Abstract
Aim:
Due to extensive treatment switching in the MAVORIC trial, lack of UK regulatory licence for the comparator, overall survival (OS) with mogamulizumab was compared with patients with previously treated advanced mycosis fungoides/Sézary syndrome (MF/SS) in real-world setting.
Design, setting & participants:
Data were from the Hospital Episode Statistics database (all patients in NHS secondary care system in 2009–2019). Patients were selected according to trial inclusion criteria, then trial and HES samples were matched on selected variables with significant imbalance.
Outcomes:
The analysis indicated significant improvement in OS for mogamulizumab treatment compared with UK clinical practice (hazard ratio: 0.36, 95% CI: 0.24, 0.53).
Conclusion:
Results suggest an OS advantage for patients with advanced MF/SS treated with mogamulizumab in MAVORIC trial compared with UK clinical practice.
Keywords: cutaneous lymphoma, humanized antibodies, mogamulizumab, monoclonal antibodies, mycosis fungoides, survival, Sézary syndrome, T-cell lymphoma, vorinostat
Plain language summary
Comparing the survival of patients treated with mogamulizumab or current standard of care for mycosis fungoides or Sézary syndrome cutaneous T-cell lymphoma in the UK.
What is this article about?
In the UK, the National Institute for Health and Care Excellence have recently recommended mogamulizumab for treating adults with Sézary syndrome with at least 1 prior systemic treatment, or advanced mycosis fungoides with at least 2 prior systemic treatments. In the pivotal MAVORIC trial, mogamulizumab was compared with vorinostat, which was not part of the UK clinical practice. There is also limited published data on treatments used in the UK to use in a comparison to the MAVORIC trial data.
As a result, to assess how long patients live when using mogamulizumab compared with when using other treatments available in the UK, mogamulizumab trial data were compared with real-world data from the UK Hospital Episode Statistics database, including all NHS England secondary care, to represent clinical practice. To ensure that patients in the two datasets were similar, patients in NHS England secondary care were selected using trial inclusion criteria, and the two data samples were then matched on selected patient characteristics.
What were the results?
The results show that patients using mogamulizumab live significantly longer than when patients used other available treatments.
What do the results mean?
Results suggest an important advantage for advanced mycosis fungoides/Sézary syndrome patients treated with mogamulizumab compared with UK clinical practice.
Cutaneous T-cell lymphomas (CTCLs), including mycosis fungoides (MF) and Sézary syndrome (SS), are a rare and heterogenous group of blood cancers that have substantial morbidity and mortality impact on the affected individuals and their families [1–3]. MF is characterised by skin lesions, such as patches and plaques in early stages, with severe disease presenting with tumors, ulceration, systemic involvement and death. SS is a rarer, more aggressive disease, defined by the occurrence of erythroderma, lymphadenopathy and leukemic involvement [4]. Both have debilitating effects on patients' quality of life [1,5]. In the early stages of the disease, patients with either MF or SS have a median survival of 21.5 years from diagnosis. This reduces drastically to 1.4 to 6 years for advanced patients (Stage IIB-IV MF and SS) depending on stage and disease typology (MF vs SS) [2,3,6,7]. The annual incidence of CTCL in the UK is 0.7 per 100,000 people [8]. MF and SS have been found to represent 55% and 2.5% of CTCLs, respectively [8], which means the annual incidence of MF/SS is 0.4 per 100,000 people [8].
In the MF I-IIA stages of the disease, topical treatments, phototherapy and localised radiotherapy are recommended [4,9]. However, there is no standard of care for stage IIB-IV MF and SS, known as advanced disease [4]. In the UK, first-line treatment for advanced disease consists of systemic therapy such as bexarotene, interferon, methotrexate, total skin electron beam therapy and extracorporeal photopheresis and mono-chemotherapies. For second line, the National Institute for Health and Care Excellence (NICE) recommends brentuximab vedotin as an option for treating CD30-positive CTCL, after at least one systemic therapy and if they have MF stage IIB or more, primary cutaneous anaplastic large cell lymphoma or SS. This accounts for a very small proportion, 15–20%, of MF patients [10], while SS patients have minimal CD-30 positivity. For those who are clinically ineligible, or refractory to brentuximab vedotin, second-line treatments include recycling first-line treatment options, off-label mono- and polychemotherapies and for a small proportion of patients, allogeneic stem cell transplant (aSCT). The efficacy of brentuximab vedotin has not been studied in the SS population specifically [4,9,10]. NICE has recently recommended mogamulizumab (Poteligeo®), a first-in-class defucosylated humanised IgG1 monoclonal antibody, as an option for treating SS in adults who have had at least 1 prior systemic treatment and as an option for treating stage IIB or above MF in adults if they have had at least two prior systemic treatments [11].
