Abstract
OBJECTIVE
Genetic and environmental factors influencing spinal development in lower vertebrates are likely to play a role in the abnormalities associated with human congenital scoliosis (CS) and idiopathic scoliosis (IS). An overview of the molecular embryology of spinal development and the clinical and genetic aspects of CS and IS are presented. Utilizing synteny analysis of the mouse and human genetic databases, likely candidate genes for human CS and IS were identified.
DESIGN
Review and synteny analysis.
METHODS
A search of the Mouse Genome Database was performed for “genes,” “markers” and “phenotypes” in the categories Neurological and neuromuscular, Skeleton, and Tail and other appendages. The Online Mendelian Inheritance in Man was used to determine whether each mouse locus had a known human homologue. If so, the human homologue was assigned candidate gene status. Linkage maps of the chromosomes carrying loci with possibly relevant phenotypes, but without known human homologues, were examined and regions of documented synteny between the mouse and human genomes were identified.
RESULTS
Searching the Mouse Genome Database by phenotypic category yielded 100 mutants of which 66 had been mapped. The descriptions of each of these 66 loci were retrieved to determine which among these included phenotypes of scoliosis, kinky or bent tails, other vertebral abnormalities, or disturbances of axial skeletal development. Forty-five loci of interest remained, and for 27 of these the comparative linkage maps of mouse and human were used to identify human syntenic regions to which plausible candidate genes had been mapped.
CONCLUSION
Synteny analysis of mouse candidate genes for CS and IS holds promise due to the close evolutionary relationship between mice and human beings. With the identification of additional genes in animal model systems that contribute to different stages of spine development, the list of candidate genes for CS and IS will continue to grow.
Keywords: Congenital scoliosis, Synteny, Idiopathic scoliosis, Spinal development, Developmental genes
INTRODUCTION
Scoliosis may be broken down into two distinct categories, congenital scoliosis (CS) and idiopathic scoliosis (IS). CS is defined as a lateral curvature of the spine due to a developmental abnormality. Scoliosis present at birth that is not associated with an underlying developmental anomaly is referred to as infantile scoliosis. An incidence of approximately 0.5 to 1/1,000 births has been observed for CS.1,2 This value was obtained by reviewing a series of 15,000 chest films in the state of Delaware (lumbar vertebral defects were not examined). Vertebral defects most commonly include hemivertebrae, block vertebrae, butterfly and wedged vertebra, and unsegmented bars (figures 1 and 2). Hemivertebrae usually represent an extra vertebral segment. Vertebral malformations that result in CS may be associated with genetic syndromes such as Alagille syndrome,3 spondylocostal dysostosis,4 and Jarcho-Levin syndrome.5 By definition, the curve in the frontal plane as viewed on a posteroanterior standing radiograph of the spine must be greater than 10 degrees.6 Although a single plane is used to establish the diagnosis, scoliosis is in fact a three dimensional deformity. As such, it results in the alteration of the normal sagittal thoracic kyphosis and lumbar lordosis.
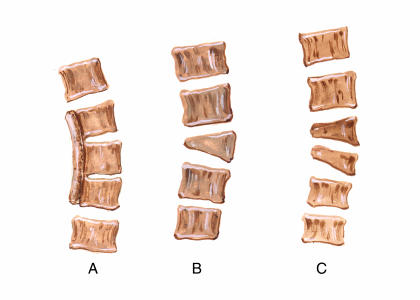
Figure 1.
Commonly encountered vertebral malformations associated with CS including an unsegmented bar (A), single hemivertebrae (B) and multiple hemivertebrae (C).
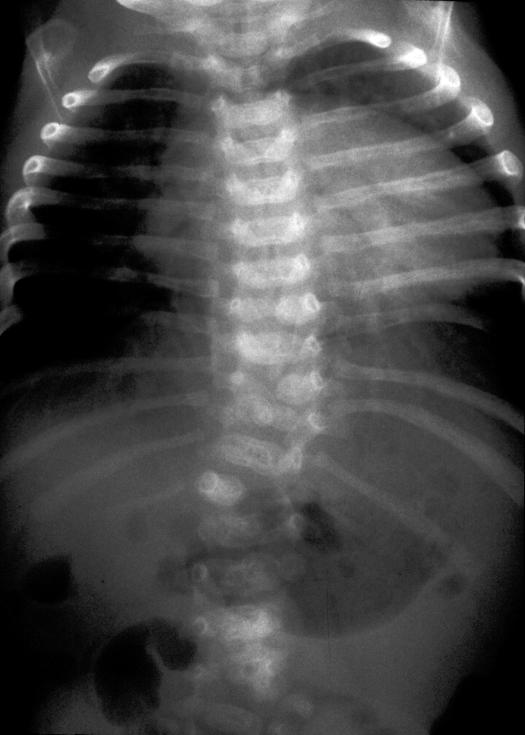
Figure 2.
Radiograph illustrating multiple segmentation abnormalities of the thoracic spine.
In contrast to this, IS is defined as a lateral curvature of the spine for which no cause can be determined. The incidence of IS in the general population ranges from 0.2% to 3%, depending on the magnitude of the curve.7 Subclassification of IS is based on the age of presentation into infantile (birth to age 3 years), juvenile (age 3 to 11 years) and adolescent (11 years and older). Evidence for a genetic contribution to IS was obtained by determining the incidence of IS of 6.94, 3.69 and 1.55%, respectively in the first, second, and third degree relatives of 114 affected individuals.8 Since the incidence of IS in the general population of Edinburgh at the time of the study was approximately 0.39%, these findings are consistent with either an autosomal dominant or multifactorial mode of inheritance. Recently, a large family with autosomal dominant IS has been identified, enabling a locus to be assigned at 17p11.9
Non-congenital scoliosis has many etiologies.10 The hereditary musculoskeletal disorders, such as osteogenesis imperfecta,11 Marfan syndrome,12 Stickler syndrome,13 Ehlers-Danlos syndrome,14 and the muscular dystrophies,15 can each include scoliosis as a manifestation. Neuromuscular diseases, such as cerebral palsy and myelomeningocele, are associated with the development of scoliosis secondary to muscle imbalance.16,17 Paralytic disorders resulting from polio or spinal trauma may lead to a progressive scoliosis. Radiation therapy, tumors and syrinx formation have also been implicated as etiologies of scoliosis. CS may also be associated with myelodysplasia.
CS is classified by orthopedists as a failure of segmentation (partial or completely fused vertebrae), failure of formation (such as hemivertebrae) and mixed defects. Each of these may cause development of a spinal curve based on asymmetric growth. The severity of the curve is related to the type of defect and whether or not the primary problem is accompanied by any compensatory developmental changes.
As the spinal curvature progresses, pulmonary function may be compromised if the deformity occurs in the thoracic region. Restrictive lung function is associated with spinal curvature that approaches 90 degrees.18 The most significant pulmonary compromise is associated with severe scoliotic curves in young children associated with multiple thoracic vertebral anomalies. Surgical treatment at an early age has been recommended in this group of patients.
The progression and ultimate prognosis are dependent upon the specific vertebral anomaly and anatomic location.19,20 Hemivertebrae will usually result in a curve that exceeds 40 degrees by 10 years, except in instances where two hemivertebrae occupy an adjacent and opposite orientation on the spinal column. Multiple hemivertebrae on the same side are associated with a more rapid progression and usually require treatment before 5 years. A unilateral unsegmented bar has a very high likelihood of resulting in severe progressive scoliosis. An unsegmented bar with a hemivertebrae is associated with the most severe prognosis, with the possibility of a spinal curve exceeding 50 degrees by 3 years of age. Not all congenital vertebral malformations result in CS. For instance, two hemivertebrae may occupy an adjacent and opposite orientation on the spinal column. In this situation progressive deformity would not occur because the vertebral malformations are balanced and together do not result in increased spinal curvature.
