Abstract
Nonpolar virB mutants of Agrobacterium tumefaciens were tested for RSF1010 mobilization and extracellular complementation. virB2 to virB11 were essential for transfer in both assays. virB1 was essential only for high frequency transfer of RSF1010 and VirE2. Coordinated transfer of a preassembled T complex is supported by these data and competition studies.
During infection of dicotyledonous plants, Agrobacterium tumefaciens transfers a segment of its Ti plasmid, the transferred DNA (T-DNA), into the plant cell. The transfer intermediate, which is termed the T strand, is composed of the single-stranded T-DNA covalently attached to the protein VirD2 (23). At least 7 of the 11 genes of the virB operon and virD4 are essential for transfer of T strands (5, 15, 16, 27). These genes, along with virE1, are also essential for transfer of the virE2-encoded single-stranded binding protein into plant cells (5, 8, 15, 25). VirE2 is essential for tumorigenesis because it coats the T strand, protecting it from degradation and assisting in its translocation of the T-DNA to the plant cell nucleus (10, 21, 28). Two models have been proposed for the simultaneous transfer of T strands and VirE2. The first model proposes that VirE2 binds to the T strand within A. tumefaciens and that a preassembled T complex is then transferred by a virB operon-virD4-encoded complex to the plant cell (29). The second model proposes that VirE2 and T strands are transferred via separate virB-virD4-encoded channels and that the T complex is assembled in the plant cell cytoplasm (5, 25).
In addition to transfer of T strands and VirE2 protein, the conjugative plasmid RSF1010 can be transferred by A. tumefaciens either to plant cells or to other agrobacteria (3, 6). Transfer of RSF1010 to either recipient is also dependent on at least two of the virB genes and virD4 (3, 15). Recently, studies of RSF1010 mobilization led to the demonstration that the 11 genes of the virB operon are also essential for the synthesis of thin, brittle pili (13, 14). In the present study, the connection between genes required for pilus assembly and transfer events was further established by characterizing nonpolar mutations in each of the 11 virB genes for function in the transfer of RSF1010 and in extracellular complementation.
Nonpolar virB mutants.
Some of the nonpolar virB operon mutants used in this study were generated by transposon mutagenesis of pKJF78, a derivative of pUC19 carrying the entire virB operon from pTiA6 as a 10.4-kb NdeI-XhoI fragment. Mutagenesis with Tn5virB (11) was done as described by de Bruijn and Lupski (12). The exact sites of transposon insertions on pKJF78 (Table 1) were mapped and sequenced, and the transposons were transferred onto pTiA6 by marker exchange (7). Additional nonpolar virB operon mutants were obtained from P. Christie (University of Texas at Houston) or from A. Binns (University of Pennsylvania). All mutants used were first confirmed to be avirulent by infection of Kalanchöe diagremontiana and for nonpolarity by trans complementation with plasmids pPC925, pPC933, pPC961, pPC975, and pPC9103 (4) obtained from P. Christie; pED52 and pED37 (11) obtained from A. Binns; and pKJF101 (18) and pKJF30 (15) (data not shown).
TABLE 1.
Function of virB2 to virB11 mutants in RSF1010 mobilization
| Straina | Relevant characteristics | Mobilization of pML122ΔKmb |
|---|---|---|
| A348 | Wild type | 1.3 × 10−3 |
| A348ΔB2 | ΔvirB2 | <6.3 × 10−7 |
| A348ΔB3 | ΔvirB3 | <7.6 × 10−7 |
| At12031 | virB3::Tn5virB (2108) | <4.2 × 10−7 |
| At12044 | virB4::Tn5virB (3549) | <6.3 × 10−7 |
| A348ΔB5 | ΔvirB5 | <8.8 × 10−7 |
| A348ΔB6 | ΔvirB6 | <5.3 × 10−7 |
| At12063 | virB6::Tn5virB (6125) | <3.3 × 10−7 |
| A348ΔB7 | ΔvirB7 | <7.1 × 10−7 |
| A348ΔB8 | ΔvirB8 | <7.0 × 10−7 |
| At12081 | virB8::Tn5virB (6779) | <5.8 × 10−7 |
| Ax42 | virB9::Tn5virB (7878) | <1.0 × 10−6 |
| A348ΔB10 | ΔvirB10 | <9.6 × 10−7 |
| At12101 | virB10::Tn5virB (8346) | <4.5 × 10−7 |
| At12102 | virB10::Tn5virB (8416) | <5.1 × 10−7 |
| At10011 | ΔvirB11 | <1.1 × 10−6 |
Construction of Tn5virB strains is described in the text, except for that of Ax42 (11), which was obtained from A. Binns. Numerical designation following Tn5virB refers to site of insertion according to the DNA sequence published by Ward et al. (26). All A348ΔB strains (4) were obtained from P. Christie. At10011 was from K. Stephens (24).
