Abstract
Transforming growth factor-β (TGF-β) proteins and their antagonists have entered clinical trials. These multi-functional regulators of cell growth and differentiation induce extracellular matrix proteins and suppress the immune system making TGF-βs useful in treatment of wounds with impaired healing, mucositis, fractures, ischemia-reperfusion injuries, and autoimmune disease. In diseases such as keloids, glomerulonephritis and pulmonary fibrosis, excessive expression of TGF-β has been implicated as being responsible for accumulation of detrimental scar tissue. In these conditions, agents that block TGF-β have prevented or reversed disease. Similarly, in carcinogenesis, blocking TGF-β activity may be valuable in stimulating an immune response towards metastasis. As these blocking agents receive approval, we will likely have new therapies for previously recalcitrant diseases.
Keywords: Fibrosis, Cancer, Immunosuppression, Knockout mice
TGF-β SYNTHESIS
Transforming growth factor-β (TGF-β) is a family of related proteins that regulate many cellular processes including growth, differentiation, extracellular matrix formation and immunosuppression.1–4 TGF-β protein is produced by nearly all normal cells and functions through a complex cell surface receptor system.5 The three mammalian isoforms of TGF-β (TGF-βs 1, 2, and 3) have similar but distinct functions and are approximately 70% identical in amino acid sequence. The TGF-βs are extremely important in the regulation of physiological homeostasis with loss of TGF-β activity being implicated in the pathogenesis of ovarian cancer,6 pancreatic cancer,7 colon cancer,8 and squamous cell carcinoma9 since TGF-β is a potent growth inhibitor. Mutations preventing TGF-β function are also causal for hereditary hemorrhagic telangiectasia,10 and corneal dystrophy.11 Over activity of TGF-β leads to Camurati-Engelmann Disease of bone,12 glomerulonephritis,13 scar formation,14 keloids,15 pulmonary fibrosis,16 and liver cirrhosis.17
The TGF-β proteins are synthesized as pre-pro-peptides which are secreted from cells in a primarily inactive form called latent TGF-β18 consisting of a 25 kD mature region surrounded by the pro region, which is also called the latency associated peptide (figure 1). Latent TGF-β resides in the extracellular spaces between cells and is available for response to cellular stress or changes in physiology. Activation of TGF-β cleaves the pro region and separates the latency peptide from the active molecule in a highly regulated manner. Agents known to activate TGF-β include, acidic microenvironments, plasmin, plasmin-like proteases, and αvβ6 integrin.
Figure 1.
TGF-β latency complex.
The structure of the inactive TGF-β complex is shown with the TGF-β dimer interacting with the latency associated peptide (LAP) and the latent TGF-β binding protein (LTBP). Arrow indicates cleavage site.
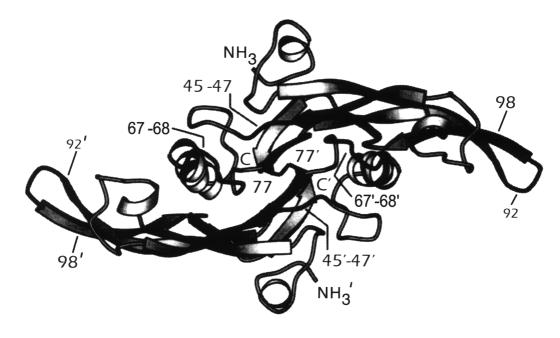
The structure of TGF-β has been determined using a combination of nuclear magnetic resonance and x-ray crystal diffraction (figure 2).19–24 Amino acids 45 and 47 interact with α2-macroglobulin, an abundant serum protein that binds and inactivates TGF-β.25,26 Amino acids 67 and 68 determine the affinity of the TGF-β isoforms for the GPI-linked protein on vascular endothelial cells.27 Amino acids 92–98 regulate binding of TGF-β to the TGF-β type II receptor (TβRII),28 with amino acid 94 directly binding TβRII.29,30
Figure 2.
Structure of TGF-β.
The crystal structure of TGF-β is shown. Amino acids important in regulating binding of TGF-β to receptors and binding proteins have been highlighted.
SIGNALING PATHWAYS
TGF-β signals through a complex membrane bound receptor and binding protein system31,32 which can include β-glycan,33 the type I and II receptors,34 endoglin,35 an uncharacterized glycosyl phosphatidylinositol-linked protein,36 and BAMBI, a naturally occurring truncated type I receptor.37 Both endoglin and β-glycan can present TGF-β to the type II receptor which complexes with and phosphorylates the type I receptor. The type I (TβRI) and II (TβRII) receptors are transmembrane serine/threonine specific kinases with TβRI interacting with and phosphorylating intracellular molecules. Intracellular signaling requires the Smad family of proteins in which Smad2 and Smad3 are directly phosphorylated by TβRI and go on to form a complex with Smad4.3,4,31,32 This complex directly enters the nucleus where it interacts with other transcription factors to regulate gene transcription. In contrast, Smad7 inhibits phosphorylation of Smad2 and Smad3 in a negative feedback loop preventing over-stimulation of the cell by TGF-β. TGF-β signaling is further regulated by the interaction of Smad proteins with a variety of transcriptional activators and repressors38 as well as by complex interactions of the Smad and MAP kinase signaling pathways.39
Through the use of microarray technology, much progress has been made in identifying the genes that are regulated by TGF-β.40 βig-h3 (TGF-β induced gene), a secreted protein that inhibits tumor formation when overexpressed, is another important protein synthesized by cells in response to TGF-β.41
TGF-β AND DISEASE
Mutations of TGF-β or components of its signal pathway are frequently found in disease (table 1). Patients with Camurati-Engelmann have a missense mutation in the latency associated peptide which changes one of three different amino acids, producing constitutively active TGF-β.12,42 This results in disregulation of TGF-β synthesis, uncoupling bone formation and resorption at the level of the osteoblasts and osteoclasts.
