Abstract
Aim:
NICE's highly specialized technology (HST) evaluations are highly restrictive in terms of entry criteria and as a consequence, the vast majority of rare disease medicines are assessed through NICE's standard, single technology appraisal (STA) route. We explored whether NICE shows flexibility and pragmatism when evaluating treatments for rare diseases through its STA process.
Materials & methods:
We matched a sample of recent, randomly selected STAs for rare diseases to STAs for non-rare diseases and conducted a thematic analysis to identify patterns in NICE's decision-making, with a specific focus on the application of NICE's published methods and the handling of uncertainty.
Results:
Three themes emerged where some flexibility was shown: ‘handling of uncertainty and discretion’, ‘application of NICE methods’ and ‘commercial arrangements’. Rare disease technologies were generally subject to longer appraisal times than those for non-rare diseases.
Conclusion:
Although NICE shows a degree of flexibility and pragmatism toward uncertainties in the evidence base for rare disease medicines, this is often off-set by a lengthy appraisal process, which can lead to delays in patients receiving vital treatment.
Keywords: flexibility, highly specialized technology, NICE, rare disease, single technology appraisal, thematic analysis
Plain language summary
What is this article about?
In England and Wales, the National Institute for Health and Care Excellence (NICE) decides whether to recommend medicines for use by the National Health Service. It has a process for evaluating very rare disease medicines – the highly specialized technology route. However, the entry criteria for this process are very restrictive, so many rare disease medicines end up in NICE's standard STA process, which is deemed inadequate to account for the complex issues associated with rare diseases. We wanted to find out whether NICE considers these issues systematically when evaluating rare disease medicines via STA.
What were the results?
We randomly selected six treatments for rare diseases and six treatments for non-rare diseases and compared information taken from documents published on NICE's website. We found three main areas where NICE showed some flexibility for rare diseases: 1) taking into account a lack of clinical evidence; 2) lenient adherence to, and interpretation of, the NICE methods guide; 3) negotiating commercial arrangements that provide access to treatment while giving the manufacturer time to gather more information. However, appraisals generally took much longer for rare diseases than for non-rare diseases.
What do the results of the study mean?
The results show that NICE shows some flexibility when evaluating rare disease medicines. However, they also show that treatments for rare diseases can be disadvantaged by going through the STA process relative to non-rare diseases, resulting in the potential for substantially delayed access for patients.
In Europe, a rare disease is generally defined as one that affects less than one person in every 2000 [1]. The small number of people affected by each individual disease creates challenges with timely diagnosis and access to services and treatments. However, when considered cumulatively, a large number of people are affected by rare diseases: it is estimated that in the UK alone, more than 3 million people will suffer from a rare disease at some point in their life [2], making these challenges significant at the population level.
Principle 4 of England's Rare Disease Action Plan 2023 commits to supporting rapid access to drugs within the National Health Service (NHS) for people with rare diseases [3]. In England, the National Institute for Health and Care Excellence (NICE) evaluates the clinical and cost–effectiveness of healthcare interventions. Treatments that NICE recommends must be funded by the NHS within 3 months of the NICE guidance being published. Most treatments undergo a single technology appraisal (STA) which, although well suited to the evaluation of therapies that are expected to benefit a large number of people, is not designed to take into account the greater uncertainty in an evidence base for the considerably smaller populations associated with treatments for rare diseases. Trials in rare diseases include small numbers of patients and often have short follow-up and unclear standards of care. These factors impact the ability to capture meaningful end points and test for statistical significance [4]. Furthermore, it is acknowledged that because of the small patient pool, the prices of rare disease technologies are generally higher than the value-based pricing to allow companies to recoup expenses on research and development [5]. Overall, these factors can present a challenge for health technology appraisal and decision making about the therapeutic value and cost–effectiveness of treatments.
In 2013, an alternative appraisal pathway for specialized treatments or treatments for very rare conditions was introduced: the highly specialized technology (HST) route. This was designed to be quicker and more pragmatic regarding the evidence base than the STA route, based on the expectation that these technologies are associated with a high degree of uncertainty when being appraised. The HST cost–effectiveness threshold (£100,000 to £300,000 per quality adjusted life year [QALY] gained) is higher than for STAs (£20,000 to £30,000 per QALY gained). In order to be considered for the HST assessment pathway, technologies are required to meet all of the following four eligibility criteria: the condition is very rare (prevalence of less than 1 in 50,000 in England); no more than 300 people in England are eligible for the technology in its licensed indication and no more than 500 across all its indications; the condition the technology is indicated for significantly shortens life or severely impairs quality of life; there are no satisfactory treatment options, or the technology is likely to offer significant additional benefit over existing treatment options.
Considering the very stringent inclusion criteria, and the fact that NICE only has capacity for a small number of such appraisals each year, rare disease topics are very often not selected for HST and are appraised via the STA route instead. It should be noted that as part of the updated NICE methods process that came into effect on 1 February 2022, the seven previous HST eligibility criteria were reduced to four to improve clarity and increase predictability for the routing of topics [6]. However, it is too early to assess the impact of this on the routing of rare disease treatments.
The inability of NICE to accommodate rare diseases has caused considerable frustration for both patient groups and pharmaceutical companies. In 2019, a debate was held in the House of Commons in which several Members of Parliament described how patients with rare diseases in their constituencies were unable to get access to treatments owing to NICE's appraisal methods [7]. Patient groups have challenged the decision to route certain treatments for rare diseases down the STA pathway, arguing that it is poorly suited for assessing the complexity of rare disease and that flexibility is needed [8–10]. In their 2019 report ‘Illuminating the Rare Reality’, Rare Disease UK states that the UK is slower to make a decision on access to treatments for rare diseases than Spain, France, Italy and Germany, and is more likely to say no [11]. As evidenced in the 2016 All Parliamentary Group on Rare, Genetic and Undiagnosed Conditions hearing on access to medicines in England, delays in making a decision can impact the health of patients and affect their families, who may see a decline in the patient's condition [12].
An analysis by Clarke et al. of appraisals submitted between 1 January 2015 and 11 March 2020 showed that NICE takes longer to reach a decision on orphan drug STAs than on non-orphan drug STAs (this is impacted by a higher proportion of orphan drug STAs needing more than one committee meeting) and that orphan drug STAs are less likely to receive a positive outcome than HST evaluations [13]. The aim of our analysis was to build on this previous research and explore whether NICE takes the rarity of a condition into account when evaluating treatments for rare diseases through the STA process. Specifically, we wanted to determine whether NICE shows any flexibility and pragmatism for rare disease technologies, given the notable challenges for clinical development and difficulties with collecting robust evidence.
Materials & methods
Appraisal selection
A set of 12 appraisals (six rare disease and six non-rare disease) for comparison and analysis was generated as follows. First, a list of STAs that had received final guidance between 1 January 2021 and 1 January 2023 was extracted from the NICE website. Appraisals for oncology drugs and treatments for acute diseases were then excluded and the remaining STAs differentiated by rarity (where rare was defined as a prevalence of <1 in 2000 [1]), NICE appraisal committee (A, B, C or D) and year of final appraisal committee meeting (ACM). A number was assigned to each rare disease appraisal and a random number generator was used to randomly select six rare disease STAs to be included in the analysis.
