Abstract
Basket, umbrella, and platform trial designs (master protocols) have emerged over the last decade to study precision medicine approaches in oncology. First-generation trials like NCI-MATCH (Molecular Analysis for Therapy Choice) have proven the principle that studying targeted therapies on a large scale is feasible both from the laboratory and clinical perspectives. However, single-agent targeted therapies have shown limited ability to control metastatic disease, despite careful matching of drug to target. As such, newer approaches employing combinations of targeted therapy, or targeted therapy with standard therapies, need to be considered. The NCI has recently embarked on three second-generation precision medicine trials to address this need: ComboMATCH, iMATCH, and myeloMATCH. The design of these trials and necessary infrastructure are discussed in the following perspective.
Introduction
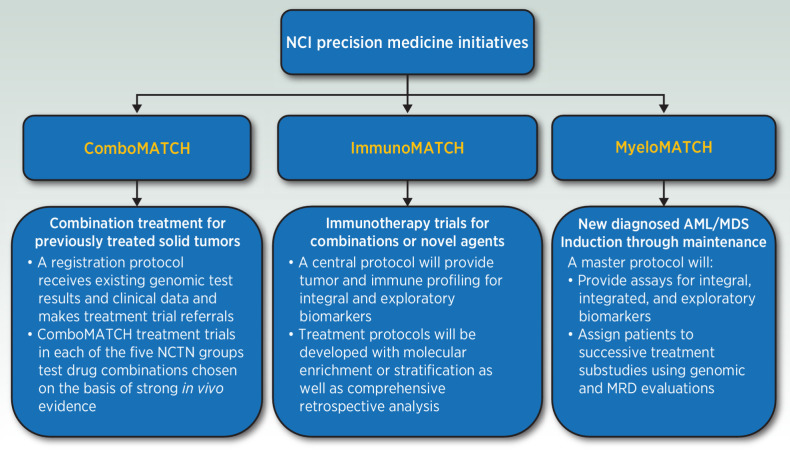
Availability of advanced molecular diagnostic tools and the development of numerous targeted agents ushered in the era of cancer precision medicine (1). Initial platform studies exploring precision medicine concepts, including the NCI-MATCH (Molecular Analysis for Therapy Choice) trial, have led to a proliferation of similar trials in the research community (2). The NCI has supported these efforts and has now developed the next generation of cancer precision medicine trials aimed at ongoing refinement of this approach. This article describes the rationale and collaborative structure of these trials (Fig. 1).
Figure 1.
The New Precision Medicine Initiatives of the National Cancer Institute.
NCI-MATCH and other precision medicine initiatives
The NCI-MATCH (Molecular Analysis for Therapy Choice) study was a precision medicine initiative (PMI) sponsored by NCI that recruited over 6,000 patients in a period of less than 2 years (3). The trial showed that it was feasible to screen and recruit patients using both central next-generation sequencing (NGS) and well-vetted community-based NGS. However, although 38% of screened patients had a potentially actionable mutation, there was a relatively low response rate to single agents. Resistance to therapy with single targeted agents has been attributed to multiple mechanisms, including resistance mutations and multi-genic or other adaptive responses, and suggests that approaches to overcome resistance are needed to achieve durable clinical benefit.
Since the original NCI-MATCH trial, the therapeutic landscape of cancer therapy has evolved substantially. The introduction of successful immunotherapies into cancer treatment has resulted in the need to understand and target underlying mechanisms of sensitivity and resistance to immune-targeted agents. In addition, the vision of precision medicine has evolved from the single target-agent paradigm to embrace more nuanced approaches to cancer treatment, in which therapies are developed based not only on driver mutations but also on observed resistance pathways. The next iteration of NCI's Precision Medicine Trials represents a multi-dimensional approach to cancer precision medicine. NCI has developed three new trials to address the areas of molecularly targeted treatment combinations, patient stratification or selection based on tumor immune characteristics, and tiered studies based on primary disease and minimal residual disease in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS; Fig. 1).
