This randomized clinical trial analyzes the safety and efficacy of auricular acupuncture as a treatment for depression.
Key Points
Question
Is auricular acupuncture a safe and effective treatment for depression?
Findings
This randomized clinical trial of 74 patients with depression undergoing 6 weeks of auricular acupuncture found no significant differences in adverse event rates between the intervention (specific auricular acupuncture) and control (nonspecific auricular acupuncture) groups, evidencing the intervention’s safety. No significant differences were found in depression recovery (50% reduction in Patient Health Questionnaire–9 score from baseline).
Meaning
These findings suggest that auricular acupuncture is safe for patients with depression, but additional studies with larger samples and longer interventions are needed for confirmation.
Abstract
Importance
Depression is a leading cause of disability worldwide, and there is increasing interest in nonpharmacological treatments. Auricular acupuncture (AA) is a simple, low-cost, and well-tolerated option, but further studies are needed to establish its efficacy and safety.
Objective
To estimate the efficacy and safety of auricular acupuncture as a treatment for depression.
Design, Setting, and Participants
This randomized clinical trial was conducted at 4 university research centers in Brazil, from March to July 2023. Eligible patients were adults aged 18 to 50 years whose score on the Patient Health Questionnaire–9 (PHQ-9) indicated moderate depression (score 10-14) or moderately severe depression (score 15-19). Exclusion criteria included previous application of AA, risk of suicidal ideation, or severe depression (PHQ-9 score >20). An intent-to-treat analysis and modified intent-to-treat analysis were conducted.
Intervention
Participants were randomized into 2 treatment groups, which included specific AA (SA) and nonspecific AA (NSA). Both groups received 12 sessions of AA with semipermanent needles with daily stimulation twice a week over 6 weeks and were followed-up for 3 months. All participants continued with their usual care for ethical reasons. The SA group’s treatment protocol consisted of 6 acupuncture points on the auricular pavilion chosen according to the diagnosis of depression by traditional Chinese medicine (Shenmen, subcortex, heart, lung, liver, and kidney). The NSA group’s acupuncture points were the external ear, the cheek and face area, and 4 nonspecific points in the helix region unassociated with mental health symptoms. A locator device was used to confirm which areas had neuroreactive points.
Main Outcomes and Measures
The primary outcome was a reduction of at least 50% in the PHQ-9 score (ie, depression recovery) at 3 months. Secondary outcomes included depression recovery at 4 and 6 weeks; depression remission (PHQ-9 score < 5) at 4 weeks, 6 weeks, and 3 months); and adverse events.
Results
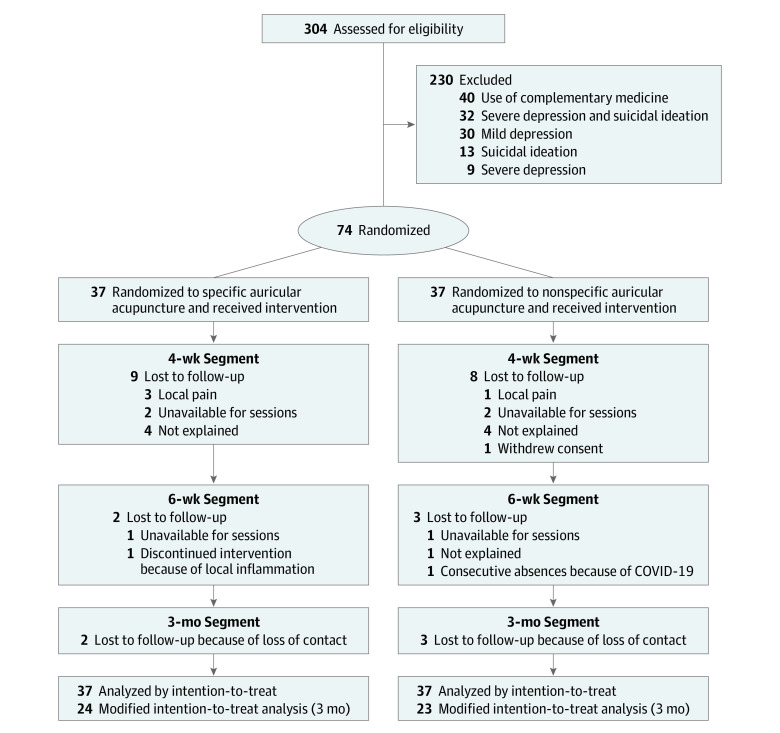
A total of 304 participants were screened, and 74 participants (62 women [84%]; median [IQR] age, 29 [23-27] years) were included in the intention-to-treat analysis, with 37 participants randomized to each group (SA and NSA). A total of 47 participants (64%) were followed-up through 3 months. The results showed no statistically significant difference in depressive recovery between the groups at 3 months (14 of 24 participants in the SA group [58%] vs 10 of 23 participants in the NSA group [43%]; risk ratio [RR], 1.34; 95% CI, 0.76-2.45; P = .38). The proportions of depression recovery and remission at 4 and 6 weeks based on the PHQ-9 were higher in the SA group (except for depression recovery at 6 weeks) with no statistically significant differences. However, a statistically significant difference was observed in symptom remission at 3 months (11 of 24 participants in the SA group [46%] vs 3 of 23 participants in the NSA group [13%]; RR, 1.99; 95% CI, 1.16-3.34; P = .02) in favor of SA. There were no significant differences in adverse event rates between the groups, evidencing the intervention’s safety. Most participants reported mild pain at the needle application site (33 patients [94%] in the SA group vs 32 patients [91%] in the NSA group). Five participants dropped out of the study due to adverse events.