Conjugation assays were done as previously described (14). Frequencies of transfer of pML122ΔKm to recipient UIA143 are expressed as transconjugants per input donor. Data are the means of triplicates from a single assay. Three independent experiments were done, with similar results.
virB2 to virB11 and virD4: a core complex for pilus assembly and transfer of three substrates.
It has previously been established that each of the 10 genes virB2 to virB11 is essential for both tumorigenesis and pilus assembly (4, 13). If pili are essential for all transfer processes, then these 10 genes should also be essential for transfer of RSF1010, VirE2, and T-DNA.
To assay RSF1010 mobilization, a derivative of RSF1010 conferring resistance to only gentamicin was created by removing the kanamycin resistance gene from pML122 (17) by digestion with SalI and religating the plasmid. This plasmid, pML122ΔKm, was mobilized into strains bearing nonpolar mutations in genes virB2 to virB11. The resulting strains were tested for their abilities to mobilize pML122ΔKm to A. tumefaciens UIA143 at 19°C and pH 5.3 as previously described (14). In three separate experiments, all strains with nonpolar mutations in the genes virB2 to virB11 did not transfer pML122ΔKm (Table 1). Thus, these 10 genes encode proteins which are absolutely essential for the conjugative transfer of RSF1010. These data expand on the results of two previous studies which showed that virB4, virB11, and virD4 are each required for conjugative transfer (3, 15).
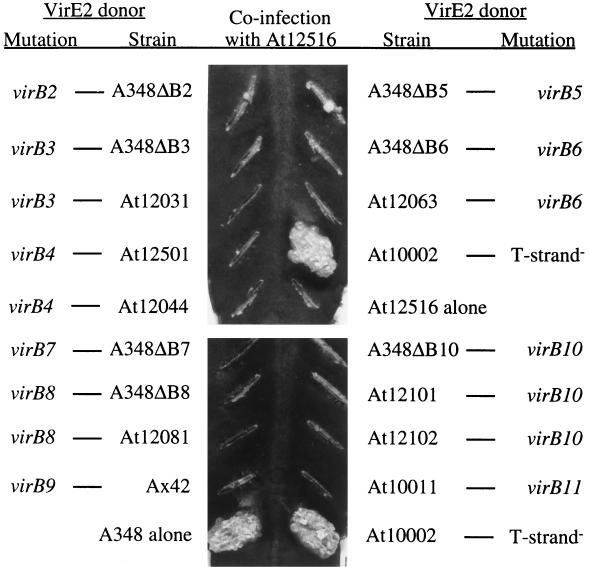
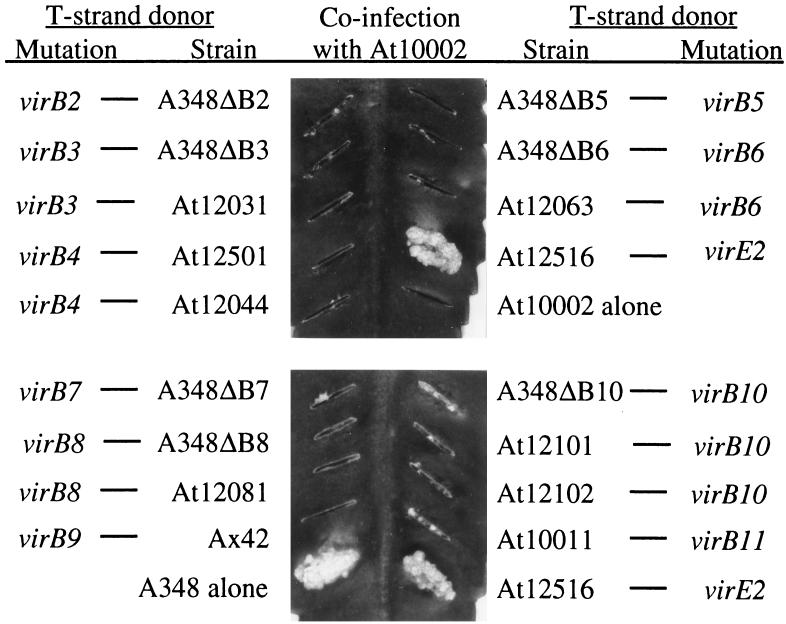
A. tumefaciens strains with mutations in the virE operon have the unusual property of having their virulence restored when coinfected with a T-DNA-defective but virE2+ strain (19). This phenomenon, which is known as extracellular complementation, is believed to occur because the T strand and VirE2 protein, when transferred separately, can associate within the plant cell cytoplasm to create a complex that can move into the nucleus (10). Previously, it had been shown that virD4 and seven genes of the virB operon, virB4 to virB6 and virB8 to virB11, are required by both the donor of the T strand and the donor of VirE2 protein to achieve extracellular complementation (5, 8, 15). To extend the characterization of the genetic requirements for extracellular