This cohort study evaluates the use of pancreatic replacement therapy for maladaptive behaviors in preschool children with autism spectrum disorder.
Key Points
Question
Does high-protease pancreatic replacement therapy in preschool children with autism spectrum disorder (ASD) improve long-term maladaptive behaviors?
Findings
In this cohort study, 190 children aged 3 to 6 years with ASD treated with high-protease pancreatic therapy improved the maladaptive behaviors of irritability and agitation on the Aberrant Behavior Checklist both in the double-blind randomized clinical trial segment and in a long-term delayed-start analysis. No safety issues emerged.
Meaning
These findings suggest improvement in maladaptive behaviors in preschool children with ASD, such as irritability or agitation, can be seen with the use of high-protease pancreatic replacement therapy.
Abstract
Importance
There is an urgent unmet need for a treatment addressing the core symptoms and associated maladaptive symptoms of autism spectrum disorder (ASD), especially in preschool populations.
Objectives
To evaluate whether treatment of children with ASD aged 3 to 6 years treated with high-protease pancreatic therapy produces long- and short-term improvements in autism-associated maladaptive behaviors.
Design, Setting, and Participants
This cohort study at 32 sites across the US used a double-blind parallel group, delayed-start design comprising a 2-week blinded placebo run-in, and a double-blind, randomized, placebo-controlled segment (12 weeks). Children were recruited into the study in 2015, with data collection continuing until 2021. The analyses were completed from June 2021 to February 2022.
Interventions
All participants were randomly assigned to receive either 900 mg high-protease pancreatic replacement therapy or placebo with food 3 times a day for 12 weeks, followed by all receiving 900 mg high-protease pancreatic replacement therapy for 24 weeks.
Main Outcomes and Measures
The primary outcome was the irritability/agitation subscale of the Aberrant Behavior Checklist (ABC-I). All potential participants were screened using the Social Communication Questionnaire (SCQ) with diagnosis confirmed by the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) for ASD and the Autism Diagnostic Inventory–Revised (ADI-R). Outcomes were measured at the conclusion of the 12-week double-blind segment and at the conclusion of the 24-week open-label segment (total 36 weeks).
Results
A total of 190 participants (150 male [79%]), aged 3 to 6 (mean [SD] age, 4.5 [0.8]) years were randomized. Mixed model for repeated measures analysis performed on ABC-I demonstrated statistically significant differences of −2.49 (95% CI, −4.66 to −0.32; Cohen d = 0.364; P = .03) at the 12-week timepoint and -3.07 (95% CI, −5.81 to −0.33; Cohen d = 0.516; P = .03) at 36-week timepoint. No convergence was noted. Our high-protease pancreatic replacement (CM-AT) was well tolerated with no emergent safety concerns or related serious adverse events noted.
Conclusions and Relevance
This cohort study of preschool children sustained cumulative reduction in the maladaptive behavior of irritability in autism. This delayed-start analysis, used to demonstrate disease and condition modification, may prove to be an important tool to evaluate treatments for ASD.
Trial Registration
ClinicalTrials.gov Identifier: NCT02410902 and NCT02649959
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder in which individuals demonstrate persistent core deficits in social communication and social interaction along with restricted, repetitive patterns of behavior, interests, or activities.1 ASD appears to be increasing in global prevalence without a clear cause.2 There is a large, global unmet need for ASD treatments, as no pharmacologic intervention has been approved in the US or worldwide for the treatment of the core symptoms of ASD.3 ASD symptoms and their severity and presentation vary widely. Individuals with ASD possess a unique combination or matrix of behaviors and corresponding levels of function that affect quality of life.4