Conclusions and Relevance
The results of this randomized clinical trial suggest that SA over 6 weeks is safe. Although there was no statistically significant difference between groups for the primary efficacy outcome, patients receiving SA did experience greater symptom remission at 3 months. A larger sample size and longer intervention are needed to further evaluate the efficacy of SA for depression.
Trial Registration
ClinicalTrials.gov Identifier: NCT05855421
Introduction
Depression is one of the leading causes of disability worldwide, affecting more than 300 million people (4.4% of the global population).1 In 2019, depressive disorders represented the highest proportion of disability-adjusted life-years among mental disorders (37.3%).2 In Brazil, the prevalence depression is among of the highest in the world, affecting 5.8% of the Brazilian population and accounting for 10.3% of years of life lost by Brazilians.3 However, less than 10% of those affected by the disease receive appropriate treatment.1,4 Nonadherence to antidepressant treatment is a concern for health care professionals; adherence to antidepressant therapy ranges from 40% to 90% across different studies, with a mean adherence rate of 65%.5
Research has shown that Australians and North Americans prefer integrative treatments for depression.6,7,8 In this sense, auricular acupuncture (AA), a type of acupuncture in which thin semipermanent needles are inserted into specific points on the auricular pavilion, may be a therapeutic option. AA is easier to implement in clinical settings than body acupuncture owing to its short application time, low technique complexity, relative safety, and daily continuous physiological stimulation.9
Several nerves are distributed in the auricular pavilions. The concha area has a rich distribution of the vagus nerve.10 Anatomical studies have shown that the auricular pavilion is the only place on the human body’s surface with a distribution of the afferent vagus nerve.11 Thus, needle stimulation on the auricular pavilion appears to lead to specific activation in the brain, mainly through the auricular branch of the vagus nerve.12,13 A systematic review14 found little evidence of the efficacy of AA for depression. The methodological quality scores showed that most analyzed studies indicated considerable methodological flaws. Additionally, no study in the review14 used semipermanent needles in the groups. Therefore, this study aims to estimate the efficacy and safety of AA for depression recovery and remission at 4 weeks, 6 weeks, and 3 months.
Methods
Study Design
This randomized clinical trial was approved by the Hospital das Clínicas ethics committee, the University of São Paulo, and the University of Southern Santa Catarina and was monitored by an independent data and safety monitoring board. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline and the Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA)15 for the design and reporting of this study. We recruited patients from the community at 4 university research centers in Santa Catarina, Brazil, from March to April 2023. All patients provided written informed consent before participation, and participants did not receive incentives or compensation to be part of the study. The study protocol and statistical analysis plan can be found in Supplement 1.
Participants
The research participants were adults aged 18 to 50 years. Eligible participants were those whose score on the Patient Health Questionnaire–9 (PHQ-9)16 indicated moderate depression (score of 10-14) or moderately severe depression (score of 15-19). Exclusion criteria included previous application of AA, risk of suicidal ideation, or severe depression (PHQ-9 score ≥20) (eTable 1 in Supplement 2). Demographic characteristics, including gender and race and ethnicity, were collected via self-report on electronic tablets using the REDCap platform. Race and ethnicity categories included Black, White, and multiracial; race and ethnicity were included in this study to align with current research.
Randomization and Blinding
A computer program performed block randomization in a 1:1 ratio, in different block sizes (4, 6, and 8), corresponding to the 2 study groups. The experimental group received specific AA (SA) for depression and usual care, and the control group received nonspecific AA (NSA) and usual care. All participants continued with their usual care for ethical reasons. Participant allocation was concealed and delivered to the acupuncturists in individual opaque sealed envelopes. Participants, evaluators, data managers, and the statistician were blinded to treatment assignment. Neither the study participants nor the investigators had any influence on randomization and allocation concealment, which an independent statistician performed. Participants were informed that there were 2 treatment protocols.
Procedures
The study assessors were trained to approach participants similarly to avoid recruitment biases. The study used a protocol of questions and procedures for all phases: prescreening, screening, data collection, and application of protocols.
Participants with severe depression levels (PHQ score ≥20) or suicidal ideation did not participate in the study and were followed and consulted by a psychiatrist. The sessions of AA application occurred twice weekly, alternating the application on the right and left auricular pavilions, over 6 weeks, totaling 12 sessions, with a duration of 15 minutes each, in reserved rooms. The acupuncturists instructed the participants to stimulate each point manually (de qi stimulation) 3 times a day (morning, afternoon, and evening) for 30 seconds or until the auricular pavilion became red or sensitive to pressure. In this study, the acupuncturists had specific training in the field, with a minimum of 1200 hours in acupuncture and a minimum of 10 years of experience. They received standardized training before the study began.
All participants answered the PHQ-9 questionnaires 4 weeks, 6 weeks, and 3 months after the study initiation and reported adverse events before each AA session (eFigure 1 in Supplement 2). Patients were asked to complete the blinding assessment and treatment efficacy perception assessment and were requested to guess which treatment they received to test the success of blinding after 3 months of the study. More details about AA procedures and outcome assessment can be found in Supplement 1.
SA Treatment
The SA group’s treatment consisted of a protocol of acupuncture points chosen according to the diagnosis of depression by traditional Chinese medicine. All SA participants used 6 preestablished points on the auricular pavilion: Shenmen, subcortex, heart, lung, liver, and kidney17,18 (eFigure 2 and eTable 2 in Supplement 2). The EL11 device (NKL) was used to find the exact location of the points; the device searches on the skin for areas of lower electrical resistance, indicating more neuroreactive points (ie, true acupuncture points).19 The size of the semipermanent needle (Complementar) used was 0.2 mm wide and 2.5 mm long. The semipermanent needles were inserted to a depth of 2.5 mm.