The efficacy and safety of mogamulizumab was assessed in the largest phase III randomized study in MF/SS, the MAVORIC trial, which was an open-label, international, phase III randomized controlled trial in patients with relapsed or refractory MF or SS [12]. In the trial, mogamulizumab led to significantly greater progression free survival (PFS, the primary end point) compared with vorinostat, with a median PFS of 7.7 months versus 3.1 months, respectively, resulting in a hazard ratio (HR) of 0.53 (95% confidence interval: 0.4, 0.7) [12]. Due to ethical concerns for the poor prognosis for non-responding patients, the MAVORIC protocol allowed patients to ‘switch’ treatments so that patients randomized to the comparator arm could benefit from the new experimental treatment [12]. Patients on vorinostat who received at least two treatment cycles and showed confirmed disease progression, or had intolerable toxicity despite dose reduction and appropriate side effect management, could access treatment with mogamulizumab. Over 70% (n = 136/186) of vorinostat patients received subsequent mogamulizumab treatment. As a result, overall survival (OS), an exploratory end point, was confounded and cannot directly be used to understand the relative impact of mogamulizumab on survival. Analysed as randomized, with no adjustment for treatment switching in the vorinostat arm, there was not a statistically significant (p < 0.05) difference in survival for mogamulizumab compared with vorinostat (HR: 0.93, 95% confidence interval: 0.61–1.43, p = 0.94) [12]. Results with crossover adjustment are reported elsewhere [13].
Vorinostat was selected as the comparator in the MAVORIC trial as it had shown response in treatment of Sézary syndrome [14]. While it is approved in the US [15], Canada and Australia for patients who have progressive, persistent or recurrent CTCL subsequent to prior systemic therapies, it is not licensed by the European Medicines Agency (EMA) [16], and is not part of current standard of care in a UK setting. With the limited second line treatment options, re-challenge with first line systemic therapies in a clinical trial setting would have been both unethical for those patients who had already received them and inappropriate by introducing selection bias into the study. The trial design, including the choice of the comparator, vorinostat, was approved by the EMA.
Due to extensive treatment switching and the use of vorinostat as the active comparator, there are uncertainties in the difference in overall survival to be expected after mogamulizumab compared with UK current clinical practice. The aim of the study was to reduce these uncertainties and support decision-making, by comparing mogamulizumab directly to patients with MF and SS previously treated with at least one prior systemic treatment in the UK real-world setting.
Materials & methods
Study design
As the comparator arm in the MAVORIC study, vorinostat, is not part of the UK clinical practice and there is limited published data for treatments which are used in the UK, the OS of mogamulizumab from the MAVORIC trial was compared to the OS observed in real-world evidence (RWE) representing UK clinical practice. The RWE was taken from the Hospital Episode Statistics (HES) [17] database maintained by the UK National Health Service (NHS). However, in the absence of common comparator between the MAVORIC trial and the HES data and therefore lack of connected network for a network meta-analysis, differences in the patient populations of the two data sources were taken into account using an unanchored indirect comparison [18]. The unanchored indirect comparison reweights the MAVORIC trial population to match the UK patient population using the available patient characteristics in the HES data for second-line, advanced MF/SS. Following this, the OS from the reweighted mogamulizumab arm of the MAVORIC trial was compared with the HES data according to the NICE guidelines by Phillippo et al. [18].
Data sources
Patients enrolled in the MAVORIC trial had histologically confirmed relapsed or refractory MF or SS, had failed (due to progression or toxicity as assessed by the principal investigator) at least one previous systemic therapy, and had an Eastern Cooperative Oncology Group (ECOG) performance score of 1 or less and adequate haematological, hepatic and renal function [19]. Detailed description of the MAVORIC trial is available elsewhere [12]. Only advanced patients (SS and MF stage IIB-IV), the population recommended for treatment by NICE, were included in this analysis. This analysis from the MAVORIC study used a data cut from 2 March 2019.
The HES database is an administrative database which reflects current UK clinical practice in advanced MF/SS [17]. It contains details of all longitudinal inpatient admissions, Accident and Emergency (A&E) attendances and outpatient appointments at NHS hospitals in England. Each HES record contains a wide range of information about an individual patient admitted to an NHS hospital including clinical information about diagnoses and procedures and patient information, such as age group, gender and ethnicity, administrative information, such as dates and methods of admission and discharge, geographical information and death. Analysis of HES data is performed on pseudonymized patient records in line with regulations and guidance from NHS Digital.
