with 7 T structural and resting fMRI data, 57 subjects) and correspond to the average functional connectivity of 57 subjects. Only the left hemisphere is displayed for visual purposes. The same subject methods as in in Lopez-Persem et al.219 were used. dACC = dorsal anterior cingulate cortex; dmPFC = dorsomedial prefrontal cortex.
Considering brain networks rather than single brain areas
The consideration and reporting of co-activated brain regions, as well as the use of multivariate analyses methods, might help to better understand the function(s) of the dmPFC/dACC. While most of our approach in this review focused on a single brain area, it is overall admitted that observing which brain networks are at work, instead of attributing a cognitive role to each brain area, is more relevant to the investigation of the brain functioning. According to this view, understanding the dmPFC/dACC does not make sense without looking at its co-activated partners. Providing the tables of activation, which is quite common in the field, will therefore greatly help to know which network is at work. Multivariate brain measures that integrate the information over multiple brain areas have also proven to be more robust.220,221 More generally, the recent development and growth of new techniques to analyse fMRI-related data, such as gradient analyses,222 may also prove key to better characterize the dmPFC/dACC activity depending on the task and network at work. It is also important to note here that the robustness of fMRI results based on the average response of a single brain region has been questioned in recent years.223 Functional MRI results seem to depend heavily on the preprocessing methods used, which vary between different teams, therefore impacting reproducibility.194 This phenomenon could partially explain why different teams obtain different results, despite testing the same hypothesis.
Digging into the link between dmPFC/dACC and physiological arousal
Numerous findings indicate a direct link between dmPFC/dACC activity and physiological arousal levels determined by the sympathetic nervous system.100 These results suggest that the dmPFC/dACC can read and directly trigger sympathetic nervous system activity, resulting in increased levels of arousal, reflected by pupil dilation, increased heartbeat, blood vessel constriction, glucose release, intestine inhibition, bladder relaxation and sweat.100 Furthermore, the dmPFC/dACC is associated with the willingness to exert higher physical25,81,152,153,224 and mental efforts.225 In other words, the dmPFC/dACC may play a role in activating the sympathetic nervous system, thereby facilitating physical and mental effort exertion.100 However, the reason why sympathetic arousal is triggered by the dmPFC/dACC activity is not straightforward and has not been thoroughly addressed by the theories discussed in the current review. This phenomenon is nevertheless compatible with most of the current accounts of the dmPFC/dACC. For example, when foraging value is high, it might be adaptive to increase the level of the sympathetic arousal to get ready to engage with further exploration of the environment by senses (vision, audition, etc.) and locomotor activity, therefore getting ready for performing higher efforts. Concerning cognitive control theories, it has been argued that ‘the contribution of [the dmPFC/dACC] to laboratory measures of cognitive control might stem from its evolutionarily older role in regulating “hot” behaviours … that are elicited by stimuli and situations with affective and nociceptive importance',103 which are not so adaptive anymore in the face of a mental challenge, such as an exam or a deadline. Moreover, others have also proposed that any physical activity is a conflict in the sense that not doing anything or relying on habitual behaviour would be the default action, thus cognitive control would be required to keep on exerting efforts that have not been reinforced.226,227 For the HER theory, it is also quite intuitive that prediction errors, which are salient events by definition, trigger more arousal. Future studies will need to determine whether the dmPFC/dACC acts solely as a driver of physiological arousal or whether it triggers sympathetic activity through one or more of the computations identified by the theories outlined in this review.
Conclusion
In summary, the dmPFC/dACC is an anatomically ill-defined brain region found active in many different cognitive scenarios. Several dmPFC/dACC theories have been proposed and developed in parallel, sometimes with contradictory results, generating a lively and fascinating debate. All authors from those studies agree that the dmPFC/dACC plays a major role in goal-directed behaviour and that its activity reflects the need for adaptation. Still, there is great variation among these theories regarding what the dmPFC/dACC computes internally and which behavioural output its activity should trigger. Our claim is not to take sides with one or the other theory, but to summarize each argument and to underline why such a debate can generate rapid advances in our knowledge about the brain. We highlighted practical and theoretical issues raised by the series of publications around the role of the dmPFC/dACC. Overall, such scientific divergences are helpful to science, and other brain regions could benefit from similar debates and diversity of approaches.
Acknowledgements
We would like to thank the Motivation Brain and Behavior team led by Dr Mathias Pessiglione, as well as Dr Jérôme Sallet and Dr Emmanuel Procyk for fruitful discussions over the last years over the topic of this review. We are very grateful to DrCéline Amiez for her precious advice on sulcal identification. We also want to warmly thank the reviewers for their helpful feedback on how to improve the current manuscript.
Contributor Information
Nicolas Clairis, Laboratory of Behavioral Genetics (LGC)- Brain Mind Institute (BMI)- Sciences de la Vie (SV), École Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland.