NSA Treatment
The existing literature acknowledges the difficulty in establishing protocols to be used as a control because of the high responsiveness and innervation of the auricular pavilion (ie, sham needling is not physiologically inert).19,20,21 As a control group strategy, our study opted for superficial needling at nonpoints or irrelevant true points, aiming to reach other neural segments. The NSA group points were the external ear, the cheek and face area, and 4 nonspecific points in the helix region (eFigure 3 in Supplement 2), which are points that are not associated with mental health symptoms (eTable 2 in Supplement 2). The EL11 locator device was used to confirm that the sham areas were not neuroreactive points. The size of the needle (Complementar) used in the control group was 0.2 mm wide and 1.0 mm long, aiming for more superficial needling. The semipermanent needles were inserted to a depth of 1.0 mm.
Outcomes
The primary outcome was the proportion of participants who showed improvement of 50% or more in depressive symptoms (ie, depression recovery) between the groups, as assessed by the PHQ-9 at 3 months. Secondary outcomes included the difference in the proportion of participants reaching depression recovery at 4 and 6 weeks; the proportion of participants who achieved a score of less than 5 on the PHQ-9 (indicating depression remission) and differences in depression scores at 4 weeks, 6 weeks, and 3 months; the difference in the proportion of reported adverse events (ie, those arising from the AA technique) and reported adverse effects (ie, the worsening of depression symptoms or the onset of suicidal ideation); and the difference in the proportion of patient blinding to randomization and perception of treatment efficacy (eTable 3 in Supplement 2).
Statistical Analysis
Data analysis in this clinical trial was conducted by an independent hired statistician, blinded to treatment groups. The sample size was designed to detect a 30% difference (experimental group, 60% and control group, 30%) in depression symptom recovery between the 2 groups. A minimum sample of 36 participants per group was estimated, considering the test as 2-tailed, with 80% power and a significance level of P < .05. An additional 10% was added, considering segment losses, totaling 40 participants in each group, for 80 participants.
Initially, a baseline comparison between the SA and NSA groups was performed with median and IQR descriptions for continuous variables and frequencies and percentages for categorical variables. Subsequently, intent-to-treat and modified intent-to-treat analyses were conducted to compare the proportions of depression recovery and remission at different time points in the study using Fisher exact test. The effect size for dichotomous variables was calculated as relative risk.
Scores were subjected to a normality test that rejected the normality hypothesis to verify sample normality. Therefore, nonparametric tests were adopted. The reduction of depression scores over time in both groups was evaluated using the median and IQR. The statistical analysis of these data was conducted using the Wilcoxon-Mann-Whitney U test.
Cochran Q and Friedman analysis of variance tests were also used to verify statistically significant differences in depression scores over time within each group. These tests were followed by post hoc tests to identify significant differences at specific time points. We performed a sensitivity analysis with missing data imputation to evaluate the robustness of the results. Multiple imputation methods were explored, including last observation carried forward, imputation using the generalized estimating equations model, and multiple imputation through the expectation-maximization lag algorithm for categorical variables; the generalized estimating equations model was applied specifically to PHQ-9 depression scores. All analyses were conducted with a significance level of P < .05. Relative and absolute frequency were used to assess treatment safety and identify adverse events. The success of blinding and the perception of treatment efficacy were assessed using Fisher exact test. Statistical analyses were performed using R statistical software version 4.1.0 (R Project for Statistical Computing).
Results
Of the 304 screened volunteers, 230 were excluded for various reasons, resulting in a cohort of 74 participants (62 women [84%]; median [IQR] age, 29 [23-37] years; 5 Black participants [7%]; 61 White participants [82%]; 8 multiracial participants [10%]) comprising the intent-to-treat sample with 37 participants randomized to SA treatment and 37 participants randomized to NSA treatment (Figure 1). At 3 months, 27 patients (36%) were lost to follow-up; a total of 47 patients (64%), including 24 patients in the SA group and 23 participants, in the NSA group, comprised the modified intent-to-treat sample. There was no difference between the study groups in the number of patients lost to follow-up (eTable 4 in Supplement 2). We conducted a comparison of baseline depression scores according to the PHQ-9 among the individuals lost to follow-up (eTable 5 in Supplement 2). The groups were similar regarding demographic characteristics, clinical diagnosis, pharmacological treatments, and health characteristics at the study’s outset, except for race and ethnicity (Table 1).
Figure 1. Flowchart of Screening, Randomization, and Follow-Up.
Table 1. Baseline Demographic and Clinical Characteristics at the Time of Randomization in the Intention-to-Treat Analysis.
