Abstract
NfsA is the major oxygen-insensitive nitroreductase of Escherichia coli, similar in amino acid sequence to Frp, a flavin reductase of Vibrio harveyi. Here, we show that a single amino acid substitution at position 99, which may destroy three hydrogen bonds in the putative active center, transforms NfsA from a nitroreductase into a flavin reductase that is as active as the authentic Frp and a tartrazine reductase that is 30-fold more active than wild-type NfsA.
A newly identified nitroreductase-flavin reductase superfamily (12, 13, 15, 16) consists of two families, A and B, which are distantly related in amino acid sequence to each other. Family A includes NfsA, the major oxygen-insensitive nitroreductase in Escherichia coli (1, 13), and Frp, a Vibrio harveyi flavin mononucleotide (FMN) reductase (3, 6), while NfsB, a minor E. coli nitroreductase (1, 9, 15, 16), and FRase I, the major FMN reductase in Vibrio (or Photobacterium) fischeri (2, 16), are included in family B. In contrast to the two luminescent bacterial enzymes, FRase I and Frp, the E. coli enzymes (NfsA and NfsB) exhibit little or no FMN reductase activity (13, 15). It may thus follow that progenitors of the NfsA/Frp and NfsB/FRase I pairs lost FMN reductase activity during evolution in E. coli cells or acquired FMN reductase activity during evolution in luminescent bacteria. In a previous experiment (14), we showed that a single amino acid substitution at position 124 of NfsB causes the transformation of NfsB from a nitroreductase into an FMN reductase that is much more active than FRase I. That 10 different single amino acid substitutions at position 124 of NfsB resulted in a similar nitroreductase-flavin reductase conversion suggests that Phe-124 in wild-type NfsB may serve as a negative selector or a physical or chemical constraint preventing FMN from gaining access to the active center (14). Indeed, a recent three-dimensional structure analysis indicated that Phe-124 is situated above the active site (or the FMN cofactor) and suggested that Phe-124 plays an essential role in the steering of a competitive inhibitor, dicoumarol, to the active site (4).
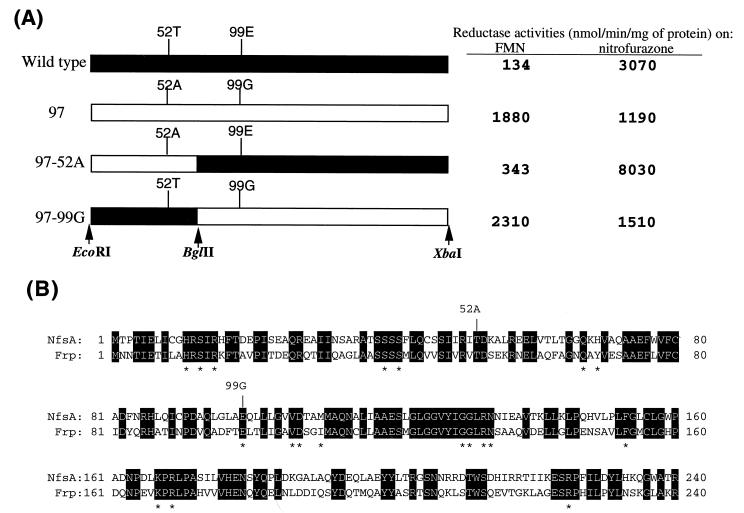
For further clarification of the relationship between nitroreductase and flavin reductase, we examined whether NfsA (family A) possesses key amino acid residues similar in property to Phe-124 in NfsB (family B). The entire NfsA coding region was subjected to PCR mutagenesis in the presence of Mn2+ (7), and an E. coli JM83 (8) cell library with a variety of mutant NfsA expression plasmids (pUC118 [11]) was constructed. As shown in Fig. 1A, the coding sequence in each construct was flanked by artificial restriction sites EcoRI and XbaI. One hundred colonies were selected at random, crude extracts were prepared, and FMN reductase activity was assayed as described previously (13, 15, 16). Exceptionally high FMN reductase activity was found in clone 97 (Fig. 1A). Nucleotide sequence analysis showed the mutant NfsA (NfsA-97) to contain nucleotide changes causing two amino acid substitutions. Thr-52 (ACC) and Glu-99 (GAA) were replaced with alanine (GCC) and glycine (GGA), respectively, in the mutant (Fig. 1B). To determine which amino acid change is responsible for the increment of FMN reductase activity, chimeras in which the two amino acid substitutions are physically separated from each other were constructed. pUC118 plasmids with wild-type or mutant NfsA sequences were digested with either EcoRI/BglII or BglII/XbaI pairs, and chimeric plasmids (NfsA-97-52A or NfsA-97-99G) were generated by subsequent ligation (Fig. 1A). Enzyme assay indicated the glutamic acid-to-glycine substitution at position 99 to be solely responsible for the increment of FMN reductase activity (Fig. 1A). The substrate specificity in NfsA-97-52A was essentially identical to that of the wild type (Fig. 1A).