complementation, cells with mutations in 10 genes, virB2 to virB11, were each tested for their abilities to complement extracellularly either the virE2 mutant At12516 (14) or the T-DNA-defective mutant At10002 (20). As expected, coinfection of At12516 with At10002 resulted in the formation of tumors on K. diagremontiana (Fig. 1 and 2). However, none of the strains with nonpolar mutations in virB2 to virB11 restored tumorigenesis to either At12516 (Fig. 1) or At10002 (Fig. 2). In one assay, severely attenuated tumors developed when a virB2 or virB5 mutant served as the donor of VirE2 protein (Fig. 1). The formation of these tumors was not reproducible, and tumors arose only when dense cell inocula were applied.
FIG. 1.
virB mutants as donors of VirE2 protein during extracellular complementation. Overnight cultures were diluted to an optical density at 600 nm of 2.0. A 5-μl volume of a 1:1 mixture (2 × 107 cells) of At12516 and the indicated virB mutants was inoculated onto a leaf surface wound created by scratching with an 18-gauge needle. The photographs were taken 30 days postinfection.
FIG. 2.
virB mutants as donors of T strands during extracellular complementation. Overnight cultures were diluted to an optical density at 600 nm of 2.0. A 5-μl volume of a 1:1 mixture (2 × 107 cells) of At10002 and the indicated virB mutants was inoculated onto a leaf surface wound created by scratching with an 18-gauge needle. The photographs were taken 30 days postinfection.
These and previous studies showed that virB2 to virB11 and virD4 are essential for transfer of T strands, VirE2 protein, and the plasmid RSF1010. Apparently, none of these genes encodes a protein which specifically functions in the movement of one but not the other substrate. Further, among the genes virB2 to virB11, there are no differences in the genes which function for transfer to plants versus bacteria, indicating that none of these genes encodes a plant-specific adhesin. Taken together, these observations suggested that virB2 to virB11 and virD4 produce a core transfer complex at the A. tumefaciens membrane. This complex is essential both for assembly of the pilus, which establishes contact with either a plant or a bacterium, and for the subsequent transfer of all three substrates: T strands, RSF1010, and VirE2.
Deletion of virB1 leads to a low level of efficiency of RSF1010 transfer.
