Abstract
The patent expiry of Humira® in 2018 opened up the current European market to eight adalimumab biosimilars – (in alphabetical order) Amgevita®, Amsparity®, Hulio®, Hukyndra®, Hyrimoz®, Idacio®, Imraldi® and Yuflyma® – for the treatment of various immune and inflammatory conditions. Amjevita, Hadlima®, Hyrimoz and Yuflyma have recently become available in the USA, with others expected to reach this market in 2023 as the US patent protection for Humira ends. Although adalimumab biosimilars demonstrate efficacy, safety and immunogenicity similar to the originator, they may differ in product excipient(s) and preservatives, along with their device type(s). Physicians may find it both difficult and time consuming to navigate their way among the array of available adalimumab biosimilars when they need to make a treatment decision. This article explores the characteristics of various adalimumab biosimilars to help clinicians navigate the various options available across Europe and the USA. In addition to drug selection, effective patient–physician communication is needed to nurture realistic patient expectations and minimise potential nocebo effects when prescribing biosimilars.
Keywords: adalimumab, biosimilar, device, dose, efficacy, formulation, nocebo, patient expectations, patient–physician communication, safety
Plain language summary
What is this article about?
This article explored the characteristics of various adalimumab biosimilars to help clinicians navigate the various options available across Europe and the USA.
What were the results?
Adalimumab biosimilars may differ between each other in product excipient(s), preservatives and device type(s). Selection of a suitable biosimilar should be supported by any characteristics which may support treatment adherence/satisfaction on a patient case-by-case basis. The use of effective patient–physician communication and shared patient–physician decision making should also be considered to support optimal therapeutic outcomes and persistence with treatment.
What do the results mean?
Careful evaluation of the characteristics of different adalimumab biosimilars could help the prescribing physician to choose the product best suited to their patient.
Adalimumab is a fully human monoclonal antibody directed against tumour necrosis factor-alpha (TNF) which is administered subcutaneously [1]. When bound to adalimumab, TNF can no longer interact with its receptors on the cell surface, thus blocking the immune and inflammatory response. Humira® was the first commercially available formulation of adalimumab with a US marketing authorisation granted in 2003, followed by European approval in the same year [2–5]. Humira indications include inflammatory bowel diseases (IBD: Crohn's disease and ulcerative colitis), chronic inflammatory rheumatism (rheumatoid arthritis [RA], non-radiographic axial spondyloarthritis, ankylosing spondylitis, psoriatic arthritis and juvenile idiopathic arthritis), hidradenitis suppurativa, skin psoriasis, and non-infectious intermediate, posterior, and diffuse (pan) uveitis. Due to its widespread indications and high cost, Humira has previously been described as the world's most lucrative drug, with sales exceeding US$20.7 billion in 2021 [6].
In 2018, patent expiry of Humira opened up the market to its biosimilars, whose competitive entry has contributed to lowering adalimumab prices [2]. By the end of 2019, approximately 35% of European patients had been switched from Humira to an adalimumab biosimilar [2]. However, the increasing availability of adalimumab biosimilars from different manufacturers is making the treatment selection a more complex task for physicians. Although adalimumab biosimilars demonstrate similar efficacy, safety and immunogenicity to the originator, they may differ in product excipient(s) and preservatives, along with their device type(s). The physician may find it both difficult and time consuming to navigate their way among the array of available adalimumab biosimilars when they need to make a treatment decision. Likewise, while patients may find the concept of biosimilars difficult to understand [7,8], the array of differing adalimumab biosimilar options may also provide an additional level of confusion.
The aim of this article is to explore the characteristics of various adalimumab biosimilars to help clinicians navigate the various options available across Europe and the USA.
Methods
For this narrative review, the PubMed database was searched for relevant articles using the search terms ‘adalimumab’ and ‘biosimilar’, as well as the specific names of adalimumab biosimilars approved in Europe and the USA (Table 1). Additional articles were identified by ad hoc searching and review of the bibliographies of identified papers. Selection of articles for inclusion was based on their relevance.
Table 1. . Characteristics of Humira® formulations and adalimumab biosimilars.
| Humira® | Humira® (citrate free) | Amgevita®/Amjevita® | Imraldi®/Hadlima® | Hyrimoz® | Hulio® | Idacio® | Amsparity® | Yuflyma® | Hukyndra® | |
|---|---|---|---|---|---|---|---|---|---|---|
| EU/US market† | EU/US | EU/US | EU/US‡ | EU/US | EU/US | EU | EU | EU | EU/US | EU |
| Needle size (G) syringe | 27 | 29 | 29 | 29 | 27 | 29 | 29 | 29 | 29 | 29 |
| Needle size (G) pen | 27 | 29 | 27 | 29 | 27 | 29 | 29 | 29 | 29 | 29 |
| Pre-filled syringe/pen | 40 mg/- | 20 mg, 40 mg, 80 mg/ 40 mg, 80 mg |
20 mg, 40 mg/40 mg |
40 mg/ 40 mg |
20 mg, 40 mg, 80 mg/ 40 mg, 80 mg |
20 mg, 40 mg/ 40 mg |
40 mg/ 40 mg |
20 mg, 40 mg/40 mg |
40 mg, 80 mg/ 40 mg, 80 mg |
40 mg, 80 mg/ 40 mg |
| Citrate | Yes | No | No | No§ | No | No | Yes | No | No | No |
| Latex | Yes | No | Yes | No | No | No | No | No | No | No |
| Volume (ml) for 40 mg dose | 0.8 | 0.4 | 0.8 | 0.4 | 0.4 | 0.8 | 0.8 | 0.8 | 0.4 | 0.4 |
| Availability of 80 mg formulation | No | Yes (since 16 Dec 2021) | No | No | No | No | No | No | Yes | Yes (syringe only) |
| Shelf life at 25°C, days | 14 | 14 | 14 | 31 | 21 | 14 (20 mg in 0.4 ml sterile solution), 56 (40 mg in 0.8 ml sterile solution) |
28 | 30 (at 30°C) | 31 | 14 |
As at 4 July 2023.