| Variable | Participants, No. (%) (N = 74) | |
|---|---|---|
| SA group (n = 37) | NSA group (n = 37) | |
| Age, median (IQR), y | 28 (22-37) | 30 (23-36) |
| Patient Health Questionnaire–9 Score, median (IQR)a | 14.0 (12.0-16.0) | 16.0 (14.0-18.0) |
| Gender | ||
| Male | 5 (13) | 6 (16) |
| Female | 32 (87) | 30 (81) |
| Nonbinary | 0 | 1 (3) |
| Race and ethnicity | ||
| Black | 0 | 5 (14) |
| Multiracial | 2 (5) | 6 (16) |
| White | 35 (95) | 26 (70) |
| Incomeb | ||
| ≤3 times the monthly minimum wage | 14 (38) | 11 (30) |
| >3 times the monthly minimum wage | 23 (62) | 26 (70) |
| Marital status | ||
| Single | 22 (59) | 17 (46) |
| Not single | 15 (41) | 20 (54) |
| Education | ||
| Less than high school up to completed high school | 10 (27) | 10 (27) |
| Incomplete college education or higher | 27 (73) | 27 (73) |
| Weekly working hours, median (IQR) (n = 49) | 42 (27-44) | 44 (22-44) |
| Previous diagnosis of depression or other mental illness | 16 (43) | 16 (43) |
| Participant used any medication for depression | 8 (22) | 4 (11) |
| Participant used medication in the last 3 mo | ||
| No | 21 (57) | 27 (73) |
| Antidepressant | 8 (22) | 5 (13) |
| Tranquilizer or sedative | 5 (13) | 4 (11) |
| Anxiolytic | 7 (19) | 3 (8) |
| Participant consumes alcoholic beverages | 26 (70) | 20 (54) |
| Participant smokes any cigarette | ||
| No | 35 (95) | 35 (96) |
| Yes | 2 (5) | 1 (2) |
| Former smoker | 0 | 1 (2) |
Abbreviations: NSA, nonspecific auricular acupuncture; SA, specific auricular acupuncture.
A score of 10 to 14 indicates moderate depression; 15 to 19, moderately severe depression; and 20 to 25, severe depression.
The monthly minimum wage in Brazil is equivalent to $250 in 2023.
Primary Outcome
Three months after randomization, 14 of the 24 participants (58%) randomized to the SA group had a reduction of at least 50% in the PHQ-9 score compared with 10 of 23 participants (43%) in the NSA group. The result was not statistically significant (risk ratio [RR], 1.34; 95% CI, 0.76-2.45; P = .38) (Table 2).
Table 2. Primary and Secondary Efficacy Outcomes in the Intention-to-Treat Analysis.
| Outcomes | Participants, No./Total No. (%) | RR (95% CI)a | P valueb | |
|---|---|---|---|---|
| SA group | NSA group | |||
| Primary outcome, depression recovery at 3 mo vs baseline (n = 47)c,d | 14/24 (58) | 10/23 (43) | 1.34 (0.76-2.45) | .38 |
| Secondary outcomes | ||||
| Depression recovery at 4 wk vs baseline (n = 57) | 12/28 (43) | 9/29 (31) | 1.28 (0.74-2.14) | .42 |
| Depression recovery at 6 wk vs baseline (n = 51) | 10/26 (38) | 10/25 (40) | 0.96 (0.53-1.65) | >.99 |
| Symptom remission at 4 wk vs baseline (n = 57)e | 8/28 (28) | 5/29 (17) | 1.35 (0.72-2.20) | .36 |
| Symptom remission at 6 wk vs baseline (n = 51) | 7/26 (27) | 5/25 (20) | 1.19 (0.61-2.00) | .74 |
| Symptom remission at 3 mo vs baseline (n = 47) | 11/24 (46) | 3/23 (13) | 1.99 (1.16-3.34) | .02 |
Abbreviations: NSA, nonspecific auricular acupuncture; RR, risk ratio; SA, specific auricular acupuncture.
Effect size was calculated as RR for dichotomous data.
Statistical significance was calculated with Fisher exact test.
The primary outcome (improvement in depression symptoms) was defined as a reduction of 50% or more from baseline Patient Health Questionnaire-9 (PHQ-9) scores. The number of cases and proportions are based on observed data.
Depressive symptoms measured by PHQ-9 scores range from 0 (least) to 27 (greatest) symptom burden.
Participants who achieved a score less than 5 on the PHQ-9, indicating symptom remission.
Secondary Outcomes
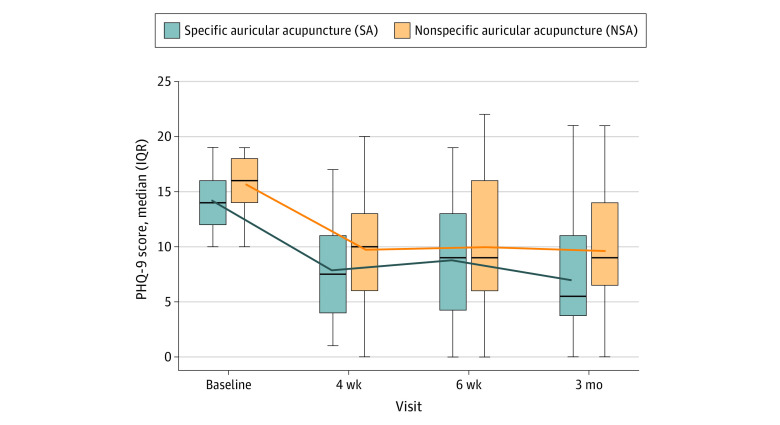
The proportions of patients with depression recovery and remission based on the PHQ-9 at 4 weeks was higher in the SA group, but not at 6 weeks, and there were no statistically significant differences between the groups at 4 or 6 weeks. A statistically significant difference in favor of the SA group was observed in depression remission after 3 months (11 of 24 participants [46%] in the SA group vs 3 of 23 participants in the NSA group [13%]; RR, 1.99; 95% CI, 1.16-3.34; P = .02) (Table 2). The median PHQ-9 scores decreased after 4 weeks, 6 weeks, and 3 months in both groups, with no statistically significant differences. Lower scores were observed in the SA group than in the NSA group (eTable 6 in Supplement 2 and Figure 2).
Figure 2. Median Depression Scores by Intention-to-Treat Analysis.
