Abstract
The potential association between antimicrobial mouthwash use and systemic health has gained attention in recent years with reports highlighting how some common systemic conditions are influenced by the use of different types of mouthwashes. In this context, links between mouthwash use and cardiovascular disease, diabetes mellitus, oral cancer, Alzheimer’s disease, and preeclampsia have been proposed, albeit with limited levels of evidence. Chlorhexidine mouthwash in particular has been the most widely studied agent while available data on other types of over-the-counter mouthwashes are generally scarce. Furthermore, there is currently no evidence-based recommendations on the appropriate use of mouthwashes during pregnancy. This article will present the current evidence on the association between mouthwash use and the aforementioned conditions with emphasis on the mechanisms that may underlie such an association.
Key words: Mouthwash, Chlorhexidine, Periodontitis, Cardiovascular disease
Introduction
Antimicrobial mouthwash use represents a common oral health care practice, as such agents are widely available. Evidence on their antiplaque actions, whether used alone or as an adjunctive treatment to mechanical oral hygiene measures, is presented in the preceding papers using the highest level of evidence available.1,2 Alongside their potential local benefits, there are some data emerging—albeit limited—that mouthwashes, like many agents, also have the potential to increase the risk of, or worsen, common systemic disorders. Here, we highlight recent evidence on the potential association between antimicrobial mouthwash use and the risk of systemic conditions (or improving them), where there is sufficient evidence to support these associations—for example, diabetes, cardiovascular disease (CVD), oral cancer, Alzheimer's disease (AD), and preeclampsia—focusing on areas of systemic health where recently discovered mechanisms underpin such associations. Most evidence at present involves chlorhexidine (CHX), although systematic reviews or Cochrane Reviews are not forthcoming for most health conditions or different types of over-the-counter (OTC) mouthwashes. Surprisingly, there is also limited evidence for appropriate OTC mouthwash use during pregnancy or links between mouthwash use and conditions such as rheumatoid arthritis and other cancers, where links have been reported between oral and systemic health. Here we outline the mechanisms that link oral health and systemic diseases, the association of oral health and CVD, oral cancer, AD, and preeclampsia.
Mechanisms linking oral health and systemic diseases
Regardless of the underlying cause, an accumulation of dental plaque predisposes to plaque-induced caries, gingivitis, and periodontitis. This last disorder is considered to be the most common noncommunicable chronic inflammatory process in humans and possibly a major contributor to early permanent tooth loss.3 Periodontitis is known to be associated with microbial dysbiosis affecting periodontal pockets.4,5
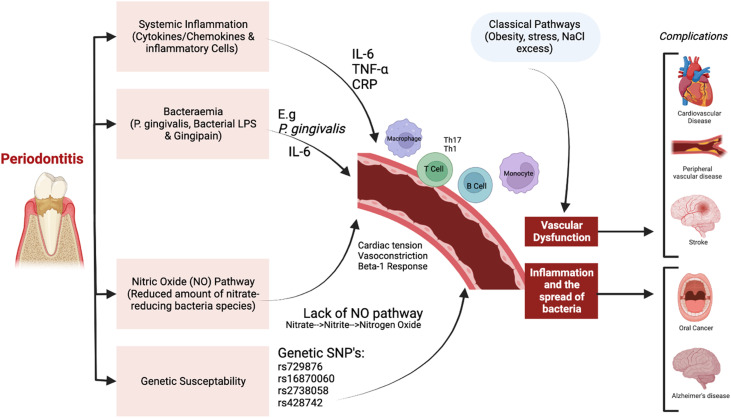
Periodontal inflammation and oral microbial dysbiosis may be a risk factor for causing, or more likely worsening, a wide range of systemic disorders, particularly CVD and diabetes mellitus. The vascular mechanisms thought to explain these links include (1) systemic inflammation due to cytokines released into the bloodstream during bacterial dysbiosis, (2) bacteraemia (pathogenic oral bacteria entering the bloodstream), (3) the oral bacterial enterosalivary pathway (with systemic nitric oxide [NO] release), and (4) genetic susceptibility. Very limited evidence is available for this latter mechanism, although there is recent work that indicated a potential genetic link between periodontitis and elevated blood pressure.6 Recently, the enterosalivary nitrate-nitrite pathway (Figure) has been demonstrated as a physiologic pathway where inorganic nitrate from exogenous (diet) and endogenous (NO metabolism) sources are reduced to nitrite in the oral cavity by the action of nitrate-reducing bacteria such as Veillonella, Actinomyces, Haemophilus, and Neisseria.7,8 Nitrite is reduced into NO in the stomach, with this reaction driven by bacteria, as mammals lack nitrate-reductase enzymes. Thus, oral nitrate-reducing bacteria may be critical in maintaining NO availability in humans and essential for both oral and cardiovascular health.9
Fig.