Alizée Lopez-Persem, FrontLab, Institut du Cerveau - Paris Brain Institute - ICM, Inserm, CNRS, Sorbonne University, AP HP, Hôpital de la Pitié Salpêtrière, 75013 Paris, France.
Funding
N.C. has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 101032219. A.L.P. has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 101026191.
Competing interests
The authors report no competing interests.
References
- 1. Kolling N, Behrens T, Wittmann M, Rushworth M. Multiple signals in anterior cingulate cortex. Curr Opin Neurobiol. 2016;37:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolling N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth MFS. Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci. 2016;19:1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander WH, Brown JW. The role of the anterior cingulate cortex in prediction error and signaling surprise. Top Cogn Sci. 2019;11:119–135. [DOI] [PubMed] [Google Scholar]
- 4. Wunderlich K, Rangel A, O’Doherty JP. Neural computations underlying action-based decision making in the human brain. Proc Natl Acad Sci U S A. 2009;106:17199–17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amiez C, Neveu R, Warrot D, Petrides M, Knoblauch K, Procyk E. The location of feedback-related activity in the midcingulate cortex is predicted by local morphology. J Neurosci. 2013;33:2217–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Procyk E, Wilson CRE, Stoll FM, Faraut MCM, Petrides M, Amiez C. Midcingulate motor map and feedback detection: Converging data from humans and monkeys. Cereb Cortex. 2016;26:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bush G. Dorsal anterior midcingulate cortex: Roles in normal cognition and disruption in attention-deficit/hyperactivity disorder. In: Cingulate neurobiology and disease. Oxford University Press; 2009:30. [Google Scholar]
- 8. Vogt BA. Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat. 2016;74:28–46. [DOI] [PubMed] [Google Scholar]
- 9. Shenhav A, Botvinick MM, Cohen JD. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19:1286–1291. [DOI] [PubMed] [Google Scholar]
- 11. Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. [DOI] [PubMed] [Google Scholar]
- 12. Pochon JB, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. J Neurosci. 2008;28:3468–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mushtaq F, Bland AR, Schaefer A. Uncertainty and cognitive control. Front Psychol. 2011;2:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yee DM, Crawford JL, Lamichhane B, Braver TS. Dorsal anterior cingulate cortex encodes the integrated incentive motivational value of cognitive task performance. J Neurosci. 2021;41:3707–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein-Flügge MC, Bongioanni A, Rushworth MFS. Medial and orbital frontal cortex in decision-making and flexible behavior. Neuron. 2022;110:2743–2770. [DOI] [PubMed] [Google Scholar]
- 16. Sun S, Zhen S, Fu Z, et al. Decision ambiguity is mediated by a late positive potential originating from cingulate cortex. NeuroImage. 2017;157:400–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clairis N, Pessiglione M. Value, confidence, deliberation: A functional partition of the medial prefrontal cortex demonstrated across rating and choice tasks. J Neurosci. 2022;42:5580–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Bouc R, Pessiglione M. A neuro-computational account of procrastination behavior. Nat Commun. 2022;13:5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Domenech P, Rheims S, Koechlin E. Neural mechanisms resolving exploitation-exploration dilemmas in the medial prefrontal cortex. Science. 2020;369:eabb0184. [DOI] [PubMed] [Google Scholar]
- 20. Alexander WH, Brown JW. A general role for medial prefrontal cortex in event prediction. Front Comput Neurosci. 2014;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: Evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. [DOI] [PubMed] [Google Scholar]
- 22. Arabadzhiyska DH, Garrod OGB, Fouragnan E, De Luca E, Schyns PG, Philiastides MG. A common neural account for social and non-social decisions. J Neurosci. 2022;42:9030–9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volz KG, Schubotz RI, von Cramon DY. Variants of uncertainty in decision-making and their neural correlates. Brain Res Bull. 2005;67:403–412. [DOI] [PubMed] [Google Scholar]
- 24. Kamiński J, Sullivan S, Chung JM, Ross IB, Mamelak AN, Rutishauser U. Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat Neurosci. 2017;20:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurniawan IT, Guitart-Masip M, Dayan P, Dolan RJ. Effort and valuation in the brain: The effects of anticipation and execution. J Neurosci. 2013;33:6160–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klein-Flugge MC, Kennerley SW, Friston K, Bestmann S. Neural signatures of value comparison in human cingulate cortex during decisions requiring an effort-reward trade-off. J Neurosci. 2016;36:10002–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kolling N, O’Reilly JX. State-change decisions and dorsomedial prefrontal cortex: The importance of time. Curr Opin Behav Sci. 2018;22:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Protopapa F, Hayashi MJ, Kulashekhar S, et al. Chronotopic maps in human supplementary motor area. PLoS Biol. 2019;17:e3000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radua J, del Pozo NO, Gómez J, Guillen-Grima F, Ortuño F. Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia. 2014;58:14–22. [DOI] [PubMed] [Google Scholar]