The figure shows the median Patient Health Questionnaire-9 (PHQ-9) scores of patients receiving specific auricular acupuncture (SA) and nonspecific auricular acupuncture (NSA) at baseline, 4 weeks, 6 weeks, and 3 months. A score of 10 to 14 indicates moderate depression; 15 to 19, moderately severe depression; and 20 to 25, severe depression. The lines inside the boxes reflect the medians, with the upper and lower ends of the boxes reflecting the IQRs. The error bars reflect the minimum and maximum values. The line plot reflects means.
The modified intent-to-treat analyses showed the similar results as the intention-to-treat analyses. Intragroup analysis was performed using the Cochran Q test, with no statistically significant differences (eTable 7 in Supplement 2). Intragroup analysis was performed using the Friedman analysis of variance test followed by post hoc tests for the differences in PHQ-9 scores at each time point compared with baseline, which revealed statistically significant differences in all evaluations, in both groups, at different assessment points (eTable 8 and eFigure 4 in Supplement 2).
Sensitivity Analyses
The sensitivity analyses revealed that there were no statistically significant differences in depression recovery across the visits (eTable 9 in Supplement 2). These analyses confirmed the statistically significant results in favor of the SA group regarding depression remission after 3 months (eTable 10 in Supplement 2). As for PHQ-9 scores, the generalized estimating equations model demonstrated favorable effect sizes of SA, and the visits had a statistically significant effect on the scores (eTable 11 and 12 in Supplement 2).
Safety
Most participants reported mild pain at the needle application site (33 patients [94%] in the SA group vs 32 patients [91%] in the NSA group). One participant had mild local inflammation in 1 of the auricular points and received appropriate treatment. Regarding adverse events, an increase in depression scores was observed during data collection in 3 participants, and 7 individuals responded positively to question 9 of the PHQ-9, which addresses suicidal ideation. These cases were carefully monitored, and the independent data monitoring and safety committee did not consider these events related to the intervention. No statistically significant difference was found between the groups in the proportion of participants with adverse effects (ie, those arising from AA) and adverse events (ie, worsening of depression symptoms or suicidal ideation). Five participants withdrew from the study as a result of adverse effects. No severe adverse events were reported (Table 3). The study participants did not change their medications or psychotropic medication dosages throughout the data collection.
Table 3. Adverse Effects and Adverse Events Listed by Treatment Group.
| Adverse event or adverse effect | Participants, No. (%) (N = 74) | |
|---|---|---|
| SA group (n = 37) | NSA group (n = 37) | |
| Mild and transient adverse effects | ||
| Mild pain | 33 (94) | 32 (91) |
| Intense pain | 1 (3) | 0 |
| Punctate bleeding | 3 (9) | 2 (6) |
| Itching in the auricle | 11 (31) | 14 (40) |
| Increased appetite | 1 (3) | 0 |
| Headache | 1 (3) | 0 |
| Hot flashes | 0 | 1 (3) |
| Difficulty sleeping | 0 | 2 (6) |
| Sensitivity to pressure | 1 (3) | 2 (6) |
| Facial skin hypersensitivity | 0 | 1 (3) |
| Moderate adverse effect: local inflammation | 1 (3) | 0 |
| Moderate adverse events | ||
| Worsening of depressive symptoms (4 wk) | 0 | 0 |
| Worsening of depressive symptoms (6 wk) | 0 | 1 (6) |
| Worsening of depressive symptoms (3 mo) | 1 (3) | 1 (6) |
| Emergence of suicidal ideation (4 wk) | 0 | 2 (6) |
| Emergence of suicidal ideation (6 wk) | 0 | 2 (6) |
| Emergence of suicidal ideation (3 mo) | 2 (6) | 2 (6) |
| Any severe adverse events and adverse effects | 0 | 0 |
| Discontinuation due to adverse effects | ||
| Local pain | 3 (9) | 1 (3) |
| Local inflammation | 1 (3) | 0 |
Abbreviations: NSA, nonspecific auricular acupuncture; SA, specific auricular acupuncture.
Blinding Quality
For results on the success of blinding, see eTable 13 in Supplement 2. No difference was found between groups in the proportion of patients who guessed correctly what kind of AA they had received and their perception of efficacy in symptom relief after the 3 months.
Discussion
The results of this randomized clinical trial showed no difference in depression recovery between the groups at 3 months. However, depression remission at 3 months was significantly higher in the SA group. In addition, no significant differences were observed in the rates of adverse events and adverse effects, and no severe adverse events were recorded, evidencing practice safety. To our knowledge, this is the first randomized clinical trial on the effectiveness of SA using a semipermanent needle with NSA directed at depression as the comparator group. However, previous studies22,23,24,25,26,27,28 using other AA techniques found favorable results in depressive symptoms, but with shorter study duration and different comparators.