Multiple causes have been hypothesized, including genetic and environmental, but none to date has been clearly identified in ASD.5,6 The recent article, “The Lancet Commission on the Future of Care and Clinical Research in Autism,”7 focused on the importance of early developmental years in the formation of autistic behaviors and the need for commencement of treatments during those early years. Ninety percent of brain growth and development occurs between the ages of 0 and 6 years; therefore, targeting treatment during this time is important for autism research and drug development.8
Disruptions of neurotransmitter systems have been associated with the pathogenesis of ASD including serotonin, dopamine, and γ-aminobutyric acid (GABA)/glutamine.5,9 Proper functioning of the serotonin and dopamine neurotransmitter systems requires essential amino acids, tryptophan and phenylalanine, that are obtained exogenously from food through protein digestion. The GABA/glutamine system requires a nonessential amino acid, glutamic acid. It has long been known that there are deficits in brain synthesis and utilization of serotonin in ASD.9,10 Previous research has demonstrated that individuals with ASD have low levels of circulating amino acids.11 Heil12 found that 65% of children with ASD have deficient levels of chymotrypsin enzyme activity. This will result in a lowered pool of essential amino acids including tryptophan (serotonin), phenylalanine (dopamine), leucine, and methionine.13,14,15,16
Serotonin is manufactured mainly in the gastrointestinal tract and in the brain from exogenous protein digestion resulting in a pool of tryptophan.17,18 Because serotonin cannot pass the blood brain barrier, it must be manufactured locally in the brain from tryptophan around the age of 2 years. Tryptophan is released from polypeptide breakdown of protein in the gut through the action of proteases, mainly chymotrypsin. Tryptophan subsequently becomes available for the brain to manufacture serotonin. Tryptophan is transported into the brain circulatory system through the action of specific receptors for tryptophan and other amino acids that facilitate transport. The enzyme chymotrypsin is responsible for cleaving essential amino acids such as phenylalanine, tryptophan, methionine, and leucine from exogenously ingested protein. This action is driven in part by the ability of chymotrypsin to cleave near aromatic residues of some amino acids, an activity not found in trypsin and elastase.18,19,20
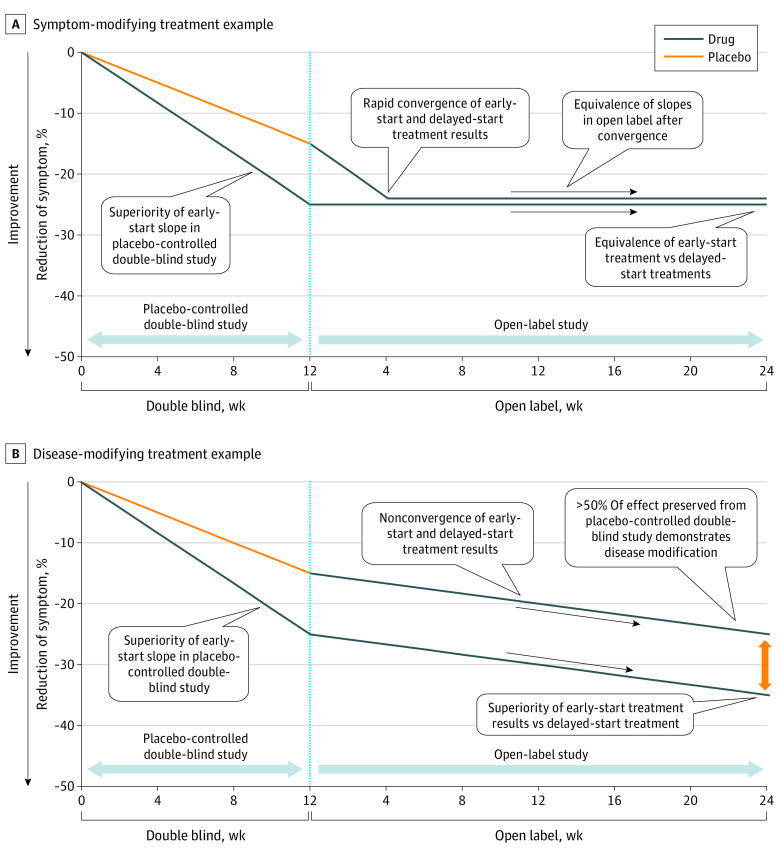
ASD presents as a complex picture of maladaptive behaviors and associated symptoms and poses unique challenges in the development of novel drug treatments. In examining potential treatments for ASD, there are 2 important concepts: looking at sustained reductions in maladaptive behaviors over time that can determine the durability of the pharmacodynamic action of the treatment, and distinguishing between symptom modification from disease or disorder modification. A delayed-start analysis used in this and other studies19,20,21 provides an appropriate context for the study of a neurodevelopmental disease in determining symptom modification from disease or disorder modification. In this type of design, the first phase of the study involves active treatment (early-start group) vs placebo (delayed-start group). In the second phase, all participants receive active treatment and the results were used to determine whether the treatment is symptom-modifying or disease-modifying.21,22,23 As illustrated in Figure 1A, when a treatment is symptom-modifying, participants receiving the active treatment will show a greater reduction in symptoms vs placebo during the double-blind segment, and during the open-label segment, both groups achieve symptom reduction. If a treatment is disease- or disorder-modifying, both groups will demonstrate continued reductions in maladaptive behaviors in the open label segment, with the early-start treatment group maintaining a greater improvement over the late start (placebo) group (Figure 1B).21,22,23,24
Figure 1. Delayed Start Analysis.
The objective of the study was to use a delayed start analysis to assess the safety, tolerability, and durability of high-protease pancreatic replacement and for long-term analysis of data for ASD. Although the delayed start analysis speaks to symptom modifying vs disease modifying, for the purpose of the paper and the nature of ASD, we will refer to the disorder-modifying outcomes of CM-AT on maladaptive behaviors in ASD.