Recent data indicate that VirB1 may facilitate contact with target plant cells, either directly or by participating in pilus assembly. A processed form of VirB1, VirB1*, can be sheared from intact cells, suggesting that this protein functions on the exterior of the bacterium (1). Other studies have shown that the N terminus of VirB1 is homologous to lysozyme (2, 18), suggesting a role in modifying the bacterial cell wall to ease assembly of the pilus or transfer structure. Consistent with these proposed roles, A348ΔB1, a strain bearing a deletion of virB1, does not make pili observable by electron microscopy (13); however, this strain does induce attenuated tumors (4). To further examine the role of VirB1 in transfer, A348ΔB1 was tested for function in transfer assays.
In the RSF1010 mobilization assay, A348ΔB1 mobilized pML122ΔKm to recipient A. tumefaciens UIA143 in only 3 of 15 assays. In the 3 positive cases, the transfer frequency was on the order of 10−6, compared to 10−3 for the wild-type strain A348. This result demonstrated that loss of VirB1 does have a major effect on transfer. If VirB1 is required for enhancing cell contacts, it is apparently also important for bacterium-to-bacterium contacts.
Deletion of virB1 is more detrimental to VirE2 protein transfer than to T-DNA transfer during extracellular complementation.
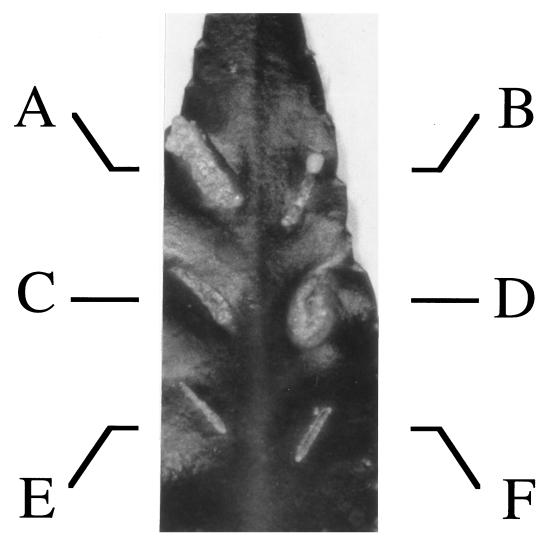
To further examine the effect of the virB1 deletion on transfer to plant cells, the effect of the mutation on extracellular complementation was tested. Since A348ΔB1 is partially virulent on K. diagremontiana (2), first either its T-DNA processing genes or its virE2 gene was deleted from it to generate avirulent VirE2 protein and T-strand donors, respectively. These deletions were generated by electroporating either pKS124 (20) or pKJF82 (14) into A348ΔB1, followed by screening for double homologous recombination as previously described for the construction of At10002 (20) and At12516 (14). Southern analysis (22) and Western blotting (15) with antibodies against VirE2, VirD2, and VirD4 confirmed that the resulting strains had the correct Ti plasmid arrangements and produced the appropriate proteins (data not shown). The resulting strains, At10002ΔB1 (virB1 and defective for T strand) and At12516ΔB1 (virB1 virE2), were completely avirulent on K. diagremontiana (Fig. 3E and F).
FIG. 3.
ΔvirB1 mutants in extracellular complementation. Overnight cultures were diluted to an optical density at 600 nm of 0.2. Strains were either mixed 1:1 for coinfections or diluted 1:1 with MG/L (7) broth for single infections. Wounds were created with an 18-gauge needle, and 5 μl (2 × 106 cells) of the indicated strains was inoculated. The photograph was taken 22 days postinfection. Inoculated A. tumefaciens strains included At12516 with At10002 (A), At12516ΔB1 with At10002ΔB1 (B), At12516 with At10002ΔB1 (C), At12516ΔB1 with At10002 (D), At12516ΔB1 (E), and At10002ΔB1 (F).
Coinfection of At10002ΔB1 with At12516ΔB1 (Fig. 3B) generated highly attenuated tumors, demonstrating that VirB1 is important for transfer of either VirE2 or T strands during extracellular complementation. Interestingly, coinfection of At12516ΔB1 with At10002 resulted in a tumor (Fig. 3D), while coinfection of At10002ΔB1 with At12516 produced highly attenuated tumors (Fig. 3C). These data indicated that loss of virB1 was more detrimental to VirE2 protein transfer than to T-strand transfer during extracellular complementation.