Aligning participants from HES database to the MAVORIC trial advanced population
The HES data and the MAVORIC trial advanced subgroup were aligned in two steps. First, patients from the HES database were selected according to the MAVORIC trial inclusion criteria (using histology, number and types of prior treatments, ECOG performance score and haematological, hepatic, and renal function), then the two samples were matched on selected variables where there was significant imbalance (details below).
To account for histology, patients from the HES data had to have the International Classification of Diseases (ICD) 10 codes for MF or SS (C84.0 for MF, 84.1 for SS) in their longitudinal records between 1 October 2010 and 31 March 2019 (Table 1). Patients were excluded if they had ICD-10 diagnosis codes for both MF (C84.0) and SS (C84.1) within their record.
Table 1. . Baseline demographic and disease characteristics of patients in MAVORIC trial [12] (ITT population) and the HES database.
| Patient characteristic | MAVORIC trial | HES data | Included in matching or selection of patients | |
|---|---|---|---|---|
| Mean age, years | 63 | 65 | Not in the base case, as they are similar. Included in SA | |
| Male, n (%) | 58% | 62% | Not in the base case, as they are similar. Included in SA | |
| Race | Not directly comparable | |||
| Disease type | MF (%) | 55% | 85% | Yes |
| SS (%) | 45% | 15% | ||
| ECOG performance status, n (%) | 0 | 210 (56.0) | Not available | Matching not possible |
| 1 | 160 (43.0) | |||
| Time from initial diagnosis (months), median (min, max) | Mogamulizumab: 41.0 (17.4–78.8) Vorinostat: 35.4 (16.2–68.2) |
Not available, only start of secondary care treatment | Matching not possible | |
| Current clinical stage | Mostly advanced (85%) | Secondary care patients receiving systemic treatment | Stage not available in HES, but are mostly advanced patients according to clinical opinion | |
| Prior systemic therapies (n) | ≥1 | 1 systemic therapy prescribed in secondary care | Patients selected from HES database at start of study to match criterion | |
| Prior systemic therapies received, mean (n) | 3.0 | 1 systemic therapy prescribed in secondary care | ||
| Prior systemic therapies | Chemotherapies Radiotherapy Phototherapy Biologic therapies |
Various treatment options | Patients selected from HES database at start of study to match criterion | |
ECOG: Eastern Cooperative Oncology Group; HES: Hospital Episode Statistics; ITT: Intention-to-treat; MF: Mycosis fungoides; SA: Sensitivity analysis; SS: Sézary syndrome.
Data were collected from the date of the first diagnosis up to either the date of death or study period end date (31 March 2019). In order to ensure that the first occurrence of the ICD-10 diagnosis code recorded within the study period represented the actual date of the patient's first ever diagnosis, patients were only included in the study if they did not have MF or SS diagnosis within the 18 months prior to the start of the study observation period.
To account for the treatment line and prior treatments, patients were selected from the HES database if they had one predefined prior systemic therapy recorded. Prior treatment options were based on the MAVORIC trial data.
Advanced MF and SS patients are seen in secondary care setting in the NHS. Therefore, although the HES data does not contain information on disease stage, patients who have received more than one line of systemic treatments in a secondary care setting can be assumed to be advanced patients.
The ECOG performance score and haematological, hepatic, and renal function, mentioned in the MAVORIC trial inclusion criteria, are not recorded in HES database. Since, patients selected from the HES database were eligible for systemic therapy, it can be inferred that they had an ECOG performance score of 1 or less and adequate haematological, hepatic and renal function.
Assumptions regarding staging, ECOG performance score and haematological, hepatic and renal function were validated by UK clinical experts who have long-term experience in treating MF/SS patients and in the use of mogamulizumab.
Variables for matching
In addition to the patient characteristics aligned using the inclusion criteria (diagnosis, inferred staging, number and type of prior treatments), additional differences in patient populations were assessed between the MAVORIC and the HES (Table 1). As this is an unanchored comparison with no common comparator, patient characteristics should be included in the matching if:
They are considered a prognostic factor or treatment effect modifier and
There are material differences between the characteristics in the studies at baseline.
These criteria are outlined in the NICE guidelines by Phillippo et al. [18].
To identify potential prognostic factors/treatment effect modifiers targeted literature searches were performed. Three publications were identified which consider the impact of potential prognostic factors in MF/SS (Table 2). Age and staging were identified by all publications as prognostic factors. Sex, laboratory values, large cell transformation and rare subtypes of MF had either limited or unclear effect. Treatment effect modifiers for PFS from the MAVORIC trial subgroup analyses were disease type (MF vs SS) and stage (IB/II vs III/IV), and these were assumed to also be relevant for OS [12]. The inclusion of these prognostic factors and treatment effect modifiers in the matching depend on the availability of their reporting in the MAVORIC trial and the HES database.