Methods
Study Design
This cohort study was conducted at 32 sites across the US (eTable 1 in Supplement 1). Children were recruited into the study in 2015, with data collection continuing until 2021. The analyses were completed in 2022. Information requiring preapproval, including all protocols and informed consent, were reviewed and approved by regional, central, or investigational center institutional review boards. The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use E6 guidelines.25 The manuscript complies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
The study used a randomized delayed-start design. All potential participants were screened for relevant inclusion and exclusion criteria and their ability to participate in the trial (eTable 2 in Supplement 1). All potential participants were screened using the Social Communication Questionnaire, and diagnosis was confirmed by the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR) for ASD and the Autism Diagnostic Inventory-Revised (ADI-R) by research-reliable raters. The Aberrant Behavior Checklist (ABC)26 was the major dependent measure, with the Irritability/Agitation score being the primary outcome variable (ABC-I). A 2-week blinded placebo run-in was commenced with those who met all eligibility criteria, including an ABC-I subscale score of 11 or greater. This level of Irritability was modeled after the RUPP study where mainly older participants (aged 5-17 years) were enrolled if they had attained an ABC-I score of 18 or greater.27
Participants were randomized to active or placebo 1:1 at the end of the placebo run-in if they (1) maintained all study protocols including inclusion and exclusionary criteria; (2) received a score on the ABC-I post run-in of 9 or greater; and (3) did not have a change in their ABC-I of more than 30% from baseline. The double-blind phase of the trial was 12 weeks, followed by an analysis at 24 weeks of the open-label segment. Study visits were every 2 weeks, followed by visits 4 weeks apart commencing at week 2 of the open-label segment. All participants, investigators, monitors, and the sponsor remained blinded as to participants’ original treatment group. An unblinded safety monitor and safety monitoring group were used. All participants or caregivers provided written informed consent. Assent was not obtained due to the age of the children (all were under age 7 years).
Study medication and placebo were manufactured at Balchem Co. The study medication consisted of a free-flowing powder supplied in a single-use trilaminar foil pouch/sachet. The active consisted of 900 mg of a microencapsulated high-protease pancreatic porcine enzyme sprinkled on food 3 times daily.
Participants
Eligible participants were children who were screened and received a diagnosis from licensed mental health professionals using the Social Communication Questionnaire, DSM-IV-TR and the ADI-R. All ADI-R administrators had obtained research reliability on this instrument.
Major exclusion criteria included (but were not limited to) prohibited medications, history of certain conditions, diagnosed psychiatric diseases excluding those comorbid to ASD, and the use of off-label medications that needed appropriate washout. Major inclusion criteria included age, ASD diagnosis, and appropriate level of ABC-I. Only 1 sibling in a family could be enrolled. Standard medical and psychosocial histories were obtained (eTable 2 in Supplement 1). Self-reported race was included as a variable because the collection of race is particularly important for efficacy studies where regulatory agencies will require this information to ensure that any treatment effects are present in different race groups. Concurrent autism-associated behavioral interventions were allowed but could not be initiated within 30 days of entering the double-blind period of the trial. Nonapproved and off-label medical interventions for ASD were prohibited during the study, along with the initiation of new interventions during the study periods (medical and/or behavioral).
Randomization and Masking
All participants were randomized using a random assignment generator used by an unblinded statistician. Catalent executed the randomization codes for the sites. During the double-blind phase, participants were randomized 1:1 into treatment (early start) and placebo (delayed start). The randomized population could include all participants who successfully completed the initial blinded placebo run-in. Participants, investigators, monitors, and the sponsor were blinded at all times during the study. The data were maintained by a third-party data management group (Target Health, Inc). The safety and study medical monitors had the ability to unblind.
Outcomes
The delayed-start analysis, which was performed on all ABC subscales, was defined by noninferiority testing where the upper limit of the 90% CI was less than 0 as defined by equation 2 in the statistical analysis section. Statistical significance was measured at the conclusion of the 12-week double-blind and at the conclusion of the 24-week open-label segment (total 36 weeks). All safety measurements including safety stool testing took place during the entire study.