Proposed causal pathways for the association between oral health and systemic complications. Constructed using Biorender (Mehrotra et al. 2021; Munoz-Aguilera et al. 2019; Oparil et al. 2018).
Oral health and CVD
In the past, interest in any links between oral disease and CVD was principally focused upon the risk of infective endocarditis arising as a consequence of bacteraemia of microbes of origin. However, over the last 3 decades, it has become evident that plaque-induced periodontal inflammation may drive certain aspects of CVD. The mechanisms that underlie the increased risk of CVD by periodontitis are well reviewed elsewhere, but centre around the effects of bacteraemia of periodontal pathogens and, possibly more importantly, a spectrum of cytokine-driven events that culminate in an increased occurrence of arteriosclerosis.10 Interleukin-6 (IL-6) is one of the foremost pro-inflammatory cytokines, with a large role in atherosclerosis development. Importantly, elevated IL-6 is associated with increased blood pressure, linking hypertension to a pro-inflammatory state.11
Increase in systemic inflammatory cytokines may also cause activation of endothelial cells and assist with development of atheroma. It has been postulated that oral host inflammation results in mediators of inflammatory pathways, such as C-reactive protein, IL-6, and tumour necrosis factor-α, effecting endothelial function.12 Other immunologic inflammatory cells such as T cells, B cells, and monocyte/macrophages are present in inflamed periodontium. These immune cells may recruit perivascular adipose tissue, leading to inflammation and damage of the blood vessel walls, consequentially causing vascular dysfunction and hypertension.13
The oral microbiome may also be important in maintaining systemic health, including the maintenance of a lower blood pressure. NO, for example, is naturally formed either through the NO synthase-dependent pathway or by the enterosalivary nitrate-nitrite-NO pathway involving bacteria.14 This pathway is important for homeostasis via oral facultative anaerobic bacteria concentrated on the posterior part of the tongue, which can reduce nitrate (from diet or excreted by salivary glands) to nitrite. Thereafter, nitrite and remaining nitrate are ingested, where nitrite is further reduced to NO either under the influence of gastric acidity or in the systemic circulation by further enzymatic and nonenzymatic pathways.15
NO is recognised to have several roles in the physiology of the cardiovascular system, including maintenance of vascular tone and control of blood pressure, endothelial integrity, inhibition of platelet aggregation, and leukocyte adhesion.15,16 Conversely, reduced NO bioavailability has been associated with impairment of endothelial function and increased risk of hypertension and cardiovascular illnesses.17 Similarly, nitrite availability within the oral cavity is important for oral health for the inhibition of acid production and maintenance of a pH balance to avoid dental caries and periodontitis.18 Additionally, impairment of NO production within diabetes mellitus may reflect the comorbidity between hypertension and diabetes mellitus. Endothelial NO synthase concentrations are reportedly low in patients with unstable angina, and reduced NO levels can indirectly increase the risk of atherosclerosis.15 Therefore, any change in NO homeostasis is considered to be critical for the risk of untoward cardiovascular events. In this context, it should be noted that NO bioavailability in medically compromised patients is easier to be disturbed as compared to healthy individuals.15
CVD and mouthwashes
There is a number of reports suggesting that antimicrobial mouthwashes may directly worsen CVD associated with the atherosclerotic process.19, 20, 21, 22 The underlying mechanisms for such an association between mouthwashes and CVD have not yet been fully elucidated; however, there is accumulating evidence on the impact of antimicrobial mouthwashes on the oral microbiome as a likely aetiology.20,23