ASSOCIATION OF CS WITH CONGENITAL MALFORMATIONS
Because CS arises from significant developmental disruptions, involvement of other organ systems is common. Spinal cord anomalies are particularly widespread, occurring in up to 20% of CS cases.10 Common associated abnormalities are found in the nervous, urogenital, gastrointestinal, and cardiovascular systems. Specific abnormalities include esophageal atresia, tracheoesophageal fistula, diastematomyelia and other congenital spinal anomalies, anal atresia, Sprengel's deformity, facial asymmetry and bladder and cloacal exstrophy.21–23 Additional associations include the Klippel-Feil syndrome (short neck, low posterior hairline, fusion of cervical vertebrae), Goldenhar's syndrome (associated with craniofacial anomalies, including microtia and epibulbar dermoids due to abnormal branchial arch development), incontinentia pigmenti (hyperpigmented whorls and streaks associated with eye, skin, hair, nail, teeth and central nervous system abnormalities), other recognizable syndromes, or the VACTERL association (Vertebral malformations, Anal atresia, Cardiac malformations, TracheoEsophageal fistula, Renal and Radial anomalies and Limb defects).
Genitourinary abnormalities have been reported to occur in 37 of 85 (43%) of patients with CS.24 Since the genitourinary system and vertebral column are both mesoderm in origin and develop during the fifth week of embryonic life, insults to the embryo during this period could result in both CS and genitourinary tract abnormalities. A 13% incidence of renal and ureteral abnormalities in patients with CS has been reported.25 Renal ectopia was observed to occur in conjunction with scoliosis with a 10-fold increase in chicks with CS induced by surgical technique.26 Several mechanisms have been proposed to explain the association between CS and renal anomalies, including a failure of spine growth interfering with normal renal ascent, teratogenic agents and intrinsic genetic defects.
There is some evidence in the literature to support the development of organ malformations at the body segment at which vertebral malformations occur.4 In a series of 26 patients with multiple vertebral segmentation defects, all patients with congenital heart disease had thoracic involvement, 4 of 6 patients with renal anomalies were found to have lumbar involvement, and all 5 patients with imperforate anus were found to have lumbar involvement.
The developmental field concept described by Opitz is useful in providing a framework for understanding multisystem involvement. Developmental field defects are defined as “any dysmorphogenetically reactive unit of the developing organism that leads to final structure.”27 If a specific pattern of malformation can be attributable to different causes, then a developmental field defect is identified. For example, the association of segmentation anomalies of the spine and ribs in a series of 110 patients with multiple etiologies, including monogenic syndromes, chromosomal abnormalities, environmental agents, and unknown causes, provides evidence that vertebral and rib anomalies constitute a developmental field defect.28 Through observation of a variable pattern of congenital malformations within the VACTERL complex associated with different etiologies, evidence has been provided for VACTERL complex representing a primary polytopic field defect.29 Identification of genes associated with CS and malformations involving the genitourinary system, central nervous system and digestive system would extend this hypothesis.
MANAGEMENT OF PATIENTS WITH CS AND IS
Once CS is identified on clinical exam, x-ray studies and evaluation by a pediatric orthopedic surgeon are indicated. Approximately 50% of patients with CS ultimately require surgical correction because of curve progression.10 Computerized tomography or magnetic resonance imaging scans may be required for further delineation of underlying vertebral and spinal cord anomalies. An evaluation for associated cardiac and renal anomalies should be performed. Early identification of CS and treatment are important for maintaining maximal pulmonary function in patients with thoracic deformities.
Infantile idiopathic scoliosis most often spontaneously regresses, whereas juvenile onset scoliosis is more likely to progress.30 Treatment includes casting and bracing when there is progression of scoliosis.31 If these conservative measures fail, surgical management with rodding for stabilization and fusion (arthrodesis) of vertebral levels to prevent growth is recommended during late adolescence.
GENETIC ASPECTS OF CS AND IS
While the absolute numbers are not large, CS is relatively common among congenital malformations. Using chromosomal deletion mapping,32 a total of 114 cases of scoliosis among 1,753 patients with multiple congenital anomalies associated with various chromosome deletions were identified. Significant associations with haploinsufficiency for chromosomal loci 2p13–15, 6q13, or 15q12 were found, implying that genes mapping to these regions play a role in the development of CS and IS.
Two studies have provided evidence for a sporadic occurrence of CS.2,33 In both studies, a sibling risk for neural tube defects of approximately 5% was reported, suggesting an etiological association between vertebral malformations and neural tube defects.
CS has also been observed in identical twins.34–37 Different mechanisms have been postulated including non-genetic factors,38 abnormalities of cleavage and somite formation,36 or abnormalities in blood supply, or to a failure of cartilage formation in the mesenchymal tissues.37 Monozygotic twin females concordant for the presence of right thoracic scoliosis and supravalvular pulmonic stenosis have been reported.35 The first twin had a lumbar hemivertebrae and absent left kidney. The second twin had no congenital vertebral malformations. This finding provides evidence for multiple genetic loci and/or modifying genes that contribute toward the development of CS and IS.
In addition, occasional familial clusters, often with associated fused ribs and segmentation defects, have been noted.39–44 These include spondylocostal dysostosis, a sporadic autosomal dominant or autosomal recessive short-trunk, short-stature syndrome that is characterized by non-progressive kyphoscoliosis, multiple hemivertebrae and rib fusion abnormalities, and spondylothoracic dysostosis, a severe and frequently lethal skeletal dysplasia which presents with a crab-like chest on x-ray. A gene for autosomal recessive spondylocostal dysostosis has been localized to chromosome 19q13.1–q13.3.44 Three mutations in three different families with autosomal recessive spondylocostal dysostosis have been described.45 These mutations include two protein truncating mutations and a missense mutation.
An autosomal recessive form of CS observed in male and female siblings of Iranian ancestry has been described.46 The children's parents were first cousins. The spinal x-rays were consistent for thoracic scoliosis, lack of segmentation of thoracic vertebrae, and multiple rib fusions. The female child had a small, abnormally shaped left kidney. Their curve patterns differed from those encountered in both spondylocostal dysplasia and spondylothoracic dysplasia.
Unlike CS, several reports in the literature support the existence of a strong hereditary component in the development of IS, but there is uncertainty regarding its mode of inheritance. Evidence for autosomal dominant with variable penetrance, multifactorial and X-linked dominant modes of inheritance has been reported.8 A locus for autosomal dominant IS has been identified corresponding to 17p11 in a three generation family of Italian ancestry with 11 affected individuals.9 Although mutations in heparin sulfotransferase genes, HS3ST3A1 and HS3ST3B1, are prevalent in this region, no mutations have been identified in these genes in two affected family members.
Interestingly, among 237 families who had at least one case of known CS, 17.3% reported having members with IS.47 This observation could be due to chance. Alternatively it is possible that CS and IS share an underlying genetic mechanism, and that a single genetic defect can result in a predisposition to different types of spinal deformities.
Because CS is usually a sporadic condition, it is generally not possible to use conventional genetic linkage studies to identify chromosome regions that contribute to the development of this disorder. Data obtained from cytogenetic studies or genetic mapping studies will have to be supplemented, either by candidate genes identified by other strategies or by a broad-based mutation screen.
OVERVIEW OF VERTEBRAL DEVELOPMENT AND SOME OF THE MAJOR GENES INVOLVED
Within the past two decades a great deal of information has been learned about the molecular embryology of spine development through the study of mouse and chick embryos. Positional information along the rostrocaudal and anterior posterior axes is laid down during gastrulation.48 The discovery of homeobox genes in Drosophila (HOMC)49 and their counterparts in vertebrates (Hox) provide strong evidence for similarities in the segmentation process in metazoa, plants and fungi.50 Hox genes are transcription factors that are involved in the specification of positional information along the rostrocaudal axis. They represent a subgroup of the homeobox gene family, which contains a 180 bp DNA sequence encoding a DNA-binding domain as part of a “homeoprotein.”49 The Hox family has been studied in great detail in multiple organisms, and homeotic mutations which resemble human dysmorphic syndromes include human synpolydactyly, associated with an in-frame insertion of polyalanine stretches in HOXD13, and human hand-foot-genital syndrome, which is due to a nonsense mutation in HOXA13.50 Hox genes are expressed in mesodermally and ectodermally derived cells along the body axis. A specific “Hox code” defined by Kessel and Gruss51 determines vertebral anatomy. There are four gene clusters, Hox A, B, C and D, containing 39 genes that are located on 4 different chromosomes. In general, genes at the 3′ end of a particular cluster are expressed earlier and, with the exception of the second genes in the cluster, occupy more anterior expression domains.49 Specification of vertebral position information is thought to be achieved by an interplay of Hox genes that are expressed at a given axial level.