Statistical Analysis
Outcome end points were analyzed for the intent-to-treat population, defined as all randomized participants who received the study drug and had at least 1 postbaseline assessment. Each ABC subscale was analyzed for change from baseline to week 12 in the double-blind segment and analyzed through week 36 and 60 for the delayed start. The analysis was performed using a mixed model for repeated measures method with terms for treatment, study center, visit, treatment by-visit interaction, and baseline scores as a covariate, and participants as a random effect. A banded Toeplitz covariance structure was used to model the within-participant errors. The Kenward-Roger approximation was used to estimate denominator degrees of freedom. The comparison of interest was the difference between the early-start and delayed-start treatment groups at weeks 12 and 36. Model-based point estimates (i.e., least square [LS] means for each treatment and the difference between treatments), 95% CIs for the difference, P value, and effect size were calculated for each time point. Effect size was calculated as the LS mean difference divided by an estimate of the pooled SD. The LS mean estimates were then further analyzed using the delayed start noninferiority methods as described in Liu-Seifert et al.21,22 For changes to be considered disease-modifying, the upper bound of the 1-sided 90% CI for Δ2–0.5 Δ1 (where Δ1 is the LS mean difference at week 12 and Δ2 is the LS mean difference at week 36) had to be less than 1, indicating a greater reduction in ABC score for those in the early start group. Statistical analyses were carried out using SAS Version 9.4 (SAS Institute). Data were analyzed from June 2021 to February 2022.
For comparison purposes, mixed-model for repeated measure statistics were generated by calculating percentage improvement for early-start group and delayed-start group mean values at each time point for early and delayed-start participants. Increasingly negative values equate to improvement (a reduction of ABC subscale symptoms). Percentage improvement was calculated as follows, using Equation 1:
| Percentage Improvement = [1 − Measurement Mean / Baseline Mean]. |
To ascertain CM-AT disorder modification, a noninferiority test was performed using LS mean difference week 12 data (end of double-blind) labeled Δ1 and mean square differences for open label weeks 4 through 24 Δ2. For each measurement time point, a noninferiority test was performed according to Equation 2:
| Δ1 – 0.5Δ2 > 0. |
An upper limit of the 90% CI was then calculated. If the upper limit of the 90% CI was less than 0, then the noninferiority criteria were preserved, illustrating disease-modification.21,22,23,24
Results
Participants
In this cohort study, a total of 361 children were screened, of whom 190 were randomized to active treatment (early-start, 92 participants) and placebo (delayed-start, 98 participants). The mean (SD) age of participants was 4.5 (0.8) years and 150 were male (79%) (eTable 3 in Supplement 1). Baseline characteristics of ASD severity were balanced in the active vs placebo groups, as demonstrated by screening ADI-R subscale scores (eTable 4 in Supplement 1). In the open label segment, 151 were eligible to participate with 119 entering the open label (62 former CM-AT and 57 former placebo)
Outcomes
Disorder modification was observed in all subscales of the ABC, except for ABC-Stereotypy, since 90% of the CI was less than zero at the end of 36 weeks. Delayed-start analysis performed on ABC-I demonstrated statistical significance during both the 12-week double-blind segment (Cohen d = 0.36; 95% CI, 1.12 to 3.40; P = .02), and during the 36-week total (Cohen d = 0.51; 95% CI, −0.33 to 5.81; P = .03). No convergence was noted. The confidence interval relates to the treatment differences only and the relationship of the early start to the delayed start groups at 36 weeks (Table 1). Statistically significant improvements were noted in 3 out of 5 subscales at the end of the double-blind segment (12 weeks): ABC-I, Hyperactivity/Noncompliance (ABC-H), and Inappropriate Speech (ABC-IS) and in 4 out of the 5 subscales after 36 weeks: ABC-I, Lethargy/Social Withdrawal (ABC-L), ABC-H, and ABC-IS (Table 2).
Table 1. Treatment Difference and Confidence Interval Limit Testing in Aberrant Behavior Checklist (ABC) Subscales to Determine Disease or Condition Modification (Total 36 Weeks).
| ABC subscale | Double-blind week 12 | Open-label week 24 | Disease-modifying test parametera | Upper limit of 90% CI | Upper limit of 90% CI <0 | ||||
|---|---|---|---|---|---|---|---|---|---|
| LS mean difference, Δ1 (95% confidence limit) | P value | Effect size, Cohen d | LS mean difference, Δ2 (95% confidence limit) | P value | Effect size, Cohen d | ||||
| Irritability/Agitation | −2.49 (−4.66, −0.32) | .03 | 0.36 | −3.07 (−5.81, −0.33) | .03 | 0.52 | −1.82 | −0.33 | Yes |
| Lethargy/Social Withdrawal | −1.03 (−3.00, 0.93) | .30 | 0.17 | −2.57 (−5.05, −0.09) | .04 | 0.48 | −2.05 | −0.09 | Yes |
| Stereotypy | −1.04 (−2.20, 0.12) | .08 | 0.28 | −1.24 (−2.72, 0.25) | .10 | 0.38 | −0.72 | 0.25 | No |
| Hyperactivity/Noncompliance | −2.72 (−5.36, −0.09) | .04 | 0.33 | −3.57 (−6.84, −0.31) | .03 | 0.50 | −2.21 | −0.31 | Yes |
| Inappropriate Speech | −1.00 (−1.77, −0.22) | .01 | 0.41 | −1.36 (−2.35, −0.36) | .01 | 0.63 | −0.86 | −0.36 | Yes |
Abbreviation: LS, least squares.