High-concentration formulation expected to reach US market in 2024.
Imraldi®/Hadlima® has been citrate-free since the beginning of 2023.
Biosimilars of adalimumab
Humira & biosimilar availability in Europe & the USA
The original Humira formulation included a phosphate/citrate buffering system, sodium chloride, mannitol, polysorbate 80, and water for injection as excipients, adalimumab 40 mg (within an 0.8 ml injection volume), and pre-filled syringes with 27-gauge (27G) needles [9]. Prior to Humira patent expiry in 2018, a new formulation was introduced which included citrate-free excipients, a reduced 0.4 ml injection volume for a 40 mg dose (100 mg/ml concentration), and 29-gauge (29G) syringe needles, which aimed to decrease the rate of injection-site reactions. Humira 20 mg (50 mg/ml concentration) is currently licensed for paediatric indications, while Humira 80 mg is licensed for a selection of adult and paediatric indications (Table 1) [2,10–28].
Biosimilars are biologic medical products that are highly similar to their reference products, but without any clinically meaningful differences in immunogenicity, safety or effectiveness [29]. While the development and approval of biosimilars require rigorous standards of quality, safety and efficacy [30,31], the regulatory process places greater emphasis on pre-clinical physicochemical and functional characterization at the earlier stages of development and less on clinical trials [30–32].
The aim of biosimilar development is to demonstrate biosimilarity, which is high similarity in terms of structure, biological activity and efficacy, safety and immunogenicity profile [31]. A biosimilar relies on the safety and efficacy experience gained with an originator (reference) medicine by demonstrating biosimilarity, therefore avoiding unnecessary repetition of any clinical trials previously undertaken with the reference medicine. Where a biosimilar is highly similar to a reference medicine, with comparable efficacy and safety in one therapeutic indication, these data can be extrapolated to other indications already approved for the reference medicine; for adalimumab biosimilars, this includes RA and psoriasis. This extrapolation needs to be supported by all scientific evidence generated in comparability studies. Variability exists between each batch of biologics, both for reference products and biosimilars, as a result of the large molecular size and complexity of biologic therapies. In addition, regulatory requirements for biosimilars do not apply to factors such as excipients, preservatives, dose volume or the type of treatment device [31].
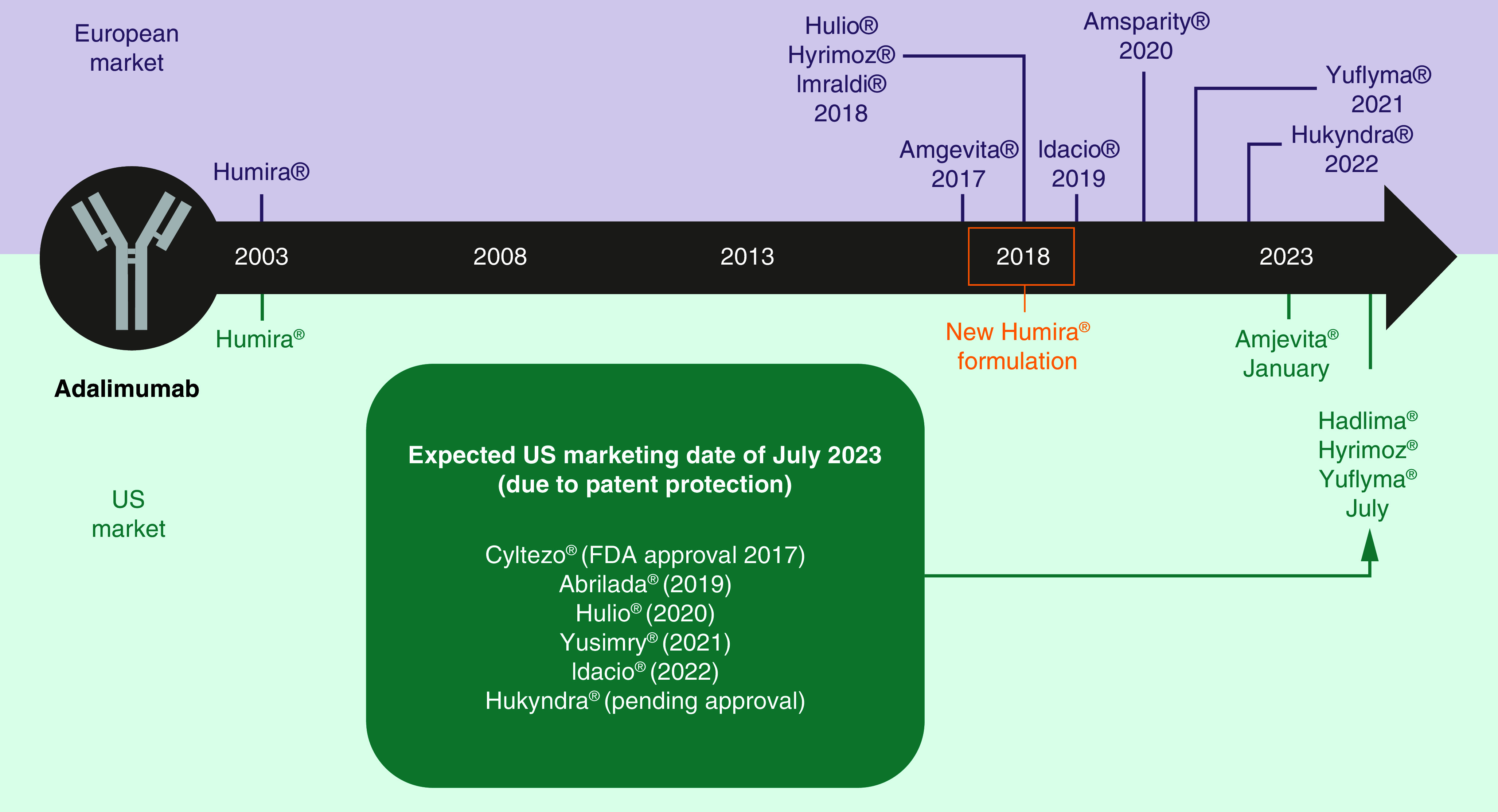
There are currently eight biosimilars of adalimumab available in Europe (Amgevita® 50 mg/ml, Imraldi® 50 and 100 mg/ml, Hyrimoz® 50 and 100 mg/ml, Hulio® 50 mg/ml, Amsparity® 50 mg/ml, Idacio® 50 mg/ml, Yuflyma® 100 mg/ml and Hukyndra® 100 mg/ml), with Amjevita® (January 2023), Hadlima® (July 2023), Hyrimoz (July 2023) and Yuflyma (July 2023) recently reaching the US market. Two adalimumab biosimilars have changed their formulations since initially reaching the European market: Imraldi is now available as a high-concentration formulation (HCF) which requires a lower volume of administration (0.4 ml vs 0.8 ml), and has also become citrate-free [33]. Similarly, Hyrimoz HCF was approved in Europe and the USA in April 2023 [34]. While the US market has lagged behind Europe with regard to adalimumab biosimilars, with delays in availability following earlier approval, all of the aforementioned remaining adalimumab biosimilars are expected to become available to US-based physicians in July 2023 due to patent protections (Figure 1).