- 30. Amiez C, Petrides M. Neuroimaging evidence of the anatomo-functional organization of the human cingulate motor areas. Cereb Cortex. 2014;24:563–578. [DOI] [PubMed] [Google Scholar]
- 31. Kolling N, Behrens TEJ, Mars RB, Rushworth MFS. Neural mechanisms of foraging. Science. 2012;336:95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC Neurosci. 2012;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci. 2004;8:539–546. [DOI] [PubMed] [Google Scholar]
- 34. Vassena E, Holroyd CB, Alexander WH. Computational models of anterior cingulate cortex: At the crossroads between prediction and effort. Front Neurosci. 2017;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate Cortex. Science. 2005;307:1118–1121. [DOI] [PubMed] [Google Scholar]
- 36. Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Top Cogn Sci. 2010;2:658–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alexander WH, Brown JW. Hierarchical error representation: A computational model of anterior cingulate and dorsolateral prefrontal cortex. Neural Comput. 2015;27:2354–2410. [DOI] [PubMed] [Google Scholar]
- 39. Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. [DOI] [PubMed] [Google Scholar]
- 40. Rushworth MF, Kolling N, Sallet J, Mars RB. Valuation and decision-making in frontal cortex: One or many serial or parallel systems? Curr Opin Neurobiol. 2012;22:946–955. [DOI] [PubMed] [Google Scholar]
- 41. Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. [DOI] [PubMed] [Google Scholar]
- 42. Joshi AA, Choi S, Liu Y, et al. A hybrid high-resolution anatomical MRI atlas with sub-parcellation of cortical gyri using resting fMRI. J Neurosci Methods. 2022;374:109566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinson H, Van Lerbeirghe J, Vanhauwaert D, Van Damme O, Hallaert G, Kalala JP. The supplementary motor area syndrome: A neurosurgical review. Neurosurg Rev. 2022;45:81–90. [DOI] [PubMed] [Google Scholar]
- 44. Tate MC, Kim C-Y, Chang EF, Polley M-Y, Berger MS. Assessment of morbidity following resection of cingulate gyrus gliomas: Clinical article. J Neurosurg. 2011;114:640–647. [DOI] [PubMed] [Google Scholar]
- 45. Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res. 1994;19:251–268. [DOI] [PubMed] [Google Scholar]
- 46. Amiez C, Sallet J, Hopkins WD, et al. Sulcal organization in the medial frontal cortex provides insights into primate brain evolution. Nat Commun. 2019;10:3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. [DOI] [PubMed] [Google Scholar]
- 49. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lopez-Persem A, Domenech P, Pessiglione M. How prior preferences determine decision-making frames and biases in the human brain. Elife. 2016;5:e20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth; 1909. Accessed 15 July 2022. http://archive.org/details/b28062449
- 52. Ding SL, Royall JJ, Sunkin SM, et al. Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol. 2016;524:3127–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caruana F, Gerbella M, Avanzini P, et al. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain. 2018;141:3035–3051. [DOI] [PubMed] [Google Scholar]
- 54. Kragel PA, Kano M, Van Oudenhove L, et al. Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nat Neurosci. 2018;21:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hänisch B, Hansen JY, Bernhardt BC, et al. Cerebral chemoarchitecture shares organizational traits with brain structure and function. eLife. 2023;12:e83843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vergani F, Lacerda L, Martino J, et al. White matter connections of the supplementary motor area in humans. J Neurol Neurosurg Psychiatry. 2014;85:1377–1385. [DOI] [PubMed] [Google Scholar]
- 59. Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. [DOI] [PubMed] [Google Scholar]
- 60. Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Loh KK, Hadj-Bouziane F, Petrides M, Procyk E, Amiez C. Rostro-Caudal organization of connectivity between cingulate motor areas and lateral frontal regions. Front Neurosci. 2018;11:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jin F, Zheng P, Liu H, Guo H, Sun Z. Functional and anatomical connectivity-based parcellation of human cingulate cortex. Brain Behav. 2018;8:e01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Du J, Rolls ET, Cheng W, et al. Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex. 2020;123:185–199. [DOI] [PubMed] [Google Scholar]
- 64. Amiez C, Wilson CRE, Procyk E. Variations of cingulate sulcal organization and link with cognitive performance. Sci Rep. 2018;8:13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jahn A, Nee DE, Alexander WH, Brown JW. Distinct regions within medial prefrontal cortex process pain and cognition. J Neurosci. 2016;36:12385–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. [DOI] [PubMed] [Google Scholar]
