Abstract
Antiserum raised against whole Helicobacter pylori cells identified a novel 94-kDa antigen. The nucleotide sequence of the gene encoding the 94-kDa antigen was determined, and analysis of the deduced amino acid sequence revealed structural features typical of the ClpB ATPase family of stress response proteins. An isogenic H. pylori clpB mutant showed increased sensitivity to high-temperature stress, indicating that the clpB gene product functions as a stress response protein in H. pylori.
Helicobacter pylori is the causative agent of chronic active gastritis and is associated with the development of peptic ulcer disease and gastric cancer (1). H. pylori is unique among bacterial pathogens in that it can tolerate exposure to a range of physiological and biological stresses encountered in the human stomach. In response to stress conditions, bacteria transiently increase the synthesis of stress response proteins which are thought to protect the cell from stress-induced damage by preventing denaturation of cellular proteins, reactivating once-inactivated proteins, and regulating the degradation of irreversibly denatured proteins (20). Studies of the immune response to stress proteins have demonstrated that they are major antigens of many bacterial pathogens (2, 11, 25), suggesting that they are abundant in the bacterial cell during infection. The HtrA (high-temperature-requirement) stress proteins are essential for the virulence of intracellular bacterial pathogens, presumably against the toxic effects of oxidative killing within host phagocytes (3, 10, 15). The Clp ATPase protein family, which comprises ClpA-, ClpB-, ClpC-, and ClpX-like proteins, includes several stress response proteins (22). Conditions which induce expression of the Clp stress proteins include high temperature, high salt or ethanol concentration, oxygen limitation, and iron limitation. Bacterial clp mutants show increased sensitivity to a range of stress conditions in vitro (13, 21–23); e.g., Listeria monocytogenes clpC mutants are sensitive to high temperature, high osmolarity, iron limitation, and oxidative stress and are attenuated in mice (21).
Despite the ability of H. pylori to survive in a stressful environment, only the urease-associated HspA and HspB and the HtrA stress proteins of H. pylori have been characterized to date (2, 4, 12, 25). This report describes the cloning and nucleotide sequence analysis of an H. pylori gene encoding a homolog of the ClpB stress response proteins, identified by screening a λ ZAP genomic library of H. pylori NCTC 11637 for clones reactive with an antiserum raised against whole H. pylori cells. To determine the role of the clpB gene product in H. pylori, an isogenic clpB mutant was constructed and compared with the parental strain for survival at high temperature.
Bacterial strains and growth conditions.
H. pylori was grown on Helicobacter-selective agar, consisting of blood agar base No. 2 (Oxoid) supplemented with 7% lysed horse blood and Dent’s selective supplement (10 μg of vancomycin/ml, 5 μg of trimethoprim/ml, 5 μg of cefsulodin/ml, and 5 μg of amphotericin/ml; Oxoid), in a microaerobic atmosphere for 48 h at 37°C. Escherichia coli strains were grown in Luria-Bertani (LB) broth or on LB agar. The antibiotics used for selection of clones were ampicillin (100 μg/ml) and kanamycin (50 μg/ml).
Screening of genomic library and recovery of pBK-CMV plasmids.