The Next Generation of PMI Trials
ComboMATCH
Most NCI-MATCH arms did not meet their clinical endpoints. This has reinforced the concept that inhibition of a single driver gene alone is not adequate to produce a clinical benefit in most malignancies. Cancers often recruit parallel or compensatory pathways to overcome inhibition of a single node, providing one plausible biological explanation for the NCI-MATCH clinical experience (4). ComboMATCH has been designed to test specific molecularly targeted combinations aimed at overcoming primary and adaptive resistance pathways.
Designing a precision medicine trial using drug combinations rather than single agents presents numerous complexities. In NCI-MATCH, the evidence threshold for testing a single drug against a single target was relatively straightforward; in ComboMATCH, the number of potential drug combinations is exponentially greater, and the number and complexity of targets is substantially enhanced. Therefore, a combination approved by the ComboMATCH Agents and Genes Working Group, made up of representation from each of the National Clinical Trial Network (NCTN) groups including the Children's Oncology Group, must demonstrate a combinatorial effect of the drug combination and a tumor response of regression or sustained stabilization in at least two relevant in vivo models to support its use, and a recommended phase II dose for the combination. Selected drug combinations without phase II dose determinations are diverted into phase I studies for later incorporation in ComboMATCH. In addition, appropriate statistical designs (typically randomized) and evidence thresholds for evaluating promising drug combinations were developed, and these are being applied uniformly to all prospective studies. Furthermore, many of the treatment arms limit the number of regimens and performance status in an effort to optimize the opportunity for treatment response.
Immunotherapy-MATCH
Immunotherapy-MATCH (iMATCH) will provide a central platform for tissue procurement and molecular testing, with the goal of enhancing immunotherapy trials through prospective patient enrichment or stratification as well as comprehensive exploratory analysis. Currently, most clinical studies of immunotherapy combinations are conducted in “all-comers” due to lack of patient selection markers, and the results are often negative and noninformative. We proposed that, while regimen-specific markers are unavailable, several clinical grade biomarkers developed for anti-PD-1/L1 monotherapy prospectively characterize elements of adaptive immunity for “biological” stratification. Examples include tumor mutational burden (TMB) as proxy for immunogenicity, and IFNγ signatures or tumor-infiltrating CD8+ lymphocyte staining as markers of tumor immunity (5, 6). It is hypothesized that composite biomarkers like TMB and tumor inflammation score (TIS) can be used to separate patients into subgroups with potentially different immune status (e.g., immune inflamed, immune excluded or immune desert), and each subgroup may have a set of immune evasion mechanisms that can be targeted with relevant combination strategies.
The iMATCH platform trial includes a central assay protocol for both integral and exploratory biomarkers. Patients will be stratified or enriched on the basis of TMB (High vs. Low), and TIS (High vs. Low). In addition, data from whole-exome sequencing (WES) and RNA sequencing (RNA-seq) will enable broader retrospective analysis for discovery of resistance mechanisms and to refine patient selection strategies in the context of specific tumor settings and treatment regimes. Treatment protocols will be developed by NCTN, with a focus on signal-seeking trials of immuno-oncology combinations. Agent/regimen selection will consider supporting data from preclinical models or clinical translational studies, as well as clinical experience and “credentials” of individual agents or the combination. We envision that most protocols under iMATCH will be histology-specific in either immunotherapy-naïve or -refractory setting, depending on feasibility and scientific hypothesis.
iMATCH addresses unique logistical, technical, and trial design challenges that set it apart from other NCI-supported precision medicine platforms in development. First, unlike in the NCI-MATCH trial, the integral markers in iMATCH will define biological subgroups not tied to specific molecular targets. Second, TMB and TIS measurements are continuous variables and require predefined cut-off points for prospective use, yet existing data are limited for identifying the optimal cutoff for all clinical settings under study (e.g., immunotherapy naïve vs. refractory setting). To address these challenges, a pilot trial is being conducted before full launch of iMATCH protocols, to resolve details of biomarker assessment as well as establish the feasibility and clinical acceptability of the turnaround times.