FIG. 1.
Amino acid changes in NfsA-97, a mutant showing a high level of FMN reductase activity. (A) Structures of wild-type NfsA, NfsA-97, and their chimeras (NfsA-97-52A and -99G). Filled boxes, sequences derived from wild-type NfsA; open boxes, sequences derived from NfsA-97. Cleavage sites for restriction endonucleases used for chimera formation are indicated by vertical arrows. Values for NADPH-FMN and nitrofurazone reductase activities in cell extracts are shown on the right. (B) Amino acid sequence homology between NfsA and Frp. Invariant amino acids are shown by white letters in black boxes. The locations of two amino acid substitutions in NfsA-97 are indicated by vertical lines labeled with the mutated amino acids. According to Tanner et al. (10), H-11, S-13, R-15, S-39, Q-67, G-131, K-167, and R-169 are involved in stabilizing the FMN cofactor. R-225 and R-133 may be required for properly steering substrates to the active center (FMN cofactor). Note that R-225 and R-133 have hydrogen bonds to E-99, which is replaced by a glycine residue in NfsA-97. Asterisks show invariant active center amino acids between Frp and NfsA (see Fig. 2).
NfsA-97-99G protein was purified to homogeneity, as described previously in the case of wild-type NfsA (13). As with wild-type NfsA (13), NfsA-97-99G was found to contain tightly associated FMN (data not shown), indicating the mutation to be irrelevant to binding of FMN cofactor. Enzyme specificity at limiting concentrations of the acceptor is reflected by Vmax/Km, while Vmax may serve as an index for substrate specificity at saturating concentrations of the acceptor. As shown in Table 1, the Vmax/Km and Vmax values for FMN of NfsA-97-99G are 4- and 50-fold, respectively, larger than the counterparts of wild-type NfsA, indicating that NfsA-97-99G is capable of reducing FMN much more effectively than the wild-type enzyme at any FMN concentration. Estimated Km values for FMN showed that wild-type NfsA has an affinity for FMN that is much stronger than those of NfsA-97-99G and Frp. The Vmax/Km and Vmax values of NfsA-97-99G for FMN were also found to be twice as large as those of the authentic flavin reductase, Frp, suggesting that NfsA-97-99G is a more active flavin reductase than Frp (Table 1). Furthermore, the mutant NfsA has a tartrazine reductase activity 30-fold higher than that of wild-type NfsA at saturating concentrations of the acceptor (tartrazine) (Table 2). Activities to reduce substrates other than FMN and tartrazine were also altered either positively or negatively by the introduction of the substitution (Table 2). It may thus follow that Glu-99 is essential for substrate recognition, and the glutamic acid-to-glycine substitution at position 99 caused the transformation of NfsA from a nitroreductase virtually lacking FMN and tartrazine reductase activity into an enzyme with high FMN and tartrazine reductase activities.