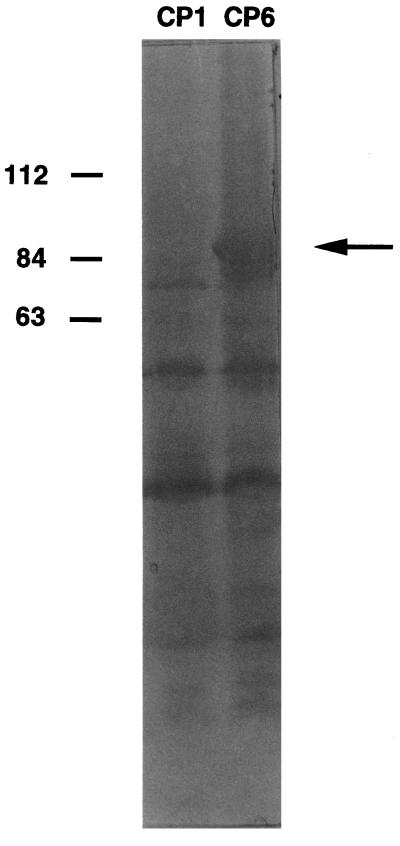
An antiserum raised against whole cells of H. pylori Roberts (16) and a λ ZAP II Express genomic library of H. pylori NCTC 11637 were kindly provided by C. W. Penn, University of Birmingham, Birmingham, United Kingdom. Anti-whole-cell antiserum was raised in two adult New Zealand White rabbits each injected intravenously twice weekly for 3 weeks with a heat-killed suspension of H. pylori containing between 5 × 106 and 1.6 × 108 bacteria. The animals were bled 2 weeks after the final dose, and the sera were pooled. The genomic library was constructed by ligation of Sau3AI partial digest fragments of 2 to 10 kb with BamHI arms of the λ ZAP II Express (Stratagene, Cambridge, United Kingdom). To screen for clones reactive with the anti-whole-cell antiserum, the library was plated on E. coli XL1-Blue MRF′ and incubated at 42°C for 3.5 h to allow plaque formation and then induced with IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C for gene expression. The plaques were transferred to nitrocellulose filters and reacted with a 1:200 dilution of anti-whole-cell antiserum as previously described (27). Of 4 × 103 plaques screened, 54 clones expressing H. pylori antigens were identified. To recover the recombinant pBK-CMV plasmids from these clones, E. coli XL1-Blue MRF′ was infected simultaneously with an f1 helper phage (Exassist; Stratagene) and the recombinant λ ZAP phage (according to the instructions provided with Exassist). Recombinant pBK-CMV plasmids were transformed into E. coli XLOLR and plated on LB-kanamycin agar. Lysates of the E. coli clones were analyzed by immunoblotting, with E. coli XLOLR containing pBK-CMV alone (CP1) used as a negative control. Of 27 E. coli XLOLR clones examined by immunoblotting, 10 clones which reacted strongly with the antiserum were characterized. Three clones encoded the structural subunits of urease (UreA and UreB) (14), two encoded UreA alone, and one each encoded the flagellar hook protein (19), catalase (18), HspB (25), and a homolog of bacterial methyl-accepting chemotaxis proteins (9). The remaining clone, E. coli CP6, produced a strongly immunoreactive reactive polypeptide of 94 kDa (Fig. 1) and was characterized further.
FIG. 1.
Immunoblot analysis of E. coli XLOLR containing either pBK-CMV with a 3.2-kb insert of H. pylori genomic DNA (CP6) or pBK-CMV alone (CP1). Blots were probed with rabbit anti-H. pylori whole-cell antiserum and detected with alkaline phosphatase-conjugated anti-rabbit antibody. The numbers to the left indicate the sizes of the protein standards in kilodaltons. The arrow to the right indicates the 94-kDa antigen expressed by E. coli CP6.
Nucleotide sequence and conservation of the gene encoding the 94-kDa antigen.
The region encoding the 94-kDa antigen was localized to a 3-kb fragment of the insert in pCP6 by immunoblot analysis of a set of nested deletion subclones, constructed by using a Promega (Southampton, United Kingdom) Erase-A-Base kit. Analysis of the nucleotide sequence of the 3-kb insert revealed an open reading frame of 2,571 nucleotides with the potential to code for 856 amino acids. The calculated molecular mass of this protein is 94 kDa, which agrees with the mass of the antigen expressed in E. coli CP6 and determined by immunoblotting. The sequence TTGAGA-15-TATTTT, which resembles an E. coli ς70 promoter (8), is located 94 bp upstream of the putative ATG start codon. Southern blot and PCR analyses of 15 H. pylori strains isolated at St. Bartholomew’s Hospital and 8 isolated at St. James’s Hospital, Dublin, Ireland, showed that the clpB gene was present in all strains examined (results not shown).
Analysis of the predicted amino acid sequence.