These preliminary findings are promising and support the use of SA in depression treatment. The rates of recovery (58%) and remission (46%) from depression in the present study are similar to those observed in meta-analyses of pharmacological treatments29,30 and superior to those for psychotherapy.31 The 33% difference in symptom remission between the groups (ie, 46% remission in the SA group vs 13% in the NSA group) was clinically meaningful; depression remission is crucial for patients, providing better psychosocial functioning and long-term prognosis, not just symptom improvement.32
The absence of statistically significant differences between the groups for depression recovery can be explained. First, the small sample size resulted in insufficient power and may have underestimated true efficacy. Second, considering the economic cost of time and participant adherence, we provided only 12 sessions over 6 weeks in this study. Therefore, adding AA doses by increasing the treatment duration (eg, 16-18 sessions over 8-12 weeks) may be an effective way to streamline the treatment program. Third, the manual stimulation of the semipermanent needle (known as de qi induction) is a factor that affects the efficacy of AA. To maintain the blinding of the study, the NSA group also used this daily stimulation, which may have contributed to an increased effect size of the technique in this group. Fourth, the study’s follow-up loss (36%) may have impacted the results. However, adherence to depression treatment remains a relevant and common challenge to overcome in clinical trials. A study by Olfson et al33 found that 36.4% of patients discontinued antidepressant treatment within the first 30 days. A meta-analysis34 reported a 36.4% dropout rate for cognitive behavioral therapy. A study35 on acupuncture in depression treatment observed a dropout rate similar to the present study. This trend of treatment discontinuation seems to be associated with the characteristics of the disease itself (apathy, hopelessness, and lethargy). Regardless of the chosen treatment, patients with depression are likely to discontinue treatment.36
First-line therapy for depression is medication, but several factors interfere with treatment access and adherence, such as system barriers and clinical and individual factors.37 Therefore, it is necessary to offer a wide range of evidence-based therapies to treat depression, aiming at person-centered mental health care.38 AA may be an option for patients who reject medication or psychotherapy because it has fewer adverse effects than pharmacological treatment. A systematic review39 of adverse events associated with AA found that most were transient, mildly intense, and tolerable. No severe adverse events were identified in our study, indicating that AA is a safe treatment.
Besides the technique’s safety, patients in the SA group had increased rates of symptom remission between 6 weeks and 3 months, which may be associated with the delayed effects of AA treatment.40 We should underscore that in AA, part of the technique’s effect is attributed to stimulating the auricular branch of the vagus nerve, increasing parasympathetic activity while simultaneously decreasing sympathetic nervous system activity.41,42 This effect may lead to an improvement in depression-related symptoms.13 A systematic review43 concluded that trigeminal nerve stimulation and transcutaneous vagus nerve stimulation improve the treatment of specific neuropsychiatric disorders, such as depression.
An additional hypothesis is that AA may reduce serotonin depletion by inhibiting the hyperactive hypothalamic-pituitary-adrenal axis, which would explain the technique’s prolonged effect at the 3-month follow-up.44 Experimental studies confirmed this hypothesis, showing that the antidepressant effect of AA is possibly associated with the normalization of this axis’ hyperactivity.45 Additionally, some studies10,13observed that AA can trigger cardioinhibitory effects similar to vagus nerve stimulation. It is essential to highlight that participants in the NSA group may have also experienced temporary inhibition of this axis due to the perception of having received adequate treatment. However, this effect tends to decrease gradually after the end of the treatment sessions.
This study has strengths. First, the research addressed the selection of AA points, providing a solid foundation for future large-scale studies. In addition, the study included a more extended study period and experienced acupuncturists who used semipermanent needles, which are considered more effective than other stimuli. The control group received needling in nonspecific areas, and blinding measures were implemented for assessors, participants, and the statistician.
Limitations
The present study also has limitations. The predominance of women in the sample precluded the detection of possible gender differences in the results. Using self-report instruments to measure the primary outcome may also introduce bias into the results. Other major limitations include the limited sample size and the relatively high loss of participants during the follow-up period. Additionally, an NSA group with positive results indicates the need to investigate better the mechanisms of action and nonspecific outcomes associated with AA.
Conclusions
The results of this randomized clinical trial suggest that SA over 6 weeks is feasible and safe. Although the differences between SA and NSA groups did not reach statistical significance for the primary outcome, SA did produce greater symptom remission at 3 months. A more extensive sample size study, more protracted intervention, and objective evaluation of the results are necessary to further investigate the efficacy of SA for depression.
Study Protocol
eTable 1. Inclusion and Exclusion Criteria
eFigure 1. Data Collection Procedures
eFigure 2. Points Applied in the Auricular Pavilion of the Specific Auricular Acupuncture (SA) Group
eTable 2. Universal Nomenclature, Location, and Indications of the Points Used in the Groups
eFigure 3. Points Applied in the Auricular Pavilion of the Nonspecific Auricular Acupuncture (NSA) Group
eTable 3. Primary and Secondary Outcomes of the Study