Table 2. . Prognostic factors relevant for the advanced MF and SS population.
| Patient/disease characteristic | Potential prognostic factor or treatment effect modifier? | Study (location, database, year, n) | MAVORIC14† | ||
|---|---|---|---|---|---|
| Agar et al. (UK, ICARSIS, 1980–2009, n = 1,502) [3] |
Kim et al. (USA, Standford University Clinic, 1958–1999, 525) [7] |
Talpur et al. (USA, MD Anderson Cancer Center, 1982–2009, 1263) [8] |
|
||
| Identified as a prognostic factor in study? | Identified as treatment effect modifier? | ||||
| Age | Yes | Yes | Yes | Yes | No (<65 vs ≥65) |
| Sex | Uncertain | Yes | No | Not tested | No |
| Laboratory values | Limited evidence | Yes (LDH) | Not tested | Yes (LDH, elevated white blood cell count, beta 2 microglobulin) | No (LDH) |
| Staging (including blood classification | Yes | Yes (presence in peripheral blood of the tumour clone without Sezary cells) | Yes (extent and type of skin involvement and presence of extracutaneous disease) | Yes (generalized vs regional tumor(s)) | Yes (IB/II vs III/IV, and MF vs SS) |
| Large-cell transformation | Yes | Yes | Not tested | Yes | Not tested |
| Rare subtypes of MF | Variable | Yes (hypopigmented MF, MF with lymphomatoid papulosis, poikilodermatous MF, folliculotropic MF) | Not tested | Yes (poikiloderma MF, MF with lymphomatoid papulosis) | Not tested |
MAVORIC trial also included race and region in predefined subgroup analysis for PFS, neither of which were identified as treatment effect modifiers.
LDH: Lactate dehydrogenase; MF: Mycosis fungoides; SS: Sézary syndrome.
Staging was included in the alignment process. Time from diagnosis, race/ethnicity, and large-cell transformation could not be matched on, as they were either not reported in in at least one of the data sources or because the reporting was not comparable between the studies (Table 2).
The proportion of patients with MF versus SS disease type, age and gender were available. Age and sex were very similar between the two data sources (mean age 63 vs 65 years, and proportion of male 58 vs 62% in the MAVORIC trial vs HES data respectively) (Table 1). Therefore, to avoid unnecessarily reducing the sample size post-matching, in the base case, only the distribution of MF and SS disease type were matched to. A sensitivity analysis including sex and age was also conducted to assess the effect of their exclusion.
Statistical analysis
For the simple matching based on disease classification (MF/SS) alone, a simple weighting was estimated based on the inverse probability of being in a given disease class in the HES dataset. In the sensitivity analyses, a more sophisticated matching-adjusted indirect comparison (MAIC) was conducted including disease type and sex using the method of moments methodology described Signorovitch et al. and the NICE Technical Support Document 18 [18,20]. A MAIC weights the individual patient data for patients randomized to mogamulizumab from MAVORIC to match the population characteristics from the HES database as closely as possible. After matching, weighted treatment outcomes are compared across more balanced populations [20,21].
Results
Prior to the adjustment, the OS Kaplan–Meier curves of the advanced patients from the MAVORIC trial (n = 150) and the HES data (n = 198) show that patients treated with mogamulizumab in MAVORIC have a better survival outlook compared with the standard of care (i.e., HES control arm) (Figures 1 & 2). The HR for OS for unadjusted mogamulizumab versus standard of care was 0.43 (95% confidence interval [CI]: 0.31, 0.60).
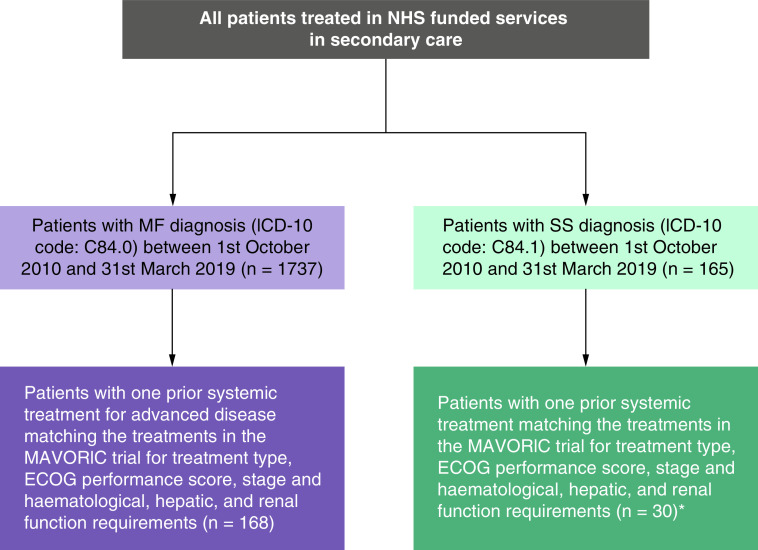
Figure 1. . Patient selection in the UK Hospital Episode Statistics database.