MyeloMATCH
MyeloMATCH is the first NCI PMI for myeloid cancers funded by the NCI intended to treat patients with myeloid cancers from diagnosis throughout their treatment journey. The NCTN Leukemia committees have committed to the myeloMATCH platform as the means through which the AML and MDS trial portfolios for initially diagnosed patients will be established and conducted. The goal is to develop a suite of studies attractive to patients, investigators, and industry collaborators in myeloid malignancies. A myeloMATCH Master Screening and Reassessment Protocol (MM-MSRP) evaluates newly diagnosed patients and assigns them to a treatment protocol based on clinical and genomic features. In general, the investigations are planned as randomized phase II trials seeking large signals within clinical or molecular subgroups to inform subsequent more precise study of selected patients with AML/MDS.
The myeloMATCH MSRP and informatics platform will facilitate cross-treatment study interrogation of genomic features and response characteristics. This will enable hypothesis generating ad hoc studies that may help identify scientific opportunities to advance effective therapeutics in AML and MDS. The MSRP design provides a platform for myeloMATCH senior laboratory experts and statisticians with specialized expertise in biomarkers and genomics to assist and advise the clinical investigators in myeloMATCH. The myeloMATCH Senior Scientific Council then approves studies that are vetted by the NCTN Leukemia Steering Committee. In this manner, myeloMATCH brings a critical mass of AML and MDS experts from throughout the United States and Canada to devise clinical trials aimed at improving precision treatment for patients with progressively decreasing disease burden of myeloid cancers undergoing successive therapeutic approaches. This effort will also generate data to inform use of various flow cytometric and molecular assays for identification and targeting of residual disease. The overarching myeloMATCH goals are to assess the impact of genomically-selected treatments, from diagnosis (Tier 1) through consolidation (Tier 2), allogeneic transplant when indicated (Tier 3), and then effective targeting of measurable residual disease (Tier 4).
NCI Resources Deployed to Support PMIs
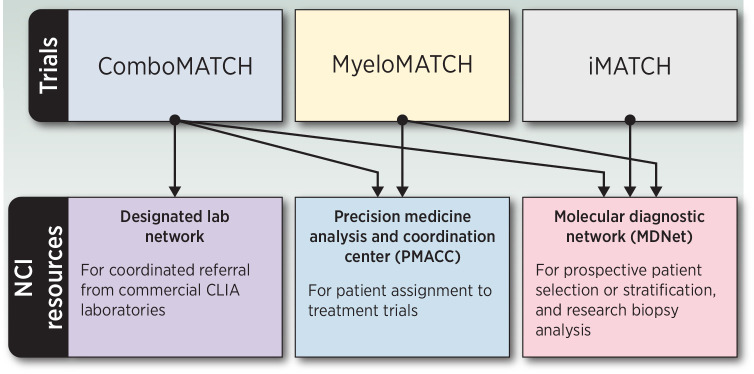
Each of these initiatives requires novel and extensive informatics and central laboratory support. Both iMATCH and myeloMATCH will require diagnostic testing with an expedited turnaround time for patient therapy assignment. Additional resources for biopsies, biobanking, and regulatory support will be provided (Fig. 2).
Figure 2.
NCI resources supporting the Precision Medicine Initiatives.
NCI-supported centralized biospecimen management, patient selection, and allocation
The molecular and immunologic diagnostic laboratory network
These new PMIs will integrate lessons learned in NCI-MATCH and take advantage of novel technologies that provide more comprehensive molecular analysis, permitting a greater depth of understanding of tumor biology. The NCI has established the Molecular and Immunologic Diagnostic Laboratory Network (MDNet) to provide both real-time diagnostic services to support iMATCH and myeloMATCH and retrospective analyses such as WES, RNA-seq, and evaluation of cell-free DNA (cfDNA) in ComboMATCH and iMATCH. The goal is not only to improve outcomes with novel therapies but also to learn more from every patient about how molecular and other characteristics can be used to optimally select therapy.