TABLE 1.
Kinetic parameters of wild-type and mutant NfsA and Frp
| Enzyme | Substrate | Km (μM) | Vmax (μmol/min/mg) | Vmax/Km |
|---|---|---|---|---|
| Wild-type NfsA | NADPHa,b | 11.0 | 85 | 7.7 |
| NADPHc | 0.8 | 1 | 1.5 | |
| Nitrofurazonea,d | 5.5 | 45 | 8.1 | |
| FMNd | 2.5 | 1 | 0.4 | |
| NfsA-97-99G | NADPHb | 17.8 | 46 | 2.6 |
| NADPHc | 2.9 | 46 | 16 | |
| Nitrofurazoned | 27.1 | 65 | 2.4 | |
| FMNd | 29.3 | 50 | 1.7 | |
| Frpe | NADPHb | 12.4 | 145 | 12 |
| NADPHc | 7.9 | 25 | 3.1 | |
| Nitrofurazoned | 19.5 | 142 | 7.3 | |
| FMNd | 25.8 | 23 | 0.9 |
TABLE 2.
Electron acceptor activities of wild-type and mutant NfsA and Frp
| Electron acceptora | Reductase activity (μmol/min/mg of protein)
|
||
|---|---|---|---|
| Wild-type NfsAb | NfsA-97-99G | Frpc | |
| Flavins | |||
| FMN | 1 | 40 | 20 |
| FAD | 3 | 37 | 19 |
| Riboflavin | 7 | 44 | 18 |
| Lumiflavin | 10 | 54 | 16 |
| Nitro compounds | |||
| Nitrofurazone | 73 | 30 | 104 |
| Methyl 4-nitrobenzoate | 24 | 48 | 57 |
| Quinones | |||
| Menadione | 24 | 77 | 119 |
| 1,4-Benzoquinone | 163 | 57 | 260 |
| Miscellaneous | |||
| Tartrazine | 0.8 | 27 | 0.1 |
| Methylene blue | 3 | 6 | 62 |
| Ferricyanide | 251 | 183 | 410 |
| DCIP | 27 | 7 | 98 |
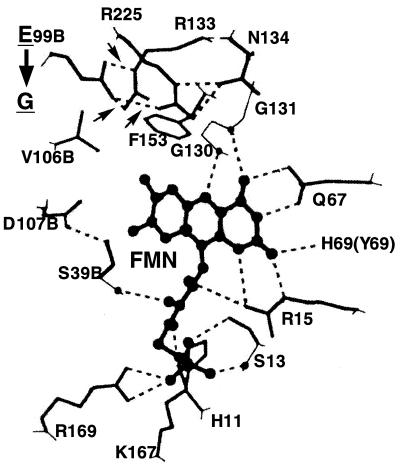
Recent crystallographic analysis (10) has revealed the three-dimensional structure of the active center of Frp. Frp is a dimer of interlocking subunits, with the FMN cofactor bound in the dimer interface (10). The active center, including the bound FMN cofactor, is formed or surrounded by 18 amino acids, 6 of which are from a subunit different from that of the remaining 12. These 18 amino acids are labeled with asterisks in Fig. 1B. Amino acid sequence alignment (Fig. 1B) indicates that 16 of the 18 amino acids are invariant between Frp and NfsA; one amino acid (I-110) is replaced with a chemically similar amino acid (methionine). Purified NfsA was shown to be eluted at a position corresponding to a 47-kDa protein in Superose 12 gel filtration, while the molecular weight of NfsA estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5) was 26,000 (13). It may thus follow that, as with Frp, NfsA is a homodimer. In addition, its FMN-containing active center may be very similar, if not identical, in three-dimensional structure to the active center of Frp, although in a previous experiment, we were unable to directly demonstrate dimer formation by NfsA in a cross-linking experiment (13). Tanner et al. (10) also suggested that Arg-225 and Arg-133, both of which form hydrogen bonds with Glu-99, may play an important role in steering substrates to the active site. Fig. 1A shows these three amino acids to be invariant between NfsA and Frp. We presume that Glu-99 and Arg-225/Arg-133 in NfsA are similarly hydrogen bonded (Fig. 2). As described above, the substitution of Glu-99 with a glycine residue resulted in conversion of NfsA to a nitroreductase associated with FMN reductase activity. Thus, it may be suggested that the loss of hydrogen bonds between the amino acid at position 99 and Arg-225/Arg-133 structurally loosens the active center so that large molecules such as FMN can be properly accommodated in the active center and recognized as efficient substrates. However, this does not necessarily mean that Glu-99 is the sole element in determining substrate specificity. Indeed, Glu-99, Arg-133, and Arg-225 are conserved in Frp, an authentic flavin reductase in V. harveyi (Fig. 1B), thus suggesting that changes in amino acids other than those described above may also be responsible for differential substrate specificity in Frp and NfsA. Note that only 50% of the amino acids of Frp are conserved in NfsA.