*All SS patients are considered advanced, so selecting patients for advanced disease was not required.
ECOG: Eastern Cooperative Oncology Group; ICD-10: International Classification of Diseases 10th Revision code; MF: Mycosis fungoides; NHS: National Health Service; SS: Sézary syndrome.
Figure 2. . Kaplan–Meier curves for overall survival.

MAIC: Matching-adjusted indirect comparison; SoC: Standard of care; tx: Treatment.
With the adjustment, the proportion of SS patients in the MAVORIC trial data reduced from 47% to 13%, which is similar to the 15% in the HES data (Table 3). The age difference increased from 2 years to five, and the difference in the proportion of males decreased from 4% to 1%. The balance of the populations post-matching suggests that the matching was successful. There were no extreme weights applied in the analysis. The effective sample size (ESS) for the MAVORIC data post-matching is 93, a reduction of 38%. After the matching mogamulizumab showed a significant OS improvement compared with UK clinical practice (Table 4 & Figure 2) with a HR of 0.36 (95%CI: 0.24, 0.53).
Table 3. . Baseline characteristics pre- and post-matching.
| Pre-matching | Post-matching | |||
|---|---|---|---|---|
| MAVORIC trial (advanced population) | HES data | MAVORIC trial (advanced population) | HES data | |
| Base case (matching to % of MF vs SS) | ||||
| n | 150 | 198 | 93 | 198 |
| % SS | 47% | 15% | 13% | 15% |
| Age, years (mean) | 63 | 65 | 60 | 65 |
| Male | 58% | 62% | 61% | 62% |
| Scenario analysis (matching for % of MF vs SS, age, sex) | ||||
| n | 150 | 198 | 85 | 198 |
| % SS | 54% | 15% | 15% | 15% |
| Age, years (mean) | 64 | 65 | 65 | 65 |
| Male | 61% | 62% | 62% | 62% |
HES: Hospital Episode Statistics; MF: Mycosis fungoides; SS: Sézary syndrome.
Table 4. . Results of the unanchored indirect comparison.
| Median survival in months (95% CI) | Hazard ratio (95% CI) | |
|---|---|---|
| Unadjusted | ||
| Mogamulizumab | 57.2 (40.1, NA) | 0.43 (0.31 to 0.60) |
| Standard of care | 17.83 (12.37, 24.03) | |
| Adjusted for MF/SS proportions | ||
| Mogamulizumab | NA (51.7, NA) | 0.36 (0.24 to 0.53) |
| Standard of care | 17.83 (12.37, 24.03) | |
| Sensitivity analyses (including MF/SS proportions, age, sex) | ||
| Mogamulizumab | NA (40.06, NA) | 0.38 (0.25 to 0.59) |
| Standard of care | 17.83 (12.37, 24.03) | |
CI: Confidence interval; MF: Mycosis fungoides; NA: Not available; SS: Sézary syndrome.
Matching for the proportion of MF/SS, sex and age in the scenario analysis resulted in balanced patient characteristics, suggesting that the matching was successful. The weights applied in the analysis were not extreme (Figure 3). The ESS post-matching is 85, a reduction of 43%. The results were very similar to that of the base case, with an OS HR for adjusted mogamulizumab versus standard of care of 0.38 (95%CI: 0.25 to 0.59) (Table 4 & Figure 2).
Figure 3. . Distribution of matching-adjusted indirect comparison weights, matching on age, sex and disease class.

Discussion
In the MAVORIC trial, mogamulizumab showed statistically significantly superior PFS compared with vorinostat in patients with relapsed or refractory MF or SS [12]. However, the trial was not designed to show a survival advantage since patients randomized to vorinostat were allowed to switch to mogamulizumab following progression or intolerable toxicity. The comparator, vorinostat, was selected as an ethical option that avoids selection bias and is reflective of agents used later in the treatment pathway. While it is licensed in the USA [15], it is not approved by the EMA [16] and is not part of current UK clinical practice. Therefore, an indirect comparison is required to compare mogamulizumab to current UK clinical practice at the second line systemic treatment setting.