CHX use in particular has been found to cause a shift in the oral microbiome leading to a possibly unfavourable decline in salivary nitrite concentration, which in turn has been postulated to lead to adverse effects on blood pressure control both in healthy adults and those with hypertension.20,23 The results of a longitudinal 3-year-study found that the daily use of OTC mouthwashes (irrespective of its constituents) represents an independent risk factor for the development of prediabetes/diabetes mellitus and hypertension.22,24 Interestingly, individuals who did not have recognised risk factors for hypertension (e.g., tobacco and/or alcohol use, high-salt diet, or old age) were more prone to the effects of mouthwash use on increasing blood pressure than those who did have identifiable risks. Notwithstanding, the researchers acknowledged the need for further studies for verification.
The risk of hospital mortality for intensive care unit patients appears to increase with the use of CHX for oral care, as shown in several reports, including meta-analyses.25, 26, 27 A reduction in the bioavailability of NO with the use of CHX has been proposed as the reason for these observations.15
The use of 0.12% CHX mouthwash may destroy more than 90% of oral nitrate-reducing bacteria with a resultant 85% reduction in the proportion of reduced nitrate.14 Several human studies have confirmed the association between the frequent use of antimicrobial mouthwashes and an increase in systolic blood pressure ranging from 2 to 5 mm Hg accompanied by a significant reduction in both salivary and plasma nitrite levels.15,19 Thus, this becomes relevant when it is known that even a sustained modest 2-mm Hg elevation in blood pressure can significantly increase the risk of mortality from stroke or ischaemic heart disease in middle-aged patients.28
Tongue cleaning may be an additional risk factor contributing to the CHX-induced increase in systolic blood pressure. Individuals who cleaned their tongue at least twice daily were found to have a higher likelihood of an elevation in their systolic blood pressure with the daily use of CHX for 1 week compared with those who did not clean the tongue.23 A plausible explanation is that tongue cleansing could allow penetration of CHX into the tongue microbiome and therefore increase the risk of disturbing the diversity of bacterial communities.
Interestingly, a recent long-term study conducted in Finland over almost 19 years showed a profound reduction of risk of cardiovascular mortality of 51% with good oral health self-care, and this effect did not differ with additional mouthwash use.29 Although further research is required to determine the role of long-term use of CHX-containing mouthwash on hypertension, it might be prudent to consider avoidance of CHX-containing mouthwashes on a long-term basis in patients at risk for or having hypertension. There appear to be no reported data of the impact of long-term use of CHX-containing agents on hypertension and indeed CVD in general.
Even if systemic availability of NO is altered by antimicrobial mouthwashes, there remain limited data that demonstrate the precise pathogenic mechanisms of any link between mouthwashes and subsequent changes in the oral microbiome and systemic levels of NO which may lead to altered blood pressure or risk of CVD. There is a need to consider the balance between the known benefits of CHX on oral disease and the limited evidence that mouthwashes do impact systemic health adversely. In all probability, the avoidance of such mouthwashes possibly has a small marginal gain on risk of hypertension, arteriosclerosis, and subsequent clinically apparent morbidity and mortality.