- 67. Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Behrens TEJ, Fox P, Laird A, Smith SM. What is the most interesting part of the brain? Trends Cogn Sci. 2013;17:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gage GJ, Parikh H, Marzullo TC. The cingulate cortex does everything. Ann Improbable Res. 2008;14:12–15. [Google Scholar]
- 70. Ebitz RB, Hayden BY. Dorsal anterior cingulate: A Rorschach test for cognitive neuroscience. Nat Neurosci. 2016;19:1278–1279. [DOI] [PubMed] [Google Scholar]
- 71. Hashiguchi M, Koike T, Morita T, Harada T, Le Bihan D, Sadato N. Neural substrates of accurate perception of time duration: A functional magnetic resonance imaging study. Neuropsychologia. 2022;166:108145. [DOI] [PubMed] [Google Scholar]
- 72. Behrens TEJ, Woolrich MW, Walton ME, Rushworth MFS. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–1221. [DOI] [PubMed] [Google Scholar]
- 73. Gloy K, Herrmann M, Fehr T. Decision making under uncertainty in a quasi realistic binary decision task - an fMRI study. Brain Cogn. 2020;140:105549. [DOI] [PubMed] [Google Scholar]
- 74. Hogan PS, Galaro JK, Chib VS. Roles of ventromedial prefrontal cortex and anterior cingulate in subjective valuation of prospective effort. Cereb Cortex. 2019;29:4277–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meder D, Kolling N, Verhagen L, et al. Simultaneous representation of a spectrum of dynamically changing value estimates during decision making. Nat Commun. 2017;8:1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chong TTJ, Apps M, Giehl K, Sillence A, Grima LL, Husain M. Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 2017;15:e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. [DOI] [PubMed] [Google Scholar]
- 79. Hare TA, Schultz W, Camerer CF, O’Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proc Natl Acad Sci U S A. 2011;108:18120–18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cereb Cortex. 2011;21:95–102. [DOI] [PubMed] [Google Scholar]
- 81. Kurniawan IT, Grueschow M, Ruff CC. Anticipatory energization revealed by pupil and brain activity guides human effort-based decision making. J Neurosci. 2021;41:6328–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de la Peña MJ, Gil-Robles S, de Vega VM, Aracil C, Acevedo A, Rodríguez MR. A practical approach to imaging of the supplementary motor area and its subcortical connections. Curr Neurol Neurosci Rep. 2020;20:50. [DOI] [PubMed] [Google Scholar]
- 83. Bonnelle V, Manohar S, Behrens T, Husain M. Individual differences in premotor brain systems underlie behavioral apathy. Cereb Cortex. 2016;26:807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Müller T, Klein-Flügge MC, Manohar SG, Husain M, Apps MAJ. Neural and computational mechanisms of momentary fatigue and persistence in effort-based choice. Nat Commun. 2021;12:4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fouragnan E, Queirazza F, Retzler C, Mullinger KJ, Philiastides MG. Spatiotemporal neural characterization of prediction error valence and surprise during reward learning in humans. Sci Rep. 2017;7:4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fouragnan E, Retzler C, Philiastides MG. Separate neural representations of prediction error valence and surprise: Evidence from an fMRI meta-analysis. Hum Brain Mapp. 2018;39:2887–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kennerley SW, Behrens TEJ, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14:1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vassena E, Deraeve J, Alexander WH. Surprise, value and control in anterior cingulate cortex during speeded decision-making. Nat Hum Behav. 2020;4:412–422. [DOI] [PubMed] [Google Scholar]
- 89. Zarr N, Brown JW. Hierarchical error representation in medial prefrontal cortex. NeuroImage. 2016;124:238–247. [DOI] [PubMed] [Google Scholar]
- 90. O’Reilly JX, Schuffelgen U, Cuell SF, Behrens TEJ, Mars RB, Rushworth MFS. Dissociable effects of surprise and model update in parietal and anterior cingulate cortex. Proc Natl Acad Sci U S A. 2013;110:E3660–E3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. [DOI] [PubMed] [Google Scholar]
- 92. Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Valenza G, Sclocco R, Duggento A, et al. The central autonomic network at rest: Uncovering functional MRI correlates of time-varying autonomic outflow. NeuroImage. 2019;197:383–390. [DOI] [PubMed] [Google Scholar]
- 94. Gillies MJ, Huang Y, Hyam JA, Aziz TZ, Green AL. Direct neurophysiological evidence for a role of the human anterior cingulate cortex in central command. Auton Neurosci. 2019;216:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Leuchs L, Schneider M, Czisch M, Spoormaker VI. Neural correlates of pupil dilation during human fear learning. Neuroimage. 2017;147:186–197. [DOI] [PubMed] [Google Scholar]
- 96. Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp. 2014;35:4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schneider M, Hathway P, Leuchs L, Sämann PG, Czisch M, Spoormaker VI. Spontaneous pupil dilations during the resting state are associated with activation of the salience network. NeuroImage. 2016;139:189–201. [DOI] [PubMed] [Google Scholar]
- 98. Schneider M, Leuchs L, Czisch M, Sämann PG, Spoormaker VI. Disentangling reward anticipation with simultaneous pupillometry/fMRI. NeuroImage. 2018;178:11–22. [DOI] [PubMed] [Google Scholar]