Comparison with protein sequences in the NBRF (National BioMedical Research Foundation) database demonstrated that the deduced amino acid sequence shows structural features typical of the Clp ATPase family, with two nucleotide-binding regions (N1 and N2) containing segment A and B nucleotide-binding motifs separated by a spacer region and enclosed between a leader sequence and a trailer sequence. The length of the spacer region of the H. pylori Clp protein is 126 amino acids, indicating that it belongs to the ClpB subfamily (22). H. pylori ClpB showed extensive amino acid identity with the ClpB proteins of Dichelobacter (Bacteroides) nodosus (17) (41% identity in an 856-amino-acid overlap), Haemophilus influenzae (6) (39.2%), and E. coli (22) (38.6%).
Construction of H. pylori clpB mutant.
The insert from pCP6 was subcloned into pUC18, generating pIP6. A 935-bp deletion and a unique BglII site were introduced into the clpB gene in pIP6 by the inverse-PCR mutagenesis (IPCRM) procedure (28) with the oligonucleotides 5′-AAAAAGAGTGGTGGGGCAAGA-3′ and 5′-CTCTTCAAACTCGCCTCTGTA-3′, each of which included a 5′-terminal BglII restriction site. For IPCRM, 0.25 to 25 ng of pIP6 was added to a PCR mixture which was subjected to 40 cycles of 1 min of denaturation at 94°C, 30 s of annealing at 55°C, and 5 min of extension at 72°C in an Omnigene thermal cycler. PCR products were digested with BglII, self-ligated (to form pIP7), and transformed into E. coli XL2-Blue (Stratagene). A 1.4-kb BamHI restriction fragment of plasmid pJMK30 containing a gene encoding resistance to kanamycin (aph3′-III [5]) was ligated in each orientation into the unique BglII site of pIP7, generating pIP18 and pIP19. These plasmids were introduced into H. pylori N6 (5) by natural transformation as previously described (7). No difference in transformation frequency was observed for the two plasmids, each giving rise to 2 × 104 kanamycin-resistant (Kmr) transformants per μg of DNA. PCR, with clpB-specific primers (5′-TTAAAAATTCCGGCTTGAAG-3′ and 5′-GTTGATAATGAATTTATTTGA-3′), on genomic DNA isolated from the parental strain and from two Kmr transformants, one obtained by using pIP18 (N6.1) and one by using pIP19 (N15.8), amplified a 2,262-bp product from N6 and a 2,827-bp product from N6.1 and N15.8, consistent with deletion of 935 bp and insertion of the 1.4-kb kanamycin cassette in strains N6.1 and N15.8. Southern blot analysis of N6.1 and N15.8 using a 1.4-kb H. pylori clpB probe provided further confirmation of allelic replacement (data not shown). The failure to isolate mutants in genes encoding stress response proteins, including hspA and hspB of H. pylori, indicates a vital role for these proteins in normal cell growth (24). Our ability to construct a clpB mutant indicates a nonessential function for this gene in H. pylori.
Characterization of H. pylori clpB mutant.