FIG. 2.
Disruption of presumed hydrogen bonds in the active center of NfsA by a Glu-to-Gly substitution at position 99. Sixteen of 17 amino acids surrounding the FMN cofactor are invariant between Frp and NfsA, strongly suggesting that the three-dimensional structure of NfsA is very similar to that of Frp. We presume the active center of NfsA to possess a hydrogen-bonding pattern virtually identical to that of Frp (10). Thus, this figure is adapted from Tanner et al. (10). As with Frp Glu-99, NfsA Glu-99 has hydrogen bonds with Arg-225 and Arg-133. These hydrogen bonds, which are labeled with three small arrows, are disrupted by the Glu-to-Gly substitution (see the thick vertical arrow) so that the structurally relaxed active center of the mutant NfsA can accommodate large molecules such as FMN as substrates. Invariant amino acids surrounding or forming the active center are His-11, Ser-13, Arg-15, Ser-39, Gln-67, Glu-99, Val-106, Asp-107, Gly-130, Gly-131, Arg-133, Asn-134, Phe-153, Lys-167, Arg-169, and Arg-225. Tyr-69 of Frp (in parentheses) is replaced by His-69 in NfsA. Residues marked with B are from the other subunit.
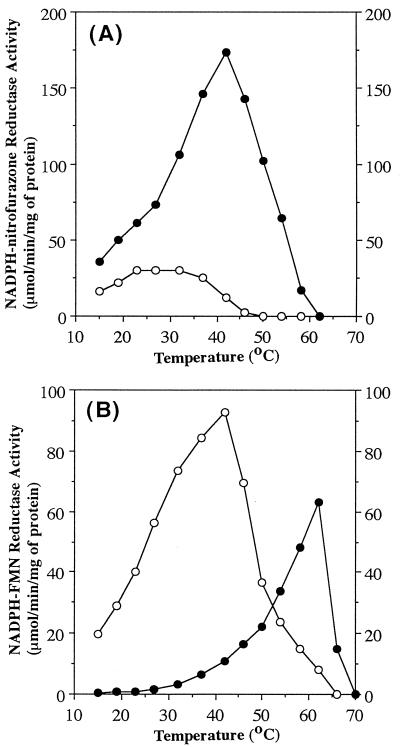
The loss of inter-amino-acid hydrogen bonds in the active center might destabilize it. Thus, we examined the temperature dependence of the activity of wild-type and mutant NfsA (Fig. 3). As expected from the hypothesis, 97-99G nitrofurazone reductase was much more labile at high temperatures than the wild type; the optimum temperature was shifted from 40 to 25°C (Fig. 3A). To our surprise, wild-type NfsA, which is associated with little or no FMN reductase activity at physiological temperatures (20 to 40°C), exhibited an apparent FMN reductase activity at a nonphysiological temperature (60°C; Fig. 3B). As in the case of 97-99G nitrofurazone reductase activity, 97-99G FMN reductase activity was temperature sensitive. The optimum temperature was shifted from 60 to 40°C. That FMN reductase activity is much more tolerant of high temperatures than nitrofurazone reductase activity may indicate again that the size of the active center is critical for determining the substrate specificity of the NfsA mutant.