- 99. Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27:885–895. [DOI] [PubMed] [Google Scholar]
- 100. Amiez C, Procyk E. Midcingulate somatomotor and autonomic functions. Handb Clin Neurol. 2019;166:53–71. [DOI] [PubMed] [Google Scholar]
- 101. Livesey AC, Wall MB, Smith AT. Time perception: Manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45:321–331. [DOI] [PubMed] [Google Scholar]
- 102. Kohoutová L, Atlas LY, Büchel C, et al. Individual variability in brain representations of pain. Nat Neurosci. 2022;25:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wager TD, Atlas LY, Botvinick MM, et al. Pain in the ACC? Proc Natl Acad Sci U S A. 2016;113:E2474–E2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2:403–405. [DOI] [PubMed] [Google Scholar]
- 107. Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. NeuroImage. 2011;54:2237–2249. [DOI] [PubMed] [Google Scholar]
- 108. Lévêque M. La psychochirurgie dans le traitement de la douleur. Douleurs Eval Diagn Trait. 2016;17:192–199. [Google Scholar]
- 109. Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol. 2011;21:195–199. [DOI] [PubMed] [Google Scholar]
- 110. Pessiglione M, Vinckier F, Bouret S, Daunizeau J, Le Bouc R. Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain. 2018;141:629–650. [DOI] [PubMed] [Google Scholar]
- 111. Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. [DOI] [PubMed] [Google Scholar]
- 112. Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. [DOI] [PubMed] [Google Scholar]
- 113. Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. [DOI] [PubMed] [Google Scholar]
- 114. MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. [DOI] [PubMed] [Google Scholar]
- 115. van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. [DOI] [PubMed] [Google Scholar]
- 116. Yeung N, Cohen JD. The impact of cognitive deficits on conflict monitoring: Predictable dissociations between the error-related negativity and N2. Psychol Sci. 2006;17:164–171. [DOI] [PubMed] [Google Scholar]
- 117. Fan J. An information theory account of cognitive control. Front Hum Neurosci. 2014;8:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wu T, Chen C, Spagna A, et al. The functional anatomy of cognitive control: A domain-general brain network for uncertainty processing. J Comp Neurol. 2020;528:1265–1292. [DOI] [PubMed] [Google Scholar]
- 119. Garrison J, Erdeniz B, Done J. Prediction error in reinforcement learning: A meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2013;37:1297–1310. [DOI] [PubMed] [Google Scholar]
- 120. Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: Neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J Neurosci. 2011;31:4178–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ide JS, Shenoy P, Yu AJ, Li C, Shan R. Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci. 2013;33:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Holroyd CB, Nieuwenhuis S, Yeung N, et al. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat Neurosci. 2004;7:497–498. [DOI] [PubMed] [Google Scholar]
- 123. Iannaccone R, Hauser TU, Staempfli P, Walitza S, Brandeis D, Brem S. Conflict monitoring and error processing: New insights from simultaneous EEG–fMRI. NeuroImage. 2015;105:395–407. [DOI] [PubMed] [Google Scholar]
- 124. Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. [DOI] [PubMed] [Google Scholar]
- 125. Brown JW. Beyond conflict monitoring: Cognitive control and the neural basis of thinking before you act. Curr Dir Psychol Sci. 2013;22:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brown JW, Alexander WH. Foraging value, risk avoidance, and multiple control signals: How the anterior cingulate cortex controls value-based decision-making. J Cogn Neurosci. 2017;29:1656–1673. [DOI] [PubMed] [Google Scholar]
- 127. Freidin E, Kacelnik A. Rational choice, context dependence, and the value of information in European starlings (Sturnus vulgaris). Science. 2011;334:1000–1002. [DOI] [PubMed] [Google Scholar]
- 128. Fouragnan EF, Chau BKH, Folloni D, et al. The macaque anterior cingulate cortex translates counterfactual choice value into actual behavioral change. Nat Neurosci. 2019;22:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat Neurosci. 2011;14:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Boorman ED, Rushworth MF, Behrens TE. Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. J Neurosci. 2013;33:2242–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Donoso M, Collins AG, Koechlin E. Foundations of human reasoning in the prefrontal cortex. Science. 2014;344:1481–1486. [DOI] [PubMed] [Google Scholar]
- 132. Hockey GRJ. A motivational control theory of cognitive fatigue. In: Ackerman PL, ed. Cognitive fatigue: Multidisciplinary perspectives on current research and future applications: American Psychological Association; 2011: 167–187. [Google Scholar]
- 133. Inzlicht M, Schmeichel BJ. What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspect Psychol Sci. 2012;7:450–463. [DOI] [PubMed] [Google Scholar]
- 134. Inzlicht M, Schmeichel BJ, Macrae CN. Why self-control seems (but may not be) limited. Trends Cogn Sci. 2014;18:127–133. [DOI] [PubMed] [Google Scholar]
- 135. Kurzban R. The sense of effort. Curr Opin Psychol. 2016;7:67–70. [Google Scholar]