In the initial stage of vertebral development (figure 3), cells from the epiblast ingress through the primitive streak and spread internally to form the endoderm followed by the mesoderm, notably the notochord which is located in the midline, and the paraxial mesoderm. Spherical clusters of mesenchymal cells called somitomeres52 precede the development of somites that in turn develop into vertebrae, ribs, skeletal muscle and dermis.53 In addition, the paraxis gene has been shown to be responsible for normal somite formation in mice.54 Paraxis codes for a basic helix-loop-helix transcription factor which is expressed in paraxial mesoderm and somites. Mice that are homozygous for a paraxis null mutation do not form somites and develop an improperly patterned axial skeleton and musculature.
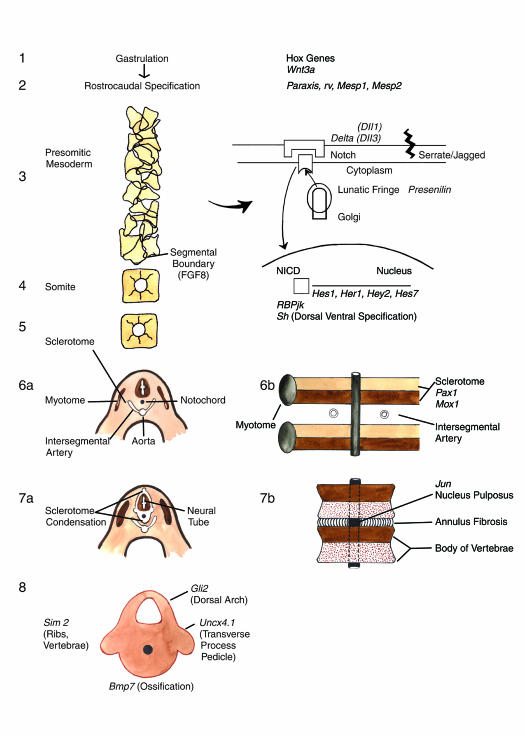
Figure 3.
A schematic diagram of murine embryologic development of somite, sclerotome and vertebral body with some of the murine genes involved at different stages. Segmentation is mediated by a molecular segmentation clock operating through the Notch signaling pathway. Segmental boundary formation is mediated by decreased concentrations of fibroblast growth factor 8. Pax1 and Mox1 are involved in sclerotome condensation and subdivision into anterior and posterior halves. Transverse embryo sections and corresponding frontal embryo sections are shown. Other genes such as Gli2, Unex4.1, BMP-7 and Jun are involved in vertebrae differentiation and ossification.
As vertebrate development progresses, a molecular segmentation clock operating through the Notch signaling pathways is postulated to be responsible for coordinated vertebrate segmentation.55 Hairy 1 oscillator regulates lunatic fringe expression, which in turn mediates the binding of Notch, a large transmembrane receptor, to Delta and Serrate, two transmembrane ligands. When Notch is proteolytically cleaved, it is translocated to the nucleus. In conjunction with the transcription factor Su(H)/RBP/jk, a series of genes of the hairy and enhancer of split family are transcribed. These genes include c-hairy 1, c-hairy 2, Hes1, Hey2, Her1 and Hes7, the Notch ligand Delta C and the glycosyl-transferase, Lunatic Fringe.56 Presenilin 1 is required for Notch signaling mediated in the paraxial mesoderm.57 Periodic pulsations of mRNA mediated by Notch signaling are responsible for the establishment of a regular array of somitic boundaries. Decreased concentrations of fibroblast growth factor 8 have been associated with segmental boundary formation in the rostral portion of developing chick embryos.58
MesP1 and MesP2 are beta-helix-loop-helix transcription factors that show segmental expression in the presomitic mesoderm.59 Studies performed in zebrafish60 have provided evidence that these genes are involved in the development of the anterior-posterior polarity within the developing somite through interaction with the fibroblast growth factor R and Delta-Notch signaling pathways.
The somite subsequently undergoes differentiation to the ventromedial and dorsomedial regions. The ventromedial region gives rise to the sclerotome (figure 3). The dorsolateral region gives rise to the dermomyotome. The posterior half of one sclerotome and the anterior half of its caudal neighbor fuse to form the nascent vertebral body. Sonic hedgehog protein61,62 has been hypothesized to play a role in sclerotome formation. Experimental evidence suggests that in the platelet-derived growth factor-a receptor is involved with normal patterning of somites. Pdgfra null mutant embryos display predominantly cervical segmentation defects, rib fusions, spina bifida occulta and truncated acromion. This phenotype has similarities to Klippel-Feil syndrome.63
Cells within the posterior half of the lateral sclerotome are destined to form the neural arch. Densely packed cells located at the anterior sclerotomal center give rise to the intervertebral disc. The less cell dense areas between the intervertebral disc regions form the anlage of the vertebral body. The Pax1 gene has been shown to be active during sclerotome formation and differentiation. Pax1 mutations have been identified in undulated mice, suggesting that sclerotome condensation is a Pax1 dependent process.64 In the mouse mutant undulated, medial sclerotome condensation does not occur at the lumbosacral level, thus preventing the formation of intervertebral discs and vertebral bodies as a result of faulty sclerotome condensation. Mox1 is also involved in the subdivision of sclerotomes into a posterior and anterior half.65 A mouse mutant “rib-vertebrae” (rv) has recently been described by Nacke et al.66 Homozygous rv mice have phenotypic features characterized by vertebral and rib defects and urogenital malformations. Somites are irregular in size. Both Pax1 and Mox1 are abnormally expressed. Based on these observations, the rv mutation results in elongation of the presomitic mesoderm and disruption of the anterior-posterior polarization of somites.
As vertebral development progresses, the prevertebrae are subsequently converted into cartilage during the sixth week of gestation. Endochondral ossification begins during the ninth week. Bone morphogenic protein (BMP) molecules are related to transforming growth factor-β and represent a group of osteoinductive cytokines from the bone matrix. Mice that are homozygous for the BMP-7 null allele display various skeletal defects, including lack of fusion of the neural spines of the atlas, twelfth thoracic, and first sacral vertebrae, openings on the side(s) of the neural arches of the third and fourth thoracic vertebrae, and absence of a lumbar vertebrae.67 Intervertebral disks have unequal thickness, resulting in tail kinks. Mutant mice have small or nonexistent ossification centers. It has been postulated that the BMP-7 protein is required for the normal ossification process to occur.
The notochord eventually regresses to form the nucleus pulposus. Jun, a major component of the heterodimeric transcription factor AP-1 is required for axial skeletogenesis through the regulation of notochord survival.68 Jun deficient mice die during midgestation and develop scoliosis due to fusion of vertebral bodies.