FIG. 3.
Temperature dependence of nitrofurazone (A) and FMN (B) reductase activities. Filled circles, wild-type NfsA; open circles, NfsA-97-99G.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Science and Culture of Japan to K.S.
REFERENCES
- 1.Bryant D W, McCalla D R, Leeksma M, Laneuville P. Type I nitroreductases of Escherichia coli. Can J Microbiol. 1981;27:81–86. doi: 10.1139/m81-013. [DOI] [PubMed] [Google Scholar]
- 2.Duane W, Hastings J W. Flavin mononucleotide reductase of luminous bacteria. Mol Cell Biochem. 1975;6:53–64. doi: 10.1007/BF01731866. [DOI] [PubMed] [Google Scholar]
- 3.Jablonski E, DeLuca M. Purification and properties of the NADH and NADPH specific FMN oxidoreductases from Beneckea harveyi. Biochemistry. 1977;16:2932–2936. doi: 10.1021/bi00632a020. [DOI] [PubMed] [Google Scholar]
- 4.Koike, H., H. Sasaki, T. Kobori, S. Zenno, K. Saigo, M. E. P. Murphy, E. T. Adman, and M. Tanokura. Unpublished data. [DOI] [PubMed]
- 5.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 6.Lei B, Liu M, Huang S, Tu S-C. Vibrio harveyi NADPH-flavin oxidoreductase: cloning, sequencing, and overexpression of the gene and purification and characterization of the cloned enzyme. J Bacteriol. 1994;176:3552–3558. doi: 10.1128/jb.176.12.3552-3558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung D W, Chen E, Goeddel D V. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique. 1989;1:11–15. [Google Scholar]
- 8.Messing J, Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982;19:269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- 9.Michael N P, Brehm J K, Anlezark G M, Minton N P. Physical characterization of the Escherichia coli B gene encoding nitroreductase and its over-expression in Escherichia coli K12. FEMS Microbiol Lett. 1994;124:195–202. doi: 10.1111/j.1574-6968.1994.tb07284.x. [DOI] [PubMed] [Google Scholar]
- 10.Tanner J J, Lei B, Tu S-C, Krause K L. Flavin reductase P: structure of a dimeric enzyme that reduces flavin. Biochemistry. 1996;36:13531–13539. doi: 10.1021/bi961400v. [DOI] [PubMed] [Google Scholar]
- 11.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 12.Zenno, S., T. Kobori, M. Tanokura, and K. Saigo. Unpublished data.
- 13.Zenno S, Koike H, Kumar A N, Jayaraman R, Tanokura M, Saigo K. Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J Bacteriol. 1996;178:4508–4514. doi: 10.1128/jb.178.15.4508-4514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zenno S, Koike H, Tanokura M, Saigo K. Conversion of NfsB, a minor Escherichia coli nitroreductase, to a flavin reductase similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri, by a single amino acid substitution. J Bacteriol. 1996;178:4731–4733. doi: 10.1128/jb.178.15.4731-4733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenno S, Koike H, Tanokura M, Saigo K. Gene cloning, purification and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri. J Biochem. 1996;120:736–744. doi: 10.1093/oxfordjournals.jbchem.a021473. [DOI] [PubMed] [Google Scholar]
- 16.Zenno S, Saigo K, Kanoh H, Inouye S. Identification of the gene encoding the major NAD(P)H-flavin oxidoreductase of the bioluminescent bacteria Vibrio fischeri ATCC 7744. J Bacteriol. 1994;176:3536–3543. doi: 10.1128/jb.176.12.3536-3543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]