Table 1.
Genetic diseases of the TGF-β system.
| Camurati-Engelmann | Latency associated peptide |
| Corneal dystrophies | βig-h3 |
| Hereditary hemorrhagic telangiectasis type I | Endoglin |
| Hereditary hemorrhagic telangiectasis type II | Activin receptor-link kinase 1 |
| Autosomal dominant juvenile polyposis | Smad4 bone morphogenetic protein receptor I |
Mutations of βig-h3, a protein involved in corneal morphogenesis, have been identified as a cause of six autosomal dominant corneal dystrophies. These include Groenouw corneal dystrophy type I, superficial variant of granular corneal dystrophy, lattice corneal dystrophy type I, lattice corneal dystrophy type IIIA, Avellino corneal dystrophy, and Reis-Bucklers corneal dystrophy.43 It appears that mutant βig-h3 protein self-polymerizes and/or incorrectly binds to other corneal components resulting in the abnormal deposits found in these dystrophies.44
Hereditary hemorrhagic telangiectasia (HHT) type 1 or Osler-Weber-Rendu disease is caused by mutations of endoglin.10 In HHT type II, mutations of activin receptor-like kinase 1 (ALK1) have been identified.45 ALK1, a membrane bound receptor that is closely related to TβRI, binds TGF-β and signals a TGF-β response. However, the preferred ligand for ALK1 is activin, a protein structurally similar to TGF-β, but distinct in its function. In both types of HHT, the endothelial cells would be expected to respond poorly to TGF-β, which could contribute to the abnormal vessel formation or altered cell adhesion properties leading to the vascular anomalies seen in this disorder.
Mutations of Smad4 have been identified as the cause of familial autosomal dominant juvenile polyposis in a subset of patients.46 Other families with this disease carry mutations in the gene encoding bone morphogenetic protein receptor 147 which is a membrane bound receptor similar to TβRI. The preferred ligand for this mutated receptor is bone morphogenetic protein, a protein similar to TGF-β with growth regulating activity. TβRII mutations have been identified in approximately 90% of colon polyps with DNA repair defects48 and 70% of gastric cancers49 with repair defects. In these patients, DNA replication errors occur due to mutations in the genes that check the copied strand of DNA for mistakes. As a result, inactivating mutations accumulate in a stretch of 10 adenine bases in TβRII, since the replication machinery does not carefully check this repetitive sequence. Additionally, deletion or inactivation of Smad4 has been shown in 30–50% of colorectal and pancreatic cancers.7,50 In all these conditions a disruption in the TGF-β signaling path-way compromises the cell's ability to respond to the growth inhibitory effects of TGF-β, thereby promoting tumorigenesis.
TGF-β KNOCKOUT MICE
Mice lacking the gene for the TGF-βs or their regulatory and signaling proteins have been generated using homologous recombination and the role of TGF-β in development and disease has been studied. Approximately 60% of mice lacking TGF-β1 die in utero due to defective yolk sac vasculogenesis and hematopoiesis, which is consistent with the role of TGF-β in embryogenesis. This defect is due to inappropriate endothelial cell differentiation.51 The remaining mice develop to term, but die in about week 3 to 4 from a rapid wasting syndrome.52,53 At birth, the knockout mice are indistinguishable from wild type litter mates, but soon develop severe, multifocal organ-dependent mixed inflammatory cell infiltration into heart, stomach, liver, diaphragm, lung, salivary gland and pancreas. These mice also have elevated antibody levels to dsDNA, ssDNA, and Sm ribonuclear protein.54 Treatment of these mice with anti-inflammatory and immune suppressive agents such as rapamycin reduces the severity of inflammation. In addition to immune system defects, the mice that are born experience delayed wound healing,55 ineffective remodeling of bone,56 and increased mitochondria in the liver in response to stress.57
TGF-β2 knockout mice exhibit primarily developmental defects in contrast to TGF-β1 mice.58 These include defects in epithelial-mesenchymal interactions, cell growth, extracellular matrix production and tissue remodeling, and affect the function of cardiac, lung, craniofacial, limb, spinal column, eye, inner ear and urogenital tissues. Analysis of eyes of TGF-β2 knockout mice show that extracellular matrix proteins, including collagen I and keratocan, are diminished and the stroma is thinner.59 TGF-β3 deficient mice also exhibit disruptions in epithelial-mesenchymal interactions as evidenced by the appearance of abnormal lung development and cleft palate.60 Unlike other models of cleft palate, these mice do not develop other craniofacial abnormalities.