Each randomly selected rare disease STA was matched to a corresponding randomly selected non-rare disease STA according to two covariables: year of final ACM and NICE committee (Table 1). As all of the rare disease STAs were for chronic conditions, we ensured that the matched non-rare disease STAs were also for chronic conditions.
Table 1. . Summary of appraisals included in the analysis.
| Generic name | Brand name | Manufacturer | Category (rare/control) | Indication under review | Appraisal year | NICE committee | Appraisal ID | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sapropterin | Kuvan | BioMarin | Rare | Hyperphenylalaninaemia in phenylketonuria | 2021 | A | TA729 | [14] |
| Secukinumab | Cosentyx | Novartis | Control | Non-radiographic axial spondyloarthritis | 2021 | A | TA719 | [15] |
| Avacopan | Tavenos | Vifor Pharma | Rare | Severe acute granulomatosis with polyangiitis or microscopic polyangiitis | 2022 | B | TA825 | [16] |
| Filgotinib | Jyseleca | Galapagos | Control | Moderately to severely active ulcerative colitis | 2022 | B | TA792 | [17] |
| Risdiplam | Evrysdi | Roche | Rare | Spinal muscular atrophy | 2021 | C | TA755 | [18] |
| Empagliflozin | Jardiance | Boehringer Ingelheim | Control | Chronic heart failure with reduced ejection fraction | 2021 | C | TA773 | [19] |
| Ravulizumab | Ultomiris | Alexion | Rare | Atypical haemolytic uraemic syndrome | 2021 | C | TA710 | [20] |
| Inclisiran | Leqvio | Novartis | Control | Primary hypercholesterolaemia or mixed dyslipidaemia | 2021 | C | TA733 | [21] |
| Mexiletine | Namuscla | Lupin | Rare | Symptoms of myotonia in non-dystrophic myotonic disorders | 2021 | D | TA748 | [22] |
| Dapagliflozin | Forxiga | AstraZeneca | Control | Chronic kidney disease | 2021 | D | TA775 | [23] |
| Crizanlizumab | Adakveo | Novartis | Rare | Prevention of sickle cell crises in sickle cell disease | 2021 | D | TA743 | [24] |
| Framenezumab | Ajovy | Teva | Control | Prevention of migraine | 2021 | D | TA764 | [25] |
NICE: National Institute for Health and Care Excellence; TA: Technology appraisal.
Data extraction
For each appraisal, the final appraisal document (FAD) was obtained from the NICE website. The following data were extracted into Microsoft Excel: generic name, brand name, manufacturer, indication under review, year of appraisal, NICE committee, date of UK marketing authorisation, date of submission to NICE, date(s) of appraisal committee meetings, date of FAD publication. We also extracted relevant content from the Committee Discussion section of the FAD for use in the thematic analysis.
Thematic analysis
A six-step reflexive thematic analysis based on the methods of Braun and Clarke [26] was carried out to identify any repeated patterns across the rare and non-rare disease appraisals. Initially, the lead author (GH) familiarised themselves with the content of the 12 FADs, extracted data relevant to the study objective, and manually generated initial codes using an inductive coding approach. The coded and extracted data were examined for potential themes, which were reviewed, defined and named by the lead author (GH). The themes were then discussed with all authors and finalised, allowing write up of the analysis to begin.
Appraisal timelines
For each appraisal, we analysed time to access (the number of days from UK marketing authorisation to the publication of final NICE guidance [27]), time in NICE (the number of days from the date of dossier submission to the publication of final NICE guidance [13]) and time between ACMs (the number of days between ACM1 and ACM2) where applicable.
Imposition of restrictions on population
For each appraisal, we captured whether the treatment had been recommended according to its full marketing authorisation or whether restrictions had been imposed on the population.
Results
Table 1 shows a summary of the 12 appraisals included in the analysis [14–25].
Thematic analysis
We identified three themes: ‘Handling of uncertainty and discretion’, ‘Application of NICE methods’ and ‘Commercial arrangements’. Figure 1 shows the themes and their associated codes, and their distribution across the 12 appraisals.
Figure 1. . Results of the thematic analysis.
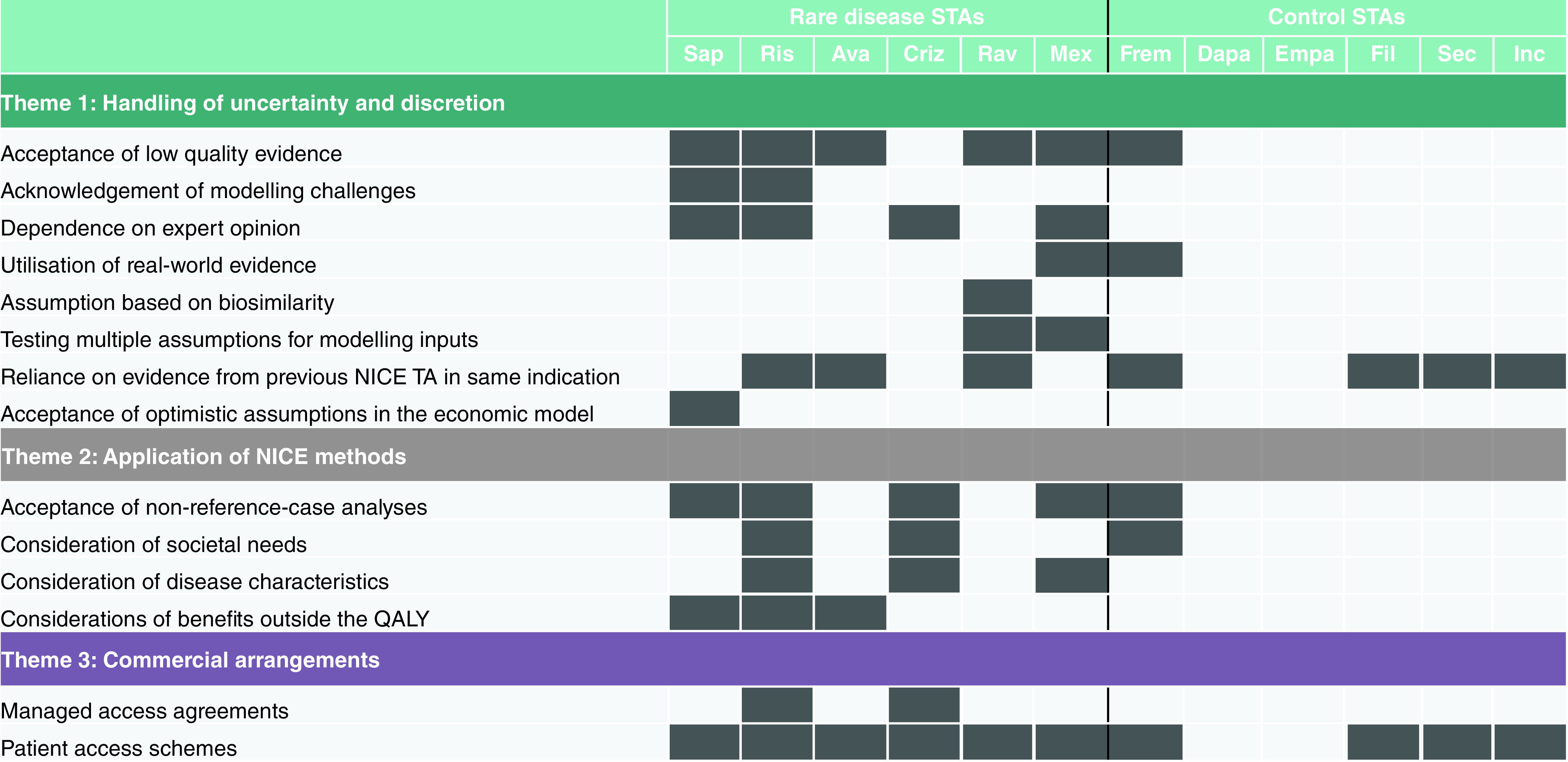
Ava: Avacopan; Criz: Crizanlizumab; Dapa: Dapagliflozin; Empa: Empagliflozin; Fil: Filgotinib; Frem: Fremanezumab; Inc: Inclisiran; Mex: Mexiletine; NICE: National Institute for Health and Care Excellence; QALY: Quality-adjusted life year; Rav: Ravulizumab; Ris: Risdiplam; Sap: Sapropterin; Sec: Secukinumab; STA: Single technology appraisal; TA: Technology appraisal.