Oral health and cancer
There were approximately 145,000 deaths and 300,000 new cases of oral cancer reported in 2021, making this one of the most common malignancies worldwide.30 Accounting for 92% to 95% of all oral cancers, oral squamous cell carcinoma (OSCC) is the most common oral malignancy.31 The overall 5-year survival of OSCC has not improved significantly beyond 50% and fewer than 1 in 5 patients who present with metastatic disease at the time of diagnosis will survive 5 years.32 Many cancers are detected at an advanced stage, when treatment options can be minimal and outcomes poor. Therefore, prevention and early detection will minimise the associated morbidity and mortality of oral cancers.
Oral cancer and mouthwashes
Alcohol consumption is a known risk factor for potentially malignant and malignant disease of the oral mucosa and possibly the gingivae. The risk of oral cancer has been shown to increase by 6.4-fold in nonsmokers who drink alcohol heavily, compared with a risk of 2.1-fold for those smokers who do not drink alcohol.33 There have been suggestions that the alcohol within many commercially available antimicrobial mouthwashes may increase the risk of oral cancer, but at present the evidence is limited and conflicting. However, the exact direct role that the alcohol present in mouthwash (with amounts up to 26%) has upon the development of oral cancer is not easily evident, with only tenuous epidemiologic data to support such a link.
Several systematic reviews and meta-analyses of multiple case-control studies do not consistently demonstrate a link between alcohol- (or indeed non-alcohol-) containing mouthwashes. However, it is recognised that the original data that such reviews have drawn from has not been notably uniform. One such study, an extensive pooled analysis of 8981 patients with head and neck cancer and 10,090 control patients from 12 case-control studies concluded that there was no overall increased risk of head and neck cancer in individuals who ever used mouthwash, but there was an association in long-term frequent users.34
The study, like others, was limited by significant methodologic issues of the available original investigations, but the trend that long-term, rather than short-term, use of mouthwashes does at least have some logic for the assocation of alcohol-containing mouthwash and oral cancer. Similarly, a recent meta-analysis demonstrated a statistically significant correlation between frequent mouthwash usage (more than once per day) and increased risk of upper aerodigestive tract cancer, with a risk increase for oral cancer more than laryngeal cancer—again suggesting logic to a link between mouthwashes and risk of epithelial dysregulation.35 Nevertheless, the lack of a consistent epidemiologic link between antimicrobial mouthwashes of any type and oral malignancy makes it challenging to suggest a mechanistic basis for any such association.
A recent investigation demonstrated that exposure to 20% alcohol causes marked cytotoxicity to oral mucosal cells even after 20 hours’ recovery.36 However, cytotoxicity is unlikely the be a driver of altered cellular function, regulation of cell turnover, and eventual OSCC. Alcohol alone is not thought to be the carcinogen, but rather its metabolic product acetaldehyde. Acetaldehyde is produced from the metabolism of alcohol by several enzymes, alcohol dehydrogenase and cytochrome P450 2E1. Subsequently, acetaldehyde is metabolised to acetate by aldehyde dehydrogenase (ALDH).37 Alcohol metabolism predominantly occurs in the liver; however, up to 30% of alcohol metabolism is extrahepatic.37 Some individuals, particularly those from East Asia, have mutations in the ALDH enzyme that result in diminished ability to metabolise acetaldehyde, with the resultant accumulation causing potential toxic effects, including skin-flushing responses, nausea, and drowsiness.38 Recently, it has been shown that acetaldehyde is produced in the oral cavity after rinsing with alcohol (not ingestion), and this occurs locally within the oral cavity and is dependent on an individual's ALDH alleles and alcohol sensitivity.38 Interestingly, in this small study of healthy young individuals, 20% of individuals “sensitive” to alcohol had measurable oral acetaldehyde levels at mutagenic levels.38 This metabolism could be occurring in both the oral mucosa as well as the oral microbiome, with many oral microbes able to metabolise acetaldehyde.39 There may, however, be a variety of factors that modulate alcohol-derived acetaldehyde exposure and its effects in the oral cavity.37 Nevertheless, it has been proposed that there is currently sufficient evidence to support the hypothesis that alcohol metabolism within the oral cavity is an independent cancer risk factor.37 Furthermore, it has been suggested that alcohol present in “nonalcoholic” beverages and food forms an epidemiologic bias in studies on alcohol-related upper digestive tract cancer.40
There are several clinical benefits for the use of mouthwash, as discussed elsewhere in this supplement. However, these benefits need to be balanced against potential risk of oral cancer. This has previously raised the question “is alcohol-containing mouthwash a justifiable course of clinical treatment to encourage patients to take?”41 Therefore, as stated previously, “While these are not definitive findings, they cannot be ignored and they further support our professional opinion and advice that high frequency and prolonged use of mouthwash is not a routine component of the maintenance of good oral health.”42 However, if required for long-term use, non-alcohol-containing mouthwashes should be recommended, with an obvious need for research required to assess the most ideal non-alcohol-containing mouthwashes or new alternatives. Ideally, a prospective large study that accurately assessed oral cavity exposure to all forms of alcohol could determine the associated risk of oral cancer development. A questionnaire that would aid in this assessment had been developed and validated43; however, any prospective study would require significant funding, and given the pressures upon publicly funded research it is likely that the costs for this would have to come from industrial sources.