Mice with deletions of genes in various components of the TGF-β signaling pathway develop additional pathological phenotypes. TβRI mice die at mid-gestation exhibiting defects in vascular development of the yolk sac and placenta with absence of red blood cells.61 TβRII mutants developed pituitary tumors when treated with chronic estradiol.62 Mice lacking Smad3 live until 8 months and die of defects in immune function.63 These mice also have an imbalance between osteoblasts and osteoclasts resulting in osteopenia63 and accelerated healing of cutaneous incisional wounds.64 Exposure of these mice to radiation-induced injury causes significantly less epidermal acanthosis and dermal influx of mast cells, macrophages, and neutrophils than wild type littermates, demonstrating that these mice have a significantly reduced fibrotic response.65 Smad4 mice present with inflammatory polyps in the glandular stomach and duodenum consistent with previous reports that Smad4 mutations are involved in a subset of familial juvenile polyposis.66
POSSIBLE USE OF TGF-β LIGANDS FOR THERAPEUTIC INTERVENTION
The well-characterized abilities of TGF-β to promote healing in both hard and soft tissues, as well as its potent immunosuppressive effects, have provided the basis for the use of TGF-β ligands as potential therapeutic agents in several disease models. Topical application of TGF-β improves the rate of healing and wound strength in cutaneous wounds in a wide variety of animal models of impaired healing including animals treated with corticosteroids, antineoplastic agents, or radiation, as well as diabetic or aged animals.67 In clinical trials TGF-β2 and TGF-β3 treatment of venous stasis and pressure ulcers, respectively, has been shown to improve healing.68,69 In a hamster model of chemotherapy-induced oral mucositis, application of TGF-β3 reduces the severity and duration of the resulting mucositis,70 and clinical trials of TGF-β3 to treat this condition are underway.71 TGF-β has also been shown to accelerate the repair of bone defects. In canine models, both TGF-β1 and TGF-β2 have been effective in increasing bone formation when applied to defects in the alveolar ridge and in the humerus, respectively.72,73 In keeping with its healing properties, TGF-β also can protect tissues from ischemia-reperfusion injury in several animal models. In rat and rabbit models of stroke, administration of TGF-β before or even 2 h after insult reduces the infarct size,74 while intravenous administration of TGF-β following coronary artery occlusion, but before reperfusion reduces cardiac necrosis.75,76 Recent studies are investigating improved delivery systems for TGF-β. Pang et al.77 report that mice receiving adenovirus overexpressing TGF-β1 showed a smaller infarct volume after middle cerebral artery occlusion followed by reperfusion.
The potent immunosuppressive effects of TGF-β make it a potential therapeutic agent in the treatment of autoimmune diseases. Indeed, treatment of rodents with TGF-β1 during the latter part of the induction phase of acute experimental allergic encephalomyelitis (EAE) (a model of multiple sclerosis) and collagen-induced arthritis prevents the development and/or exacerbation of disease symptoms.78,79 Again, novel delivery systems for administration of TGF-β are being developed. A genetically engineered retrovirus transduced with cDNA for latent TGF-β delays and ameliorates EAE development,80 and intranasal administration of a TGF-β1 plasmid prevents the development of T helper cell type 1-mediated experimental colitis.81 Additionally, intramuscular injections of adenoviral TGF-β1 into rodent recipients of lung transplants attenuates acute rejection.82
ANTAGONISTS OF TGF-β FOR DISEASE TREATMENT
Even though TGF-β has great potential as a therapeutic agent, there are a number of fibroproliferative disorders where unregulated expression of TGF-β plays a causal role in the condition. Numerous immunohistochemical studies have demonstrated the overexpression of TGF-β in glomerulonephritis, pulmonary fibrosis, liver cirrhosis, and keloids suggesting that molecules that antagonize TGF-β may be useful in the treatment of these diseases.83 In addition, since many tumors express increased amounts of TGF-β, agents that block TGF-β may be valuable in stimulating an immune response toward metastases.84 Mechanisms to block TGF-β activity include soluble TβRII fragments,85 decorin,86 tranilast,87 neutralizing antibodies,88 threonine kinase inhibitors,89 and RNA expression inhibitors such as anti-sense expression vectors or blocking oligonucleotides.90
Impressive results have been obtained in animal models of fibrosis using TGF-β antagonists. Glomerulonephritis in rats has been essentially cured using antibodies to TGF-β,91 administration of decorin protein,92 and gene therapy using a decorin gene expressed in muscle tissue that circulates to act on kidney.13 Moreover, anti-TGF-β2 antibody and a TGF-β1 antisense oligonucleotide attenuate kidney fibrosis in the diabetic rat and in unilateral ureteral obstructions, respectively.88,93 Hepatic fibrosis in rodents can be inhibited by soluble TβRII administered intraperitoneally94 or by intramuscular injections of an adenovirus expressing the ectodomain of TβRII fused to the Fc portion of IgG.95 Adenoviral soluble TβRII can also inhibit constrictive remodeling after coronary angioplasty in pigs.96 Pulmonary fibrosis has been successfully treated using adenovirus expression of decorin in the airway.86 Administration of TGF-β antibodies prevents skin and lung fibrosis in murine sclerodermatous graft-vs-host disease.97 Fibrotic scar tissue is successfully treated using antibodies to TGF-β1 or antibodies to TGF-β2, but paradoxically, topical application of TGF-β3 protein.14 Both decorin98 and TGF-β antibodies99 attenuate gliotic scar formation following injury to the rat CNS. In a mouse model of intestinal radiation enteropathy, intraperitoneal injection of soluble TβRII preserves mucosal surface area with less intestinal wall fibrosis than in controls.100 Mice injected intramuscularly with an adenovirus vector expressing the soluble extra-cellular TGF-β binding domain of TβRII are protected from developing corneal opacification, edema, and angiogenesis induced by silver nitrate.85
Several rodent carcinogenesis models have demonstrated the efficacy of TGF-β antagonists to inhibit tumor growth and metastasis. Antisense oligonucleotides to TGF-β2 inhibit the growth of mouse malignant melanomas84 and fibrosarcomas.101 TGF-β antibody inhibits metastasis of tumorigenic human xenotransplants in nude mice,102 while ectopic expression of decorin in rat C6 glioma cells inhibits tumor formation.103 Furthermore, oral administration of tranilast, an anti-allergic compound which can inhibit TGF-β, inhibits the growth of experimental 9L rat gliomas.104 A phase I clinical trial of TGF-β antagonists for the treatment of metastatic cancer is currently in progress.105 In this trial, autologous tumor cells are removed from patients and treated in vitro with TGF-β2 anti-sense DNA to suppress TGF-β expression from tumor cells. This treatment blocks the immunosuppressive activity of TGF-β enhancing immune system recognition of tumor cells. TGF-β2 blocked cells are then injected back into the patient as antigens for the immune system. Five patients with progressive glioblastoma multi-form and one with progressive gliosarcoma are enrolled in the trial and treated with 2 to 7 subcutaneous injections of approximately 1x107 modified tumor cells. TGF-β levels were decreased by 50%–98%. Two patients had partial regressions and one patient had stable disease following therapy supporting further clinical evaluation of TGF-β modified anti-sense tumor cells for treatment of incurable metastatic disease.