A differential pattern of T-strand transfer compared to VirE2 protein transfer during extracellular complementation would be expected if VirB1 is required for improving cell contacts, and, thereby, for increasing the overall frequency of transfer events. A strain with a poor overall transfer efficiency would be less likely to provide the 600 individual molecules of VirE2 protein necessary to coat a 20-kb T strand within the cytoplasm of the plant cell (9). However, the transfer efficiency is probably sufficient for a single transfer event required to donate a T strand during coinfection.
These results contradict other studies which concluded that infection of plant cells normally occurs by separate transfer of VirE2 and T strands (5, 25). If this were the case, A348ΔB1 could not induce even attenuated tumors because VirE2 protein transfer would be severely hampered, just as it is during extracellular complementation. Thus, in order for A348ΔB1 to induce attenuated tumors, it must transfer both T strands and VirE2 protein in a single transfer event, as does a T strand during extracellular complementation.
Competition studies support a model for preassociation of the T complex.
Further evidence supporting the preassociation of VirE2 with T strands and their transfer as a single event was found in competition assays. Strain At10002 does not make T strands due to a deletion of the T-DNA processing genes virD1 and virD2 (20). When assayed for RSF1010 mobilization between bacteria, this mutant had a slight but reproducible reduction in conjugation frequency (Table 2). As the deletion in At10002 altered the genetic organization of the virD operon, it is possible that reduced synthesis of the essential protein VirD4 caused the observed decrease in the transfer frequency. However, Western blotting showed that the steady-state levels of VirD4 protein were similar in wild-type A348 and the mutant At10002, demonstrating that synthesis of VirD4 is not affected by the upstream deletion in At10002 (data not shown).
TABLE 2.
Mobilization of pML122 from T-strand- and virE2-defective strains
| Strain | Relevant genotype | Conjugation frequency (mean ± SD) (10−4)a |
|---|---|---|
| A348 | Wild type | 28 ± 3 |
| At10002 | ΔvirD1 -D2 | 15 ± 2 |
| At12516 | ΔvirE2 | 34 ± 2 |
| At10005 | ΔvirD1 -D2 ΔvirE2 | 29 ± 2 |
Data are expressed as transconjugants per input donor. Numbers are the means and standard deviations of triplicates from a single assay. Five independent experiments were performed, with similar results.
Competition studies have shown that transfer of VirE2 protein to plant cells is inhibited by the presence of RSF1010 (5). Based on this finding, it is possible that the inhibition of RSF1010 transfer from strain At10002 was due to the presence of VirE2 protein. If this is the case, deletion of the virE2 gene from At10002 should relieve the inhibition of pML122 transfer and restore transfer to wild-type levels. To test this possibility, the virE2 gene was deleted from At10002 as described above for the construction of At12516ΔB1. The resulting strain, At10005, mobilized pML122 to the A. tumefaciens recipient UIA143 at a frequency similar to that measured for A348 (Table 2). Thus, elimination of virE2 from At10002 promoted pML122 transfer.
These data show that VirE2 protein can inhibit RSF1010 mobilization between bacteria in the absence of T strands. However, the virE2 deletion mutant At12516 showed little increase in the frequency of RSF1010 mobilization compared to wild-type A348 (Table 2). Thus, the inhibitory effect of VirE2 protein on RSF1010 mobilization occurs primarily when T strands are absent.
These observations are consistent with models that favor preassociation of the T strand and the VirE2 protein within the bacterium. In the presence of T strands, free VirE2 protein would be sequestered away from the transfer complex, allowing uninhibited transfer of RSF1010 between bacteria.