Oral health and AD
AD is neurodegenerative disorder that arises in individuals older than 65 years and is characterised by a spectrum of signs and symptoms that include those of dementia. It accounts for up to 75% of all dementias, with the disease doubling in prevalence every 5 years beyond the age of 65 years.44 Estimates vary, but possibly 40 million people are living with AD and by 2050 it is possible that 65 million individuals will be alive with this disorder.44,45 The 2 major pathologic features of AD are the presence of amyloid plaques and neurofibrillary tangles within the central nervous system, with risk factors of AD being wide-ranging and including hypertension, diabetes, and CVD.
Recently, several studies have attempted to evaluate a potential correlation between periodontal disease and AD. It was reported that the bacteria involved in periodontal disease, particularly Gram-negative anaerobic species, produce inflammatory mediators and toxins that can contribute to the development, maintenance, and worsening of systemic diseases in AD.46,47
Some of the key bacterial species present in the biofilm of periodontal disease, such as Porphyromonas gingivalis, Prevotella intermedia, Treponema denticola, and Fusobacterium nucleatum, have been shown to be present in the brain tissue of patients with AD.46 Furthermore, the production of inflammatory mediators by these bacteria can induce neuroinflammation and neurodegeneration,46,48,49 and recent novel research using a murine model suggests that bacteria from distant sites (eg, the gastrointestinal tract) may play a role in neurodegeneration of AD.50
A systematic review by Borsa et al51 found that in addition to the existence of a correlation between periodontal disease and AD, the treatment of periodontal disease may help prevent the progression of AD. Kamer et al52 also highlighted this likely association and recommended that dental surgeons should be a part of the multidisciplinary team treating patients with AD, particularly as there is some evidence that the presence of periodontal inflammation/disease may contribute to cognitive decline in AD. However, this limited evidence also suggests that the oral bacteria of patients with periodontal disease and AD are not notably different to those without AD, and overall there remains little robust evidence to support the notion that periodontal disease is a key causative factor for AD.46,48,49 Clearly, well-designed, randomised, and controlled studies are warranted to determine the exact role, if any, of plaque-induced periodontal inflammation on the risk or progression of AD. Bearing in mind that dementia can be linked to vascular disease (and diabetes mellitus amongst others), an association between periodontal disease and AD would perhaps not be unexpected, but whether there is a direct link amongst the oral microbiome, the consequent local periodontal disease and eventual neurodegeneration seems unlikely as AD does not have as strong links with socioeconomic status as does periodontal disease. Salivary estimation of amyloid beta and tubulin-associated unit (TAU) proteins may be a noninvasive means of monitoring the progression of AD,53 but importantly, regardless of whether oral disease drives neurodegeneration, oral health is essential for people with AD and allied disorders. In particular, strategies must be in place to lessen the risk of plaque-induced disease; caries (and hence pain) and periodontal disease (and hence dysgeusia, oral malodour, dysarthria, compromised eating, and social embarrassment) as well as the need for potentially stressful invasive oral health care.