Figure 1. . Marketing dates of adalimumab biosimilars in US and Europe.
Efficacy & safety of adalimumab biosimilars
Clinical data on the efficacy and tolerability of adalimumab biosimilars (Amgevita/Amjevita, Imraldi/Hadlima, Hyrimoz, Hulio, Idacio, Amsparity, Yuflyma and Hukyndra) approved by the European Medicines Agency (EMA) and US FDA have been previously described [35–46].
All of the currently available adalimumab biosimilars have been compared with Humira in phase III randomized, double-blind studies in patients with RA or plaque psoriasis (Table 2) [36–42,44,46]. In all these studies, the biosimilars had clinical equivalence to Humira [36–42,44,46]. In the RA studies, clinical equivalence was based on the proportion of patients who achieved a 20% improvement in American College of Rheumatology criteria (ACR20) at 12 weeks (in one study with Amsparity) [39] or at 24 weeks (in the other studies with Amgevita/Amjevita, Imraldi/Hadlima, Hulio and Yuflyma) [37,40,42,46], whereas in the psoriasis studies, clinical equivalence was based on the change from baseline in Psoriasis Area and Severity Index (PASI) at week 16 [38,44] or the proportion of patients achieving a 75% reduction in PASI score (PASI75) at week 16 [36,41]. In all of the studies (RA and psoriasis), the criteria for equivalence were based on the between-group difference in the primary end point falling within predefined limits as set out by regulatory guidelines.
Table 2. . randomized phase 3 trials with adalimumab biosimilars, in which their clinical activity was compared with originator adalimumab (Humira®).
| Author | Investigated biosimilar | Study design | Patients | n | Duration, weeks | Primary end point | Primary end point relative to Humira® | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rheumatoid arthritis | ||||||||
| Cohen et al. 2017 | ABP 501 (Amjevita®/ Amgevita®) | R, DB, P | Moderate to severe active RA on MTX | 526 | 24 | ACR20 at week 24 | RR 1.039 (90% CI 0.954, 1.133) | [37] |
| Fleischmann et al. 2018 | PF-06410293 (Amsparity®) | R, DB, P | Active RA on MTX | 597 | 78 | ACR20 at week 12 | Adjusted difference -2.98% (95% CI -10.38, 4.44; 90% CI -9.25, 3.28) | [39] |
| Weinblatt et al. 2018 | SB5 (Imraldi®/ Hadlima®) | R, DB, P | Moderate to severe active RA on MTX | 544 | 52 | ACR20 at week 24 | Adjusted difference 0.1% (95% CI -7.83, 8.13), in PPS (n = 476) | [46] |
| Genovese et al. 2019 | FKB327 (Hulio®) | R, DB, P | Active RA on MTX | 728 | 24–54 | ACR20 at week 24 | Adjusted difference -1.6%† (95% CI -7.9, 4.7; 90% CI -7.3, 3.6), in FAS (n = 721) | [40] |
| Kay et al. 2021 | CT-P17 (Yuflyma®) | R, DB, P | Active RA on MTX | 648 | 24–52 | ACR20 at week 24 | Adjusted difference 0%‡ (95% CI -5.94, 5.94) | [42] |
| Plaque psoriasis | ||||||||
| Papp et al. 2017 | ABP 501 (Amjevita®/ Amgevita®) | R, DB, P | Moderate to severe psoriasis (PASI ≥12), not responsive to or tolerant of conventional systemic therapy | 350 | 16–52 | % change from baseline PASI score at week 16 | LSM difference -2.18% (95% CI -7.39, 3.02) | [44] |
| Blauvelt et al. 2018 | GP2017 (Hyrimoz®) | R, DB, P | Moderate to severe psoriasis (PASI ≥12), previously treated with phototherapy or conventional systemic therapy | 465 | 51 | PASI75 at week 16 | Adjusted difference 1.8% (95% CI -7.46, 11.15) in PPS (n = 393) | [36] |
| Hercogová et al. 2020 | MSB11022 (Idacio®) | R, DB, P | Moderate to severe psoriasis (PASI ≥12), previously treated with phototherapy or conventional systemic therapy | 443 | 52 | PASI75 at week 16 | Difference –1.9% (95% CI -7.82, 4.07) in PPS (n = 394) | [41] |
| Feldman et al. 2021 | AVT02 (Hukyndra®) | R, DB, P | Moderate to severe psoriasis (PASI ≥12), not responsive to or tolerant of ≥1 non-biologic systemic therapy | 413 | 52 | % change from baseline PASI score at week 16 | LSM difference 2.3% (95% CI -1.34, 5.88; 90% CI -0.76, 5.29) in FAS (n = 412) | [38] |
Crude difference in proportions calculated from data reported in the paper, namely ACR20 response rate of 74.1% with FKB327 and 75.7% with adalimumab.