THERAPEUTIC APPLICATIONS: PROBLEMS AND PERSPECTIVES
The preceding sections highlight the many types of diseases in which alterations in the TGF-β signaling pathway may have pathological consequences. While restoration of the proper flux through the pathway by administration of either ligand or antagonists may alleviate the pathology, accomplishing this in only the affected tissue or cell type will be difficult. Normal homeostatic actions of TGF-β in uncompromised cells may also be altered by treatment agents leading to unwanted and unexpected complications. The pleiotropic actions of TGF-β are illustrated by its role in carcinogenesis, where TGF-β can have tumor suppressor, as well as prooncogenic activities.106 TGF-β normally inhibits growth of epithelial cells, but tumorigenesis is often accompanied by a loss of responsiveness to this growth inhibition coupled with increased production of TGF-β, which in turn facilitates prooncogenic effects of TGF-β on stroma. These effects can include increased cell motility, enhanced angiogenesis and suppression of immune surveillance. An ideal treatment for cancer would involve restoring TGF-β signaling in epithelial cells and inhibiting its action in stromal cells, necessitating the development of cell type specific delivery systems.
Since the mode of delivery of TGF-β ligand or antagonist will be critical in affecting only the desired system, local, as opposed to systemic, delivery is probably preferable. For example, in a phase I trial for treatment of chronic progressive multiple sclerosis, intravenous administration of active TGF-β2107 resulted in anemia and reversible nephrotoxicity in some patients with no change in expanded disability status score of magnetic resonance imaging lesions during treatment. In contrast, murine experimental allergic encephalomyelitis was ameliorated by administration of myelin basic protein-activated T cells transduced with latent TGF-β1.80 In this regard administration of adenoviral vectors of TGF-β or TGF-β antagonists is being widely used in animal models.13,77,81,95,96 The administration of active-vs.-latent TGF-β is also an important consideration. Active TGF-β has a much shorter half-life than the latent form,108 and active TGF-β may result in more side effects, since it is not subject to the activation step which is tightly controlled in vivo. Additional complications of administration of active TGF-β are its reported inactivation by proteases present in wound fluid from venous leg ulcers.109 This proteolytic degradation of ligand may have contributed to the inefficacy of topical TGF-β3 in treating chemotherapy-induced oral mucositis in patients with lymphomas and solid tumors.71
In spite of these problems, some current clinical modalities may be acting by decreasing TGF-β signaling. The success of angiotensin-converting-enzyme inhibitors in treating diabetic nephropathy, interferon-α in treating hepatic fibrosis, azathioprine and prednisone in treating autoimmune hepatitis, and interferon-γ in treating pulmonary fibrosis is due, in part, to the ability of these agents to reduce serum levels of TGF-β.110 The search for small molecules that target the TGF-β system is currently underway using combinatorial chemistry and high-throughput drug screening.
CONCLUSION
TGF-β and its antagonists have tremendous potential for the treatment of diseases that currently do not respond well to conventional therapy. The development of additional analogs of TGF-β and antagonists of TGF-β, as well as further studies into the cell biology of this important cytokine, may enable us to develop new therapeutics.
Contributor Information
Kathleen C. Flanders, Laboratory of Cell Regulation and Carcinogenesis, National Institutes of Health, Bethesda, Maryland.
James K. Burmester, Personalized Medicine Research Center, Marshfield Medical Research Foundation, Marshfield, Wisconsin.