Mouthwashes and AD
As cognition and motor function are key to individuals being able to physically clean the mouth, mouthwashes may become the only simple means of patients with AD, or indeed their carers, to reduce the oral bacterial (and fungal) load and hence lessen the risk of plaque-induced disease of the teeth and periodontal tissues. As noted in earlier sections, it may be that mouthwashes have a marginal deleterious effect on vascular function and oral mucosal biology, but this is not significant enough to suggest that individuals at risk for or having neurodegenerative disorders such as AD not use or receive therapy with commercially available mouthwashes.
Several methods can help to control the periodontal biofilm, with special emphasis on the chemical-mechanical control.2 Appropriate hygiene measures with mechanical cleaning and toothpaste are certainly the best method of preventing periodontal disease; however, the use of mouthwashes can also be extremely important, especially in patients with motor difficulties and those who have risk factors such as diabetes or smoking.2
The use of antimicrobial mouthwashes in the prevention or control of periodontal disease may help to attenuate the progression of AD; however, there is currently insufficient evidence to support or refute this notion, with further studies needed. Although the presence of similar bacteria in periodontal disease and AD is observed in several studies, there is no robust scientific evidence in the literature that supports a cause-and-effect relationship between periodontal disease and AD.46,48,49 Therefore, it would not be sensible to indicate the constant use of mouthwashes for the prevention of AD. Well-designed, randomised, and controlled studies should be carried out in order to determine the exact association between periodontal disease and AD and to better define whether the control of periodontal disease is effective in the onset and/or control of AD.
Preeclampsia and oral health
Preeclampsia is a potentially life-threatening, multisystemic disorder clinically characterised by sudden-onset hypertension beyond 20 weeks’ gestation and at least 1 other associated complication, including proteinuria, maternal organ dysfunction, or uteroplacental dysfunction. Preeclampsia affects about 4 million women globally each year and may account for about 70,000 maternal deaths and 500,000 child deaths.54 It is thus one of the most significant complications of pregnancy. A key pathophysiologic feature is the development of an abnormal placenta in which the spiral arteries in utero become fibrous and narrow, preventing blood flow to the placenta. Due to hypoperfusion of the placenta, pro-inflammatory protein release is stimulated into the mother's circulation, leading to endothelial cell dysfunction and essentially resulting in vasoconstriction and thus hypertension and kidney damage due to the retention of salt.54,55
Several causative mechanisms have been described linking periodontitis and preeclampsia, particularly in lower-middle-income countries. Future studies will determine whether maternal amelioration of periodontitis prevents preeclampsia; however, further investigation is required to confirm causation.56 Nevertheless, it is yet to be shown whether treatment of periodontitis can prevent preeclampsia.
Preeclampsia and mouthwashes
There is very little information on mouthwash use during “normal” pregnancy, as controlled studies have not been done; most toxicity studies relate to animals where teratogenic effects have not been observed. Across the globe, CHX use is not contraindicated during pregnancy, and it is postulated that its use is appropriate when the benefits outweighs the risk. Considering the aforementioned link, It may be desirable to improve oral health in women at risk for preeclampsia. Currently, however, there is conflicting evidence as to the use of antimicrobial mouthwashes and its effect within foetal gestation and periodontitis associated with preeclampsia.
There is limited evidence of the concurrent use of CHX mouthwash with periodontal therapy in pregnancy. A recent systematic review and meta-analysis has reported that for pregnant women with periodontal disease, using CHX with conventional periodontal treatment, such as scaling, diminished adverse birth outcomes, with lower risks of preterm birth and low birth weight.57 There were significant biases in the studies incorporated into this meta-analysis, and there is a need for further well-conducted randomised controlled trials to ascertain this potential benefit of additional use of mouthwash after periodontal therapy for pregnant women with periodontitis.