Theme 1: Handling of uncertainty & discretion
This focuses on how the NICE Committees discuss evidence associated with uncertainty, and what approaches are taken to address uncertainty given the lack of available evidence. We found that at least one approach to address uncertainty was used in all six rare disease STAs, compared with four of the six control STAs. For the rare disease STAs, acceptance of low-quality evidence was the most common way of handling uncertainty (used in five of the six appraisals). For example, in their appraisal of mexiletine (TA748), the committee decided that in the absence of a burden of disease study, two alternative sources of evidence could be used to derive utility values, despite uncertainties with both sources: “In the absence of a burden of disease study, the committee considered both the vignette approach and the Statland et al. mapping in their decision making, and agreed that the utility increase from mexiletine would be somewhere between the values generated by these 2 approaches” [22].
For the control STAs, uncertainty was most commonly handled by reliance on evidence from previous appraisals within the same indication. For example, in their assessment of fremanazumab for preventing migraine (TA764), the Committee stated: “It is acknowledged that better day-to-day functioning overall would lead to increased quality of life and utility values, and that a similar rationale had been accepted by the committee in other appraisals” [25].
In four of the six rare disease appraisals, the Committee placed a degree of dependence on expert opinion to help their decision-making process; this was not evident in the control STAs. Similarly, the committees for the rare disease STAs acknowledged modelling changes and testing of multiple assumptions for modelling inputs, accepted assumptions based on biosimilarity, and accepted optimistic assumptions in the economic model.
Theme 2: Application of NICE methods
This focuses on how flexible the Committees are regarding adherence to, and interpretation of, the NICE methods guide, which is applied to ensure consistency between appraisals for different indications. We found that in five of the six rare disease appraisals, the Committee showed flexibility around the NICE methods guide, compared with two of the control STAs. The most common areas of flexibility were in ‘acceptance of non-reference case analyses’, ‘consideration of disease characteristics’ and ‘consideration of benefits outside the QALY’. For example, in their assessment of risdiplam for spinal muscular atrophy (TA755), the Committee acknowledged that the economic model did not produce plausible estimates of overall survival in order to identify whether the treatment met NICE's end-of-life criterion: “It noted that it usually assesses whether this criterion is met by referring to the mean survival predicted by the model. However, it accepted the limitations of the model in this case mean that estimates from the literature are more robust” [18]. In two of the rare disease appraisals, the rarity of the disease was acknowledged by the Committees: for risidiplam (TA755), “The committee acknowledged the difficulty of appraising drugs for very rare conditions…decision making took into account the rarity and severity of the disease” [18] and for mexiletine (TA748), “…the evidence base is necessarily weaker for some technologies, such as those used to treat rare diseases” [22].
Theme 3: Commercial arrangements
This focuses on the use of strategies to allow improvement in the value proposition in the presence of uncertainties in the evidence base that could not be resolved. Patient access schemes (PAS) are classed as simple (involving a fixed pricing agreement or percentage discount from the list price) or complex (such as outcomes-based agreements, dose caps, rebates, or upfront free stock) [28]. Managed access agreements (MAAs) can take a variety of forms, including agreements with subpopulation restrictions and real-world evidence collection [29]. MAAs can be complex, but have several advantages for manufacturers, including providing market access while allowing time to strengthen the evidence base for further reimbursement discussions [29]. We found that all rare disease STAs were associated with patient access schemes, compared with four of the six controls. Two of the rare disease STAs (risdiplam and crizanlizumab) were also associated with MAAs, compared with none of the controls. For risdiplam (TA755), the MAA was designed to allow collection of further data that would inform the cost–effectiveness results [18]. For crizanlizumab (TA743), the Committee stated that a MAA would allow the manufacturer to “…gather enough evidence on longer-term effectiveness…” [24].
Appraisal timelines
Figure 2 and Table 2 show the time to access and the time in NICE for the appraisals included in the analysis. The mean time to access was 118 days longer for rare disease STAs than for controls (504 days vs 386 days). The median time to access was 54.5 days longer for rare disease STAs (371 days; range 138 to 1079 days) than for controls (256.5 days; range 134 to 1042 days). Sapropterin had a time to access of 4677 days and was excluded from the analysis of time to access as an outlier (it was rejected for routine commissioning for over a decade after marketing authorisation before being appraised and recommended by NICE).
Figure 2. . Time to access and time in NICE.