However, these findings seem to contradict the work of Binns et al. (5), who concluded that RSF1010 entry into plant cells was dependent on virE2. In concordance with their results, the particular virE2 mutant used in their studies does not mobilize RSF1010 between bacteria (data not shown). However, this defect was attributed to the fact that this particular mutant produces a VirE2::β-galactosidase fusion protein (5) since a deletion of virE2 had no effect on transfer (Table 2). The results presented here thus concur with the earlier findings of Buchanan-Wollaston et al. (6), who demonstrated that transfer of RSF1010 into plant cells is not dependent on virE2. These data are further consistent with the observation of Binns et al. (5) that overexpression of VirE2 in a wild-type background inhibited RSF1010 transfer to plant cells by threefold (5).
Summary.
These studies have shown that VirB2 to VirB11, along with VirD4, comprise a core transfer complex which is required for both pilus assembly and transfer events. This core complex functions to transfer three diverse substrates without regard for the nature of the recipient. Differential transfer was noted in the absence of VirB1, in agreement with its proposed role in facilitating cell contacts. Recently, virB1 to virB11 and virD4 were shown to be the only Ti plasmid genes essential for pilus assembly and transfer (13). However, it is possible that unidentified chromosomally encoded proteins are essential either as integral members of the transfer complex or as accessory proteins required for assembly of the pilus and transfer structures.
Although it was clear that RSF1010, T strands, and VirE2 utilized the same transfer complex, questions remain as to whether the T strand and VirE2 associate within the bacterium or within the plant cytoplasm. The data presented here on extracellular complementation of virB1 mutants and competition of RSF1010 and VirE2 for the transfer complex support a model for the coordinated movement of T strands and VirE2 protein into plant cells as a single, preassembled T complex.
Acknowledgments
I thank Peter Christie and Andrew Binns for providing nonpolar virB mutants and complementing plasmids; Denis Bougarel and Lin Lee for their technical assistance; and Joe Don Heath, Trevor Charles, Kathryn Stephens, Wanyin Deng, and Eugene Nester for their helpful suggestions.
This work was supported by Public Health Service National Research Service award 5T32 GM07270-21 from the National Institute of General Medical Sciences and by the University of Washington Graduate School Committee for Plant-Molecular Integration and Function.
REFERENCES
- 1.Baron C, Llosa M, Zhou S, Zambryski P C. VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens, is processed to a C-terminal secreted product, VirB1*. J Bacteriol. 1997;179:1203–1210. doi: 10.1128/jb.179.4.1203-1210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer M, Eferl R, Zellnig G, Terferle K, Dijkstra A, Karaimann G, Högenauer G. Gene 19 of plasmid R1 is required for both efficient conjugative DNA transfer and bacteriophage R17 infections. J Bacteriol. 1995;177:4279–4288. doi: 10.1128/jb.177.15.4279-4288.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beijersbergen A, Dulk-Ras A, Schilperoort R, Hooykaas P. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science. 1992;256:1324–1326. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- 4.Berger B R, Christie P J. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binns A N, Beaupré C E, Dale E M. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan-Wollaston V, Passiatore J E, Cannon F. The effect of vir mutations on plasmid transfer into plants. In: Palcios R, Verma D P S, editors. Molecular genetics of plant-microbe interactions. St. Paul, Minn: APS Press; 1988. pp. 281–282. [Google Scholar]
- 7.Cangelosi G A, Best E A, Martinetti G, Nester E W. Genetic analysis of Agrobacterium. Methods Enzymol. 1991;204:384–397. doi: 10.1016/0076-6879(91)04020-o. [DOI] [PubMed] [Google Scholar]
- 8.Christie P J, Ward J E, Winans S C, Nester E W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988;170:2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citovsky V, De Vos G, Zambryski P. Single-stranded DNA binding protein encoded by the virE locus of A. tumefaciens. Science. 1988;240:501–504. doi: 10.1126/science.240.4851.501. [DOI] [PubMed] [Google Scholar]