Conclusion
Key questions remain regarding definitive proof of the role of mouthwash use and its beneficial or detrimental effects on systemic health. A number of different key research questions are warranted, focused more on epidemiology than establishing pathologic mechanisms: (1) What is the definitive evidence that prolonged use of CHX increases the number of cardiac events and/or lessens the quality of life of those living with CVD? (2) Are non-alcohol-containing antimicrobial mouthwashes as effective as those with alcohol in lessening plaque-induced oral disease? (3) Does regular use of mouthwashes definitively increase or lessen the progression of dementias such as that of AD? However, mouthwashes that lessen plaque-induced oral disease may, in some instances, be the sole mechanism to diminish intraoral plaque load. There is some evidence that antimicrobial mouthwashes may cause harm to the systemic health of an individual, with most evidence relating to CVD; however, currently there is no consistent objective information to definitively suggest that any mouthwash-linked harm outweighs benefit.
Conflict of interest
None disclosed.
Acknowledgments
Funding
The authors have not received any commercial sponsorship directly or indirectly for this review. The narrative review reflects the authors opinions based on evidence considered of the active ingredients of the more widely available mouthwashes. The authors’ views should not necessarily be interpreted as the views of their faculties, universities, or associated organisations.
Author contributions
All contributed equally and industriously.
Footnotes
This article is published as part of a supplement sponsored by FDI World Dental Federation.
REFERENCES
- 1.Ciancio SG. Mouthwashes: rationale for use. Am J Dent. 2015;28 (Spec No A):4a–8a. [PubMed] [Google Scholar]
- 2.James P, Worthington HV, Parnell C, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;3(3) doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan A, Palmer RM. A clinical guide to periodontology: pathology of periodontal disease. Brit Dent J. 2014;216(8):457–461. doi: 10.1038/sj.bdj.2014.299. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz-Carrillo JL, Hernández-Reyes VE, García-Huerta OE, et al. Periodontal disease-diagnostic and adjunctive non-surgical considerations. IntechOpen; 2019. Pathogenesis of periodontal disease. [Google Scholar]
- 5.Gasner NS, Schure RS. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL: 2023. Periodontal disease. 2022. [Google Scholar]
- 6.Munz M, Richter GM, Loos BG, et al. Meta-analysis of genome-wide association studies of aggressive and chronic periodontitis identifies two novel risk loci. Eur J Human Genet. 2019;27(1):102–113. doi: 10.1038/s41431-018-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosier BT, Moya-Gonzalvez EM, Corell-Escuin P, Mira A. Isolation and characterization of nitrate-reducing bacteria as potential probiotics for oral and systemic health. Frontiers in Microbiology. 2020;11 doi: 10.3389/fmicb.2020.555465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blekkenhorst LC, Bondonno NP, Liu AH, et al. Nitrate, the oral microbiome, and cardiovascular health: a systematic literature review of human and animal studies. Am J Clin Nutrition. 2018;107(4):504–522. doi: 10.1093/ajcn/nqx046. [DOI] [PubMed] [Google Scholar]
- 9.Bryan NS, Tribble G, Angelov N. Oral microbiome and nitric oxide: the missing link in the management of blood pressure. Curr Hypertens Rep. 2017;19:1–8. doi: 10.1007/s11906-017-0725-2. [DOI] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazquez-Oliva G, Fernandez-Real JM, Zamora A, Vilaseca M, Badimon L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Human Hypertens. 2005;19(6):457–462. doi: 10.1038/sj.jhh.1001845. [DOI] [PubMed] [Google Scholar]