References
- 1.Roberts AB, Sporn MB. The transforming growth factor-βs. In: Sporn MB, Roberts AB, editors. Peptide Growth Factors and Their Receptors. Handbook of Experimental Pharmacology. Vol. 1, Chapter 8. Berlin, Heidelberg: Springer; 1995. pp. 419–472. [Google Scholar]
- 2.Massagué J. The transforming growth factor-β family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 3.Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 4.Verrecchia F, Mauviel A. Transforming growth factor-β signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 5.Massagué J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Kanuma T, Mizunuma H, Takama F, Ibuki Y, Wake N, Mogi A, Shirtara Y, Takenoshita S. Analysis of specific gene mutations in the transforming growth factor-β signal transduction pathway in human ovarian cancer. Cancer Res. 2000;60:4507–4512. [PubMed] [Google Scholar]
- 7.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Rozenblum E, Weinstein CL, Fischer A, eo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 9.Garrigue-Antar L, Muñoz-Antonia T, Antonia SJ, Gesmonde J, Vellucci VF, Reiss M. Missense mutations of the transforming growth factor β type II receptor in human head and neck squamous carcinoma cells. Cancer Res. 1995;55:3982–3987. [PubMed] [Google Scholar]
- 10.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 11.Mashima Y, Yamamoto S, Inoue Y, Yamada M, Konishi M, Watanabe H, Maeda N, Shimomura Y, Kinoshita S. Association of autosomal dominantly inherited corneal dystrophies with Big-h3 gene mutations in Japan. Am J Ophthalmol. 2000;130:516–517. doi: 10.1016/s0002-9394(00)00571-7. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Kinoshita A, Yoshiura Ki, Makita Y, Wakui K, Honke K, Niikawa N, Taniguchi N. Domain-specific mutations of a transforming growth factor-(TGF)β1 latency-associated peptide cause Camurati-Englemann disease because of the formation of a constitutively active form of TGF-β1. J Biol Chem. 2001;276:11469–11472. doi: 10.1074/jbc.C000859200. [DOI] [PubMed] [Google Scholar]
- 13.Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 14.Shah M, Foreman DM, Ferguson MWJ. Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1022. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 15.Lee TY, Chin GS, Kim WJ, Chau D, Gittes GK, Longaker MT. Expression of transforming growth factor β1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179–184. [PubMed] [Google Scholar]
- 16.Khalil N, Greenberg AH. The role of TGF-β in pulmonary fibrosis. Ciba Found Symp. 1991;157:194–207. doi: 10.1002/9780470514061.ch13. [DOI] [PubMed] [Google Scholar]
- 17.Castilla A, Prieto J, Fausto N. Transforming growth factors β1 and α in chronic liver disease. N Engl J Med. 1991;324:933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence DA. Latent-TGF-β: an overview. Mol Cell Biochem. 2001;219:163–170. doi: 10.1023/a:1010819716023. [DOI] [PubMed] [Google Scholar]
- 19.Daopin S, Li M, Davies DR. Crystal structure of TGF-β 2 refined at 1.8 A resolution. Proteins. 1993;17:176–192. doi: 10.1002/prot.340170207. [DOI] [PubMed] [Google Scholar]
- 20.Archer SJ, Bax A, Roberts AB, Sporn MB, Ogawa Y, Piez KA, Weatherbee JA, Tsang ML, Lucas R, Zheng Bl, et al. Transforming growth factor-β1: NMR signal assignments of the recombinant protein expressed and isotopically enriched using Chinese hamster ovary cells. Biochemistry. 1993;32:1152–1163. doi: 10.1021/bi00055a021. [DOI] [PubMed] [Google Scholar]
- 21.Daopin S, Piez KA, Ogawa Y, Davies DR. Crystal structure of transforming growth factor-β2. An unusual fold for the superfamily. Science. 1992;257:369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- 22.Hinck AP, Archer SJ, Qian SW, Roberts AB, Sporn MB, Weatherbee JA, Tsang ML, Lucas R, Zhang BL, Wenker J, Torchia DA. Transforming growth factor-β1: Three-dimensional structure in solution and comparison with the X-ray structure of transforming growth factor-β2. Biochemistry. 1996;35:8517–8534. doi: 10.1021/bi9604946. [DOI] [PubMed] [Google Scholar]
- 23.Mittl PRE, Priestle JP, Cox DA, McMaster G, Cerletti N, Grutter MC. The crystal structure of TGF-β3 and comparison to TGF-β2: Implications for receptor binding. Protein Sci. 1996;5:1261–1271. doi: 10.1002/pro.5560050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlunegger MP, Grutter MC. An unusual feature revealed by the crystal structure at 2.2 A resolution of human transforming growth factor-β2. Nature. 1992;358:430–434. doi: 10.1038/358430a0. [DOI] [PubMed] [Google Scholar]
- 25.Burmester JK, Qian SW, Roberts AB, Huang A, Amatayakul-Chantler S, Suardet L, Odartchenko N, Madri JA, Sporn MB. Characterization of distinct functional domains of transforming growth factor-β. Proc Natl Acad Sci. 1993;90:8628–8632. doi: 10.1073/pnas.90.18.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb DJ, Atkins TL, Crookston KP, Burmester JK, Qian SW, Gonias SL. Transforming growth factor-β isoform 2-specific high affinity binding to native α2-macroglobulin. Chimeras identify a sequence that determines affinity for native but not activated α2-macroglobulin. J Biol Chem. 1994;269:30402–30406. [PubMed] [Google Scholar]
- 27.Zhang KQ, Polga D, Salzman SA, Burmester JK. Amino acids 67 and 68 of TGF-β regulate binding to a GPI-linked membrane protein on vascular endothelial cells. Cytokines Cell Mol Ther. 2001 Mar;7((1)):25–30. doi: 10.1080/13684730216399. [DOI] [PubMed] [Google Scholar]
- 28.Qian SW, Burmester JK, Tsang ML, Weatherbee JA, Hinck AP, Ohlsen DJ, Sporn MB, Roberts AB. Binding affinity of transforming growth factor-β for its type II receptor is determined by the C-terminal region of the molecule. J Biol Chem. 1996;271:30656–30662. doi: 10.1074/jbc.271.48.30656. [DOI] [PubMed] [Google Scholar]