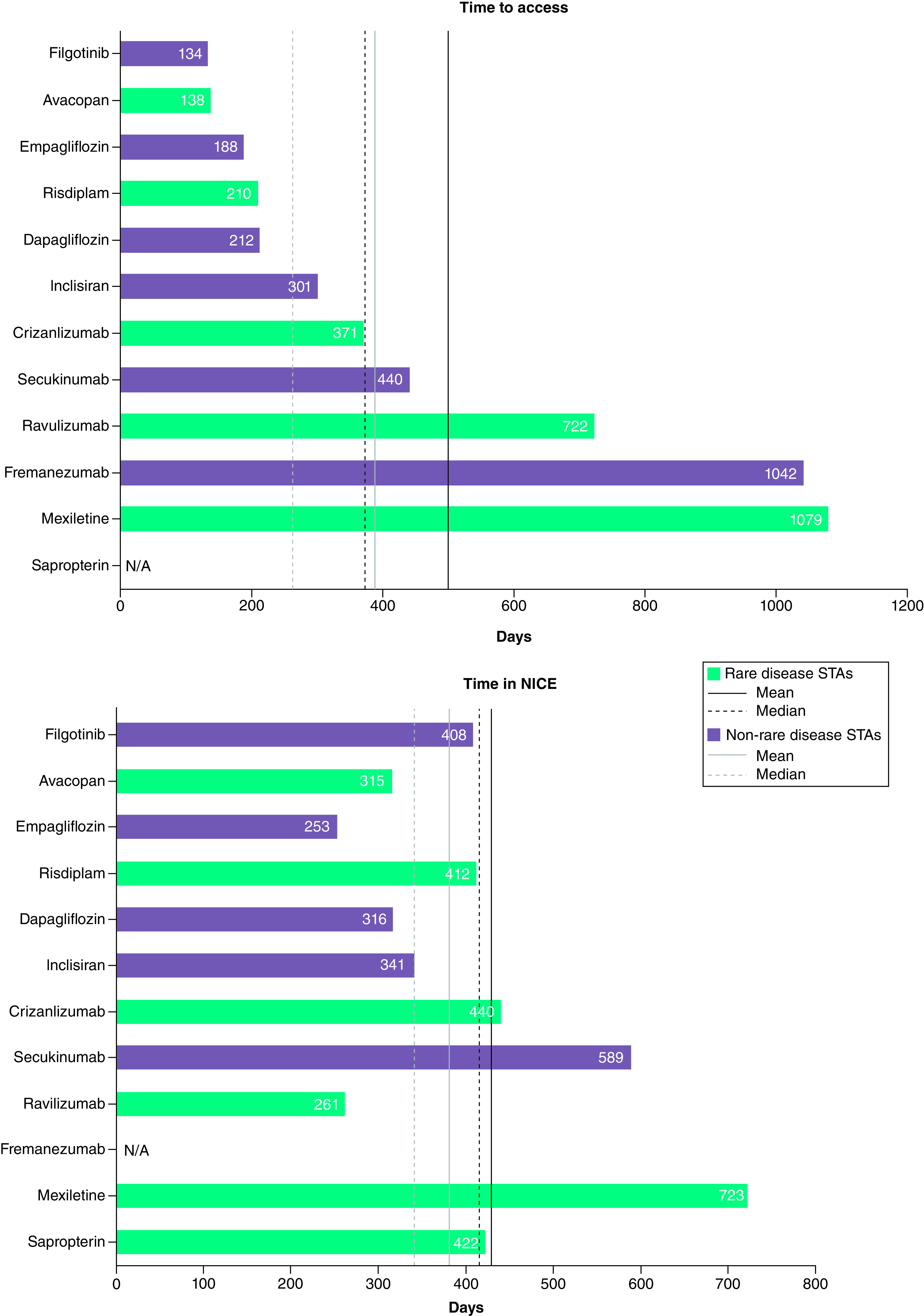
The time to access analysis does not include sapropterin, which was considered an anomaly with a time to access of 4677 days. The time in NICE analysis does not include fremanezumab, which was reappraised in a rapid review process (105 days).
NICE: National Institute for Health and Care Excellence; STA: Single technology appraisal.
Table 2. . Appraisal committee meetings.
| Technology | Category (rare/control) | Number of ACMs | Mean time between ACM1 and ACM2 (days) |
|---|---|---|---|
| Sapropterin | Rare | 3 | 64 |
| Secukinumab | Control | 2 | 65 |
| Avacopan | Rare | 2 | 63 |
| Filgotinib | Control | 1 | – |
| Risdiplam | Rare | 2 | 63 |
| Empagliflozin | Control | 1 | – |
| Ravulizumab | Rare | 1 | – |
| Inclisiran | Control | 1 | – |
| Mexiletine | Rare | 2 | 345 |
| Dapagliflozin | Control | 2 | 62 |
| Crizanlizumab | Rare | 2 | 252 |
| Fremanezumab | Control | 1 | – |
ACM: Appraisal committee meeting.
Two other treatments had a time to access of >1000 days: mexiletine (rare disease sample; TA748) and fremanezumab (control sample; TA764). For mexiletine, this was due, at least in part, to the extended time between ACM1 and ACM 2 (345 days), however the reason for the delay between meetings is unclear. For fremanezumab, the initial recommendation only covered the chronic migraine indication, with a non-recommendation in episodic migraine owing to uncertainties in the economic modelling with regards to stopping treatment and quality of life. NICE invited the manufacturer to submit additional evidence focusing on differential utilities after concluding that they should be included in the economic modelling of migraine [30]. It is likely that the time needed to gather this additional evidence contributed to the extended time to access. New final guidance (following a rapid review process) then recommended framenezumab for both chronic and episodic migraine [25].
The mean time in NICE was 48 days longer for rare disease STAs than for controls (429 days vs 381 days). The median time in NICE was 76 days longer for rare disease STAs (417 days; range 261 to 723 days) than for controls (341 days; range 253 to 589 days). Fremanezumab was excluded from the analysis of average time in NICE as it was re-appraised for episodic migraine in a rapid review process following its initial restricted recommendation in chronic migraine.
Rare disease STAs, on average, required more ACMs (2.0) than control STAs (1.3). The mean time between ACM1 and ACM2 was longer for rare disease STAs (157 days; range 62 to 345 days) than for controls (64 days; range 62 to 65 days).
Imposition of restrictions on population
In our sample, one rare disease STA (sapropterin; TA729) had a recommendation that covered only part of the marketing authorisation, compared with four of the control STAs (secukinumab [TA719], empagliflozin [TA773], inclisiran [TA733], dapagliflozin [TA775]) (Table 3). Two of these were subject to a commercial arrangement (sapropterin and inclisiran). All seven technologies (five rare and two control) that were recommended in line with their marketing authorisation were subject to a commercial arrangement.
Table 3. . Restrictions on recommended populations.
| Generic name | Category (rare/control) | Appraisal ID | Population in final scope | Recommended population in FAD | Ref. |
|---|---|---|---|---|---|
| Sapropterin | Rare | TA729 | People with PKU whose HPA has been shown to be responsive to sapropterin dihydrochloride therapy | Option for treating hyperphenylalaninaemia that responds to sapropterin (response as defined in the summary of product characteristics) in people with PKU, only if they are under 18 and a dose of 10 mg/kg is used, only using a higher dose if target blood phenylalanine levels cannot be achieved at 10 mg/kg, or aged 18 to 21 inclusive, continuing the dose they were having before turning 18 or at a maximum dose of 10 mg/kg, or are pregnant (from a positive pregnancy test until birth). Sapropterin is recommended only if the company provides it according to the commercial arrangement | [14] |
| Secukinumab | Control | TA719 | People with non-radiographic axial spondyloarthritis with objective signs of inflammation, whose disease has responded inadequately to, or who are intolerant to, NSAIDs | Option for treating active non-radiographic axial spondyloarthritis with objective signs of inflammation (shown by elevated C-reactive protein or MRI) that is not controlled well enough with NSAIDs in adults. It is recommended only if TNF-alpha inhibitors are not suitable or do not control the condition well enough and the company provides secukinumab according to the commercial arrangement | [15] |
| Empagliflozin | Control | TA773 | Adults for the treatment of symptomatic chronic heart failure with reduced ejection fraction | Option for treating symptomatic chronic heart failure with reduced ejection fraction in adults, only if it is used as an add-on to optimised standard care with an ACE inhibitor or ARB, with a beta blocker and, if tolerated, an MRA, or sacubitril valsartan with a beta blocker and, if tolerated, an MRA | [19] |
| Incisiran | Control | TA733 |
Primary prevention population - Adults who are primary prevention with elevated risk with serum LDL-C ≥2.6 mmol/L despite maximally tolerated statins or adults with a history of HeFH without ASCVD and serum LDL-C ≥2.6 mmol/L despite maximally tolerated statins. Secondary prevention population - Adults with ASCVD (including HeFH) and serum LDL-C ≥2.6 mmol/L despite maximally tolerated statins |
Option for treating primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia as an adjunct to diet in adults. It is recommended only if there is a history of certain cardiovascular events and LDL-C concentrations are persistently 2.6 mmol/l or more, despite maximum tolerated lipid-lowering therapy, that is maximum tolerated statins with or without other lipid-lowering therapies other lipid-lowering therapies when statins are not tolerated or are contraindicated, and the company provides inclisiran according to the commercial arrangement | [21] |
| Dapagliflozin | Control | TA775 | Adults with CKD who are receiving individually optimised standard care | Option for treating CKD in adults. It is recommended only if it is an add-on to optimised standard care including the highest tolerated licensed dose of ACE inhibitors or ARBs, unless these are contraindicated, and people have an eGFR of 25 ml/min/1.73 m2 to 75 ml/min/1.73 m2 at the start of treatment and have type 2 diabetes or have a uACR of 22.6 mg/mmol or more | [23] |
ACE: Angiotensin-converting enzyme; ARB: Angiotensin-receptor blocker; ASCVD: Atherosclerotic cardiovascular disease; CKD: Chronic kidney disease; eGFR: Estimated glomerular filtration rate; HeFH: Heterozygous familial hypercholesterolaemia; HPA: Hyperphenylalaninaemia; LDL-C: Low-density lipoprotein cholesterol; MRA: Mineralocorticoid receptor antagonist; MRI: Magnetic resonance imaging; NSAIDs: Non-steroidal anti-inflammatory drugs; PKU: Phenylketonuria; TNF: Tumour necrosis factor; uACR: Urine albumin-to-creatinine ratio.