- 136. Brown JW. Conflict effects without conflict in anterior cingulate cortex: Multiple response effects and context specific representations. Neuroimage. 2009;47:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Walton ME, Devlin JT, Rushworth MFS. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. [DOI] [PubMed] [Google Scholar]
- 138. Kennerley SW, Walton ME, Behrens TEJ, Buckley MJ, Rushworth MFS. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. [DOI] [PubMed] [Google Scholar]
- 139. Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. [DOI] [PubMed] [Google Scholar]
- 140. Walton ME, Bannerman DM, Rushworth MFS. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Walton ME, Bannerman DM, Alterescu K, Rushworth MFS. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. [DOI] [PubMed] [Google Scholar]
- 143. Rushworth MFS, Buckley MJ, Behrens TEJ, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. [DOI] [PubMed] [Google Scholar]
- 144. Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. [DOI] [PubMed] [Google Scholar]
- 145. Walton ME, Croxson PL, Behrens TEJ, Kennerley SW, Rushworth MFS. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(Suppl 2):T142–T154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Holroyd CB, McClure SM. Hierarchical control over effortful behavior by rodent medial frontal cortex: A computational model. Psychol Rev. 2015;122:54–83. [DOI] [PubMed] [Google Scholar]
- 147. Silvetti M, Seurinck R, Verguts T. Value and prediction error in medial frontal cortex: Integrating the single-unit and systems levels of analysis. Front Hum Neurosci. 2011;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Anderson CA, Arciniegas DB, Huddle DC, Leehey MA. Akinetic mutism following unilateral anterior cerebral artery occlusion. J Neuropsychiatry Clin Neurosci. 2003;15:385–386. [DOI] [PubMed] [Google Scholar]
- 149. Freemon FR. Akinetic mutism and bilateral anterior cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 1971;34:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Laplane D, Degos JD, Baulac M, Gray F. Bilateral infarction of the anterior cingulate gyri and of the fornices. Report of a case. J Neurol Sci. 1981;51:289–300. [DOI] [PubMed] [Google Scholar]
- 151. Cairns H, Oldfield RC, Pennybacker JB, Whitteridge D. Akinetic mutism with an epidermoid cyst of the 3rd ventricle. Brain. 1941;64:273–290. [Google Scholar]
- 152. Darby RR, Joutsa J, Burke MJ, Fox MD. Lesion network localization of free will. Proc Natl Acad Sci U S A. 2018;115:10792–10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Le Bouc R, Borderies N, Carle G, et al. Effort avoidance as a core mechanism of apathy in frontotemporal dementia. Brain. 2023;146:712–726. [DOI] [PubMed] [Google Scholar]
- 154. Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013;80:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Trevisi G, Eickhoff SB, Chowdhury F, et al. Probabilistic electrical stimulation mapping of human medial frontal cortex. Cortex. 2018;109:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zacharopoulos G, Shenhav A, Constantino S, Maio GR, Linden DEJ. The effect of self-focus on personal and social foraging behaviour. Soc Cogn Affect Neurosci. 2018;13:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sheth SA, Mian MK, Patel SR, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nieuwenhuis S, Schweizer TS, Mars RB, Botvinick MM, Hajcak G. Error-likelihood prediction in the medial frontal cortex: A critical evaluation. Cereb Cortex. 2007;17:1570–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:266–277. [DOI] [PubMed] [Google Scholar]
- 160. Yeung N, Nieuwenhuis S. Dissociating response conflict and error likelihood in anterior cingulate cortex. J Neurosci. 2009;29:14506–14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jahn A, Nee DE, Brown JW. The neural basis of predicting the outcomes of imagined actions. Front Neurosci. 2011;5:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shenhav A, Straccia MA, Cohen JD, Botvinick MM. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci. 2014;17:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Shenhav A, Straccia MA, Botvinick MM, Cohen JD. Dorsal anterior cingulate and ventromedial prefrontal cortex have inverse roles in both foraging and economic choice. Cogn Affect Behav Neurosci. 2016;16:1127–1139. [DOI] [PubMed] [Google Scholar]
- 164. Kolling N, Scholl J, Chekroud A, Trier HA, Rushworth MFS. Prospection, perseverance, and insight in sequential behavior. Neuron. 2018;99:1069–1082.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Goussi-Denjean C, Fontanier V, Stoll FM, Procyk E. The differential weights of motivational and task performance measures on medial and lateral frontal neural activity. J Neurosci. 2023;43:4329–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Stoll FM, Fontanier V, Procyk E. Specific frontal neural dynamics contribute to decisions to check. Nat Commun. 2016;7:11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Tervo DGR, Proskurin M, Manakov M, et al. Behavioral variability through stochastic choice and its gating by anterior cingulate cortex. Cell. 2014;159:21–32. [DOI] [PubMed] [Google Scholar]
- 168. Tervo DGR, Kuleshova E, Manakov M, et al. The anterior cingulate cortex directs exploration of alternative strategies. Neuron. 2021;109:1876–1887.e6. [DOI] [PubMed] [Google Scholar]