Crude difference in proportions calculated from data reported in the paper, namely ACR20 response rate of 82.7% in each group.
ACR20: Proportion of patients achieving a 20% improvement in American College of Rheumatology criteria; CI: Confidence interval; DB: Double-blind; FAS: Full analysis set; LSM: Least squares mean; MTX: Methotrexate; P: Parallel; PASI: Psoriasis Area and Severity Index; PPS: Per protocol set; R: Randomized; RA: Rheumatoid arthritis; RR: Risk ratio.
In addition to equivalence in the primary end point, these studies consistently showed comparable results with regard to secondary end points, safety/tolerability and immunogenicity [36–42,44,46]. In each study, the incidence of any adverse events (AEs), serious AEs, AEs of special interest or discontinuation due to AEs was similar in the group receiving the biosimilar and the group receiving Humira [36–42,44,46]. Similarly, the frequency of anti-drug antibody development was similar with each biosimilar and Humira, as was the proportion of patients who developed neutralising antibodies [36–42,44,46].
Most of the phase III trials included an extension phase after the assessment of the primary end point to evaluate the 52-week efficacy and safety of the biosimilar as well as the effects of switching from Humira to the biosimilar [36,38,40,41,47–50]. In most studies, this involved a re-randomisation of the patients who were originally assigned to Humira to remain on Humira or to switch to the biosimilar, at week 26 in the RA studies [40,47,48] or at week 16 in the psoriasis studies [38,41,49]. However, in one of the RA studies, all of the patients who had been taking Humira were switched to the biosimilar (in this case, Imraldi/Hadlima) at week 24 [50], and in one of the psoriasis studies, patients in both groups were re-randomized to continue on their current treatment (Humira or Hyrimoz) or to undergo multiple switches (6 weeks of the alternative, then back to their original treatment for 6 weeks, then back to the alternative for 6 weeks then back to their original treatment for the remainder of the trial) [36]. Irrespective of the study design, all the studies demonstrated that the biosimilar and Humira showed consistent efficacy and safety/tolerability after switching [36,38,40,41,47–50].
Switching to an adalimumab biosimilar
Switching from originator adalimumab (i.e., Humira) to an adalimumab biosimilar is typically seen as a way of providing a lower-cost alternative, along with the potential to increase treatment availability for both biologically-naive and bioexperienced patients [51]. In clinical practice, physicians may consider switching from an originator to a biosimilar, or even from one biosimilar to another, for numerous valid reasons such as convenience/administration or tolerability issues, or the switch may be driven by third parties, such as payers [52]. Thus, knowledge of available biosimilars needs to be integrated with what physicians know about their patients and their disease on a case-by-case basis. While data on switching patients from one adalimumab biosimilar to another remain limited, available evidence suggests a reassuring profile of effectiveness when switching between an originator biologic and a biosimilar [53]. Multiple successive switches from originator infliximab to infliximab biosimilars are both effective and safe in patients with IBD, regardless of the number of switches [54].
Switching patients from a 40 mg/0.8 ml formulation of adalimumab to a lower volume (40 mg/0.4 ml) dose with fewer excipients and delivery using a smaller gauge needle has been evaluated [55]. The 40 mg/0.4 ml adalimumab formulation was well tolerated and associated with less injection site-related pain than the 40 mg/0.8 ml adalimumab formulation. While it remains unclear which feature(s) of the 40 mg/0.4 ml formulation (composition, volume and/or needle size) were most responsible for the reported reduction in injection-site pain, such findings indicate that numerous factors have the potential to reduce this type of pain and improve tolerability.
A nationwide switching program in Iceland demonstrated the importance of thorough patient information and the possible need for individualized ‘introduction’ programs when planning a switch to biosimilars [56]. The study assessed the impact of switching from Humira to Imraldi among patients with inflammatory disorders (RA, IBD, or psoriasis). More patients received individualized instruction on using the Humira pen compared with the Imraldi pen (90.5 vs 18.2%, respectively). Patients treated regularly with adalimumab preferred the Humira injection device over the Imraldi device, even though adalimumab dosing did not vary. In addition, nearly half (46.6%) of patients found it more difficult to use the Imraldi pen than the Humira pen due to a more painful insertion of the needle (62.2%) and overall differing injection process (63.0%). These data indicate that patients require individualized instructions specific to the biosimilar, and the patient's healthcare team needs to be familiar with any differences in device type between agents.
Physicians should remain aware that the efficacy and tolerability of active treatments, such as adalimumab biosimilars, can be jeopardised by nocebo effects and their underlying psychological and neurobiological mechanisms, in addition to a multitude of individual factors [57]. Nocebo effects represent new or worsening symptoms or adverse events that typically occur as a consequence of patients' negative expectations and not the pharmacologic action of the treatment itself. Nocebo effects can therefore negatively affect treatment adherence in patients switching from originator biologics to biosimilars, with subsequent impairment of quality of life and higher treatment costs, and potential to damage the patient-clinician relationship. Enhanced physician-patient communication, such as informing all patients directly about the decision to switch to a biosimilar and the reasons for this (e.g., lower cost, fewer injection-site reactions), appears to be a useful mitigation strategy for minimizing potential nocebo effects (and improve rates of patient acceptance and persistence); ‘soft skills’ training on the nocebo effect for medical and pharmacy staff may also be beneficial. Productive patient–physician communication also provides the opportunity for patients to discuss any treatment-related issues, such as injection-site pain [58].