- 29.Burmester JK, Qian SW, Ohlsen D, Sporn MB, Roberts AB. Mutational analysis of a transforming growth factor-β receptor binding site. Growth Factors. 1998;15:231–242. doi: 10.3109/08977199809002119. [DOI] [PubMed] [Google Scholar]
- 30.Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. Crystal structure of the human TβR2 ectodomain — TGβ3 complex. Nat Struct Biol. 2002;3:203–208. doi: 10.1038/nsb766. [DOI] [PubMed] [Google Scholar]
- 31.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 32.Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol. 2002;15:731–740. [PubMed] [Google Scholar]
- 33.López-Casillas F, Wrana JL, Massagué J. Betaglycan presents ligand to the TGF-β signaling receptor. Cell. 1993;73:1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 34.Massagué J, Like B. Cellular receptors for type β transforming growth factor. J Biol Chem. 1985;260:2636–2645. [PubMed] [Google Scholar]
- 35.Cheifetz S, Bellón T, Calés C, Vera S, Bernabeu C, Massagué J, Letarte M. Endoglin is a component of the transforming growth factor-β receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 36.Morello JP, Plamondon J, Meyrick B, Hoover R, O'Connor-McCourt MD. Transforming growth factor-β receptor expression on endothelial cells: Heterogeneity of type III receptor expression. J Cell Physiol. 1995;165:201–211. doi: 10.1002/jcp.1041650123. [DOI] [PubMed] [Google Scholar]
- 37.Onichtchouk D, Chen Y-G, Dosch R, Gawantka V, Delius H, Massagué J, Niehrs C. Silencing of TGF-β signaling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 38.de Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-beta signaling in cancer. J Natl Cancer Inst. 2000;92:1388–1402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- 39.Yue J, Mulder KM. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 40.Akiyoshi S, Ishii M, Nemoto N, Kawabata M, Aburatani H, Miyazono K. Targets of transcriptional regulation by transforming growth factor-β: Expression profile analysis using oligonucleotide arrays. Jpn J Cancer Res. 2001;92:257–268. doi: 10.1111/j.1349-7006.2001.tb01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skonier J, Bennett K, Rothwell V, Kosowski S, Plowman G, Wallace P, Edelhoff S, Disteche C, Neubauer M, Marquardt H, et al. βig-h3: A transforming growth factor-β-responsive gene encoding a secreted protein that inhibits cell attachment in vitro and suppresses the growth of CHO cells in nude mice. DNA Cell Biol. 1994;13:571–584. doi: 10.1089/dna.1994.13.571. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita A, Saito T, Tomita H, Makita Y, Yoshida K, Ghadami M, Yamada K, Kondo S, Ikegawa S, Nishimura G, Fukushima Y, Nakagomi T, Saito H, Sugimoto T, Kamegaya M, Hisa K, Murray JC, Taniguchi N, Niikawa N, Yoshiura K. Domain-specific mutations in TGFβ1 result in Camurati-Engelmann disease. Nat Genet. 2000;26:19–20. doi: 10.1038/79128. [DOI] [PubMed] [Google Scholar]
- 43.Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 44.Streeten BW, Qi Y, Klintworth GK, Eagle RC, Jr, Strauss JA, Bennett K. Immunocalization of βig-h3 protein in 5q31-linked corneal dystrophies and normal corneas. Arch Ophthalmol. 1999;117:67–75. doi: 10.1001/archopht.117.1.67. [DOI] [PubMed] [Google Scholar]
- 45.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon S-J, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 46.Howe JR, Roth S, Ringold JC, Summers RW, Järvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, Stone EM, Aaltonen LA. Mutations in the Smad4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- 47.Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, Velculescu VE, Traverso G, Vogelstein B. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- 48.Parsons R, Myeroff LL, Liu B, Wilson JK, Markowitz SD, Kinzler KW, Vogelstein B. Microsatellite instability and mutations of the transforming growth factor β Type II receptor gene in colorectal cancer. Cancer Res. 1995;55:5548–5550. [PubMed] [Google Scholar]
- 49.Myeroff LL, Parsons R, Kim SJ, Hedrick L, Cho KR, Orth K, Mathis M, Kinzler KW, Lutterbaugh J, Park K, et al. A transforming growth factor-β receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res. 1995;55:5545–5547. [PubMed] [Google Scholar]
- 50.Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Wilson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 51.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knockout mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 52.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dang H, Geiser AG, Letterio JJ, Nakabayashi T, Kong L, Fernandes G, Talal N. SLE-like autoantibodies and Sjogren's syndrome-like symphoproliferation in TGF-β knockout mice. J Immunol. 1995;155:3205–3212. [PubMed] [Google Scholar]
- 55.Crowe MJ, Doetschman T, Greenhalgh DG. Delayed wound healing in immunodeficient TGF-β1 knockout mice. J Invest Dermatol. 2000;115:3–11. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 56.Geiser AG, Zeng QQ, Sato M, Helvering LM, Hirano T, Turner CH. Decreased bone mass and bone elasticity in mice lacking the transforming growth factor-β1 gene. Bone. 1998;23:87–93. doi: 10.1016/s8756-3282(98)00078-7. [DOI] [PubMed] [Google Scholar]
- 57.Williams AO, Knapton AD, Geiser A, Letterio JJ, Roberts AB. The liver in transforming growth factor-β1 (TGF-β1) null mutant mice. Ultrastruct Pathol. 1996;20:477–490. doi: 10.3109/01913129609016352. [DOI] [PubMed] [Google Scholar]
