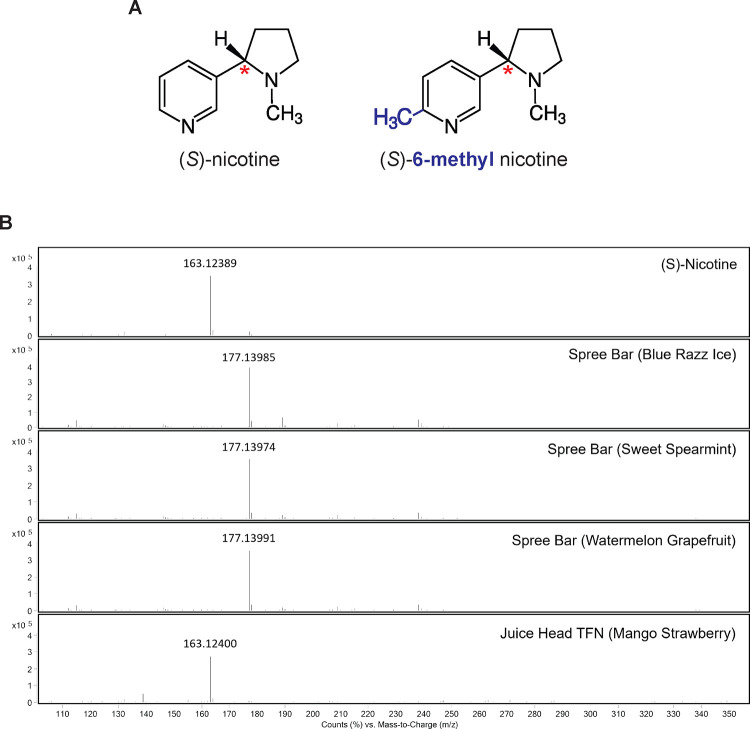
Figure 3.
A. Chemical structures of (S)-nicotine (left), the major form of nicotine in tobacco, and (S)-6-methyl nicotine (right), trademarked as “Metatine”, with a methyl group substitution (blue) at position 6 of the pyridine ring. The red asterisk indicates the chiral center of nicotine. B. Mass spectra from extracted ion chromatograms for pure (S)-nicotine and four e-liquids. The major observed ion in each spectrum corresponds to the expected [M+H]+ ion for either nicotine (163.1230 m/z) or 6-methylnicotine (177.1386 m/z).