Injection pain
Factors contributing to pain with a subcutaneous injection can be split into three distinct groups linked to the product, injection, or patient [58]. Factors linked to the product include formulation (ingredients, pH, buffers, delivery volume, needle size, and type of device [syringe/pen]). Factors linked to the injection process include injection speed, viscosity of product fluid, injection angle and technique, frequency of injection and site, temperature of product and constituent allergens (e.g., latex). Factors linked to the patient include low body weight, injection anxiety/phobia, dramatic perception of pain, nocebo effect, female gender, patients' expectations, movement(s) during the injection process and the presence of specific diseases/conditions (e.g., severe RA, fibromyalgia or depression).
Given that originator adalimumab and its biosimilars are all administered subcutaneously, this mode of therapy can be associated with a subjective level of local pain and irritation from the needle puncture [58]. Injection-site reactions, which are frequently reported with originator adalimumab use, include erythema and/or itching, bleeding, pain and swelling [55]. While injection-site pain may only be a concern to some patients, any pain and discomfort associated with injections has the potential to negatively impact on medication adherence and overall patient experience [58,59]. In contrast, revised drug formulations and less invasive administration devices, which may improve the overall experience of subcutaneous administration, have the potential to positively impact on patients' convenience and adherence [59].
A higher frequency of injections which are deemed to be painful by the patient is correlated with a larger needle diameter [60]; thus, pain can be minimized via the use of narrower gauge needles. In addition, pain can be minimized and patient comfort improved during subcutaneous dosing by using short (4–8 mm) and thin-wall needles, conveniently lubricated and with sharp tips [60–64]. Anecdotal evidence suggests that, while all needles are sharp, some patients may deem some to be ‘sharper’ or ‘more blunt’, which may impact on injection-site pain [58].
The needle size of available adalimumab biosimilars varies but is typically 29G, with Hyrimoz and Amgevita using larger 27G needles (Table 1). For any patient who has a fear of needles (or ‘needle phobia’), an autoinjector pen allows injections to be self-administered without the needle being seen [65].
The prescribing physician's choice of adalimumab biosimilar will be influenced by differences in excipients, dosing formulations, and delivery devices (pens, pre-filled syringes) (Table 1), although some patient-specific factors may also require careful consideration, such as the presence of latex allergy, patient's access (e.g., formulary availability via insurer, reimbursement or cost/co-pays), or the need for prolonged stability at room temperature (e.g., for patients who are travelling or living in student halls of residence).
Other product-related factors for consideration
Product-related factors vary widely between originator adalimumab and biosimilars, and even between biosimilars themselves (Table 1). The differing formulations of adalimumab biosimilars need to be carefully considered for patient suitability. Buffers, such as citrate, are frequently added to parenteral formulations to optimize solubility and stability by pH adjustment [58], and some adalimumab biosimilars contain citrate (Table 1). The use of citrate in the formulation of an adalimumab biosimilar may increase the sensation of injection-site pain in some patients [55,59,66,67].
Higher volumes of injection, typically required with lower concentration formulations, are typically associated with increased patient discomfort and sometimes pain at the site of administration, with reduced injection-site pain reported where the use of a lower volume is possible [55,68–70]. Of note, the revised formulation of Humira which became available in 2018 is a higher concentration formulation than the originator, thus requiring a lower volume of administration (0.4 ml instead of 0.8 ml to deliver the same 40 mg dose) to improve tolerability [3]. Use of a 0.4 ml injection (versus 0.8 ml) to deliver a 40 mg dose of adalimumab was associated with less injection site-related pain [55]. The revised formulation of Humira also includes fewer excipients, no longer contains citrate or latex, and the injection devices have a smaller needle (29G vs 27G). Two randomized, crossover phase II studies in patients with RA demonstrated improved tolerability based on injection-site pain with the revised Humira formulation (versus the original formulation) [67]. However, the characteristic(s) of the current Humira formulation (composition, volume, needle size) most responsible for the reported decrease in injection-site pain remains unknown.
Availability of 80 mg formulations of adalimumab biosimilars remains important, particularly for patients with IBD, given that therapeutic doses may need to be 80 mg or greater [71]; this allows the need for only one injection to deliver the required 80 mg dose, and may be particularly important for those patients with a needle phobia who want to avoid the burden of an increased number of injections and also opt for an autoinjector pen or a device with a needle shield.
Latex hypersensitivity to injection devices for biologic therapies have been reported, but remain rare [72,73]. Adalimumab biosimilars which contain latex as part of the device should be avoided in patients with a latex allergy due to the potential for increasingly severe allergic reactions [73]; a general lack of knowledge among providers and nurses regarding this contraindication to therapy has been reported.
Discussion & expert opinion
When considering the use of adalimumab biosimilars, the prescribing physician may consider treatment choice based on suitability and aim to select the best option to support adherence and optimize patient outcomes. As all currently available adalimumab biosimilars have efficacy and tolerability similar to that of reference adalimumab, selection of a biosimilar should be supported by any characteristics which may support treatment adherence/satisfaction on a case-by-case basis. In addition, shared patient–physician decision making should also be considered to support optimal patient outcomes and persistence with treatment. For patients with a needle phobia, the use of autoinjector pens with concealed finer/shorter needles may be preferred. For patients with a lower pain tolerance/threshold, any factors such as reduced injection volume/frequency and the inclusion of fewer excipients, which may reduce the overall sensation of injection-site pain may be helpful. For patients with a latex allergy, adalimumab biosimilars containing latex in their construction may be contraindicated. For physicians and healthcare staff, adalimumab biosimilars with a longer shelf life than others may be helpful within the clinical setting. Likewise, those biosimilars with prolonged stability (>30 days vs 14 days) at room temperature (i.e., 25°C) may be deemed more convenient by patients who travel and do not have access to cooler storage during extended periods. For patients with IBD where a dose of adalimumab 80 mg may be needed, those biosimilars with an 80 mg formulation allow the convenience of a single injection, which may be particularly preferred by individuals with a needle phobia, particularly if administration is via an autoinjector pen.
For any adalimumab biosimilar, patients should be comfortable administering treatment to themselves and be provided with educational support to ensure attainment of a good injection technique to minimise any injection-site pain [58]; the patient should be able to operate the selected treatment device with relative ease, and any established patient condition which may negatively affect this requires careful consideration by the physician.