- 58.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGF-β2 knockout mice have multiple developmental defects that are non-overlapping with other TGF-β knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saika S, Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGF-β2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- 60.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 61.Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential TGF-β type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shida N, Ikeda H, Yoshimoto T, Oshima M, Taketo MM, Miyoshi I. Estrogen-induced tumorigenesis in the pituitary gland of TGF-β ± knockout mice. Biochim Biophys Acta. 1998;407:79–83. doi: 10.1016/s0925-4439(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 63.Borton AJ, Frederick JP, Datto MB, Wang XF, Weinstein RS. The loss of Smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J Bone Miner Res. 2001;16:1754–1764. doi: 10.1359/jbmr.2001.16.10.1754. [DOI] [PubMed] [Google Scholar]
- 64.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and impaired. 1999;59:6113–6117. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 65.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–68. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takaku K, Miyoshi H, Matsunaga A, Oshima M, Sasaki N, Taket MM. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 1999;59:6113–7. [PubMed] [Google Scholar]
- 67.Roberts AB, Sporn MB. Transforming growth factor-β. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. pp. 275–308. [Google Scholar]
- 68.Robson MC, Phillip LG, Cooper DM, Lyle WG, Robson LE, Odom L, Hill DP, Pharm D, Hanham AF, Ksander GA. Safety and effect of transforming growth factor-β2 for treatment of venous stasis ulcers. Wound Rep Reg. 1995;3:157–167. doi: 10.1046/j.1524-475X.1995.30207.x. [DOI] [PubMed] [Google Scholar]
- 69.Hirshberg J, Coleman J, Marchant B, Rees RS. TGF-β3 in the treatment of pressure ulcers: a preliminary report. Adv Skin Wound Care. 2001;14:91–95. doi: 10.1097/00129334-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Sonis ST, Lindquist L, Van Vugt A, Stewart AA, Stam K, Qu GY, Iwata KK, Haley JD. Prevention of chemotherapy-induced ulcerative mucositis by transforming growth factor-β3. Cancer Res. 1994;54:1135–1138. [PubMed] [Google Scholar]
- 71.Foncuberta MC, Cagnoni PJ, Brandts CH, Mandanas R, Fields K, Derigs HG, Reed E, Sonis ST, Fay J, LeVeque F, Pouillart P, Schrezenmeier H, Emmonds R, Thiel E. Topical transforming growth factor-β3 in the prevention or alleviation of chemotherapy-induced oral mucositis in patients with lymphomas or solid tumors. J Immunother. 2001;24:384–388. doi: 10.1097/00002371-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 72.Ruskin JD, Hardwick R, Buser D, Dahlin C, Schenk RK. Alveolar ridge repair in a canine model using rh TGF-β1 with barrier membranes. Clin Oral Implants Res. 2000;11:107–115. [PubMed] [Google Scholar]
- 73.Sumner DR, Turner TM, Urban RM, Leven RM, Hawkins M, Nichols EH, McPherson JM, Galante JO. Locally delivered rhTGF-β2 enhances bone ingrowth and bone regeneration at local and remote sites of skeletal injury. J Orthop Res. 2001;19:85–94. doi: 10.1016/S0736-0266(00)00015-2. [DOI] [PubMed] [Google Scholar]
- 74.Flanders KC, Ren RF, Lippa CF. Transforming growth factor-βs in neurodegenerative disease. Prog Neurobiol. 1998;54:71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 75.Lefer AM, Tsao P, Aoki N, Palladino MA., Jr Mediation of cardioprotection by transforming growth factor-β. Science. 1990;249:61–64. doi: 10.1126/science.2164258. [DOI] [PubMed] [Google Scholar]
- 76.Lefer AM, Ma XL, Weyrich AS, Scalia R. Mechanism of the cardioprotective effect of transforming growth factor-β1 in feline myocardial ischemia and reperfusion. Proc Natl Acad Sci USA. 1993;90:1018–1022. doi: 10.1073/pnas.90.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- 78.Racke M, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-β1. J Immunol. 1991;146:3012–3017. [PubMed] [Google Scholar]
- 79.Thorbecke GJ, Shah R, Leu CH, Kuruvilla AP, Hardison AM, Palladino MA. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor-β during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen LZ, Hochwald GM, Huang C, Dakin G, Tao H, Cheng C, Simmons WJ, Dranoff G, Thorbecke GJ. Gene therapy in allergic encephalomyelitis using myelin basic protein-specific T cells engineered to express latent transforming growth factor-β1. Proc Natl Acad Sci USA. 1998;95:12516–12521. doi: 10.1073/pnas.95.21.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitani A, Fuss IJ, Nakamura K, Schwartz OM, Usui T, Strober W. Treatment of experimental (Trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor-(TGF)β1 plasmid: TGF-β1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor β2 chain down regulation. J Exp. Med. 2000;192:41–52. doi: 10.1084/jem.192.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suda T, D'Ovidio F, Daddi N, Ritter JH, Mohanakumar T, Patterson GA. Recipient intramuscular gene transfer of active transforming growth factor-β1 attenuates acute lung rejection. Ann Thorac Surg. 2001;71:1651–1656. doi: 10.1016/s0003-4975(01)02528-0. [DOI] [PubMed] [Google Scholar]
- 83.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 84.Fakhrai H, Dorigo O, Shawler DL, Lin H, Mercola D, Black KL, Royston I, Sobol RE. Eradication of established intracranial rat gliomas by transforming growth factor β antisense gene therapy. Proc Natl Acad Sci USA. 1996;93:2909–2914. doi: 10.1073/pnas.93.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakamoto T, Ueno H, Sonoda K, Hisatomi T, Shimizu K, Ohashi H, Inomata H. Blockade of TGF-β by in vivo gene transfer of a soluble TGF-β type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Ther. 2000;7:1915–1924. doi: 10.1038/sj.gt.3301320. [DOI] [PubMed] [Google Scholar]