eTable 4. Loss to Follow-Up Throughout the Study
eTable 5. Baseline Depression Score Analysis for Participants Who Did Not Complete the Study
eTable 6. Secondary Outcomes of Depression Scores by Intention-to-Treat Analysis
eTable 7. Secondary Efficacy Outcomes in Depression Recovery and Remission by Modified Intent-to-Treat Analysis
eTable 8. Secondary Outcomes of Depression Scores by Modified Intent-to-Treat Analysis
eFigure 4. Median Depression Scores by Modified Intent-to-Treat Analysis
eTable 9. Sensitivity Analysis for Depression Recovery With Missing Data Imputation
eTable 10. Sensitivity Analysis for Symptom Remission With Missing Data Imputation
eTable 11. Sensitivity Analysis for Depression Score With Missing Data Imputation
eTable 12. Adjustment of the Generalized Estimating Equations (GEE) Model
eTable 13. Blinding Assessment and Perception of Treatment Efficacy
Data Sharing Statement
References
- 1.World Health Organization . Depression and other common mental disorders: global health estimates. 2017. Accessed October 30, 2023. https://apps.who.int/iris/handle/10665/254610
- 2.GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137-150. doi: 10.1016/S2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World mental health report: Transforming mental health for all. June 16, 2022. Accessed July 30, 2023. https://www.who.int/publications/i/item/9789240049338
- 4.World Health Organization . Mental health. Accessed July 30, 2023. https://www.who.int/health-topics/mental-health#tab=tab_1
- 5.de Fátima Cunha M, de Cássia Gandini R. Compliance and non compliance to the pharmacological treatment for depression. Psic Teor e Pesq. 2009;25(3):409-418. doi: 10.1590/S0102-37722009000300015 [DOI] [Google Scholar]
- 6.Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294. doi: 10.1176/appi.ajp.158.2.289 [DOI] [PubMed] [Google Scholar]
- 7.Jorm AF, Korten AE, Jacomb PA, Christensen H, Rodgers B, Pollitt P. “Mental health literacy”: a survey of the public’s ability to recognise mental disorders and their beliefs about the effectiveness of treatment. Med J Aust. 1997;166(4):182-186. doi: 10.5694/j.1326-5377.1997.tb140071.x [DOI] [PubMed] [Google Scholar]
- 8.Jorm AF, Medway J, Christensen H, Korten AE, Jacomb PA, Rodgers B. Public beliefs about the helpfulness of interventions for depression: effects on actions taken when experiencing anxiety and depression symptoms. Aust N Z J Psychiatry. 2000;34(4):619-626. doi: 10.1080/j.1440-1614.2000.00761.x [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Wen J, Hong J. The effects of auricular therapy for cancer pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:1618767. doi: 10.1155/2020/1618767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Marca R, Nedeljkovic M, Yuan L, Maercker A, Elhert U. Effects of auricular electrical stimulation on vagal activity in healthy men: evidence from a three-armed randomized trial. Clin Sci (Lond). 2010;118(8):537-546. doi: 10.1042/CS20090264 [DOI] [PubMed] [Google Scholar]
- 11.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15(1):35-37. doi: 10.1002/ca.1089 [DOI] [PubMed] [Google Scholar]
- 12.Carreno FR, Frazer A. The allure of transcutaneous vagus nerve stimulation as a novel therapeutic modality. Biol Psychiatry. 2016;79(4):260-261. doi: 10.1016/j.biopsych.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 13.Wu C, Liu P, Fu H, et al. Transcutaneous auricular vagus nerve stimulation in treating major depressive disorder: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(52):e13845. doi: 10.1097/MD.0000000000013845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrêa HP, Moura CC, Azevedo C, Bernardes MFVG, Mata LRFPD, Chianca TCM. Effects of auriculotherapy on stress, anxiety and depression in adults and older adults: a systematic review. Rev Esc Enferm USP. 2020;54:e03626. [DOI] [PubMed] [Google Scholar]
- 15.MacPherson H, Altman DG, Hammerschlag R, et al. ; STRICTA Revision Group . Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 2010;7(6):e1000261. doi: 10.1371/journal.pmed.1000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Focks C. Atlas de Acupuntura: Com Sequência de Fotos e Ilustrações, Textos Didáticos e Indicações Clínicas. Manole; 2005. [Google Scholar]
- 18.Lv X, Wang B, Jianbin B, Ye J. Clinical observation of depression after breast cancer operation treated with aurieular point sticking therapy. Article in Chinese. Zhongguo Zhen Jiu. 2015;35(5):447-450. [PubMed] [Google Scholar]
- 19.Zhang CS, Yang AW, Zhang AL, May BH, Xue CC. Sham control methods used in ear-acupuncture/ear-acupressure randomized controlled trials: a systematic review. J Altern Complement Med. 2014;20(3):147-161. doi: 10.1089/acm.2013.0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.do Prado JM. Aplicação da acupuntura auricular verdadeira e sham no tratamento do estresse. In: Enfermeiros. Escola de Enfermagem da Universidade de São Paulo; 2014. [Google Scholar]
- 21.Dincer F, Linde K. Sham interventions in randomized clinical trials of acupuncture—a review. Complement Ther Med. 2003;11(4):235-242. doi: 10.1016/S0965-2299(03)00124-9 [DOI] [PubMed] [Google Scholar]
- 22.Valiani M, Mansourian M, Ashtari F. The effect of auriculotherapy on stress, anxiety, and depression in MS patients: a double blind randomized clinical control trial (parallel design). Acta Med Mediter. 2018;34(2):561-567. doi: 10.19193/0393-6384_2018_2s_89 [DOI] [Google Scholar]
- 23.Carter K, Olshan-Perlmutter M, Marx J, Martini JF, Cairns SB. NADA ear acupuncture: an adjunctive therapy to improve and maintain positive outcomes in substance abuse treatment. Behav Sci (Basel). 2017;7(2):37. doi: 10.3390/bs7020037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergdahl L, Broman JE, Berman AH, Haglund K, von Knorring L, Markström A. Auricular acupuncture and cognitive behavioural therapy for insomnia: a randomised controlled study. Sleep Disord. 2016;2016:7057282. doi: 10.1155/2016/7057282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Mansilla J, González López-Arza MV, Varela-Donoso E, Montanero-Fernández J, González Sánchez B, Garrido-Ardila EM. The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: a randomized controlled trial. Clin Rehabil. 2015;29(7):683-693. doi: 10.1177/0269215514554240 [DOI] [PubMed] [Google Scholar]