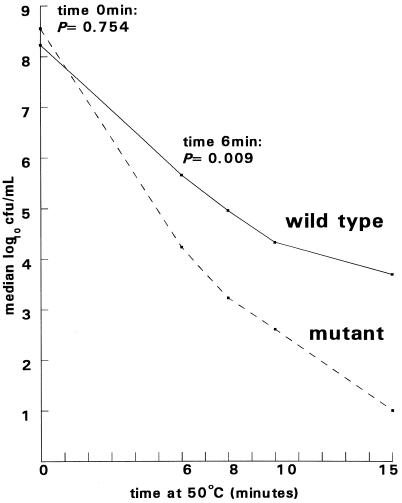
The H. pylori clpB mutant N6.1 was microaerophilic and oxidase, catalase, and urease positive. No difference in the rate of growth was apparent between mutant and wild-type bacteria on Helicobacter-selective agar at 30, 37, or 40°C. For temperature stress experiments, wild-type and mutant bacteria, grown on Helicobacter-selective agar at 30°C for 48 h, were harvested, washed, and resuspended in phosphate-buffered saline (pH 7.24). Approximately 108 bacteria (in 50 μl) were transferred to 1-ml volumes of phosphate-buffered saline, (pH 7.24) prewarmed to 50°C, and were incubated for 15 min. Samples (20 μl) were removed at 6, 8, 10, and 15 min, diluted 10-fold serially, and plated on Helicobacter-selective agar to determine the number of CFU at increasing time intervals. Statistical significance was determined by using the Wilcoxon rank sum test (STATA statistical package; Stata Corporation, College Station, Tex.). In five independent experiments the sizes of the initial inocula of mutant and wild-type bacteria did not differ significantly; the median values (ranges) were 1.7 × 108 CFU/ml (1.0 × 108 to 2.4 × 108) and 1.8 × 108 CFU/ml (9.5 × 107 to 4.2 × 108) for the wild type and mutant, respectively (P = 0.754) (Fig. 2). In each of the five experiments, the viable count for the clpB mutant was lower than that of the wild-type strain at each subsequent sampling time (Fig. 2). The difference in viability was most apparent in the first 6 min: the median viable counts (ranges) for the wild type and mutant were 4.5 × 105 CFU/ml (8.2 × 104 to 1.2 × 108) and 1.7 × 104 CFU/ml (1.1 × 104 to 2.2 × 104), respectively (P = 0.009). Thereafter the difference in viability was less marked, although the counts for the mutant remained significantly lower than those for the wild type at 8, 10, and 15 min.
FIG. 2.
Survival at 50°C of wild-type H. pylori (N6) and the H. pylori clpB mutant (N6.1). Median values for five experiments are shown.
Summary.
This work has identified a conserved H. pylori gene encoding a novel immunodominant antigen. The predicted amino acid sequence of this antigen has structural features typical of the ClpB stress response proteins. The clone encoding the H. pylori ClpB protein was identified in a λ ZAP genomic library by virtue of its reactivity with antiserum raised against H. pylori whole cells. Our ability to construct a clpB mutant indicates that the clpB gene is not essential for growth of H. pylori. The clpB mutant shows increased sensitivity to high-temperature stress, indicating that the clpB gene product is a stress response protein which may be important for survival of H. pylori in the hostile environment of the human stomach. As H. pylori lacks a heat shock sigma 32 (26), transcriptional control of this gene is likely to be different from that in E. coli. Further experiments are required to define the promoter for this gene.
Nucleotide sequence accession number.
The nucleotide sequence of the H. pylori clpB gene has been submitted to the EMBL database under accession no. YO8238.
Acknowledgments
We gratefully acknowledge Charles Penn for his generous gift of the H. pylori λ ZAP II Express library and rabbit anti-whole cell antiserum. We are also grateful to C. A. O’Morain for donating clinical isolates and to Richard Ferrero for H. pylori N6. We thank Nick Dorrell for help with IPCRM and Brendan Wren for critical review of the manuscript.
This work was supported in part by Oravax Inc., Cambridge, Mass.