- 86.Kolb M, Margetts PJ, Galt T, Sime PJ, Xing Z, Schmidt M, Gauldie J. Transient transgene expression of decorin in the lung reduces the fibrotic response to Bleomycin. Am J Respir Crit Care Med. 2001;163:770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 87.Kosuga K, Tamai H, Ueda K, Hsu YS, Ono S, Tanaka S, Doi T, Myou- U W, Motohara S, Uehata H. Effectiveness of tranilast on restenosis after directional coronary atherectomy. Am Heart J. 1997;134:712–718. doi: 10.1016/s0002-8703(97)70055-3. [DOI] [PubMed] [Google Scholar]
- 88.Hill C, Flyvbjerg A, Rasch R, Bak M, Logan A. Transforming growth factor-β2 antibody attenuates fibrosis in the experimental diabetic rat kidney. J Endocrinol. 2001;170:647–651. doi: 10.1677/joe.0.1700647. [DOI] [PubMed] [Google Scholar]
- 89.Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A, Olson BA, Weinstock J, Laping NJ. Identification of novel inhibitors of the transforming growth factor β1 (TGF-β1) Type 1 receptor (ALK5) J Med Chem. 2002;45:999–1001. doi: 10.1021/jm010493y. [DOI] [PubMed] [Google Scholar]
- 90.Maggard M, Meng L, Ke B, Allen R, Devgan L, Imagawa DK. Antisense TGF-β2 immunotherapy for hepatocellular carcinoma: Treatment in a rat tumor model. Ann Surg Oncol. 2001;8:32–37. doi: 10.1007/s10434-001-0032-6. [DOI] [PubMed] [Google Scholar]
- 91.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 92.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 93.Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M. Transforming growth factor-β1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int. 2000;58:1885–1892. doi: 10.1111/j.1523-1755.2000.00360.x. [DOI] [PubMed] [Google Scholar]
- 94.Yata Y, Gotwals P, Koteliansky V, Rockey DC. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-β soluble receptor: Implications for antifibrotic therapy. Hepatology. 2002;35:1022–1030. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- 95.Ueno H, Sakamoto T, Nakamura T, Qi Z, Astuchi N, Takeshita A. A soluble transforming growth factor-β receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum Gene Ther. 2000;11:33–42. doi: 10.1089/10430340050016139. [DOI] [PubMed] [Google Scholar]
- 96.Kingston PA, Sinha S, David A, Castro MG, Lowenstein PR, Heagerty AM. Adenovirus-mediated gene transfer of a secreted transforming growth factor-β type II receptor inhibits luminal loss and constrictive remodeling after coronary angioplasty and enhances advertitial collagen deposition. Circulation 2001. 104:2595–25601. doi: 10.1161/hc4601.099405. [DOI] [PubMed] [Google Scholar]
- 97.McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-β treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol. 1999;163:5693–5699. [PubMed] [Google Scholar]
- 98.Logan A, Baird A, Berry M. Decorin attenuates gliotic scar formation in the rat cerebral hemisphere. Exp Neurol. 1999;159:504–510. doi: 10.1006/exnr.1999.7180. [DOI] [PubMed] [Google Scholar]
- 99.Moon LD, Fawcett JW. Reduction in CNS scar formation without concomitant increase in axon regeneration following treatment of adult rat brain with a combination of antibodies to TGF-β1 and β2. Eur J Neurosci. 2001;14:1667–1677. doi: 10.1046/j.0953-816x.2001.01795.x. [DOI] [PubMed] [Google Scholar]
- 100.Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer-Jensen M. Recombinant soluble transforming growth factor-β type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286–1296. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- 101.Spearman M, Taylor WR, Greenberg AH, Wright JA. Antisense oligodeoxyribonucleotide inhibition of TGF-β1 gene expression and alterations in the growth and malignant properties of mouse fibrosarcoma cells. Gene. 1994;149:25–29. doi: 10.1016/0378-1119(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 102.Hoefer M, Anderer FA. Anti-(transforming growth factor-β) antibodies with predefined specificity inhibit metastasis of highly tumorigenic human xenotransplants in nu/nu mice. Cancer Immunol Immunother. 1995;41:302–308. doi: 10.1007/BF01517218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stander M, Naumann U, Dumitrescu L, Heneka M, Loschmann P, Gulbins E, Dichgans J, Weller M. Decorin gene transfer-mediated suppression of TGF-β synthesis abrogates experimental malignant glioma growth in vivo. Gene Ther. 1998;5:1187–1194. doi: 10.1038/sj.gt.3300709. [DOI] [PubMed] [Google Scholar]
- 104.Platten M, Wild-Bode C, Wick W, Leitlein J, Dichgans J, Weller M. N-[3,4-dimethoxycinnamoyl]-anthranilic acid (tranilast) inhibits transforming growth factor-β releases and reduces migration and invasiveness of human malignant glioma cells. Int J Cancer. 2001;93:53–61. doi: 10.1002/ijc.1289. [DOI] [PubMed] [Google Scholar]
- 105.Fakhrai H, Mantil J, Liu L, Nicholson G, Satter CM, Ruppert J, Krause G, Saadatmandi N, Shawler DL. Treatment of glioma with a TGFβ antisense-modified tumor cell vaccine; Proc Am Assoc Cancer Res 93rd Annual Mtg. San Francisco, CA; 2002. Apr 06–10, Abst 714. [Google Scholar]
- 106.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 107.Calabresi PA, Fields NS, Maloni HW, Hanham A, Carlino J, Moore J., Levin MC, Dhib-Jalbut S, Tranquill LR, Austin H, McFarland HF, Racke MK. Phase 1 trial of transforming growth factor beta 2 in chronic progressive MS. Neurology. 1998;51:289–292. doi: 10.1212/wnl.51.1.289. [DOI] [PubMed] [Google Scholar]
- 108.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990;86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schimid P, Itin P, Cox D. Proteolytic inactivation of transforming growth factor beta 3 by elastase in venous leg ulcers: implications for clinical trials using topically-applied peptide growth factors. Br J Dermatol. 1999;140:1170–2. [PubMed] [Google Scholar]
- 110.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Eng J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]