- 26.Jiao Y, Han Y, Li X, et al. Comparison of body, auricular, and abdominal acupuncture treatments for insomnia differentiated as internal harassment of phlegm-heat syndrome: an orthogonal design. Evid Based Complement Alternat Med. 2015;2015:578972. doi: 10.1155/2015/578972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohr P, Rodriguez M, Slavíčková A, Hanka J. The application of vagus nerve stimulation and deep brain stimulation in depression. Neuropsychobiology. 2011;64(3):170-181. doi: 10.1159/000325225 [DOI] [PubMed] [Google Scholar]
- 28.Wang XR, Song DD, Tao TQ, et al. Qi-regulating and blood circulation-promoting therapy improves health status of stable angina pectoris patients with depressive symptoms. Evid Based Complement Alternat Med. 2021;2021:7319417. doi: 10.1155/2021/7319417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trivedi MH, Rush AJ, Wisniewski SR, et al. ; STAR*D Study Team . Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40. doi: 10.1176/appi.ajp.163.1.28 [DOI] [PubMed] [Google Scholar]
- 30.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi: 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 31.Cuijpers P, Karyotaki E, Ciharova M, Miguel C, Noma H, Furukawa TA. The effects of psychotherapies for depression on response, remission, reliable change, and deterioration: a meta-analysis. Acta Psychiatr Scand. 2021;144(3):288-299. doi: 10.1111/acps.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rush AJ, Kraemer HC, Sackeim HA, et al. ; ACNP Task Force . Report by the ACNP task force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841-1853. doi: 10.1038/sj.npp.1301131 [DOI] [PubMed] [Google Scholar]
- 33.Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry. 2006;163(1):101-108. doi: 10.1176/appi.ajp.163.1.101 [DOI] [PubMed] [Google Scholar]
- 34.Fernandez E, Salem D, Swift JK, Ramtahal N. Meta-analysis of dropout from cognitive behavioral therapy: magnitude, timing, and moderators. J Consult Clin Psychol. 2015;83(6):1108-1122. doi: 10.1037/ccp0000044 [DOI] [PubMed] [Google Scholar]
- 35.Mischoulon D, Brill CD, Ameral VE, Fava M, Yeung AS. A pilot study of acupuncture monotherapy in patients with major depressive disorder. J Affect Disord. 2012;141(2-3):469-473. doi: 10.1016/j.jad.2012.03.023 [DOI] [PubMed] [Google Scholar]
- 36.de Lorent L, Agorastos A, Yassouridis A, Kellner M, Muhtz C. Auricular acupuncture versus progressive muscle relaxation in patients with anxiety disorders or major depressive disorder: a prospective parallel group clinical trial. J Acupunct Meridian Stud. 2016;9(4):191-199. doi: 10.1016/j.jams.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association . American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders: Compendium 2006. American Psychiatric Publishing; 2006. [Google Scholar]
- 38.World Health Organization . WHO traditional medicine strategy 2014-2023. May 13, 2013. Accessed October 30, 2023. https://www.who.int/publications/i/item/9789241506096
- 39.Tan JY, Molassiotis A, Wang T, Suen LKP. Adverse events of auricular therapy: a systematic review. Evid Based Complement Alternat Med. 2014;2014:506758. doi: 10.1155/2014/506758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Qin W, Guo Q, et al. Divergent neural processes specific to the acute and sustained phases of verum and SHAM acupuncture. J Magn Reson Imaging. 2011;33(1):33-40. doi: 10.1002/jmri.22393 [DOI] [PubMed] [Google Scholar]
- 41.Rong PJ, Zhao JJ, Li YQ, et al. Auricular acupuncture and biomedical research: a promising Sino-Austrian research cooperation. Chin J Integr Med. 2015;21(12):887-894. doi: 10.1007/s11655-015-2090-9 [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Hernando D, Fernández-de-Las-Peñas C, Pareja-Grande JA, García-Esteo FJ, Mesa-Jiménez JA. Management of auricular transcutaneous neuromodulation and electro-acupuncture of the vagus nerve for chronic migraine: a systematic review. Front Neurosci. 2023;17:1151892. doi: 10.3389/fnins.2023.1151892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiozawa P, Silva ME, Carvalho TC, Cordeiro Q, Brunoni AR, Fregni F. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review. Arq Neuropsiquiatr. 2014;72(7):542-547. doi: 10.1590/0004-282X20140061 [DOI] [PubMed] [Google Scholar]
- 44.Le JJ, Yi T, Qi L, Li J, Shao L, Dong JC. Electroacupuncture regulate hypothalamic-pituitary-adrenal axis and enhance hippocampal serotonin system in a rat model of depression. Neurosci Lett. 2016;615:66-71. doi: 10.1016/j.neulet.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 45.Liu RP, Fang JL, Rong PJ, et al. Effects of electroacupuncture at auricular concha region on the depressive status of unpredictable chronic mild stress rat models. Evid Based Complement Alternat Med. 2013;2013:789674. doi: 10.1155/2013/789674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eTable 1. Inclusion and Exclusion Criteria
eFigure 1. Data Collection Procedures
eFigure 2. Points Applied in the Auricular Pavilion of the Specific Auricular Acupuncture (SA) Group
eTable 2. Universal Nomenclature, Location, and Indications of the Points Used in the Groups
eFigure 3. Points Applied in the Auricular Pavilion of the Nonspecific Auricular Acupuncture (NSA) Group
eTable 3. Primary and Secondary Outcomes of the Study
eTable 4. Loss to Follow-Up Throughout the Study
eTable 5. Baseline Depression Score Analysis for Participants Who Did Not Complete the Study
eTable 6. Secondary Outcomes of Depression Scores by Intention-to-Treat Analysis
eTable 7. Secondary Efficacy Outcomes in Depression Recovery and Remission by Modified Intent-to-Treat Analysis
eTable 8. Secondary Outcomes of Depression Scores by Modified Intent-to-Treat Analysis
eFigure 4. Median Depression Scores by Modified Intent-to-Treat Analysis
eTable 9. Sensitivity Analysis for Depression Recovery With Missing Data Imputation
eTable 10. Sensitivity Analysis for Symptom Remission With Missing Data Imputation
eTable 11. Sensitivity Analysis for Depression Score With Missing Data Imputation
eTable 12. Adjustment of the Generalized Estimating Equations (GEE) Model
eTable 13. Blinding Assessment and Perception of Treatment Efficacy
Data Sharing Statement