Disease-modifying test parameter described in Statistical Analysis section.
Table 2. Treatment Difference Statistical Significance to Effect Size to and Confidence Interval in Aberrant Behavior Checklist (ABC) Subscales (Total 36 Weeks).
| Analysis and time point | ABC subscale score | ||||
|---|---|---|---|---|---|
| Irritability/Agitation | Lethargy/Social Withdrawal | Stereotypy | Hyperactivity/Noncompliance | Inappropriate Speech | |
| Baseline | |||||
| Drug baseline value (early-start) | 22.9 | 17.4 | 9.4 | 30.7 | 5.6 |
| Placebo baseline value (delayed-start) | 22.6 | 16.6 | 9.3 | 29.0 | 5.6 |
| Double-blind week 12 | |||||
| ABC MMRM LS mean change drug (early-start) | −7.78 | −7.72 | −3.19 | −9.22 | −1.74 |
| ABC MMRM LS mean change placebo (late-start) | −5.29 | −6.68 | −2.15 | −6.49 | −0.74 |
| Treatment difference (95% CI) | −2.49 (−4.66 to −0.32) | −1.03 (−3.00 to 0.93) | −1.04 (−2.20 to 0.12) | −2.72 (−5.36 to −0.09) | −1.00 (−1.77 to −0.22) |
| P value | .03 | .30 | .08 | .04 | .01 |
| Effect size | 0.364 | 0.167 | 0.283 | 0.328 | 0.406 |
| Open-label week 24 (36 weeks total) | |||||
| ABC MMRM LS mean change drug/drug (early-start) | −9.80 | −9.56 | −3.83 | −11.19 | −1.91 |
| ABC MMRM LS mean change placebo/drug (late-start) | −6.73 | −6.99 | −2.59 | −7.62 | −0.56 |
| P value | 0.028 | 0.042 | 0.103 | 0.032 | 0.008 |
| Effect Size | 0.516 | 0.476 | 0.383 | 0.504 | 0.628 |
| 95% CI | (−5.81 to −0.33) | (−5.05 to −0.09) | (−2.72 to 0.25) | (−6.84 to −0.31) | (−2.35 to −0.36) |
Abbreviations: LS, least squares; MMRM, mixed model for repeated measures.
Delayed-start outcomes are often depicted graphically, as shown in examples in Figure 1, by first calculating the slopes from double-blind segment mean baseline value to the mean week 12 for both early-start (active) and delayed-start (placebo) groups. The result of the open-label LS zero-intercept linear data fits were concatenated onto the double-blind week 12 mean values for their respective groups and plotted on a linear time scale. Slopes were also determined and plotted for both the delayed-start and early-start group means from the double-blind portion of the trial for each ABC subscale.21,22,23,24
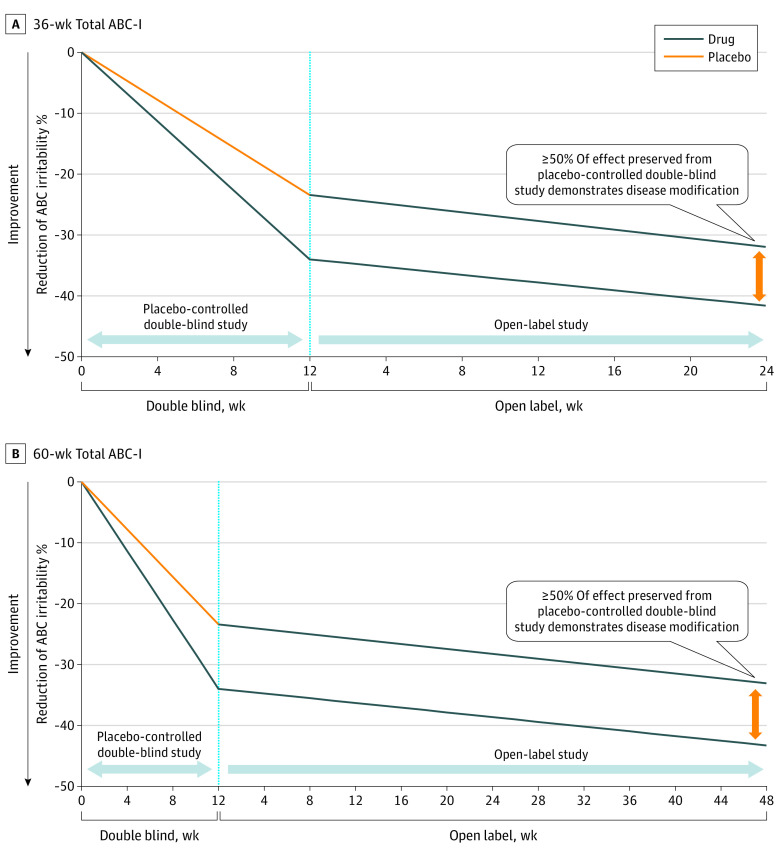
Figure 2 shows 2 delayed start time points of the ABC-I subscale total 36 weeks (Figure 2A) and 60 weeks (Figure 2B). At both time points, disease or disorder modification was demonstrated. The slopes demonstrate disorder modification and statistical significance at both the 12-week conclusion of the double-blind segment and at the end of the 24-week open-label active phase. The slopes of the linear fits for the percentage change improvements of the early-start and delayed-start groups in the open-label segment do not converge, remaining essentially parallel for the duration of the analysis. The parallel improvement of both groups and the preservation of the treatment change of more than 50% of the early-start group was further demonstrative of the disorder-modification of CM-AT on children with ASD aged 3 to 6 years.