USE OF SYNTENY ANALYSIS TO IDENTIFY CANDIDATE GENES FOR HUMAN CS AND IS
The first major goal of the “genome project” was to identify and map thousands of markers in the human and mouse genomes. This task has been completed.69,70 Work to date has made it apparent that family relationships among genes have been conserved over evolution. One practical result of these findings is that genes harboring functionally important mutations in model organisms become candidates for corresponding human diseases, sharing features of the mutant phenotypes. Moreover, we have learned that chromosomal organization is highly conserved in these two species, with the preservation of linkage arrangements among groups of neighboring genes over evolution. Only about 200 chromosome rearrangements are thought to have occurred since the human and murine lineages diverged.71,72 A second practical consequence of the genome project's progress, therefore, is that genetic mapping data from the mouse can be used to predict the locations of corresponding human genes. An extensive collection of conserved synteny data is conveniently accessible via the National Center for Biotechnology Information's Human/Mouse Homology Relationships Web site.73
Synteny conservation was used as the basis to identify potential human candidate genes by map position. We therefore undertook a systematic review of mouse mutations with skeletal, tail, or neuromuscular phenotypes with the goal of identifying potential human candidate genes for CS and IS.74 When the human homologue of the mouse mutant gene was known, this became a candidate. When such a homologue was not known, the established syntenic relationships between the two species were used to identify additional human candidate genes by their chromosomal locations. We report the results of this review, which have subsequently been updated, yielding a relatively small number of human candidate genes meeting our criteria for inclusion.
METHODS
A search of the Mouse Genome Database on the Mouse Genome Informatics Web site75 was performed for “genes,” “markers” and “phenotypes” in the categories Neurological and neuromuscular, Skeleton, and Tail and other appendages. Retrieved loci were reviewed for possible scoliotic phenotypes, choosing at this point to err in being more, rather than less inclusive. Either the Online Mendelian Inheritance in Man Web site,76 or the Genome Database Web site was used.77 The Online Mendelian Inheritance in Man site was used to determine whether each mouse locus has a known human homologue. If so, the human homologue was assigned candidate gene status. Linkage maps of the chromosomes carrying loci with possibly relevant phenotypes, but without known human homologues, were examined and regions of documented synteny between the mouse and human genomes were identified. Mouse loci mapping to regions in which synteny is not well-conserved or mapping close to syntenic region endpoints were excluded from further analysis. The Genome Database Web site77 was then used to identify human genes mapping within ∼5% of the map position specified by the synteny map. The descriptions of the identified human genes were reviewed, and once again liberal criteria was used for assignment of candidate status.
RESULTS
Searching the Mouse Genome Database by phenotypic category yielded 100 mutants of which 66 had been mapped. Next the descriptions of each of the 66 loci were retrieved to determine which among these included phenotypes of scoliosis, kinky or bent tails, other vertebral abnormalities, or disturbances of axial skeletal development. Forty-five loci of interest remained at this point, and for 27 of these the comparative linkage maps of mouse and human were used to identify human syntenic regions to which plausible candidate genes had been mapped (table 1). For each of these ‘synteny candidates,’ the mouse locus symbol, locus name, map position, corresponding human map position, and human candidate gene(s) symbol are listed. Thus, by reading across each entry in the table, the process used for assessing each locus is briefly recapitulated.
Table 1.
Synteny-Defined Candidates for IS and CS.
| Mouse mutant or locus | Map position (chromosome, cM) | Human syntenic region | Human candidate(s) | Human syndrome(s) |
| Dbf (Pax3, Ihh) | 1, 40 | 2q35 | PAX3, IHH | Waardenburg, CFDH |
| Gli2 | 1, 63 | 2q14 | GLI2 | |
| Lmx1a | 1, 88.2 | 1q21–q23 | LMX1.1 | |
| Ltap | 1, 93.4 | 1q21–q23 | VANGL2 | |
| us and Lmx1b | 2, 14 and 21 | 9q34 | LMXIB | NP |
| rh and Hoxd | 2, 38 and 45 | 2q31 | HOXD cluster | |
| Pax1 and dm | 2, 82 and 80 | 20p11 | PAX1 | |
| Jun | 4, 44.6 | 1p31–32 | JUN | |
| sks and sno | 4, 54.6 and 58.3 | 1p33-p32.2 | COL9A2 | MED type 2 |
| ct | 4, 69 | 1p35 | PAX7, CRTM | |
| lx | 5, 22 | 4p16.1 | MSX1 | Wolf-Hirschorn |
| hop | 6, 13 | 7q22-qter | PTN, PAX4 | |
| tc | 6, 35.6 | 2p13-pII | TGFA | |
| Dll3 | 7, 10 | 19q13.2–q13.3 | DLL3 | |
| Tks | 9, 9 | llq22–q24 or 19p13.3-PI3.2 | MMP cluster or ACP5 | |
| lu | 9, 23 | llq22–q24 | MMP cluster | |
| Aft | 9, 32 | 15q23–q25 | CSK, PML | |
| tk | 9, 48 | 6q12–q13 | COL12A1 | |
| Ky | 9, 56 | 3q21 | MYLK | |
| Wnt3a | 11, 32 | 1q42 | WNT3A | |
| Ts | 11, 73.5 | 17q25 | TIMP2 | |
| Rbt | 11, 74 | 17q25 | TIMP2, CBX2 | |
| Bst | 16, 31.5 | 3q13.2 | COL8A1 | |
| Sim2 | 16, 67.6 | 21q22.2 | SIM2 | |
| mctl | 17, 18.5 | 6p21.3 | COL11A2, RXRB | type 2 Stickler, OSMED |
| Fbn-2 | 18, 29 | 5q23.3–q31 | FBN2 | CCA |
| ocd | 19, 6 | llql3 | LTBP3 |
CFDH, craniofacial deafness-hand syndrome; NP, nail-patella syndrome; MED, multiple epiphyseal dysplasia; OSMED, otospondylomegaepiphyseal dysplasia syndrome; CCA, congenital contractural arachnodactyly.
DISCUSSION
For further details regarding each mouse locus, the interested reader is referred to “mouse genome informatics.” Specific lesions in cloned mouse genes have been shown to cause scoliosis. Seven of these are briefly discussed below:
Pax1
Three mutant alleles (undulated, undulated-extreme and undulated-short tail) of Pax1 have been described in the mouse. All three mutations reduce or eliminate Pax1 gene expression and cause deficient development of the anterior vertebral element.78 Scoliosis, split and fused vertebrae, and hypomorphic intervertebral discs, more prominently in the more posterior regions of the body, result. Pax1 appears to be important in specifying ventromedial differentiation of the sclerotome, thus accounting for the vertebral abnormalities seen in the various undulated mutant alleles. Murine Pax1 maps to 80 cM on chromosome 2,79 while the human homologue maps to 20p11 by somatic cell hybrid and fluorescence in situ hybridization analysis.80 The mutant diminutive (dm) which causes decreased viability, decreased body size, macrocytic anemia, extra ribs, vertebral deformations, and short, kinked tails,81 maps to 82 cM,82 and may be an additional Pax1 allele. However mutation analysis of Pax1 in dm/dm mice failed to find any coding-region lesions.