ComboMATCH will initially use DNA sequencing performed by commercial and academic laboratories (known as the Designated Laboratory Network); generally, only one actionable mutation of interest (aMOI) will be used to select therapy although some arms do have exclusionary variants. MDNet will perform WES to assess molecular concordance with aMOIs detected by the Designated Laboratories. For iMATCH, MDNet will provide prospective TMB and TIS data as well as actionable mutations that will be used to define molecular subgroups for clinical testing of specific treatment arms. For myeloMATCH, MDNet assays will also be used to evaluate responses, and potentially reassign responding patients to the next myeloMATCH treatment protocol, proceeding roughly parallel to the standard approach for these diseases, including transplant and maintenance therapy. In addition, MRD will be assessed using both flow cytometry and double-strand NGS to improve methods for determination of this important treatment endpoint and for protocol assignment. These approaches will eventually amass sufficient data to allow for both longitudinal and cross-study assessments of the natural history of AML and MDS.
All three precision medicine studies will also include more detailed retrospective molecular characterization (WES and RNA-seq, cfDNA, protein studies) for further exploration. These investigations will be performed on the bone marrow biopsies for myeloMATCH, and from tumor biopsies for iMATCH and ComboMATCH.
The Precision Medicine Analysis and Coordination Center
To support the simultaneous development of multiple PMI trials, NCI recognized the need to leverage a unified informatics methodology. NCI established the Precision Medicine Analysis and Coordination Center (PMACC) as a data and bioinformatics support center to implement a high percentage of infrastructure commonality across initiatives. The primary goal of the PMACC is to facilitate the assignment of patients to the highest priority treatment without delay and with full traceability.
On the basis of lessons learned from NCI-MATCH and new, more sophisticated initiative designs, the PMACC is enhancing the existing NCTN clinical trial infrastructure. These enhancements include both modifications and standardized configurations to the patient registration system, clinical data management system, specimen tracking approach, terminology services, and integration layers. At the heart of ComboMATCH and myeloMATCH is the NCI-MATCHBox precision medicine platform. This platform houses the treatment assignment algorithms, coupled with a clinical verification team, and is fully integrated with the NCTN clinical trial infrastructure.
ComboMATCH will take advantage of capabilities developed to consume data from the Designated Laboratory Network under NCI-MATCH. These capabilities support the automated ingestion and harmonized annotation of molecular sequencing data. For myeloMATCH, the MATCHBox will consume assay data from MDNet to derive treatment assignments. All three of the new initiatives will leverage the existing PMACC Data Warehouse for analysis and reporting.
Regulatory support
NCI recognized that the PMI trials would have to be conducted under the Cancer Therapy Evaluation Program (CTEP) Investigational New Drug (IND) process to be feasible, as only NCI could provide the centralized infrastructure support needed for such initiatives. To facilitate these trials, NCI leadership allowed expeditious negotiation of collaborative research agreements with pharma partners for agents approved by the PMI trial governance committees. This decision greatly streamlined the ability of NCI to access agents required for all three PMI trials.
Challenges for This Next Generation of PMI Trials
Statistical design
The three PMI trials (ComboMATCH, iMATCH, and myeloMATCH) differ substantially in the types of clinical and translational science questions they address. Accordingly, different statistical designs are needed to generate interpretable and convincing evidence of treatment efficacy and biomarker utility, including “intended use” and “fit for purpose” assays.
ComboMATCH is a platform trial comprising multiple subprotocols, each investigating a specific drug combination, that are clustered into cassettes run by different NCTN groups (7). Subprotocols may include multiple cohorts, differing by one or more molecular characteristic, tumor histology, and history of prior treatments and outcomes. Cohorts comprised of patients who never received either agent in the combination generally include a randomization between combination and single agents with a primary endpoint of progression-free survival unless background evidence strongly suggests limited efficacy of one or both single agents in similar cohorts. Nonrandomized single-arm two-stage designs with objective response endpoint are used to study drug combinations for cohorts of patients who have progressed on one or both agents, or when strong background evidence suggests limited single-agent activity. Appropriate, standardized designs for ComboMATCH have been facilitated by a statistical design working group which includes members from both the NCTN network groups and NCI.