The current study used RWE from the UK HES dataset to estimate the OS impact of mogamulizumab compared with treatments received in UK clinical practice in patients with advanced MF and SS who had one prior systemic therapy. The HES dataset provided a unique opportunity to assess survival in MF/SS in the second line setting and to construct a comparator arm of current UK clinical practice. It is comprised of all MF/SS patients treated in the NHS in England's secondary care system between 2009 and 2019. To ensure the compatibility of the HES and the MAVORIC trial data, an unanchored indirect comparison was conducted to match the patient populations according to NICE guidelines [22].
The unanchored indirect comparison accounted for the available prognostic factors and treatment modifiers in two steps: using the MAVORIC trial inclusion criteria to select patients from the HES dataset to align with the MAVORIC trial and matching the MAVORIC trial population to the resulting HES population. The results showed that mogamulizumab was associated with a significant survival advantage, both before (HR: 0.43, 95%CI: 0.31, 0.60) and after adjustment for patient characteristics (HR: 0.36, 95% CI: 0.24, 0.53 in the base case and HR: 0.38, 95% CI: 0.25 to 0.59 in scenario analysis).
This approach provides a direct comparison of mogamulizumab against current UK clinical practice represented by RWE, reflects the current UK MF/SS population, and does not require any adjustment for treatment switching with its attendant uncertainties, as no patients in current clinical practice received mogamulizumab. Regulatory bodies have recognized the utility of RWE for supplementing and supporting clinical trial data in new drug applications [23–25], and the acceptance of RWE is increasing particularly in oncology and rare diseases [26].
The results are in line with the MAVORIC trial results, where mogamulizumab, in addition to the PFS advantage, demonstrated significantly improved responses in three compartments of the disease, including the blood and skin compartments which are known to be key prognostic factors and predictors of reduced survival [12]. Such improvements in response could translate into improved survival for patients.
There are some limitations with this analysis. In an unanchored comparison, adjustments are made for important known differences between the patient populations [22]. However, not all potential prognostic factors and treatment effect modifiers identified from the literature were reported in both HES and MAVORIC datasets. Diagnosis, number and type of prior treatment, proportion of MF/SS patients, age and sex were available and taken into account. However, the HES population had had one prior treatment compared with a median of three prior treatments in the MAVORIC population, suggesting that the HES population may have a better prognosis, leading to potentially conservative estimates. Some missing patient characteristics, such as staging and ECOG performance status can be inferred from the available data, while others, such as large cell transformation, rare subtypes of MF and laboratory values are not available. However, in general, it is not expected that RWE databases record patient characteristics as extensively as clinical trials. In addition, the HES data were extracted by a third party and aggregate sufficient statistics supplied for these analyses. As such, it was not possible to assess the extent and potential impact of missing data.
Furthermore, some patients, most likely those with less severe disease, may have been diagnosed and managed outside of the secondary care setting. Additionally, it was not possible to verify the date of first ever diagnosis of CTCL, and, as is common in studies involving HES, it is also possible that the clinical terminology and classification codes assigned to patients are not true representations of what happened in clinical practice. Nevertheless, the HES dataset is used by the NHS for financial management and, while it excludes private hospitals, it provides a relatively comprehensive longitudinal dataset for England with a long follow-up. According to NICE, “the HES data provide the best available source of evidence” for this patient population and was consequently used as the basis of the NICE guidance [11,27].
Additional research using comparative data for mogamulizumab versus clinical practice without mogamulizumab would provide further information, once the data are available.
Conclusion
Mogamulizumab is a novel monoclonal based immuno-oncology treatment with an innovative mode of action which has demonstrated superior PFS and response in MF/SS, alongside significant improvements in health-related quality of life and a well-tolerated safety profile compared with vorinostat, as reported in the MAVORIC trial, a phase III RCT [12].
The MAVORIC trial comparator, vorinostat, was a reasonable comparator option in this heavily pre-treated population, who have a rare haematological malignancy and are already refractory to the limited standard agents. However, it is not approved in the UK, where standard of care consists of multiple treatment options.
To compare mogamulizumab to current UK clinical practice in advanced MF and SS patients an unanchored indirect comparison was conducted to the HES data comprising of all MF/SS patients treated in the NHS secondary care system between 2009 and 2019. After matching the patient populations, the HRs were 0.36 (95% CI: 0.24, 0.53) and 0.38 (95% CI: 0.25–0.59) depending on the patient characteristics included in the matching.