Discussion
Our analysis of published NICE documentation suggests that NICE appraisal committees may use a degree of flexibility and pragmatism when assessing the uncertainties associated with the evidence base for rare diseases appraised via the STA route. A previous analysis showed that the majority of rare disease technologies appraised via the STA route between 2018 and 2021 received a positive recommendation from NICE [31], which implies that the STA route is suitable for appraisal of rare disease technologies. However, upon closer inspection, this process is offset by the length of time required to deliver a successful outcome. Discussions between NICE and manufacturers can be protracted and demonstrating cost–effectiveness is challenging within the constraints of the £20–30,000 ICER threshold. As a result, manufacturers may concede to a less favourable pricing agreement, or narrow their target patient population, in order to meet the cost–effectiveness threshold and achieve a positive recommendation from NICE. The lengthy discussions between stakeholders prevent decisions from being made in a timely manner and can ultimately delay access to treatment for patients.
There was no clear pattern in the application of flexibility across the rare disease appraisals regarding the themes of ‘Handling of uncertainty and discretion’ and ‘Application of NICE methods’. In the case of ‘Handling of uncertainty and discretion’, application of flexibility was inconsistent across the appraisals because of differences in the diseases and the level of evidence submitted. Similarly, inconsistency in the ‘Application of NICE methods’ could be linked to the level of evidence submitted by the manufacturer: the greater the uncertainty in the evidence, the more likely it is that the Committee will need to take other factors, such as rarity, into consideration. Therefore, when solely considering the narrative of the published FAD, it appears that NICE Committees do provide a degree of flexibility for rare disease technologies appraised via the STA pathway. However, the flexibility applied is still limiting for rare diseases appraised through STA.
Within our sample of STAs, we found no evidence to suggest that one appraisal Committee showed more flexibility than another, nor that their final decision was dictated by the Evidence Review Group. Similarly, McCann & Plested (2012) found that the likelihood of a positive or negative recommendation did not vary by the committee assessing the submission [32]. We also found no evidence that rare disease medicines were more likely to receive a restricted recommendation in the STA process than non-rare disease medicines. This is consistent with the findings of Clarke et al. (2021) [13]. In contrast, a previous analysis found that between 2013 and 2017, half of the rare disease medicines that completed STA review were given a restricted recommendation, compared with around one-fifth of non-rare disease medicines [33]. In the same period, only 13% of rare disease medicines were recommended for the full licensed population, compared with more than two-thirds of non-rare disease medicines [33].
It is important to note that our analysis and conclusions are based on information available in the public domain. This gives an indication of how NICE applies flexibility, but does not reveal the full picture; there may have been other factors and confidential aspects considered during the dialogue between NICE and the manufacturers that were not noted in the appraisal consultation documents and committee papers. These details are not frequently published, however there are several high-profile examples of rare disease manufacturers who have shared their experiences about the protracted and difficult negotiations encountered with NICE (Box 1). These case studies highlight the complexity of the STA process with rare disease technologies and how the achievement of a positive recommendation can be fraught with challenges, which require significant time and stakeholder input to resolve.
Box 1. Case studies of rare disease treatments appraised via the STA route.
Case study 1: sapropterin for treating hyperphenylalaninaemia in PKU – access delayed and patient population heavily restricted. BioMarin was invited to submit sapropterin for appraisal in October 2018, but initially refused after the NICE topic selection panel determined the STA route was most appropriate, stating that the process was a “waste of time and money” [34]. The NICE routing panel determined that the target population was estimated to be larger, and the condition was chronic but not severely disabling given that children could be managed with a controlled diet, and thus was not eligible for appraisal via the HST programme [9]. In response to this, BioMarin stated that the “burden and severity of PKU as a disease in the UK is not recognized by NICE or the NHS” [35]. In April 2020, BioMarin agreed to re-engage with the process following pressure from the patient community. Following three ACMs, sapropterin was finally recommended as an option for treating hyperphenylalaninaemia that responds to sapropterin in patients with PKU by NICE, albeit in a heavily restricted population. In addition, BioMarin also had to concede to an 80% discount on the list price to meet the cost–effectiveness criteria outlined in the STA appraisal process [36]. Thus, given the constraints of the STA pathway, demonstrating cost–effectiveness proved incredibly difficult for BioMarin.
Case study 2: nusinersen for treating spinal muscular atrophy – access delayed by protracted decision making. NICE began assessing nusinersen after an 8-month delay in the decision regarding whether it would consider the treatment via the STA or HST process [37]. Although Biogen argued that it was unlikely that an STA could capture the full clinical benefit for patients with SMA who would be suitable for treatment with nusinersen [38], NICE believed that this pathway was most appropriate. After an initial non-recommendation, Biogen once again expressed reservations about the suitability of the STA pathway for evaluating medicines such as nusinersen, stating that “the decision and the lengthy timeframe of the whole process highlights the UK challenge in providing access to rare disease medicines in a timely manner, similar to other leading economies” [37]. At ACM1, the committee found that the NHS would have to pay £400,000 - £600,000 per QALY gained to be considered cost-effective based on the drug's list price, and that “even with a proposed confidential price reduction, the cost of nusinersen is too high for it to be considered a cost-effective use of NHS resources” [39]. Acknowledging the constraints imposed by the STA route given the high cost of treatment, Biogen began working with NICE and NHS England to discuss a managed access agreement (MAA) framework to make nusinersen available while continuing to gather further data [37]. At ACM2, an updated managed access proposal and economic model was insufficient to overrule the committee's initial decision. Further updates to these were required before NICE issued a positive recommendation [40].