Figure 2. Aberrant Behavior Irritability/Agitation (ABC-I) Delayed Start 36-Week and 60-Week Totals (ABC).
Safety
No serious adverse events (SAEs) related or unrelated were seen during the double-blind segment. No related SAEs were seen during the open label segment. Table 3 displays all SAEs and adverse events (AEs) that emerged over 5%. No CM-AT–associated safety signals emerged. Other outcomes, including participant disposition (eTable 5 in Supplement 1), fecal chymotrypsin measurements (eTable 6 in Supplement 1), responder analysis (eTable 7 in Supplement 1), CGI-Severity (eTable 8 in Supplement 1), and CGI-Improvement (eTable 9 and eTable 10 in Supplement 1) can be found in the eAppendix in Supplement 1.
Table 3. Serious Adverse Events (SAE) and AEs (Double-Blind Week 12 to Open-Label Week 24).
| Adverse event | Participants, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Double-blind (12 weeks) | Open label 24 weeks | ||||||
| SAE | AE | SAE | AE among all participants (n = 172) | ||||
| Related | Unrelated | Drug (n = 92) | Placebo (n = 98) | Related | Unrelated | Total AEs | |
| Total AEs | Total AEs | ||||||
| Infection (including colds, flu, croup, ear, sinus, and other) | 0 | 0 | 62 (22) | 72 (26) | 0 | 0 | 15 (9) |
| Gastrointestinal (including vomiting, constipation, diarrhea, flatulence, and upset) | 0 | 0 | 41 (15) | 63 (23) | 0 | 0 | 6 (3) |
| Respiratory (cough, nasal congestion, asthma, and sore throat) | 0 | 0 | 0 | 17 (6) | 0 | 0 | 3 (2) |
| Psychiatric (sleep disturbances, irritability, repetitive behaviors, aggression, and anxiety) | 0 | 0 | 10 (4) | 15 (5) | 0 | 0 | 4 (2) |
Discussion
The nature of ASD as a neurodevelopmental disorder presents some unique challenges. Disorder modification across multiple subscales of the ABC, as demonstrated in this study, could represent a significant improvement in the study and assessment of ASD treatments.
A delayed-start analysis can distinguish symptom modification from disease or disorder modification.21,22 As illustrated in Figure 2, CM-AT demonstrated that it is a disorder-modifying intervention as the early-start group maintained a benefit over the delayed-start group. Both continued to improve on active treatment in the open-label segment while preserving at least 50% of the improvement. The early-start group received a lasting head start over the delayed-start group. In this study, the delayed-start group never converged with the early-start group vs the typical symptom-modifying treatment as illustrated in Figure 1A. At no time was the slope of improvement of the delayed-start group steeper than the slope of improvement of the early-start group over the duration of the study.21,22,23,24
This delayed-start analysis demonstrated disorder modification on 4 of 5 subscales of the ABC. Sustained improvement in children with ASD is an important consideration in clinical trials and studies. A disease or disorder–modifying treatment has the potential of delaying or improving the severity of maladaptive behaviors with early treatment rather than simply modifying the behaviors once they have emerged.
Previous research has demonstrated that individuals with ASD have low levels of circulating amino acids.12 Deficient levels of chymotrypsin in their GI tract may contribute to the lowered pool of amino acids especially tryptophan (serotonin), phenylalanine (dopamine), leucine, and methionine.13,14
Serotonin synthesis peaks at age 6 years in the brains of neurotypical children, but brain synthesis is significantly reduced in children with ASD, suggesting a disruption in serotonergic mechanisms during a time of early growth and development.9,10 Because serotonin cannot pass the blood brain barrier after approximately the age of 2 years, it must be manufactured locally in the brain from the essential amino acid tryptophan. It has been found in ASD that the production of serotonin during the peak manufacture ages of 0 to 6 is only 60% that of neurotypical children10 (eFigure in Supplement 1).