These results showed evidence of an OS advantage for patients treated with mogamulizumab in the MAVORIC trial compared with current UK clinical practice and has demonstrated that mogamulizumab represents an important treatment option for second-line advanced stage MF/SS patients.
RWE can complement RCTs and can help to address potential areas of uncertainties in clinical trials. One such example is their use as an external control arm, which, after adjustment of the patient population, can provide estimates of comparative effectiveness to current clinical practice.
Summary points
The pivotal MAVORIC trial compared mogamulizumab to vorinostat in patients with mycosis fungoides (MF) or Sézary syndrome (SS) cutaneous T-cell lymphoma.
Due to extensive treatment switching from treatment to control (over 70%) in the trial and the lack of access to vorinostat in UK clinical practice, the overall survival (OS) of patients randomized to mogamulizumab was compared directly to survival for patients with advanced MF/SS previously treated with at least one prior systemic treatment in the UK real-world setting.
Mogamulizumab data were taken from the advanced subgroup of the MAVORIC trial and comparator data representing current UK clinical practice was from the Hospital Episode Statistics (HES) database, including all MF/SS patients treated in NHS England secondary care between 2009 and 2019.
Patients from the HES database were selected according to the MAVORIC trial inclusion criteria, aligning histology, number and types of prior treatments, inferred staging and performance status.
The two samples were matched on additional available variables with significant imbalance according to the National Institute of Health and Care Excellence guideline (proportion of MF/SS).
A scenario analyses was also conducted including also variables, that were balanced between populations (age and sex).
With the adjustment, mogamulizumab showed a significant OS improvement compared with UK clinical practice with a hazard ratio (HR) of 0.36 (95% confidence interval (CI): 0.24, 0.53).
The scenario analysis resulted in similar OS improvement, with a HR of 0.38 (95% CI: 0.25–0.59).
These results provide evidence of an OS advantage in patients treated with mogamulizumab in the MAVORIC trial compared with current UK clinical practice.
RWE can complement trial data and can help to address potential areas of uncertainties in clinical trials.
Footnotes
Author contributions
N Hawkins led the conception, design of the analysis, interpretation of results and was involved in the analysis of data and the drafting of the paper. N Hawkins was responsible for the final approval of the version to be published. N Muszbek participated in the conceptualization and interpretation of the analysis and has drafted the manuscript with R Evans. R Evans was involved in the analyses, interpretation of results and drafted the paper with N Muszbek. L McNamara and T Jones were involved in the conception, interpretation of results and the drafting of the paper. All authors agreed to be accountable for all aspects of the work.
Financial & competing interests disclosure
This study was funded by K Kirin. N Hawkins, N Muszbek and R Evans are partners/employees of Visible Analytics Ltd, which designed and conducted this analysis and received consultancy fees from Kyowa Kirin. L McNamara and T Jones are employees of Kyowa Kirin. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of the MAVORIC trial data that have been shared with them and the Hospital Episode Statistics data that was shared in aggregate format, and that the use of this shared data is in accordance with the terms agreed upon their receipt.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Demierre MF, Gan S, Jones J, Miller DR. Significant impact of cutaneous T-cell lymphoma on patients' quality of life: results of a 2005 National Cutaneous Lymphoma Foundation Survey. Cancer 107(10), 2504–2511 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Scarisbrick JJ, Prince HM, Vermeer MH et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J. Clin. Oncol. 33(32), 3766–3773 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides survival from diagnosis, prognostic for the largest cohort of advanced mycosis fungoides and Sézary Syndrome patients markers.
- 3.Agar NS, Wedgeworth E, Crichton S et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J. Clin. Oncol. 28(31), 4730–4739 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Trautinger F, Eder J, Assaf C et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome – Update 2017. Eur. J. Cancer 77, 57–74 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Orlowska D, Selman LE, Beynon T et al. “It's a traumatic illness, traumatic to witness”: a qualitative study of the experiences of bereaved family caregivers of patients with cutaneous T-cell lymphoma. Br. J. Dermatol. 179(4), 882–888 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch. Dermatol. 139(7), 857–866 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Talpur R, Singh L, Daulat S et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin. Cancer Res. 18(18), 5051–5060 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Public Health England. National Cancer Registration and Analysis Services. Registration of Cutaneous T-Cell Lymphoma (CTCL) in England. http://www.ncin.org.uk/view?rid=3275
- 9.Gilson D, Whittaker SJ, Child FJ et al. British Association of Dermatologists and UK Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018. Brit. J. Dermatol. 180(3), 496–526 (2019). [DOI] [PubMed] [Google Scholar]; • British clinical guideline for the management of primary cutaneous lymphomas.