Dll3
The Dll3 gene is a homologue of the Drosophila Notch ligand Delta83 and is located at 6 cM on mouse chromosome 7.84 This locus was recently shown to harbor the pudgy (pu) gene.83 The mutation disrupts the proper formation of morphological borders in early somite development and formation of rostral-caudal compartment boundaries within somites. The mutation may have arisen as a consequence of x-ray exposure and leads to multiple axial skeletal abnormalities in affected animals. The vertebral column, which is drastically shortened along its entire length, is composed of a mixture of irregular fused vertebrae and incompletely developed vertebral bodies interspersed with occasional normal vertebrae. Multiple rib fusions occur which involve the sternum, particularly in the caudal half of the thoracic region. In embryonic development, animals have indistinct, late-appearing, and shortened somites. Segmentation does not occur as far distally as in normal embryos. Adults have shortened, toad-like bodies and both viability and fertility are impaired.85 An underlying abnormality in the process of segmentation appears to be the mechanism underlying the pu/pu phenotype. Crowe et al86 demonstrated that the Notch and Delta ligands are involved in patterning development of feather buds in the chick embryo, highlighting their role in numerous cell fate decisions in different organisms. Alagille syndrome is associated with vertebral segmentation defects and mutations in the jagged gene (JAG1), a ligand of the Notch receptor.87
The human region syntenic to the mouse Dll3 is 19q13.2–13.3.73,88 On the basis of genome-wide scanning by homozygosity mapping in a large consanguineous Arab-Israeli family with six affected family members, a gene for autosomal recessive spondylocostal dysostosis has been localized to this region,44 corresponding to a gene coding the Notch ligand Delta-like 3 (Dll3). Mutations in three families with autosomal recessive spondylocostal dysostosis have been identified, including two protein truncating mutations and a missense mutation.45
Wnt3a
This gene is a member of a moderate-sized multigene family comprised of at least 12 members in humans and the mouse. Genes in this family function both in establishing the body plan in development and as potential oncogenes.89 The Wnt proteins are relatively insoluble, have affinity for extracellular proteoglycans, particularly heparin sulfate, and are secreted inefficiently.90
This affinity for extracellular matrix components coupled with their growth-promoting actions provides a simple mechanistic framework for understanding their role in directing establishment of the body plan by promoting proliferation of cells over a limited anatomic region. Wnt3a is necessary for generation of the posterior portion of the neuraxis, as knockout mice fail to develop a tailbud and are truncated from a point slightly anterior to the hindlimbs.91 A spontaneous mouse mutant, vestigial tail (vt), has a similar but less severe phenotype. Homozygous (vt/vt) mice develop a severely hypomorphic tail, with either a small filament only, or a short stump-like tail. Usually, there is absence of caudal vertebrae, with the remaining vertebrae exhibiting morphologic abnormalities. In some instances, presacral vertebrae are absent and two ossification centers are present in the caudal, lumbar and sacral vertebrae. Caudal neural tube anomalies were observed in regions corresponding to defective somite formation. Embryos display a reduction in the ventral ectodermal ridge of the tail at the tenth day of gestation. Accessory neural tubes are also observed.92 The Wnt3a gene has been localized to mouse chromosome 11 at 32cM. The human orthologue, Wnt3a, has been mapped to human chromosome 1q42 by fluorescence in situ hybridization analysis.93 It is approximately 53.0 kb long and composed of 4 exons.
Ky
A degenerative myopathy precedes the development of kyphoscoliosis in the ky mouse.94 An absence of muscle hypertrophy in response to increased demand is not observed in ky mice. Muscles obtained from ky/ky mice are smaller and have slower and weaker muscle contractions as compared to control mice. A shift to the expression of slow muscle contractile protein isoforms including, myosin heavy chains and myosin light chains, occurs. The Ky gene has been localized on chromosome 9 at 56cM. The encoded gene is a novel muscle specific protein, not one of the myosin chains. A GC deletion in codon 24 that leads to a premature stop codon at position 125 has been observed in ky/ky mice. The human Ky orthologue falls into a conserved synteny region on 3q21. Myosin light polypeptide kinase is localized within this region and could possibly be a candidate gene for CS and IS.
Lmx1a
The phenotypic mutant Dr (dreher) falls within the Lmx1a: gene. Dreher mice have absence of interneurons in the dorsal spinal cord and granule neurons in the cerebellar cortex that result from a failure of roof plate development.95 There is also a failure of formation of the dorsal neural arches. Lmx1a: serves an important function in the specification of dorsal cell fates in the central nervous system and in developing vertebrae. Using Northern analysis, Lmx1a: has been localized to the roof plate and immediate neighboring structures during early central nervous system development. Lmx1a: maps to a conserved synteny region, 1q22–q23, and stimulates transcription of insulin.96
Fbn-2
The shaker-with-syndactylism (sy) phenotype is associated with auditory and vestibular defects, fusion of digits and early lethality.97 A reduction in the caliber of the shafts of long bones and decreased bone density of the long bones of the limbs, girdles, and vertebrae have been observed. Loss of function mutations in Fbn-2 occurring outside of a “conserved neonatal region” has been observed in sy, syfp and syfp-2J.98 In humans, Fbn-2 maps to a conserved synteny region on 5q23.3-3.1. Mutations in Fbn-2 are associated with congenital contractural arachnodactyly.99 The association of scoliosis with congenital contractural arachnodactyly supports the candidacy of Fbn-2 for IS and CS.
Sim2
Sim2 mice have CS resulting from unequal sizes of ribs and vertebrae.100 They die shortly after birth because of lung atelectasis and breathing failure. They also have diaphragm hypoplasia, rib protrusions and abnormal intercostal muscle attachments. It has been suggested that rib overgrowth in Sim2 mice is responsible for the development of CS. The expression of Sim2 in the vertebral body, and not the somites, provides evidence for its contribution toward the later stages of vertebral development by regulating their growth. Sim2 is localized to human chromosome 21q22.2 that shares synteny conservation with the 67.6 region of mouse chromosome 16.101 The 21q22.2 region is associated with many of the features of Down's syndrome. Sim2 belongs to a family of transcription factors characterized by a basic helix-loop-helix-PAS (PER, ARNT, SIM) domain.102 Sim is responsible for midline central nervous system development in the fly.103
ENVIRONMENTAL FACTORS WHICH MAY BE ASSOCIATED WITH THE DEVELOPMENT OF CS
Vertebral malformations in humans have been reported to occur in association with alcohol,104 anticonvulsant medications including valproic acid105 and dilantin,106 hyperthermia,107 and maternal insulin dependent diabetes mellitus.108 No epidemiologic studies have been performed to evaluate the association between environmental teratogens and vertebral malformations.
The association of spinal cord anomalies in humans and neural tube defects in mice with CS as described in the preceding sections, raises the possibility that similar etiologic mechanisms are involved in the development of these malformations. Folic acid use has been associated with the prevention of neural tube defects.109 Further studies need to be performed to determine the possible effects antenatal folic acid use may have on the development of congenital vertebral malformations.
An association of vertebral malformations has been reported in Sprague-Dawley rat fetuses exposed to I(Kr)-blockers (class III antiarrhythmic agent) in utero.110 A temporary induction of hypoxia and reoxygenation injury via the induction of embryonic cardiac arrhythmia has been proposed as the mechanism of teratogenicity.
Thoracic vertebral malformations have been induced in a dose dependent fashion in mice with maternal exposure to carbon monoxide at 9 days of gestation.111 Possible mechanisms for vertebral malformation include alteration of expression of homeobox genes or sonic hedgehog by carbon monoxide, or through direct action of carbon monoxide on the cartilaginous skeleton.
Rats exposed to boric acid on day 9 of gestation develop 6, rather than the normal number of 7 cervical vertebrae.112 This has been associated with an alteration of hox gene expression pattern, presumably mediated by boric acid.
The influence of various teratogens on the expression of genes that influence vertebrate development has not been well studied. One possible model for the development of CS is multifactorial. An understanding of possible environmental influences on vertebrate development may help to provide more complete genetic counseling for families.
DIRECTIONS FOR FUTURE WORK
It is likely that different approaches will enable an elucidation of the genetic and environmental basis of CS. Recent advances in understanding spine development provide evidence for a genetic basis in the determination of a particular vertebrate identity during development. Since CS is due to localized alterations in vertebral body development as opposed to a more widespread distribution of vertebral malformations, a model needs to be developed that incorporates position identity and failure of segmentation. The association of renal, cardiac, skeletal and spinal cord malformations with CS may reflect the involvement of different genes that are associated with developmental pathways in several organs. The usual sporadic nature of CS could be due to tissue mosaicism. Synteny analysis of mouse candidate genes for CS holds some promise due to the close evolutionary relationship between mice and human beings. With the identification of additional genes in animal model systems that contribute to different stages of spine development, the list of candidate genes for CS will continue to grow. As genes are identified that contribute toward the development of IS, allelic mutations in these genes may also be responsible for the development of CS. Epidemiologic studies can provide assistance in determining whether various environmental factors contribute to CS. Microarray expression analysis in developing mice embryos may be used to investigate influences of various environmental factors on genes associated with spine development.
Acknowledgments
The authors thank Shirley Thompson for her work on the figures, and also the Marshfield Clinic Research Foundation for its support through the assistance of Alice Stargardt, Doreen Luepke and Dr. Graig Eldred in the preparation of this manuscript.
Contributor Information
Philip F. Giampietro, Medical Genetics Services, Marshfield Clinic, Marshfield, Wisconsin.