As a trophic factor, serotonin regulates diverse and developmentally critical processes, including cell division, differentiation, migration, myelination, synaptogenesis, neurogenesis, and dendritic pruning. Low levels of serotonin may be associated with differing features of ASD, including deficits in socialization, sleep, and cognition, as well as the presence of anxiety, obsessive compulsive behaviors, depression, and suicidality.9,28 This lack of serotonin production is likely to create what appears to be a neurodevelopmental gap (eFigure 1 in Supplement 1).
Enzyme replacement, therefore, appears to be a more efficient and potentially effective way for the body to take up the amino acids necessary for growth, repair, and other protein functions, especially essential amino acids which need to be obtained exogenously from the diet. Many professionals in the ASD field have tried to establish the connection between the gut and the brain in ASD by connecting maladaptive behaviors with the presence of comorbid GI symptoms. Treatment with CM-AT, a specific high-protease porcine enzyme that demonstrates disorder modification in ASD, may provide an association between gut and brain physiology.9,28
Limitations
The limitations of this study included the narrow age range studied, modest number of participants, duration of study participation, heterogeneity of ASD population, dosing considerations, and instruments used. The lack of validated outcome measures should cultivate the continued development of treatment outcome measures which will help the field identify and compare efficacious interventions and tailored treatments for children with ASD.29
Conclusions
This cohort study of children aged 3 to 6 years with ASD demonstrated sustained cumulative reductions in the maladaptive behavior of irritability. Reductions in maladaptive behaviors that interfere with activities of daily living for this preschool population of children with ASD may potentially improve their quality of life. This delayed-start analysis, used to demonstrate disease or condition modification, may prove to be an important tool used to evaluate treatments for ASD in the future.
eTable 1. Clinical Trial Sites
eTable 2. Inclusion/Exclusion Criteria
eTable 3. Participant Demographics
eTable 4. Autism Diagnostic Interview Revised (ADI-R): Subscale Scores at Baseline
eFigure. Neurodevelopmental Gap in Brain Synthesis Capacity in Autistic Children Compared With Non-Autistic Children
eTable 5. Participant Disposition
eTable 6. Fecal Chymotrypsin Data
eTable 7. Responder Analysis
eTable 8. CGI: Severity
eTable 9. CGI: Improvement
eTable 10. CGI: Improvement—Most Severe
Data Sharing Statement
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; 2000. [Google Scholar]
- 2.Maenner MJ, Warren Z, Williams AR, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill Summ. 2023;72(2):1-14. doi: 10.15585/mmwr.ss7202a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeidan J, Fombonne E, Scorah J, et al. Global prevalence of autism: a systematic review update. Autism Res. 2022;15(5):778-790. doi: 10.1002/aur.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadeem MS, Al-Abbasi FA, Kazmi I, et al. Multiple risk factors: a challenge in the management of autism. Curr Pharm Des. 2020;26(7):743-754. doi: 10.2174/1381612826666200226101218 [DOI] [PubMed] [Google Scholar]
- 5.Marotta R, Risoleo MC, Messina G, et al. The neurochemistry of autism. Brain Sci. 2020;10(3):163. doi: 10.3390/brainsci10030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauer AK, Stanton JE, Hans S, Grabrucker AM. Autism spectrum disorders: etiology and pathology. National Library of Medicine . 2022. Accessed October 22, 2023. https://www.ncbi.nlm.nih.gov/books/NBK573613/
- 7.Lord, C., Charman, T., Havdahl, A., et al. The Lancet Commission on the future of care and clinical research in autism. Lance. 2022;399(10321):271-334. doi: 10.1016/S0140-6736(21)01541-5 [DOI] [PubMed] [Google Scholar]
- 8.Tierney AL, Nelson CA III. Brain development and the role of experience in the early years. Zero Three. 2009;30(2):9-13. [PMC free article] [PubMed] [Google Scholar]
- 9.Kepser L-J, Homberg J. The neurodevelopmental effects of serotonin: a behavioural perspective. Behav Brain Res. 2014;227:3-13. [DOI] [PubMed] [Google Scholar]
- 10.Chugani DC, Muzik O, Behen M, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45(3):287-295. doi: [DOI] [PubMed] [Google Scholar]