Case study 3: betibeglogene autotemcel for treating transfusion-dependent beta-thalassaemia – difficult negotiations leading to withdrawal of product from Europe. NICE routed bluebird bio's betibeglogene autotemcel, with a UK list price of £1.45 million, via the STA pathway. Protracted discussions had taken place between bluebird bio, NICE and NHS England regarding the suitability of the STA pathway as opposed to a HST appraisal. In a statement, bluebird bio stated that they were concerned about the treatment being evaluated through STA but reluctantly accepted the perspective of NICE, hoping that the committee would apply “appropriate discretion to the complexity of the disease area, small trial populations and management of uncertainty” [41]. Unsurprisingly, given the high-list price of betibeglogene autotemcel, the most plausible ICER, using the committee's preferred assumptions, was considerably higher than the £30,000 QALY threshold [42]. Betibeglogene autotemcel was not recommended by NICE in draft guidance [43]. The appraisal was suspended shortly after ACM2 when bluebird bio withdrew its operations from Europe, citing the challenges of “achieving appropriate value recognition and market access” for its treatment in the UK and the rest of Europe [44].
We analysed topics that were assessed before NICE implemented its methods and process update in February 2022 [45]. The update was intended to allow more flexibility for Committees when evidence generation is difficult and provide clearer inclusion criteria for the HST route. It was also designed to give additional weight to health benefits in the most severe conditions using a severity modifier that considers two measures of disease severity: the absolute QALY shortfall (the number of future QALYs that are lost by people living with the disease) and proportional QALY shortfall (the proportion of future QALYs that are lost by people living with the disease). An analysis of three orphan medicine STAs showed that if these had been appraised using the severity modifier, the recommendations would not have been affected, but by using alternative methods (based on achievement of an incremental cost–effectiveness ratio below standard thresholds), the appraisals would have been considerably shorter [46]. The authors concluded that the updated NICE processes are unlikely to result in faster reimbursement of orphan medicines [46]. Uncertainty about the impact of the severity modifier and the fact that the discount rate remained unchanged means that the analysis of topics before the methods and process update should not confound the outcomes of our research. We look forward to reviewing the impact of the methods and process update on rare disease appraisals.
It is also important to note that there is a second decision modifier in the HST process: size of benefit. This considers the size of the incremental QALY gain in relation to the additional weight that would need to be assigned to the QALY benefits for cost–effectiveness to fall within £100,000 per QALY. Rare diseases routing to STA will miss out on this.
The inclusion of rarity as a modifier in NICE's decision making has previously been considered on the grounds that there may be a moral and ethical justification for applying a greater weight when an unmet need or health inequality is a result of the disease being rare [47]. However, the decision was taken not to include rarity as the evidence strongly suggested that the public do not consider it to be an important modifier. In addition, it was argued that certain characteristics of rare diseases (such as burden of illness, severity, age of the population and the desire to reduce health inequality) can be captured in other modifiers.
The current landscape for bringing new drugs to market in the UK is troubling. A 2022 report from the Association of the British Pharmaceutical Industry (ABPI) revealed that the number of clinical trials initiated in the UK fell by 41% between 2017 and 2021 [48]. This prompted the initiation of an independent review by the government into the UK commercial clinical trials landscape to identify the key steps needed to rebuild competitiveness in this area [49]. Reduced access to clinical trials has serious implications for patients, particularly those with rare diseases who have limited treatment options via routine care [48]. The number of MHRA approvals of new medicines has also fallen in recent years [50]. The Voluntary Scheme for Branded Medicines Pricing and Access (VPAS) was established in 2019 with the intention of supporting innovation in the life sciences industry and improving access to the most effective and best value medicines, while keeping NHS expenditure on branded medicines affordable [51]. Members of the scheme make payments, which are calculated by adding a payment percentage to their eligible sales. However, the payment percentage rates have rapidly escalated, being set at 26.5% for 2023. A survey conducted in 2022 by the Ethical Medicines Interest Group (EMIG) showed the potential impact of VPAS rebate increases on UK pharmaceutical investment: 50% of respondents said they will reduce supply of medicines to the UK and a further 40% are considering a reduction [52]. More than 75% of respondents predicted a 10–20% reduction in investment in UK clinical research [52]. In June 2022, NHS England launched the Innovative Medicines Fund (IMF), a £340 million programme designed to provide managed access to innovative, non-oncology medicines not recommended for routine reimbursement. Eligible technologies with the potential to address a high unmet need must be associated with a significant uncertainty surrounding the cost–effectiveness results which can be addressed with further evidence generation in the 2 to 3 years post-evaluation [53]. Unfortunately, since the programme became operational, not a single medicine has been put through for reimbursement consideration, which is a concern. Although industry is generally in support of the IMF, there is significant consternation among companies regarding the commercial risks of participating in the process [54] with one company stating that ‘it is highly probable that many companies would opt to disengage from the IMF process and could reconsider launching specific therapies in the UK entirely’ [55]. With these factors, and the protracted NICE STA appraisal process for drugs that do make it through trials and regulatory approval, it is not surprising that some manufacturers of drugs for rare diseases are looking to downscale their UK activities.
In 2022, NICE announced that it is developing a proportionate approach to technology appraisals, which is designed to increase capacity and allow NICE to produce more guidance [56]. Medicines that are deemed not to need a full appraisal will go through a simpler, faster process, allowing NICE flexibility to allocate its limited resources and to take a more pragmatic approach where appropriate. We hope that this in turn will allow more timely and efficient reviews of medicines for rare diseases and will address the concerns of manufacturers, patients and those who care for and treat them.
Limitations
Our analysis had a number of limitations. Our sample size was smaller than previous analyses [13,27]. Nevertheless, our observations on longer timelines for rare disease STAs are consistent with these previous reports. Unlike the analysis by Clarke et al. 2019 [13], we excluded appraisals of oncology medicines. However, our results are consistent with those of Clarke et al., showing a longer time in NICE and a requirement for more committee meetings for rare disease STAs compared with non-rare disease STAs. Data extraction was carried out by a single author, which may have introduced some bias. Finally, we only included appraisals that received draft guidance after 2021; it is possible that any delays were, at least in part, due to the COVID-19 pandemic.
Commercial arrangements were identified as a theme, but these are commonplace with treatments for rare diseases, which are typically associated with high acquisition costs. This theme could be explained by NHS England's requirement for the cost-effective use of resources, rather than by any flexibility shown because the disease is rare. An analysis of 47 orphan drugs for chronic (non-oncology) conditions appraised by NICE found that 90% were subject to at least one type of commercial arrangement [29]. A survey of ABPI member companies showed that in a sample of 30 terminated NICE appraisals from 2016 to 2021 (15 of which were for orphan or ultra-orphan drugs), 34% were terminated owing to a stated lack of flexibility to pursue differential net pricing or complex schemes, with consequent risk to previously approved indications [57].
Conclusion
Despite NICE showing a degree of flexibility and pragmatism toward uncertainties in the evidence base, rare disease technologies are subjected to a lengthy appraisal process through the standard STA pathway. These delays can unfairly disadvantage patients with rare conditions. NICE and manufacturers should work together to find ways to shorten the appraisal process and to allow patients faster access to treatments for rare diseases.
Summary points
Despite the existence of a process for the evaluation of highly specialized technologies, many medicines for rare diseases are routed through NICE's standard single technology appraisal (STA) process. NICE states that there is no absolute limit on the number of technologies that can be routed through highly specialized technology (HST), however the criteria for HST are highly selective and many rare disease topics fail to meet them.