Robert D. Blank, Section of Endocrinology, Department of Medicine, University of Wisconsin-Madison, Madison, Wisconsin.
Cathleen L. Raggio, Department of Pediatric Orthopedics, Hospital for Special Surgery, New York, New York.
Sajid Merchant, Medical Genetics Services, Marshfield Clinic, Marshfield, Wisconsin.
F. Stig Jacobsen, Department of Orthopedic Spine Surgery, Marshfield Clinic, Marshfield, Wisconsin.
Thomas Faciszewski, Department of Orthopedic Spine Surgery, Marshfield Clinic, Marshfield, Wisconsin.
Sanjay K. Shukla, Clinical Research Center, Marshfield Clinic Research Foundation, Marshfield, Wisconsin.
Anne R. Greenlee, Reproductive Toxicology, Marshfield Clinic Research Foundation, Marshfield, Wisconsin.
Cory Reynolds, Clinical Research Center, Marshfield Clinic Research Foundation, Marshfield, Wisconsin.
David B. Schowalter, Medical Genetics, Mayo Clinic, Rochester, Minnesota.
References
- 1.Shands AR, Eisberg HB. The incidence of scoliosis in the state of Delaware. A study of 50,000 minifilms of the chest made during a survey for tuberculosis. J Bone Joint Surg. 1955;37A:1243. [PubMed] [Google Scholar]
- 2.Wynne-Davies R. Congenital vertebral anomalies: aetiology and relationship to spina bifida cystica. J Med Genet. 1975;12:280–288. doi: 10.1136/jmg.12.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alagille D, Odievre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63–71. doi: 10.1016/s0022-3476(75)80706-2. [DOI] [PubMed] [Google Scholar]
- 4.Mortier GR, Lachman RS, Bocian M, Rimoin DL. Multiple vertebral segmentation defects: analysis of 26 new patients and review of the literature. Am J Med Genet. 1996;61:310–319. doi: 10.1002/(SICI)1096-8628(19960202)61:4<310::AID-AJMG3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Jarcho S, Levin PM. Hereditary malformations of the vertebral bodies. Johns Hopkins Med J. 1938;62:216. [Google Scholar]
- 6.Scoliosis Research Society. A glossary of scoliosis terms. Spine. 1976;1:57–58. [Google Scholar]
- 7.Weinstein SL. The thoracolumbar spine. In: Weinstein SL, Buckwalter JA, editors. Turek's orthopedics: Principles and their application. Philadelphia: Lippincott Company; 1994. pp. 447–484. [Google Scholar]
- 8.Wynne-Davies R. Familial (idiopathic) scoliosis. A family survey. J Bone Joint Surg Br. 1968;50:24–30. [PubMed] [Google Scholar]
- 9.Salehi LB, Mangino M, De Serio S, De Cicco D, Capon F, Semprini S, Pizzuti A, Novelli G, Dallapiccola B. Assignment of a locus for autosomal dominant idiopathic scoliosis (IS) to human chromosome 17p11. Hum Genet. 2002;111:401–404. doi: 10.1007/s00439-002-0785-4. [DOI] [PubMed] [Google Scholar]
- 10.Winter RB. Posterior spinal fusion in scoliosis: indications, techniques, and results. Orthop Clin North Am. 1979;10:787–800. [PubMed] [Google Scholar]
- 11.Sillence DO, Barlow KK, Cole WG, Dietrich S, Garber AP, Rimoin DL. Osteogenesis imperfecta type III. Delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet. 1986;23:821–832. doi: 10.1002/ajmg.1320230309. [DOI] [PubMed] [Google Scholar]
- 12.Pyeritz RE, McKusick VA. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979;300:772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad NN, Ala-Kokko L, Knowlton RG, Jimenez SA, Weaver EJ, Maguire JI, Tasman W, Prockop DJ. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy) Proc Natl Acad Sci USA. 1991;88:6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmann BU, Royce PM, Superti-Furga A. The Ehlers-Danlos syndrome. In: Royce PM, Steinmann BU, editors. Connective tissue and its heritable disorders: Molecular, genetic, and medical aspects. 5th ed. New York: Wiley-Liss; 1993. pp. 351–407. [Google Scholar]
- 15.Emery AE. X-linked muscular dystrophy with early contractures and cardiomyopathy (Emery-Dreifuss type) Clin Genet. 1987;32:360–367. doi: 10.1111/j.1399-0004.1987.tb03302.x. [DOI] [PubMed] [Google Scholar]
- 16.DeLuca PA. The musculoskeletal management of children with cerebral palsy. Pediatr Clin North Am. 1996;43:1135–1150. doi: 10.1016/s0031-3955(05)70454-5. [DOI] [PubMed] [Google Scholar]
- 17.Sarwark JF. Spina bifida. Pediatr Clin North Am. 1996;43:1151–1158. doi: 10.1016/s0031-3955(05)70455-7. [DOI] [PubMed] [Google Scholar]
- 18.Day GA, Upadhyay SS, Ho EK, Leong JC, Ip M. Pulmonary function in congenital scoliosis. Spine. 1994;19:1027–1031. doi: 10.1097/00007632-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 19.McMaster M, Ohtsuka K. The natural history of congenital scoliosis. A study of two hundred and fifty-one patients. J Bone Joint Surg Am. 1982;64:1128–1147. [PubMed] [Google Scholar]
- 20.Winter RB, Moe JH, Eilers VE. Congenital scoliosis: a study of 234 patients treated and untreated. J Bone Joint Surg (Am) 1968;50:1–47. [Google Scholar]
- 21.Winter RB, Lonstein JE, Denis F. Pain patterns in adult scoliosis. Orthop Clin North Am. 1988;19:339–345. [PubMed] [Google Scholar]
- 22.Chetcuti P, Dickens DR, Phelan PD. Spinal deformity in patients born with oesophageal atresia and tracheooesophageal fistula. Arch Dis Child. 1989;64:1427–1430. doi: 10.1136/adc.64.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loder RT, Dayioglu MM. Association of congenital vertebral malformations with bladder and cloacal exstrophy. J Pediatr Orthop. 1990;10:389–393. doi: 10.1097/01241398-199005000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Vitko RJ, Cass AS, Winter RB. Anomalies of the genitourinary tract associated with congenital scoliosis and congenital kyphosis. J Urol. 1972;108:655–659. doi: 10.1016/s0022-5347(17)60831-0. [DOI] [PubMed] [Google Scholar]
- 25.Cowell HR, MacEwen GD, Hubben C. Incidence of abnormalities of the kidney and ureter in congenital scoliosis. Birth Defects Orig Artic Ser. 1974;10:142–145. [PubMed] [Google Scholar]
- 26.Maizels M, Stephens FD. The induction of urologic malformations. Understanding the relationship of renal ectopia and congenital scoliosis. Invest Urol. 1979;17:209–217. [PubMed] [Google Scholar]
- 27.Opitz JM. The developmental field concept in clinical genetics. J Pediatr. 1982;101:805–809. doi: 10.1016/s0022-3476(82)80337-5. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Frias ML, Urioste M. Segmentation anomalies of the vertebras and ribs: a developmental field defect: epidemiologic evidence. Am J Med Genet. 1994;49:36–44. doi: 10.1002/ajmg.1320490109. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Frias ML, Frias JL. VACTERL as primary, polytopic developmental field defects. Am J Med Genet. 1999;83:13–16. doi: 10.1002/(sici)1096-8628(19990305)83:1<13::aid-ajmg4>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66:1061–1071. [PubMed] [Google Scholar]
- 31.Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Study. J Bone Joint Surg Am. 1995;77:815–822. doi: 10.2106/00004623-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Brewer C, Holloway S, Zawalnyski P, Schinzel A, Fitzpatrick D. A chromosomal deletion map of human malformations. Am J Hum Genet. 1998;63:1153–1159. doi: 10.1086/302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connor JM, Conner AN, Connor RA, Tolmie JL, Yeung B, Goudie D. Genetic aspects of early childhood scoliosis. Am J Med Genet. 1987;27:419–424. doi: 10.1002/ajmg.1320270220. [DOI] [PubMed] [Google Scholar]