- 11.Arnold GL, Hyman SL, Mooney RA, Kirby RS. Plasma amino acids profiles in children with autism: potential risk of nutritional deficiencies. J Autism Dev Disord. 2003;33(4):449-454. doi: 10.1023/A:1025071014191 [DOI] [PubMed] [Google Scholar]
- 12.Heil M, Pearson D, Fallon J. Low endogenous fecal chymotrypsin: a possible biomarker for autism? International Meeting for Autism Research; May 2014. Accessed November 7, 2023. https://www.researchgate.net/publication/268131323_Low_Endogenous_Fecal_Chymotrypsin_A_Possible_Biomarker_for_Autism
- 13.Tirouvanziam R, Obukhanych TV, Laval J, et al. Distinct plasma profile of polar neutral amino acids, leucine, and glutamate in children with autism spectrum disorders. J Autism Dev Disord. 2012;42(5):827-836. doi: 10.1007/s10803-011-1314-x [DOI] [PubMed] [Google Scholar]
- 14.Garbarino V, Gilman T, Daws LC, Gould GG. Extreme enhancement or depletion of serotonin transporter function and serotonin availability in autism spectrum disorder. Pharmacol Res. 2019;140:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyck W, Ammann R, Avraham R. Quantitative determination of fecal chymotrypsin as a screening test for pancreatic exocrine insufficiency. Am J Dig Dis. 1965;10:530-544. doi: 10.1007/BF02233046 [DOI] [PubMed] [Google Scholar]
- 16.Cavallini G, Benini L, Brocco G, et al. The fecal chymotrypsin photometric assay in the evaluation of exocrine pancreatic capacity. Comparison with other direct and indirect pancreatic function tests. Pancreas. 1989;4(3):300-304. doi: 10.1097/00006676-198906000-00005 [DOI] [PubMed] [Google Scholar]
- 17.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002-1012. doi: 10.1038/nrn1256 [DOI] [PubMed] [Google Scholar]
- 18.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF. Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience. 2017;342:212-231. doi: 10.1016/j.neuroscience.2016.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang CJ, Tan HP, Du YJ. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience. 2014;267:1-10. doi: 10.1016/j.neuroscience.2014.02.021 [DOI] [PubMed] [Google Scholar]
- 20.Abdulamir HA, Abdul-Rasheed OF, Abdulghani EA. Serotonin and serotonin transporter levels in autistic children. Saudi Med J. 2018;39(5):487-494. doi: 10.15537/smj.2018.5.21751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu-Seifert H, Andersen SW, Lipkovich I, Holdridge KC, Siemers E. A novel approach to delayed-start analyses for demonstrating disease-modifying effects in Alzheimer’s disease. PLoS One. 2015;10(3):e0119632. doi: 10.1371/journal.pone.0119632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu-Seifert H, Siemers E, Holdridge KC, et al. Delayed-start analysis: mild Alzheimer’s disease patients in solanezumab trials, 3.5 years. Alzheimers Dement. 2015;1(2):111-121. doi: 10.1016/j.trci.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agostino RB Sr. The delayed-start study design. N Engl J Med. 2009;361(13):1304-1306. doi: 10.1056/NEJMsm0904209 [DOI] [PubMed] [Google Scholar]
- 24.Olanow CW, Rascol O, Hauser R, et al. ; ADAGIO Study Investigators . A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361(13):1268-1278. doi: 10.1056/NEJMoa0809335 [DOI] [PubMed] [Google Scholar]
- 25.ICH . ICH Guidelines. 2009. Accessed October 21, 2023. https://www.ich.org/page/quality-guidelines
- 26.Aman MG, Singh NN. Aberrant Behavior Checklist: Community Supplementary Manual. In: Volkmar FR, ed. Encyclopedia of Autism Spectrum Disorders. Slosson Educational Publications; 1994: 10-17. [Google Scholar]
- 27.McCracken JT, McGough J, Shah B, et al. ; Research Units on Pediatric Psychopharmacology Autism Network . Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347(5):314-321. doi: 10.1056/NEJMoa013171 [DOI] [PubMed] [Google Scholar]
- 28.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi: 10.3390/nu8010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grzadzinski R, Janvier D, Kim SH. Recent developments in treatment outcome measures for young children with autism spectrum disorder (ASD). Semin Pediatr Neurol. 2020;34:100806. doi: 10.1016/j.spen.2020.100806 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Clinical Trial Sites
eTable 2. Inclusion/Exclusion Criteria
eTable 3. Participant Demographics
eTable 4. Autism Diagnostic Interview Revised (ADI-R): Subscale Scores at Baseline
eFigure. Neurodevelopmental Gap in Brain Synthesis Capacity in Autistic Children Compared With Non-Autistic Children
eTable 5. Participant Disposition
eTable 6. Fecal Chymotrypsin Data
eTable 7. Responder Analysis
eTable 8. CGI: Severity
eTable 9. CGI: Improvement
eTable 10. CGI: Improvement—Most Severe
Data Sharing Statement