The iMATCH trial provides prospective tumor characterization that can enable therapeutic trials in molecularly enriched or stratified patient populations. A phased approach and innovative statistical design were needed to address uncertainty about the turnaround times, cut-off points, feasibility, and predictive ability for candidate biomarkers and signatures. iMATCH has begun with a pilot study of nivolumab and cabozantinib in advanced refractory melanoma and head and neck squamous cell carcinoma which will assess the feasibility of prospective testing as well as efficacy in molecular subgroups based on TMB and TIS (TMB/TIS: High/High, High/Low, Low/High, and Low/Low). The study will have two stages with interim analyses and early stopping rules based on assay turnaround time, subgroup distribution and efficacy within subgroups, and the results from the first stage will determine whether and how stage 2 of the trial will proceed. Retrospective molecular analyses will be planned using WES, RNA-seq, NanoString PanCancer IO 360 panel and multiplex immunofluorescence to further explore optimal cutoffs for biological classification and to identify predictive markers for the specific regimen. The pilot trial will generate valuable evidence to aid in planning future treatment arms and biomarker signatures to be tested within the iMATCH trial platform.
MyeloMATCH aims to conduct studies to improve care for patients diagnosed with AML and MDS throughout the course of their disease. The MM-MSRP will be used to collect and coordinate genomic and other biomarker data to enroll eligible patients on trials at diagnosis and in subsequent phases of their treatment. MyeloMATCH subprotocols will primarily employ independent randomized phase II clinical trial designs powered to detect large signals for promising, new therapies. Patients’ eligibility to enroll on specific trial protocols will be based on phase of therapy (e.g., initial treatment, transplant, maintenance), age group, fitness, and genomic targets. Biospecimens collected under the MM-MSRP will also be leveraged to conduct prespecified and post hoc biomarker analyses using cutting-edge MRD technologies with the goal of clinically validating additional predictive biomarkers.
Complexity of cross-NCTN collaboration
The original NCI-MATCH trial was led by the ECOG-ACRIN network group with representation of other network groups as sub study principal Investigators. The current generation of PMI trials is intended to involve all the network groups equally. This requires an unparalleled collaborative effort, with each group having representation on the governance committees of each PMI and an active role in protocol authoring. This also requires collaboration for the NCTN operations offices and enrolling sites about data sharing and specimen tracking and allocation and requires software solutions for communication between PMACC and MDNet labs. The PMI collaboration has also motivated additional discussions between several of the network groups to formulate additional best practices and standards for data collection beyond the PMIs.
Each PMI trial also has its own governance structure to collaboratively review and approve subprotocols prior to submission to CTEP, NCI, and to manage the study development and implementation process.
For ComboMATCH, an effort was made to minimize the number of committees involved in protocol development. The Agents and Genes Working Group (C-AGWG), with representation from each of the NCTN groups, established the levels of evidence required for ComboMATCH studies, and C-AGWG review became the first step in study development. Concepts initially approved by C-AGWG are presented to the proposed collaborating pharmaceutical partners; if informally approved, then the study concept is presented to the Statistical Design Development Working Group, again composed of representatives from each of the NCTN groups, which critically evaluates the designs and their underlying assumptions for the cohorts within each subprotocol as described above. Subprotocols reaching this point are then presented to the ComboMATCH Steering Committee. Three other committees provided operational oversight for the ComboMATCH study development process: a Protocol Logistics Working Group, the PMACC, and the Molecular Biomarker and Specimen Management Committee.
For iMATCH, the pilot will be managed by the SWOG Cancer Research Network with other groups participating in the full-scale initiative.
For myeloMATCH, senior leadership comprises representatives from each of the NCTN Groups, including the Group Leukemia Committee Chairs. Individual NCTN investigators develop clinical trial concepts with guidance and input from leadership. Once a concept is endorsed by the senior myeloMATCH leadership, it is further developed within the Leukemia Committee of the NCTN group that will lead that specific treatment trial. Each protocol includes study champions from each NCTN group.
Each of the PMIs encourages participation of early and mid-career investigators to provide opportunities for collaboration and career development in clinical trials methodology. Young NCTN investigators receive mentoring throughout this process.