- 169. Newman LA, Creer DJ, McGaughy JA. Cognitive control and the anterior cingulate cortex: How conflicting stimuli affect attentional control in the rat. J Physiol Paris. 2015;109(1–3):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Carr JK, Fournier NM, Lehmann H. Increased task demand during spatial memory testing recruits the anterior cingulate cortex. Learn Mem. 2016;23:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kuwabara M, Mansouri FA, Buckley MJ, Tanaka K. Cognitive control functions of anterior cingulate cortex in macaque monkeys performing a Wisconsin Card Sorting Test analog. J Neurosci. 2014;34:7531–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gluth S, Rieskamp J, Büchel C. Neural evidence for adaptive strategy selection in value-based decision-making. Cereb Cortex. 2014;24:2009–2021. [DOI] [PubMed] [Google Scholar]
- 173. Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. [DOI] [PubMed] [Google Scholar]
- 174. Shenhav A, Musslick S, Botvinick MM, Cohen JD. Misdirected vigor: Differentiating the control of value from the value of control. PsyArXiv. [Preprint]. 2020. doi: 10.31234/osf.io/5bhwe [DOI]
- 175. Bush KA, James GA, Privratsky AA, Fialkowski KP, Kilts CD. Action-value processing underlies the role of the dorsal anterior cingulate cortex in performance monitoring during self-regulation of affect. PLoS One. 2022;17:e0273376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jahn A, Nee DE, Alexander WH, Brown JW. Distinct regions of anterior cingulate cortex signal prediction and outcome evaluation. Neuroimage. 2014;95:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. Conflict, error likelihood, and RT: Response to Brown & Yeung et al. NeuroImage. 2011;57:320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. NeuroImage. 2011;57:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Carp J, Fitzgerald KD, Taylor SF, Weissman DH. Removing the effect of response time on brain activity reveals developmental differences in conflict processing in the posterior medial prefrontal cortex. NeuroImage. 2012;59:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci U S A. 2010;107:7922–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Neta M, Schlaggar BL, Petersen SE. Separable responses to error, ambiguity, and reaction time in cingulo-opercular task control regions. NeuroImage. 2014;99:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wu T, Dufford AJ, Egan LJ, et al. Hick–Hyman law is mediated by the cognitive control network in the brain. Cereb Cortex. 2018;28:2267–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yeung N, Cohen JD, Botvinick MM. Errors of interpretation and modeling: A reply to Grinband et al. Neuroimage. 2011;57:316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lee DG, Daunizeau J, Pezzulo G. Evidence or confidence: What is really monitored during a decision? Psychon Bull Rev. 2023;30:1360–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lee D, Daunizeau J. Trading mental effort for confidence in the metacognitive control of value-based decision-making. eLife. 2021;10:e63282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol. 2000;83:1701–1709. [DOI] [PubMed] [Google Scholar]
- 187. Brown JW. Medial prefrontal cortex activity correlates with time-on-task: What does this tell us about theories of cognitive control? NeuroImage. 2011;57:314–315. [DOI] [PubMed] [Google Scholar]
- 188. Forster SE, Brown JW. Medial prefrontal cortex predicts and evaluates the timing of action outcomes. Neuroimage. 2011;55:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacol. 2011;36:3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Coull JT, Vidal F, Burle B. When to act, or not to act: That’s the SMA’s question. Curr Opin Behav Sci. 2016;8:14–21. [Google Scholar]
- 191. Rushworth MFS, Kennerley SW, Walton ME. Cognitive neuroscience: Resolving conflict in and over the medial frontal cortex. Curr Biol. 2005;15:R54–R56. [DOI] [PubMed] [Google Scholar]
- 192. Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Vassena E, Deraeve J, Alexander WH. Predicting motivation: Computational models of PFC can explain neural coding of motivation and effort-based decision-making in health and disease. J Cogn Neurosci. 2017;29:1633–1645. [DOI] [PubMed] [Google Scholar]
- 194. Botvinik-Nezer R, Holzmeister F, Camerer CF, et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Anderson ML. Neural reuse: A fundamental organizational principle of the brain. Behav Brain Sci. 2010;33:245–266. [DOI] [PubMed] [Google Scholar]
- 196. Bergeron V. Anatomical and functional modularity in cognitive science: Shifting the focus. Philos Psychol. 2007;20:175–195. [Google Scholar]
- 197. Salehi M, Greene AS, Karbasi A, Shen X, Scheinost D, Constable RT. There is no single functional atlas even for a single individual: Functional parcel definitions change with task. Neuroimage. 2020;208:116366. [DOI] [PubMed] [Google Scholar]