However, rare disease medicines find it more challenging to meet the cost–effectiveness requirements of the STA process due to the complexities of the disease and the limited evidence base, which can generate large amounts of clinical and economic uncertainty.
We explored whether NICE takes rarity into account and shows flexibility and pragmatism when evaluating medicines for rare disease via the STA process.
Six STAs for rare disease treatments that received draft guidance after 1 January 2021 were randomly selected and matched with six STAs for non-rare diseases according to year of final appraisal committee meeting and NICE appraisal committee (appraisals in oncology and acute conditions were excluded).
A six-step thematic analysis was carried out to identify any repeated patterns in NICE decision making across the 12 appraisals.
We also analysed time to access (time from marketing authorisation to publication of final NICE guidance), time in NICE (time from dossier submission to publication of NICE guidance) and time between appraisal committee meetings.
We identified three themes related to NICE decision making, where flexibility had been shown by the committees: ‘handling of uncertainty and discretion’, ‘application of NICE methods’ and ‘commercial arrangements’.
Our results suggest that NICE shows a degree of flexibility and pragmatism when assessing treatments for rare diseases via the STA route.
However, treatments for rare diseases that are routed through STA often experience a protracted decision process.
NICE and manufacturers need to work together to shorten the STA process and allow patients faster access to treatments for rare diseases.
The implementation of the updated NICE methods should be reviewed in the future to understand its applicability and usability to address uncertainties in rare diseases, and to decipher whether STA routing is fit for purpose for rare disease technologies.
Acknowledgments
The authors thank L McNamara and K Lock (both formerly of Kyowa Kirin International plc) for their helpful insights in interpretation of the data and reviewing the manuscript.
Footnotes
Author contributions
G Hale: design of the work; acquisition, analysis and interpretation of the data; revising the work for important intellectual content; final approval. J Morris: design of the work; interpretation of the data; revising the work for important intellectual content; final approval. J Barker-Yip: acquisition and interpretation of the data; revising the work for important intellectual content; final approval.
Financial disclosure
Kyowa Kirin International plc provided funding for this research. Writing and editorial assistance was provided by Joanna Todd (Cogentia Healthcare Consulting Ltd); this was funded by Kyowa Kirin International plc. The authors have received no other financial and/or material support for this research or the creation of this work apart from that disclosed.
Competing interests disclosure
G Hale, J Morris and J Barker-Yip are employees of Cogentia Healthcare Consulting Ltd, which received consulting fees from Kyowa Kirin International plc to perform the research described in this manuscript. The authors have no other competing interests or relevant affiliations with any organization/entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance wasutilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Department of Health and Social Care. The UK rare diseases framework (2021). https://www.gov.uk/government/publications/uk-rare-diseases-framework/the-uk-rare-diseases-framework#introduction.
- 2.Department of Health. The UK strategy for rare diseases (2013). https://www.gov.uk/government/publications/rare-diseases-strategy
- 3.Department of Health and Social Care. England rare diseases action plan 2023: main report (2023). https://www.gov.uk/government/publications/england-rare-diseases-action-plan-2023/england-rare-diseases-action-plan-2023-main-report
- 4.Mellerio JE. The challenges of clinical trials in rare diseases. Br. J. Dermatol. 187(4), 453–454 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Drummond MF, Wilson DA, Kanavos P, Ubel P, Rovira J. Assessing the economic challenges posed by orphan drugs (2007). https://www.cambridge.org/core/journals/international-journal-of-technology-assessment-in-health-care/article/abs/assessing-the-economic-challenges-posed-by-orphan-drugs/9279ADA1E6557B1C6CAF8242A53F96DC [DOI] [PubMed]
- 6.National Institute for Health and Care Excellence. NICE health technology evaluation topic selection: the manual (2022). https://www.nice.org.uk/process/pmg37/resources/nice-health-technology-evaluation-topic-selection-the-manual-pdf-72286780924357
- 7.UK Parliament (Hansard). NICE appraisals: rare disease treatments. (2019). https://hansard.parliament.uk/Commons/2019-03-21/debates/735963B4-2640-4CB1-98CF-290E2736796B/NICEAppraisalsRareDiseasesTreatments
- 8.National Institute for Health and Care Excellence. Risdiplam for treating spinal muscular atrophy. Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) (2020). https://www.nice.org.uk/guidance/ta755/documents/scope-consultation-comments-and-responses
- 9.National Institute for Health and Care Excellence. Sapropterin for treating phenylketonuria. Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) (2018). https://www.nice.org.uk/guidance/ta729/documents/scope-consultation-comments-and-responses
- 10.National Institute for Health and Care Excellence. Mexiletine for treating symptomatic myotonia in adults with non-dystrophic myotonic disorders. Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) (2019). https://www.nice.org.uk/guidance/ta748/documents/scope-consultation-comments-and-responses
- 11.Rare Disease UK. Illuminating the rare reality (2019). https://www.raredisease.org.uk/wp-content/uploads/sites/7/2019/02/Illuminating-the-rare-reality-2019.pdf
- 12.Rare Disease UK. All party parliamentary group hearing on access to medicines in England (2016). https://www.raredisease.org.uk/our-work/coordination-of-care/all-party-parliamentary-group-hearing-on-access-to-medicines-in-england/
- 13.Clarke S, Ellis M, Brownrigg J. The impact of rarity in NICE's health technology appraisals. Orphanet. J. Rare. Dis. 16(1), 218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Analysis of STA appraisals of orphan and non-orphan medicines
- 14.National Institute for Health and Care Excellence. Sapropterin for treating phenylketonuria [TA729] (2021). https://www.nice.org.uk/guidance/ta729
- 15.National Institute for Health and Care Excellence. Secukinumab for treating non-radiological axial spondyloarthritis [TA719] (2021). https://www.nice.org.uk/guidance/ta719
- 16.National Institute for Health and Care Excellence. Avacopan for treating severe active granulomatosis with polyangiitis or microscopic polyangiitis [TA825] (2022). https://www.nice.org.uk/guidance/ta825
- 17.National Institute for Health and Care Excellence. Filgotinib for treating moderately to severely active ulcerative colitis [TA792] (2022). https://www.nice.org.uk/guidance/ta792
- 18.National Institute for Health and Care Excellence. Risdiplam for treating spinal muscular atrophy [TA755] (2021). https://www.nice.org.uk/guidance/ta755
- 19.National Institute for Health and Care Excellence. Empagliflozin for treating chronic heart failure with reduced injection fraction [TA773] (2022). https://www.nice.org.uk/guidance/ta773
- 20.National Institute for Health and Care Excellence. Ravulizumab for treating atypical haemolytic uraemic syndrome [TA710] (2021). https://www.nice.org.uk/guidance/ta710
- 21.National Institute for Health and Care Excellence. Inclisiran for treating primary hypercholesterolaemia or mixed dyslipidaemia [TA733] (2021). https://www.nice.org.uk/guidance/ta733