- 10.Citovsky V, Zupan J, Warnick D, Zambryski P. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science. 1992;256:1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- 11.Dale E M, Binns A N, Ward J E., Jr Construction and characterization of Tn5virB, a transposon that generates nonpolar mutations, and its use to define virB8 as an essential virulence gene in Agrobacterium tumefaciens. J Bacteriol. 1993;175:887–891. doi: 10.1128/jb.175.3.887-891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bruijn F J, Lupski J R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids—a review. Gene. 1984;27:131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 13.Fullner K J, Lara J C, Nester E W. Pilus assembly by Agrobacterium T-DNA transfer genes. Science. 1996;273:1007–1009. doi: 10.1126/science.273.5278.1107. [DOI] [PubMed] [Google Scholar]
- 14.Fullner K J, Nester E W. Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J Bacteriol. 1996;178:1498–1503. doi: 10.1128/jb.178.6.1498-1504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fullner K J, Stephens K M, Nester E W. An essential virulence protein of Agrobacterium tumefaciens, VirB4, requires an intact mononucleotide binding domain to function in transfer of T-DNA. Mol Gen Genet. 1994;245:705–715. doi: 10.1007/BF00297277. [DOI] [PubMed] [Google Scholar]
- 16.Grimsley N, Hohn B, Ramos C, Kado C, Rogowsky P. DNA transfer from Agrobacterium to Zea mays or Brassica by agroinfection is dependent on bacterial virulence functions. Mol Gen Genet. 1989;217:309–316. doi: 10.1007/BF02464898. [DOI] [PubMed] [Google Scholar]
- 17.Labes M, Pühler A, Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene. 1990;89:37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- 18.Mushegian A R, Fullner K J, Koonin E V, Nester E W. A family of lysozyme-like virulence factors in bacterial pathogens of plants and animals. Proc Natl Acad Sci USA. 1996;93:7321–7326. doi: 10.1073/pnas.93.14.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otten L, De G H, Leemans J, Hain R, Hooykaas P, Schell J. Restoration of virulence of vir region mutants of Agrobacterium tumefaciens strain B6S3 by coinfection with normal and mutant Agrobacterium strains. Mol Gen Genet. 1984;195:159–163. [Google Scholar]
- 20.Piers K L, Heath J D, Liang X, Stephens K M, Nester E W. Agrobacterium-mediated transformation of yeast mimics plant transformation. Proc Natl Acad Sci USA. 1996;93:1613–1618. doi: 10.1073/pnas.93.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi L, Hohn B, Tinland B. Integration of complete transferred DNA units is dependent on the activity of virulence E2 protein of Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 1996;93:126–130. doi: 10.1073/pnas.93.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 23.Stachel S E, Timmerman B, Zambryski P. Activation of Agrobacterium tumefaciens vir gene expression generates multiple single-stranded T-strand molecules from the pTiA6 T-region: requirement for 5′ virD gene products. EMBO J. 1987;6:857–863. doi: 10.1002/j.1460-2075.1987.tb04831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens K M, Roush C, Nester E. Agrobacterium tumefaciens VirB11 protein requires a consensus nucleotide-binding site for function in virulence. J Bacteriol. 1994;177:27–36. doi: 10.1128/jb.177.1.27-36.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundberg C, Meek L, Carroll K, Das A, Ream W. VirE1 protein mediates export of the single-strand DNA-binding protein VirE2 from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1996;178:1207–1212. doi: 10.1128/jb.178.4.1207-1212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward J E, Akiyoshi D E, Regier D, Datta A, Gordon M P, Nester E W. Characterization of the virB operon from an Agrobacterium tumefaciens Ti plasmid. J Biol Chem. 1988;263:5804–5814. . (Correction, 265:4678, 1990.) [PubMed] [Google Scholar]
- 27.Yusibov V M, Steck T R, Gupta V, Gelvin S B. Association of single-stranded transferred DNA from Agrobacterium tumefaciens with tobacco cells. Proc Natl Acad Sci USA. 1994;91:2994–2998. doi: 10.1073/pnas.91.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zupan J R, Citovsky V, Zambryski P. Agrobacterium VirE2 protein mediates nuclear uptake of single-stranded DNA in plant cells. Proc Natl Acad Sci USA. 1996;93:2392–2397. doi: 10.1073/pnas.93.6.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zupan J R, Zambryski P. Transfer of T-DNA from Agrobacterium to the plant cell. Plant Physiol. 1995;107:1041–1047. doi: 10.1104/pp.107.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]