Physicians may consider ways of minimizing or avoiding any nocebo effects associated with the switch from originator adalimumab to a biosimilar (or from one adalimumab biosimilar to another, when required). Specific approaches include avoidance of negative instructions and expectations, framing information to focus on the positive attributes of treatment, and the promotion of shared decision-making and patient empowerment.
Switching from one adalimumab biosimilar to another may be required in some clinical situations, where, for example, adherence to treatment may be suboptimal. This switch may be related to the formulation and/or device, or where a dose of adalimumab may need to change, but without the need for additional injections.
Conclusion
In conclusion, the number of adalimumab biosimilars reaching the European and US markets will continue to increase, particularly within the USA in the second half of 2023. Careful evaluation of the characteristics of different biosimilars could help a practitioner to choose the product best suited to their patient. In addition, the use of effective patient–physician communication, along with fully explaining the reasons for a switch to an adalimumab biosimilar and a shared decision for switching, will support the overall goal of optimizing therapeutic outcomes.
Executive summary
Adalimumab biosimilars are available as an alternative, cost-effective treatment to Humira®, with similar efficacy and tolerability.
Adalimumab biosimilars differ between each other in product excipient(s), preservatives and device type(s).
Physicians should carefully consider which adalimumab biosimilar is most suitable for their patients on an individual ‘case-by-case’ basis.
Effective patient–physician communication should support realistic patient expectations and clearly explain the reasons for switching to an adalimumab biosimilar.
Physicians must consider ways of minimizing or avoiding any nocebo effects associated with the switch from Humira to a biosimilar (or biosimilar to biosimilar).
Biosimilars with reduced injection volume/frequency and the inclusion of fewer excipients may help to reduce the overall sensation of injection-site pain.
The use of autoinjector pens with concealed finer/shorter needles may be preferred for patients with a needle phobia.
Physicians should ensure that patients are comfortable administering treatment to themselves and maintain good injection technique.
Shared patient–physician decision making should be considered to support optimal patient outcomes and persistence with treatment.
Acknowledgments
The authors thank Matt Joynson who provided editorial support during the development of this manuscript on behalf of Springer Healthcare Communications.
Footnotes
Author contributions
All authors were responsible for the conceptualisation and visualisation of the manuscript and preparation/writing of the original draft, along with the reviewing and editing of the final version. All authors have read and agreed to the published version of the manuscript. All authors contributed equally to this work.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
Competing interests disclosure
V Abitbol has contributed to training courses and conferences, and acted as a consultant for Takeda, Amgen, Viatris, Sandoz, Janssen, Fresenius, Gilead, Tillotts, Celltrion, Pfizer, Galapagos and Nordic Pharma. S Benkhalifa and C Habauzit are employees of Celltrion Healthcare France. H Marotte has contributed to training courses and conferences, and acted as a consultant for AbbVie, Amgen, BMS, Celltrion Healthcare, Lilly, MSD, Nordic Pharma, Novartis, Pfizer, Sandoz and Sanofi. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing and editorial support were provided by Matt Joynson of Springer Healthcare Communications, and were funded by Celltrion Healthcare France.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ellis CR, Azmat CE. Adalimumab. In: statPearls. StatPearls Publishing, FL, USA: (2022) (Internet). [PubMed] [Google Scholar]
- 2.Coghlan J, He H, Schwendeman AS. Overview of Humira® biosimilars: current European landscape and future implications. J. Pharm. Sci. 110(4), 1572–1582 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Electronic Medicines Compendium. Humira 40mg/0.4ml solution (2021). https://www.medicines.org.uk/emc/product/2150/smpc
- 4.US Food and Drug Administration. Humira: summary of product characteristics (2008). https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125057s0110lbl.pdf
- 5.US Food and Drug Administration. Purple book database of licensed biological products. USFDA, MD, USA: (2021). https://purplebooksearch.fda.gov [Google Scholar]
- 6.Gibbons JB, Laber M, Bennett CL. Humira: the first $20 billion drug. Am. J. Manag. Care. 29(2), 78–80 (2023). [DOI] [PubMed] [Google Scholar]; • Provides a concise overview of Humira®.
- 7.Gall S, Kiltz U, Kobylinski T et al. Patient knowledge about biosimilars and satisfaction with the education provided by rheumatologists or nurse specialists in a biosimilar multiswitch scenario - the perception study. Semin. Arthritis Rheum. 57, 152119 (2022). [DOI] [PubMed] [Google Scholar]; •• Highlights that patients may find the concept of biosimilars difficult to understand.
- 8.Gibofsky A, Evans C, Strand V. Provider and patient knowledge gaps on biosimilars: insights from surveys. Am J Manag Care. 28(Suppl. 12), S227–S233 (2022). [DOI] [PubMed] [Google Scholar]; •• Highlights that physicians and patients lack knowledge of biosimilars.