- 34.Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- 35.McKinley LM, Leatherman KD. Idiopathic and congenital scoliosis in twins. Spine. 1978;3:227–229. doi: 10.1097/00007632-197809000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Ogden JA, Southwick WO. Congenital and infantile scoliosis in triplets. Clin Orthop. 1978;(136):176–178. [PubMed] [Google Scholar]
- 37.Pool RD. Congenital scoliosis in monozygotic twins. Genetically determined or acquired in utero? J Bone Joint Surg Br. 1986;68:194–196. doi: 10.1302/0301-620X.68B2.3958001. [DOI] [PubMed] [Google Scholar]
- 38.Hattaway GL. Congenital scoliosis in one of monozygotic twins: a case report. J Bone Joint Surg Am. 1977;59:837–838. [PubMed] [Google Scholar]
- 39.Cantu JM, Urrusti J, Rosales G, Rojas A. Evidence for autosomal recessive inheritance of costovertebral dysplasia. Clin Genet. 1971;2:149–154. doi: 10.1111/j.1399-0004.1971.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 40.Rivera H, Perez-Salas JM, Nazara Z, Ramirez ML. A probably distinct autosomal recessive thoraco-limb dysplasia. J Med Genet. 1988;25:619–622. doi: 10.1136/jmg.25.9.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenz P, Rupprecht E. Spondylocostal dysostosis: dominant type. Am J Med Genet. 1990;35:219–221. doi: 10.1002/ajmg.1320350215. [DOI] [PubMed] [Google Scholar]
- 42.Giacoia GP, Say B. Spondylocostal dysplasia and neural tube defects. J Med Genet. 1991;28:51–53. doi: 10.1136/jmg.28.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romeo MG, Distefano G, Di Bella D, Mangiagli A, Caltabiano L, Roccaro S, Mollica F. Familial Jarcho-Levin syndrome. Clin Genet. 1991;39:253–259. doi: 10.1111/j.1399-0004.1991.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 44.Turnpenny PD, Bulman MP, Frayling TM, Abu-Nasra TK, Garrett C, Hattersley AT, Ellard S. A gene for autosomal recessive spondylocostal dysostosis maps to 19q13.1–q13.3. Am J Hum Genet. 1999;65:175–182. doi: 10.1086/302464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 2000;24:438–441. doi: 10.1038/74307. [DOI] [PubMed] [Google Scholar]
- 46.Langer LO, Jr, Moe JH. A recessive form of congenital scoliosis different from spondylothoracic dysplasia. Birth Defects Orig Artic Ser. 1975;11:83–86. [PubMed] [Google Scholar]
- 47.Purkiss SB, Driscoll B, Cole WG, Alman B. Idiopathic scoliosis in families of children with congenital scoliosis. Clin Orthop. 2002;(401):27–31. doi: 10.1097/00003086-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Chevallier A. [Role of the somitic mesoderm in the development of the rib cage of bird embryos. I. Origin of the sternal component and conditions for the development of the ribs (author's transl)] J Embryol Exp Morphol. 1975;33:291–311. [PubMed] [Google Scholar]
- 49.McGinnis W, Garber RL, Wirz J, Kuroiwa A, Gehring WJ. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984;37:403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- 50.Mark M, Rijli FM, Chambon P. Homeobox genes in embryogenesis and pathogenesis. Pediatr Res. 1997;42:421–429. doi: 10.1203/00006450-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Kessel M, Gruss P. Murine developmental control genes. Science. 1990;249:374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson AG. Somitomeres: mesodermal segments of vertebrate embryos. Development. 1988;104:209–220. doi: 10.1242/dev.104.Supplement.209. (Suppl) [DOI] [PubMed] [Google Scholar]
- 53.Dietrich S, Kessel M. The vertebral column. In: Thorogood P, editor. Embryos, genes and birth defects. Hoboken, New Jersey: John Wiley & Sons; 1997. pp. 282–302. [Google Scholar]
- 54.Burgess R, Rawls A, Brown D, Bradley A, Olson EN. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature. 1996;384:570–573. doi: 10.1038/384570a0. [DOI] [PubMed] [Google Scholar]
- 55.Palmeirium I, Henrique D, Ish-Horowicz D, Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 56.Pourquie O. Vertebrate somitogenesis. Annu Rev Cell Dev Biol. 2001;17:311–350. doi: 10.1146/annurev.cellbio.17.1.311. [DOI] [PubMed] [Google Scholar]
- 57.Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;398:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 58.Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- 59.Saga Y, Hata N, Koseki H, Taketo MM. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation and initiation. Genes Dev. 1997;11:1827–1839. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- 60.Sawada A, Fritz A, Jiang Y, Yamamoto A, Yamasu K, Kuroiwa A, Saga Y, Takeda H. Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and Mesp-b confers the anterior identity to the developing somites. Development. 2000;127:1691–1702. doi: 10.1242/dev.127.8.1691. [DOI] [PubMed] [Google Scholar]
- 61.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 62.Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- 63.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 64.Dietrich S, Gruss P. Undulated phenotypes suggest a role of Pax-1 for the development of vertebral and extravertebral structures. Dev Biol. 1995;167:529–548. doi: 10.1006/dbio.1995.1047. [DOI] [PubMed] [Google Scholar]
- 65.Candia AF, Hu J, Crosby J, Lalley PA, Noden D, Nadeau JH, Wright CV. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992;116:1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- 66.Nacke S, Schafer R, Habre de Angelis M, Mundlos S. Mouse mutant “rib-vertebrae” (rv): a defect in somite polarity. Dev Dyn. 2000;219:192–200. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1046>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 67.Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res. 1997;230:28–37. doi: 10.1006/excr.1996.3411. [DOI] [PubMed] [Google Scholar]
- 68.Behrens A, Haigh J, Mechta-Grigoriou F, Nagy A, Yaniv M, Wagner EF. Impaired intervertebral disc formation in the absence of Jun. Development. 2003;130:103–109. doi: 10.1242/dev.00186. [DOI] [PubMed] [Google Scholar]
- 69.Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996;380:152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- 70.Venter JC, Adams MD, Martin-Gallardo A, McCombie WR, Fields C. Genome sequence analysis: scientific objectives and practical strategies. Trends Biotechnol. 1992;10:8–11. doi: 10.1016/0167-7799(92)90158-r. [DOI] [PubMed] [Google Scholar]
- 71.Nadeau JH. Maps of linkage and synteny homologies between mouse and man. Trends Genet. 1989;5:82–86. doi: 10.1016/0168-9525(89)90031-0. [DOI] [PubMed] [Google Scholar]
- 72.Eppig JT, Nadeau JH. Comparative maps: the mammalian jigsaw puzzle. Curr Opin Genet Dev. 1995;5:709–716. doi: 10.1016/0959-437x(95)80002-m. [DOI] [PubMed] [Google Scholar]
- 73.Seldin MF. [January 23, 2003];Human/Mouse Homology Relationships Web site. Available at: http://www.ncbi.nlm.nih.gov/Omim/Homology/
- 74.Giampietro PF, Raggio CL, Blank RD. Synteny-defined candidate genes for congenital and idiopathic scoliosis. Am J Med Genet. 1999;83:164–177. [PubMed] [Google Scholar]
- 75. [January 23, 2003];Mouse Genome Database (MGD), Mouse Genome Informatics Web site, The Jackson Laboratory, Bar Harbor, Maine. Available at: http://www.informatics.jax.org/