- 198. Salehi M, Karbasi A, Barron DS, Scheinost D, Constable RT. Individualized functional networks reconfigure with cognitive state. Neuroimage. 2020;206:116233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Raichle ME. The restless brain. Brain Connect. 2011;1:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Hutzler F. Reverse inference is not a fallacy per se: Cognitive processes can be inferred from functional imaging data. Neuroimage. 2014;84:1061–1069. [DOI] [PubMed] [Google Scholar]
- 201. Krueger JI, Heck PR. The heuristic value of p in inductive statistical inference. Front Psychol. 2017;8:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Shenhav A, Botvinick M. Uncovering a missing link in anterior cingulate research. Neuron. 2015;85:455–457. [DOI] [PubMed] [Google Scholar]
- 203. Woo JH, Azab H, Jahn A, Hayden B, Brown JW. The PRO model accounts for the anterior cingulate cortex role in risky decision-making and monitoring. Cogn Affect Behav Neurosci. 2022;22:952–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5:115–133. [PubMed] [Google Scholar]
- 205. Khamassi M, Lallée S, Enel P, Procyk E, Dominey PF. Robot cognitive control with a neurophysiologically inspired reinforcement learning model. Front Neurorobot. 2011;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. LeCun Y. A path towards autonomous machine intelligence. Open Review. Published 27 June 2022 (version 0.9.2). https://openreview.net/pdf?id=BZ5a1r-kVsf [Google Scholar]
- 207. Khamassi M, Enel P, Dominey PF, Procyk E. Medial prefrontal cortex and the adaptive regulation of reinforcement learning parameters. Prog Brain Res. 2013;202:441–464. [DOI] [PubMed] [Google Scholar]
- 208. Kool W, Botvinick M. Mental labour. Nat Hum Behav. 2018;2:899–908. [DOI] [PubMed] [Google Scholar]
- 209. Fine JM, Zarr N, Brown JW. Computational neural mechanisms of goal-directed planning and problem solving. Comput Brain Behav. 2020;3:472–493. [Google Scholar]
- 210. Zarr N, Brown JW. Foundations of human spatial problem solving. Sci Rep. 2023;13:1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Petrides M, Mackey S. The orbitofrontal cortex: Sulcal and gyral morphology and architecture. In: Zald D, Rauch S, eds. The orbitofrontal cortex: Oxford University Press; 2006: 19–38. [Google Scholar]
- 212. Chiavaras MM, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. J Comp Neurol. 2000;422:35–54. [PubMed] [Google Scholar]
- 213. Imada Y, Takumi T, Aoyama H, Sadatomo T, Kurisu K. Morphological classification of the medial frontal cortex based on cadaver dissections: A guide for interhemispheric approach. Neurol Med Chir(Tokyo). 2021;61:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Li Y, Sescousse G, Amiez C, Dreher JC. Local morphology predicts functional organization of experienced value signals in the human orbitofrontal cortex. J Neurosci. 2015;35:1648–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Lopez-Persem A, Verhagen L, Amiez C, Petrides M, Sallet J. The human ventromedial prefrontal cortex: Sulcal morphology and its influence on functional organization. J Neurosci. 2019;39:3627–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Smith SM, Beckmann CF, Andersson J, et al. Resting-state fMRI in the human connectome project. NeuroImage. 2013;80:144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Sallet J, Mars RB, Noonan MP, et al. The organization of dorsal frontal cortex in humans and macaques. J Neurosci. 2013;33:12255–12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Van Essen DC, Ugurbil K, Auerbach E, et al. The human connectome project: A data acquisition perspective. Neuroimage. 2012;62:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Lopez-Persem A, Roumazeilles L, Folloni D, et al. Differential functional connectivity underlying asymmetric reward-related activity in human and nonhuman primates. Proc Natl Acad Sci U S A. 2020;117:28452–28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Han X, Ashar YK, Kragel P, et al. Effect sizes and test-retest reliability of the fMRI-based neurologic pain signature. NeuroImage. 2022;247:118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kragel PA, Han X, Kraynak TE, Gianaros PJ, Wager TD. Functional MRI can be highly reliable, but it depends on what you measure: A commentary on Elliott et al. (2020). Psychol Sci. 2021;32:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Margulies DS, Ghosh SS, Goulas A, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A. 2016;113:12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Elliott ML, Knodt AR, Ireland D, et al. What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol Sci. 2020;31:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Umemoto A, Lin H, Holroyd CB. Electrophysiological measures of conflict and reward processing are associated with decisions to engage in physical effort. Psychophysiology. 2023;60:e14176. [DOI] [PubMed] [Google Scholar]
- 225. Soutschek A, Nadporozhskaia L, Christian P. Brain stimulation over dorsomedial prefrontal cortex modulates effort-based decision making. Cogn Affect Behav Neurosci. 2022;22:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shenhav A, Musslick S, Lieder F, et al. Toward a rational and mechanistic account of mental effort. Annu Rev Neurosci. 2017;40:99–124. [DOI] [PubMed] [Google Scholar]
- 227. Kool W, Botvinick M. A labor/leisure tradeoff in cognitive control. J Exp Psychol Gen. 2014;143:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]