- 9.Pilunni D, Santuccio C, Sottosanti L, Felicetti P, Navarra P. Relationship between injection site reactions and different adalimumab formulations. Analysis of the adverse events reported in Italy in the period 2016–2019. Eur. Rev. Med. Pharmacol Sci. 25(8), 3300–3305 (2021). [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Amgevita: EPAR – all authorized presentations (2018). https://www.ema.europa.eu/en/documents/all-authorised-presentations/amgevita-epar-all-authorised-presentations_en.pdf
- 11.European Medicines Agency. Amsparity: EPAR – all authorized presentations (2020). https://www.ema.europa.eu/en/documents/all-authorised-presentations/amsparity-epar-all-authorised-presentations_en.pdf
- 12.European Medicines Agency. Idacio: EPAR – all authorized presentations (2020). https://www.ema.europa.eu/en/documents/all-authorised-presentations/idacio-epar-all-authorised-presentations_en.pdf
- 13.European Medicines Agency. Amsparity: summary of product characteristics (2021). https://www.ema.europa.eu/en/documents/product-information/amsparity-epar-product-information_en.pdf
- 14.European Medicines Agency. Humira: EPAR – all authorized presentations (2021). https://www.ema.europa.eu/en/documents/all-authorised-presentations/humira-epar-all-authorised-presentations_en.pdf
- 15.European Medicines Agency. Hukyndra: EPAR – all authorized presentations (2022). https://www.ema.europa.eu/en/documents/all-authorised-presentations/hukyndra-epar-all-authorised-presentations_en.pdf
- 16.European Medicines Agency. Yuflyma: EPAR – all authorized presentations (2022). https://www.ema.europa.eu/en/documents/all-authorised-presentations/yuflyma-epar-all-authorised-presentations_en.pdf
- 17.European Medicines Agency. Idacio: summary of product characteristics (2022). https://www.ema.europa.eu/en/documents/product-information/idacio-epar-product-information_en.pdf
- 18.European Medicines Agency. Hulio: EPAR – all authorized presentations (2022). https://www.ema.europa.eu/en/documents/all-authorised-presentations/hulio-epar-all-authorised-presentations_en.pdf
- 19.European Medicines Agency. Imraldi: EPAR – all authorized presentations (2022). https://www.ema.europa.eu/en/documents/all-authorised-presentations/imraldi-epar-all-authorised-presentations_en.pdf
- 20.European Medicines Agency. Humira: summary of product characteristics (2022). https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf
- 21.European Medicines Agency. Hukyndra: summary of product characteristics (2023). https://www.ema.europa.eu/en/documents/product-information/hukyndra-epar-product-information_en.pdf
- 22.European Medicines Agency. Yuflyma: summary of product characteristics (2023). https://www.ema.europa.eu/en/documents/product-information/yuflyma-epar-product-information_en.pdf
- 23.European Medicines Agency. Hulio: summary of product characteristics (2023). https://www.ema.europa.eu/en/documents/product-information/hulio-epar-product-information_en.pdf
- 24.European Medicines Agency. Hymiroz: EPAR – all authorized presentations (2023). https://www.ema.europa.eu/en/documents/all-authorised-presentations/hyrimoz-epar-all-authorised-presentations_en.pdf
- 25.European Medicines Agency. Hymiroz: summary of product characteristics (2023). https://www.ema.europa.eu/en/documents/product-information/hyrimoz-epar-product-information_en.pdf
- 26.European Medicines Agency. Imraldi: summary of product characteristics (2023). https://www.ema.europa.eu/en/documents/product-information/imraldi-epar-product-information_en.pdf
- 27.European Medicines Agency. Amgevita: summary of product characteristics (2023). https://www.ema.europa.eu/en/documents/product-information/amgevita-epar-product-information_en.pdf
- 28.US Food and Drug Administration. Amgevita: summary of product characteristics (2016). https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761024lbl.pdf
- 29.Kirchhoff CF, Wang XM, Conlon HD, Anderson S, Ryan AM, Bose A. Biosimilars: key regulatory considerations and similarity assessment tools. Biotechnol. Bioeng. 114(12), 2696–2705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency. Guideline on similar biological medicinal products (2014). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf
- 31.European Medicines Agency. Biosimilars in the EU. Information guide for healthcare professionals (2019). https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf
- 32.Declerck P, Farouk Rezk M. The road from development to approval: evaluating the body of evidence to confirm biosimilarity. Rheumatology (Oxford). 56(Suppl. 4), iv4–iv13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biogen. lmraldi™ low-volume, citrate-free formulation (2023). https://lowvolume.imraldi.eu/en/home/low-volume.html
- 34.Sandoz. Sandoz receives European Commission approval for biosimilar Hyrimoz® (adalimumab) (2018). https://www.sandoz.com/news/media-releases/sandoz-receives-european-commission-approval-biosimilar-hyrimoz-adalimumab [DOI] [PubMed]
- 35.Bellinvia S, Cummings JRF, Ardern-Jones MR, Edwards CJ. Adalimumab biosimilars in Europe: an overview of the clinical evidence. BioDrugs. 33(3), 241–253 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Blauvelt A, Lacour JP, Fowler JF Jr et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br. J. Dermatol. 179(3), 623–631 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Genovese MC, Choy E et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomized, double-blind, phase III equivalence study. Ann. Rheum. Dis. 76(10), 1679–1687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldman SR, Reznichenko N, Pulka G et al. Efficacy, safety and immunogenicity of AVT02 versus originator adalimumab in subjects with moderate to severe chronic plaque psoriasis: a multicentre, double-blind, randomized, parallel group, active control, phase III study. BioDrugs. 35(6), 735–748 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleischmann RM, Alten R, Pileckyte M et al. A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira®) in the treatment of active rheumatoid arthritis. Arthritis Res. Ther. 20(1), 178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genovese MC, Glover J, Greenwald M et al. FKB327, an adalimumab biosimilar, versus the reference product: results of a randomized, phase III, double-blind study, and its open-label extension. Arthritis Res. Ther. 21(1), 281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hercogová J, Papp KA, Chyrok V, Ullmann M, Vlachos P, Edwards CJ. AURIEL-PsO: a randomized, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Br. J. Dermatol. 182(2), 316–326 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kay J, Jaworski J, Wojciechowski R et al. Efficacy and safety of biosimilar CT-P17 versus reference adalimumab in subjects with rheumatoid arthritis: 24-week results from a randomized study. Arthritis Res. Ther. 23(1), 51 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee A, Shirley M. PF-06410293: an adalimumab biosimilar. BioDrugs. 34(5), 695–698 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Papp K, Bachelez H, Costanzo A et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J. Am. Acad. Dermatol. 76(6), 1093–1102 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Somers M, Bossuyt P, Ferrante M, Peeters H, Baert F. Belgian IBD Research Group [BIRD] Position Statement 2019 on the use of adalimumab biosimilars in inflammatory bowel diseases. J. Crohns Colitis. 14(5), 680–685 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Weinblatt ME, Baranauskaite A, Niebrzydowski J et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 70(1), 40–48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleischmann RM, Alvarez DF, Bock AE et al. Randomized study of PF-06410293, an adalimumab (ADL) biosimilar, compared with reference ADL for the treatment of active rheumatoid arthritis: results from weeks 26–52, including a treatment switch from reference ADL to PF-06410293. RMD Open. 7(2), e001578 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furst DE, Jaworski J, Wojciechowski R et al. Efficacy and safety of switching from reference adalimumab to CT-P17 (100 mg/ml): 52-week randomized, double-blind study in rheumatoid arthritis. Rheumatology (Oxford). 61(4), 1385–1395 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papp K, Bachelez H, Costanzo A et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 177(6), 1562–1574 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Weinblatt ME, Baranauskaite A, Dokoupilova E et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol. 70(6), 832–840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruda RC, Kelly KA, Feldman SR. Real-world outcomes following switching from anti-TNF reference products to biosimilars for the treatment of psoriasis. J. Dermatolog. Treat. 34(1), 2140569 (2023). [DOI] [PubMed] [Google Scholar]; • Provides real-world evidence to support switching patients to biosimilars.