- 76. [January 23, 2003];OMIM™ Online Mendelian Inheritance in Man Web site. Available at: http://www.ncbi.nlm.nih.gov/Omim/
- 77. [January 23, 2003];GDB Human Genome Database Web site. Toronto (Ontario, Canada): The Hospital for Sick Children, Baltimore (Maryland, USA): Johns Hopkins University, 1990-. Updated daily. Available at: http://gdb.org/
- 78.Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- 79.Balling R, Deutsch U, Gruss P. Undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax 1. Cell. 1988;55:531–535. doi: 10.1016/0092-8674(88)90039-6. [DOI] [PubMed] [Google Scholar]
- 80.Stapleton P, Weith A, Urbanek P, Kozmik Z, Busslinger M. Chromosomal localization of seven PAX genes and cloning of a novel family member, PAX-9. Nat Genet. 1993;3:292–298. doi: 10.1038/ng0493-292. [DOI] [PubMed] [Google Scholar]
- 81.Stevens L, Mackensen J. The inheritance and expression of a mutation in the mouse affecting blood formation, the axial skeleton, and body size. J Hered. 1958;49:153–160. [Google Scholar]
- 82.Mackensen JA. Position of diminutive, dm, in linkage group V. Mouse News Lett. 1962;27:38–39. [Google Scholar]
- 83.Kusumi K, Sun ES, Kerrebrock AW, Bronson RT, Chi DC, Bulotsky MS, Spencer JB, Birren BW, Frankel WN, Lander ES. The mouse pudgy mutation disrupts Delta homologue Dll3 and initiation of early somite boundaries. Nat Genet. 1998;19:274–278. doi: 10.1038/961. [DOI] [PubMed] [Google Scholar]
- 84.St Amand W, Cupp MB. Linkage mapping of Ht and pu. Mouse News Lett. 1957;17:88. [Google Scholar]
- 85.Gruneberg H. Genetical studies on the skeleton of the mouse. XXIX. Pudgy. Genet Res. 1961;2:384–393. [Google Scholar]
- 86.Crowe R, Henrique D, Ish-Horowicz D, Niswander L. A new role for Notch and Delta in cell fate decisions: patterning the feather array. Development. 1998;125:767–775. doi: 10.1242/dev.125.4.767. [DOI] [PubMed] [Google Scholar]
- 87.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trash BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 88.DeBry RW, Seldin MF. Human/mouse homology relationships. Genomics. 1996;33:337–351. doi: 10.1006/geno.1996.0209. [DOI] [PubMed] [Google Scholar]
- 89.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 90.Burrus LW, McMahon AP. Biochemical analysis of murine Wnt proteins reveals both shared and distinct properties. Exp Cell Res. 1995;220:363–373. doi: 10.1006/excr.1995.1327. [DOI] [PubMed] [Google Scholar]
- 91.Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 92.Gruneberg H. Genetical studies on the skeleton of the mouse. XVI. Tail-kinks. J Genet. 1955;53:536–550. [Google Scholar]
- 93.Saitoh T, Hirai M, Katoh M. Molecular cloning and characterization of WNT 3A and WNT14 clustered in human chromosome 1q42 region. Biochem Biophys Res Commun. 2001;284:1168–1175. doi: 10.1006/bbrc.2001.5105. [DOI] [PubMed] [Google Scholar]
- 94.Blanco G, Coulton GR, Biggin A, Grainge C, Moss J, Barrett M, Berquin A, Marechal G, Skynner M, van Mier P, Nikitopoulou A, Kraus M, Ponting CP, Mason RM, Brown SD. The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum Mol Genet. 2001;10:9–16. doi: 10.1093/hmg/10.1.9. [DOI] [PubMed] [Google Scholar]
- 95.Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- 96.German MS, Wang J, Fernald AA, Espinosa R, 3rd, Le Beau MM, Bell GI. Localization of the genes encoding two transcription factors, LMX1 and CDX3, regulating insulin gene expression to human chromosomes 1 and 13. Genomics. 1994;24:403–404. doi: 10.1006/geno.1994.1639. [DOI] [PubMed] [Google Scholar]
- 97.Gruneberg H. Genetical studies on the skeleton of the mouse. XXXII. The development of shaker with syndactylism. Genet Res. 1962;3:157–166. [Google Scholar]
- 98.Chaudhry SS, Gazzard J, Baldock C, Dixon J, Rock MJ, Skinner GC, Steel KP, Kielty CM, Dixon MJ. Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum Mol Genet. 2001;10:835–843. doi: 10.1093/hmg/10.8.835. [DOI] [PubMed] [Google Scholar]
- 99.Putnam EA, Zhang H, Ramirez F, Milewicz DM. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet. 1995;11:456–458. doi: 10.1038/ng1295-456. [DOI] [PubMed] [Google Scholar]
- 100.Goshu E, Jin H, Fasnacht R, Sepenski M, Michaud JL, Fan CM. Sim2 mutants have developmental defects not overlapping with those of Sim1 mutants. Mol Cell Biol. 2002;22:4147–4157. doi: 10.1128/MCB.22.12.4147-4157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dahmane N, Charron G, Lopes C, Yaspo ML, Maunoury C, Decorte L, Sinet PM, Bloch B, Delabar JM. Down syndrome-critical region contains a gene homologous to Drosophila sim expressed during rat and human central nervous system development. Proc Natl Acad Sci USA. 1995;92:9191–9195. doi: 10.1073/pnas.92.20.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crews ST, Fan CM. Remembrance of things PAS: regulation of development by bHLH-PAS proteins. Curr Opin Genet Dev. 1999;9:580–587. doi: 10.1016/s0959-437x(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 103.Crews S, Franks R, Hu S, Matthews B, Nambu J. Drosphila single-minded gene and the molecular genetics of CNS midline development. J Exp Zool. 1992;261:234–244. doi: 10.1002/jez.1402610303. [DOI] [PubMed] [Google Scholar]
- 104.Tredwell SJ, Smith DF, Macleod PJ, Wood BJ. Cervical spine anomalies in fetal alcohol syndrome. Spine. 1982;7:331–334. doi: 10.1097/00007632-198207000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Bantz EW. Valproic acid and congenital malformations. A case report. Clin Pediatr (Phila) 1984;23:352–353. doi: 10.1177/000992288402300611. [DOI] [PubMed] [Google Scholar]
- 106.Hanold KC. Teratogenic potential of valproic acid. J Obstet Gynecol Neonatal Nurs. 1986;15:111–116. doi: 10.1111/j.1552-6909.1986.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 107.Edwards MJ. Hyperthermia as a teratogen: a review of experimental studies and their clinical significance. Teratog Carcinog Mutagen. 1986;6:563–582. doi: 10.1002/tcm.1770060610. [DOI] [PubMed] [Google Scholar]
- 108.Ewart-Toland A, Yankowitz J, Winder A, Imagire R, Cox VA, Aylsworth AS, Golabi M. Oculoauriculovertebral abnormalities in children of diabetic mothers. Am J Med Genet. 2000;90:303–309. [PubMed] [Google Scholar]
- 109.Lewis DP, Van Dyke DC, Stumbo PJ, Berg MJ. Drug and environmental factors associated with adverse pregnancy outcomes. Part II: Improvement with folic acid. Ann Pharmacother. 1998;32:947–961. doi: 10.1345/aph.17298. [DOI] [PubMed] [Google Scholar]
- 110.Skold AC, Wellfelt K, Danielsson BR. Stage-specific skeletal and visceral defects of the I(Kr)-blocker almokalant: further evidence for teratogenicity via a hypoxia-related mechanism. Teratology. 2001;64:292–300. doi: 10.1002/tera.1084. [DOI] [PubMed] [Google Scholar]
- 111.Farley FA, Loder RT, Nolan BT, Dillon MT, Frankenburg EP, Kaciroti NA, Miller JD, Goldstein SA, Hensinger RN. Mouse model for thoracic congenital scoliosis. J Pediatr Orthop. 2001;21:537–540. [PubMed] [Google Scholar]
- 112.Wéry N, Narotsky MG, Pacico N, Kavlock RJ, Picard JJ, Gofflot F. Defects in cervical vertebrae in boric acid-exposed rat embryos are associated with anterior shifts of hox gene expression domains. Birth Defects Res. 2003;67:59–67. doi: 10.1002/bdra.10031. [DOI] [PubMed] [Google Scholar]