REFERENCES
- 1.Blaser M J. Helicobacter pylori: its role in disease. Clin Infect Dis. 1992;15:386–393. doi: 10.1093/clind/15.3.386. [DOI] [PubMed] [Google Scholar]
- 2.Dunn B E, Roop II R M, Sung C C, Sharma S A, Perez-Perez G I, Blaser M J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elzer P H, Phillips R W, Kovach M E, Peterson K M, Roop R M., II Characterization and genetic complementation of a Brucella abortus high-temperature-requirement A (htrA) deletion mutant. Infect Immun. 1994;62:4135–4139. doi: 10.1128/iai.62.10.4135-4139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans D J, Jr, Evans D G, Engstrand L, Graham D Y. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrero R L, Cussac V, Courcoux P, Labigne A. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J Bacteriol. 1992;174:4212–4217. doi: 10.1128/jb.174.13.4212-4217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 7.Haas R, Meyer T F, van Putten J P M. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol Microbiol. 1993;8:753–760. doi: 10.1111/j.1365-2958.1993.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 8.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promotor DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazelbauer G L. Bacterial chemoreceptors. Curr Opin Struct Biol. 1992;2:505–510. [Google Scholar]
- 10.Johnson K, Charles I, Dougan G, Pickard D, O’Gaora P, Costa G, Miller T A I, Hormaeche C E. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann S H E, Schoel B. Heat shock proteins as antigens in immunity against infection and self. In: Morimoto R I, Tisieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 495–531. [Google Scholar]
- 12.Kleanthous H, Clayton C L, Morgan D D, Pallen M J, Tabaqchali S. Molecular cloning and nucleotide sequence determination of htrA, a gene encoding a 48-kDa stress protein of Helicobacter pylori. In: Gasbarrini G, Pretolani S, editors. Basic and clinical aspects of H. pylori infection. Berlin, Germany: Springer-Verlag; 1993. pp. 195–202. [Google Scholar]
- 13.Kruger E, Volker U, Hecker M. Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance. J Bacteriol. 1994;176:3360–3367. doi: 10.1128/jb.176.11.3360-3367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labigne A F, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. Infect Immun. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S-R, Dorrell N, Everest P H, Dougan G, Wren B W. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infect Immun. 1996;64:2088–2094. doi: 10.1128/iai.64.6.2088-2094.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luke C J, Kubiak E, Cockayne A, Elliott T S J, Penn C W. Identification of flagellar and associated polypeptides of Helicobacter (formerly Campylobacter) pylori. FEMS Microbiol Lett. 1990;71:225–230. doi: 10.1016/0378-1097(90)90061-t. [DOI] [PubMed] [Google Scholar]
- 17.Mattick J S, Anderson B J, Cox P T, Dalrymple B P, Bills M M, Hobbs M, Egerton J R. Gene sequences and comparison of the fimbrial subunits representative of Bacteroides nodosus serogroups A to I: class I and class II strains. Mol Microbiol. 1991;5:561–573. doi: 10.1111/j.1365-2958.1991.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Odenbreit S, Wieland B, Haas R. Cloning and genetic characterization of Helicobacter pylori catalase and construction of a catalase-deficient mutant strain. J Bacteriol. 1996;178:6960–6967. doi: 10.1128/jb.178.23.6960-6967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Toole P W, Kostrzynska M, Trust T J. Non-motile mutants of Helicobacter pylori defective in flagellar hook production. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 20.Parsell D A, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 21.Rouquette C, Ripio M T, Pellegrini E, Bolla J M, Tascon R I, Vazquez-Boland J A, Berche P. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol Microbiol. 1996;21:977–987. doi: 10.1046/j.1365-2958.1996.641432.x. [DOI] [PubMed] [Google Scholar]
- 22.Squires C, Squires C L. The Clp proteins: proteolysis regulators or molecular chaperones? J Bacteriol. 1992;174:1081–1085. doi: 10.1128/jb.174.4.1081-1085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squires C L, Pedersen S, Ross B M, Squires C. ClpB is the Escherichia coli heat shock protein F84.1. J Bacteriol. 1991;173:4254–4262. doi: 10.1128/jb.173.14.4254-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suerbaum, S., and A. Labigne. 1993. Helicobacter pylori hspA-B heat shock operon: nucleotide sequence, expression, and putative role. Acta Gastro-enterol. Belg. 56(Suppl.):51.
- 25.Suerbaum S, Thiberge J M, Kansau I, Ferrero R L, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol Microbiol. 1994;14:959–974. doi: 10.1111/j.1365-2958.1994.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 27.Wren B W, Clayton C L, Mullany P, Tabaqchali S. Molecular cloning and expression of Clostridium difficile toxin A in Escherichia coli K12. FEBS Lett. 1987;225:82–86. doi: 10.1016/0014-5793(87)81135-3. [DOI] [PubMed] [Google Scholar]
- 28.Wren B W, Henderson J, Ketley J M. A PCR-based strategy for the rapid construction of defined bacterial deletion mutants. BioTechniques. 1994;16:994–996. [PubMed] [Google Scholar]