- 22.National Institute for Health and Care Excellence. Mexiletine for treating symptomatic myotonia in adults with non-dystrophic myotonic disorders [TA748] (2021). https://www.nice.org.uk/guidance/ta748
- 23.National Institute for Health and Care Excellence. Dapagliflozin for treating chronic kidney disease [TA775] (2022). https://www.nice.org.uk/guidance/ta775
- 24.National Institute for Health and Care Excellence. Crizanlizumab for preventing sickle cell crises in sickle cell disease [TA743] (2021). https://www.nice.org.uk/guidance/ta743
- 25.National Institute for Health and Care Excellence. Fremanezumab for preventing migraine [TA764] (2022). https://www.nice.org.uk/guidance/ta764
- 26.Braun V, Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 3(2), 77–101 (2006). [Google Scholar]; • Describes the methods of the six-step thematic analysis
- 27.Trim J, Nair M, Large S. Uncovering the hidden rare disease gap within NICE appraisals. Value. Health. 25(12), S343 (2022). [Google Scholar]; • Analysis of STA appraisals of orphan and non-orphan medicines
- 28.National Institute for Health and Care Excellence. Patient Access Schemes Liaison Unit. (2023). https://www.nice.org.uk/about/what-we-do/patient-access-schemes-liaison-unit
- 29.Pharmaphorum. How does rare disease prevalence impact drug pricing in England? (2022). https://pharmaphorum.com/market-access-2/how-does-rare-disease-prevalence-impact-drug-pricing-england/
- 30.National Institute for Health and Care Excellence. Fremanezumab for preventing migraine (rapid review of TA631 [ID3952]). Committee papers (2021). https://www.nice.org.uk/guidance/ta764/documents/committee-papers-3
- 31.Foxon G, Craddy P, Wilson C. Does orphan drug status confer any benefits for products undergoing HTA appraisal by NICE in England? Value. Health. 25(1), S180 (2022). [Google Scholar]
- 32.McCann E, Plested M. Exploring the role of the committee in the NICE appraisal process: how consistent are decisions across committees? Value. Health. 15(7), A318 (2012). [Google Scholar]
- 33.McConaghie A. ‘Inflexible’ NICE blocking access to rare disease drugs (2019). https://www.pmlive.com/pharma_news/inflexible_nice_blocking_access_to_rare_disease_drugs_1278747
- 34.Bryson R. Cait Cotter loses High Court battle for life-changing drug (2020). https://www.gazette-news.co.uk/news/18271320.cait-cotter-loses-high-court-battle-life-changing-drug
- 35.Cohen D. MPs call for ‘life-changing’ Kuvan to be made affordable (2019). https://www.bbc.co.uk/news/health-48218737
- 36.Cohen D. UK patients who tested Kuvan for phenylketonuria will get supplies after trials end, says company. BMJ 365, l1874 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Spinal News International. NICE draft guidance ‘minded no’ for access to Spinraza for SMA treatment disappoints Biogen (2018). https://spinalnewsinternational.com/nice-spinraza-biogen/
- 38.National Institute for Health and Care Excellence. Nusinersen for treating spinal muscular atrophy. Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) (2018). https://www.nice.org.uk/guidance/ta588/documents/scope-consultation-comments-and-responses
- 39.Luxner L. SMA groups outraged over UK rejection of Spinraza coverage as too expensive (2018). https://smanewstoday.com/news/sma-groups-outraged-over-uk-rejection-of-spinraza-coverage/
- 40.National Institute for Health and Care Excellence. Nusinersen for treating spinal muscular atrophy. Chair's presentation. 2nd appraisal committee meeting (2018). https://www.nice.org.uk/guidance/ta588/documents/1-2
- 41.National Institute for Health and Care Excellence. Zynteglo for treating transfusion-dependent beta-thalassaemia. Consultation comments on the draft scope (2019). https://www.nice.org.uk/guidance/gid-ta10334/documents/scope-consultation-comments-and-responses
- 42.Lightning Health. NICE rejects gene therapy Zynteglo (2021). https://www.lightning.health/news/nice-rejects-gene-therapy-zynteglo/
- 43.National Institute for Health and Care Excellence. Betibeglogene autotemcel for treating transfusion-dependent beta-thalassaemia [ID968] (2022). https://www.nice.org.uk/guidance/discontinued/gid-ta10334
- 44.Dunleavy K. With the pricing situation ‘untenable’ in Europe, bluebird will wind down its operations in the ‘broken’ market (2021). https://www.fiercepharma.com/pharma/situation-untenable-bluebird-will-wind-down-its-operations-broken-europe
- 45.National Institute for Health and Care Excellence. NICE publishes new combined methods and processes manual and topic selection manual for its health technology evaluation programmes (2022). https://www.nice.org.uk/news/article/nice-publishes-new-combined-methods-and-processes-manual-and-topic-selection-manual-for-its-health-technology-evaluation-programmes
- 46.Lee D, McCarthy G, Saeed O, Allen R, Malottki K, Chandler F. The challenge for orphan drugs remains: three case studies demonstrating the impact of changes to NICE methods and processes and alternative mechanisms to value orphan products. Pharmacoecon. Open. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Analysis of the impact of the 2022 NICE methods and process update on evaluation of orphan medicines.
- 47.National Institute for Health and Care Excellence. CHTE methods review. Modifiers. Task and finish group report (2020). https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/nice-guidance/chte-methods-consultation/Modifiers-task-and-finish-group-report.docx
- 48.Association of the British Pharmaceutical Industry. Rescuing patient access to industry clinical trials in the UK (2022). https://www.abpi.org.uk/publications/rescuing-the-uk-industry-clinical-trials/
- 49.Office for Life Sciences. Lord O'Shaughnessy to lead independent review into UK clinical trials (2023). https://www.gov.uk/government/news/lord-oshaughnessy-to-lead-independent-review-into-uk-clinical-trials
- 50.Kollewe J. UK approved fewer new drugs than EU and US in year after Brexit transition (2022). https://www.theguardian.com/business/2022/jul/14/uk-approved-fewer-new-drugs-eu-us-year-after-brexit-transition
- 51.Department of Health and Social Care. Policy paper: voluntary scheme for branded medicines pricing and access (2018). https://www.gov.uk/government/publications/voluntary-scheme-for-branded-medicines-pricing-and-access
- 52.CSL. EMIG surveys member investment intentions related to 2023 VPAS rebate level (2022). https://www.csl-uk.com/emig-surveys-member-investment-intentions-related-to-2023-vpas-rebate-level/
- 53.NHS England. The Innovative Medicines Fund principles (2022). https://www.england.nhs.uk/wp-content/uploads/2022/06/B1686-the-innovate-medicines-fund-principles-june-2022.pdf
- 54.Association of the British Pharmaceutical Industry. The Innovative Medicines Fund - good news for patients but has an opportunity been missed to be more ambitious? (2022). https://www.abpi.org.uk/media/blogs/2022/june/the-innovative-medicines-fund-good-news-for-patients-but-has-an-opportunity-been-missed-to-be-more-ambitious/
- 55.IPSEN. IPSEN's response to the Innovative Medicines Fund Consultation (2022). https://www.ipsen.com/uk-ireland/ipsens-response-to-the-innovative-medicines-fund-consultation/
- 56.National Institute for Health and Care Excellence. Taking a proportionate approach to technology appraisals (2022). https://www.nice.org.uk/about/what-we-do/proportionate-approach-to-technology-appraisals
- 57.Association of the British Pharmaceutical Industry. ABPI member survey: Reasons for NICE ‘optimised’ recommendations and terminated appraisals (2021). https://www.abpi.org.uk/media/udnpxhjx/abpi-member-survey_nice-optimised-and-terminated-appraisals.pdf