- 10.National Institute for Health and Care Excellence (NICE). Brentuximab vedotin for treating CD30-positive cutaneous T-cell lymphoma | Guidance [TA577] (2019). https://www.nice.org.uk/guidance/ta577/documents/html-content-3
- 11.National Institute for Health and Care Excellence. Mogamulizumab for previously treated mycosis fungoides and Sézary syndrome [TA754] | Guidance| NICE. https://www.nice.org.uk/guidance/ta754 ; •• Guidance for mogamulizumab by the National Institute for Health and Care Excellence (NICE) in the UK.
- 12.Kim YH, Bagot M, Pinter-Brown L et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomized, controlled phase III trial. Lancet Oncol. 19(9), 1192–1204 (2018). [DOI] [PubMed] [Google Scholar]; •• Pivotal trial publication for mogamulizumab for patients with previously treated mycosis fungoides and Sézary Syndrome.
- 13.Hawkins N, Muszbek N, Evans R, Dequen-O'Byrne P, Jones T, McNamara L. Adjusting for treatment crossover in the MAVORIC trial: survival in advanced mycosis fungoides and Sézary syndrome. J. Comp. Eff. Res. 11(11), 805–813 (2022). [DOI] [PubMed] [Google Scholar]; • Results of the crossover adjustment of the overall survival in the mogamulizumab pivotal trial.
- 14.Mann BS, Johnson JR, He K et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin. Cancer Res. 13(8), 2318–2322 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration (FDA). FDA approves mogamulizumab-kpkc for mycosis fungoides or Sézary syndrome. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-mogamulizumab-kpkc-mycosis-fungoides-or-sezary-syndrome
- 16.European Medicines Agency (EMA). Withdrawal assessment report for Vorinostat MSD 100 mg hard capsules (2009). https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-vorinostat-msd_en.pdf
- 17.NHS Digital. Hospital Episode Statistics (HES). NHS Digital (2020). https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics
- 18.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU TECHNICAL SUPPORT DOCUMENT 18: population-adjusted indirect comparisons (MAIC and STC) – NICE Decision Support Unit (2016). http://nicedsu.org.uk/technical-support-documents/population-adjusted-indirect-comparisons-maic-and-stc/ ; • Methodological guidelines for population adjusted indirect comparisons from the Decision Support Unit of the National Institute for Health and Care Excellence (NICE) in the UK.
- 19.Kim YH, Ortiz-Romero PL, Pro B et al. Time to next treatment in patients with previously treated cutaneous T-cell lymphoma (CTCL) receiving mogamulizumab or vorinostat: A MAVORIC post-hoc analysis. Internati. Con. Malig. Lymph. Hematolog. Oncol. (2019). https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.7539 [Google Scholar]
- 20.Signorovitch JE, Wu EQ, Yu AP et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics 28(10), 935–945 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Signorovitch JE, Sikirica V, Erder MH et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health 15(6), 940–947 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Latimer NR, Abrams KR. NICE DSU Technical Support Document 16: Adjusting survival time estimates in the presence of treatment switching. Dec. Supp. Unit, ScHARR. 57 (2014). https://www.sheffield.ac.uk/nice-dsu/tsds/treatment-switching [PubMed] [Google Scholar]; • Methodological guidelines for adjusting for treatment switching from the Decision Support Unit of the NICE in the UK.
- 23.Chan K, Nam S, Evans B et al. Developing a framework to incorporate real-world evidence in cancer drug funding decisions: the Canadian Real-world Evidence for Value of Cancer Drugs (CanREValue) collaboration. BMJ Open 10(1), e032884 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Innovative Medicines Initiative (IMI) IMI1 Final Project Report Public Summary: incorporating real-life clinical data into drug development (GETREAL) (2017). https://www.imi.europa.eu/sites/default/files/uploads/documents/115546_GetReal_Final_Report.pdf
- 25.U.S. Food and Drug Administration (FDA). Framework for FDA's Real-World Evidence Program (2018). https://www.fda.gov/media/120060/download
- 26.O'Donnell JC, Le TK, Dobrin R et al. Evolving use of real-world evidence in the regulatory process: a focus on immuno-oncology treatment and outcomes. Future Oncol. 17(3), 333–347 (2021). [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence (NICE). Single Technology Appraisal – Appeal hearing: advice on mogamulizumab for previously treated mycosis fungoides and Sezary syndrome [ID1405]. Decision of the panel (2021). https://www.nice.org.uk/guidance/ta754/history