Summary and Conclusions
Our understanding of precision cancer medicine has changed dramatically in the decade between the planning of the original MATCH trial and the planning of its successor trials described in this article. To address the therapeutic challenges that initial precision medicine studies revealed, ComboMATCH, myeloMATCH, and iMATCH have been developed. Each involves multiple partners from the pharmaceutical industry, academia, NCI cooperative group networks, and NCI staff. This collaboration is necessary to assure that the carefully designed studies using well-characterized, analytically validated assays in appropriate patient populations will achieve the dual goals of optimizing individualized therapy for patients, while at the same time discovering approaches that have an impact on the treatment of future patients. The ability to conduct trials of this complexity is made possible by a strong commitment across the oncology community and will reap rewards for years to come.
Authors' Disclosures
C.D. Blanke reports grants from NCI during the conduct of the study. H.P. Erba reports grants and other support from AbbVie, Agios, Daiichi Sankyo, Glycomimetics, and Jazz; grants from ALX Oncology, Amgen, Forma, Forty Seven, Gilead, ImmunoGen, MacroGenics, Novartis, and PTC; and other support from Celgene/BMS, Incyte, Astellas, Genentech, Kura Oncology, Novartis, Syros, Takeda, and Trillium outside the submitted work. J.M. Ford reports grants from Genentech and Merus outside the submitted work. R.J. Gray reports grants from NCI during the conduct of the study. M.L. LeBlanc reports grants from NIH during the conduct of the study. S. Hu-Lieskovan reports personal fees from Regeneron, Ascendis, BMS, Merck, Amgen, Astellas, Genmab, Nektar, Novartis, Vaccinex, and Xencor during the conduct of the study. S.M. Luger reports personal fees from AbbVie, Amgen, and BMS and grants from Celgene and Hoffman-La Roche outside the submitted work. F. Meric-Bernstam reports personal fees from AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, Daiichi Sankyo Co. Ltd., Calibr (a division of Scripps Research), DebioPharm, Ecor1 Capital, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., GT Apeiron, Genentech Inc., Harbinger Health, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, Menarini Group, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Protai Bio Ltd, Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks, Black Diamond, Biovica, Eisai, Fog Pharma, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Sanofi, Silverback Therapeutics, Spectrum Pharmaceuticals, Thera Technologies, and Zentalis; grants from Aileron Therapeutics, Inc., AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co.; and other support from European Organization for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO), and Cholangiocarcinoma Foundation outside the submitted work. K. Politi reports grants from NCI via SWOG during the conduct of the study as well as grants and personal fees from AstraZeneca and Roche/Genentech, grants from Boehringer Ingelheim and D2G Oncology, and personal fees from Janssen and Halda Therapeutics outside the submitted work; in addition, K. Politi has a patent related to EGFR T790M mutation testing licensed and with royalties paid from MSKCC/MolecularMD. No disclosures were reported by the other authors.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
References
- 1. Mateo J, Steuten L, Aftimos P, André F, Davies M, Garralda E, et al. Delivering precision oncology to patients with cancer. Nat Med 2022;28:658–65. [DOI] [PubMed] [Google Scholar]
- 2. Middleton G, Robbins H, Andre F, Swanton C. A state-of-the-art review of stratified medicine in cancer: towards a future precision medicine strategy in cancer. Ann Oncol 2022;33:143–57. [DOI] [PubMed] [Google Scholar]
- 3. Flaherty KT, Gray R, Chen A, Li S, Patton D, Hamilton SR, et al. The molecular analysis for therapy choice (NCI-MATCH) trial: lessons for genomic trial design. J Natl Cancer Inst 2020;112:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flaherty KT, Gray RJ, Chen AP, Li S, McShane LM, Patton D, et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J Clin Oncol 2020;38:3883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. [DOI] [PubMed] [Google Scholar]
- 6. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meric-Bernstam F, Ford JM, O'Dwyer PJ, Shapiro GI, McShane LM, Freidlin B, et al. National cancer institute combination therapy platform trial with molecular analysis for therapy choice (ComboMATCH). Clin Cancer Res 2023;29:1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]