- 52.Mysler E, Azevedo VF, Danese S et al. Biosimilar-to-biosimilar switching: what is the rationale and current experience? Drugs 81(16), 1859–1879 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scrivo R, Castellani C, Mancuso S et al. Effectiveness of non-medical switch from adalimumab bio-originator to SB5 biosimilar and from ABP501 adalimumab biosimilar to SB5 biosimilar in patients with chronic inflammatory arthropathies: a monocentric observational study. Clin. Exp. Rheumatol. 41(3), 613–619 (2023). [DOI] [PubMed] [Google Scholar]
- 54.Gros B, Plevris N, Constantine-Cooke N et al. Multiple infliximab biosimilar switches appear to be safe and effective in a real-world inflammatory bowel disease cohort. United European Gastroenterol. J. 11(2), 179–188 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nash P, Vanhoof J, Hall S et al. Randomized crossover comparison of injection site pain with 40 mg/0.4 or 0.8 ml formulations of adalimumab in patients with rheumatoid arthritis. Rheumatol. Ther. 3(2), 257–270 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karlsdottir K, Gunnarsdottir AI, Grondal G et al. A patients' perspective towards the injection devices for Humira® and Imraldi® in a nationwide switching program. Front. Med. (Lausanne). 9, 799494 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front. Pharmacol. 10, 1372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the clinical implications of nocebo effects when using biosimilars.
- 58.St Clair-Jones A, Prignano F, Goncalves J, Paul M, Sewerin P. Understanding and minimizing injection-site pain following subcutaneous administration of biologics: a narrative review. Rheumatol. Ther. 7(4), 741–757 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the need to minimize injection-site pain following subcutaneous administration of biologics.
- 59.Gely C, Marín L, Gordillo J et al. Impact of pain associated with the subcutaneous administration of adalimumab. Gastroenterol Hepatol. 43(1), 9–13 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens. Mot. Res. 23(1–2), 37–43 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Hirsch L, Gibney M, Berube J, Manocchio J. Impact of a modified needle tip geometry on penetration force as well as acceptability, preference, and perceived pain in subjects with diabetes. J. Diabetes Sci. Technol. 6(2), 328–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kreugel G, Beijer HJM, Kerstens MN, ter Maaten JC, Sluiter WJ, Boot BS. Influence of needle size on metabolic control and patient acceptance. Eur. Diabetes Nurs. 4(2), 51–55 (2007). [Google Scholar]
- 63.Petersen C, Zeis B. Syringe siliconisation trends, methods and analysis procedures. Int. Pharm. Ind. 7, 78–84 (2015). [Google Scholar]
- 64.Watt RP, Khatri H, Dibble ARG. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int. J. Pharm. 554, 376–386 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Fernandez JM, Madsen S, Krase JM, Shi VY. Classification and mitigation of negative injection experiences with biologic medications. Dermatol. Ther. 33(2), e13240 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Rosembert D, Malaviya A, How J et al. Different failure rates after non-medical switching of 744 patients from adalimumab originator to 2 different adalimumab biosimilars at Cambridge University Hospitals, UK: real-world experience [journal abstract]. J Crohns Colitis. 14(Suppl. 1), S438–S439 (2020). [Google Scholar]
- 67.Zbacnik TJ, Holcomb RE, Katayama DS et al. Role of buffers in protein formulations. J. Pharm. Sci. 106(3), 713–733 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Jørgensen JT, Rømsing J, Rasmussen M, Møller-Sonnergaard J, Vang L, Musaeus L. Pain assessment of subcutaneous injections. Ann. Pharmacother. 30(7–8), 729–732 (1996). [DOI] [PubMed] [Google Scholar]
- 69.Mathaes R, Koulov A, Joerg S, Mahler HC. Subcutaneous injection volume of biopharmaceuticals-pushing the boundaries. J. Pharm. Sci. 105(8), 2255–2259 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Zijlstra E, Jahnke J, Fischer A, Kapitza C, Forst T. Impact of injection speed, volume, and site on pain sensation. J. Diabetes Sci. Technol. 12(1), 163–168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Crohn's and Colitis Organisation (ECCO). Adalimumab. Interventions (2022). http://www.e-guide.ecco-ibd.eu/interventions-therapeutic/adalimumab
- 72.Johnson C, Zumwalt M, Anderson N. Latex hypersensitivity to injection devices for biologic therapies in psoriasis patients. Cutis 102(2), 116–118 (2018). [PubMed] [Google Scholar]
- 73.Zbehlik AJ, Brown LA. Underappreciated medication contraindication. Arthritis Care Res (Hoboken). 62(12), 1815 (2010). [DOI] [PubMed] [Google Scholar]