- 12.Orlandi M, Suvan J, Petrie A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236(1):39–46. doi: 10.1016/j.atherosclerosis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113(9):1009–1023. doi: 10.1093/cvr/cvx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro KB, Hotchkiss JH, Roe DA. Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chem Toxicol. 1991;29(11):751–755. doi: 10.1016/0278-6915(91)90183-8. [DOI] [PubMed] [Google Scholar]
- 15.Blot S. Antiseptic mouthwash, the nitrate-nitrite-nitric oxide pathway, and hospital mortality: a hypothesis generating review. Intensive Care Med. 2021;47(1):28–38. doi: 10.1007/s00134-020-06276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollenberg SM, Cinel I. Bench-to-bedside review: nitric oxide in critical illness–update 2008. Crit Care. 2009;13(4):218. doi: 10.1186/cc7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutrition. 2013;143(6):818–826. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 18.Raizada MK, Joe B, Bryan NS, et al. Report of the National Heart, Lung, and Blood Institute Working Group on the role of microbiota in blood pressure regulation: current status and future directions. Hypertens. 2017;70(3):479–485. doi: 10.1161/HYPERTENSIONAHA.117.09699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bescos R, Ashworth A, Cutler C, et al. Effects of chlorhexidine mouthwash on the oral microbiome. Sci Rep. 2020;10(1):5254. doi: 10.1038/s41598-020-61912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palka L, Nowakowska-Toporowska A, Dalewski B. Is chlorhexidine in dentistry an ally or a foe? A narrative review. Healthcare (Basel) 2022;10(5) doi: 10.3390/healthcare10050764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshipura K, Munoz-Torres F, Fernandez-Santiago J, Patel RP, Lopez-Candales A. Over-the-counter mouthwash use, nitric oxide and hypertension risk. Blood Press. 2020;29(2):103–112. doi: 10.1080/08037051.2019.1680270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tribble GD, Angelov N, Weltman R, et al. Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front Cell Infect Microbiol. 2019;9:39. doi: 10.3389/fcimb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshipura KJ, Munoz-Torres FJ, Morou-Bermudez E, Patel RP. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide. 2017;71:14–20. doi: 10.1016/j.niox.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014;174(5):751–761. doi: 10.1001/jamainternmed.2014.359. [DOI] [PubMed] [Google Scholar]
- 26.Price R, MacLennan G, Glen J, Su DC. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ. 2014;348:g2197. doi: 10.1136/bmj.g2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parreco J, Soe-Lin H, Byerly S, et al. Multi-center outcomes of chlorhexidine oral decontamination in intensive care units. Surg Infect (Larchmt) 2020;21(8):659–664. doi: 10.1089/sur.2019.172. [DOI] [PubMed] [Google Scholar]
- 28.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 29.Janket SJ, Lee C, Surakka M, et al. Oral hygiene, mouthwash usage and cardiovascular mortality during 18.8 years of follow-up. Br Dent J. 2023:1–6. doi: 10.1038/s41415-023-5507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 31.Ganesh D, Sreenivasan P, Öhman J, et al. Potentially malignant oral disorders and cancer transformation. Anticancer Res. 2018;38(6):3223–3229. doi: 10.21873/anticanres.12587. [DOI] [PubMed] [Google Scholar]
- 32.Johnson NW, Warnakulasuriya S, Gupta PC, et al. Global oral health inequalities in incidence and outcomes for oral cancer: causes and solutions. Adv Dent Res. 2011;23(2):237–246. doi: 10.1177/0022034511402082. [DOI] [PubMed] [Google Scholar]
- 33.Maasland DH, van den Brandt PA, Kremer B, Goldbohm RA, Schouten LJ. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: results from the Netherlands Cohort Study. BMC Cancer. 2014;14:187. doi: 10.1186/1471-2407-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boffetta P, Hayes RB, Sartori S, et al. Mouthwash use and cancer of the head and neck: a pooled analysis from the International Head and Neck Cancer Epidemiology Consortium (INHANCE) Eur J Cancer Prev. 2016;25(4):344. doi: 10.1097/CEJ.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahrens W, Pohlabeln H, Foraita R, et al. Oral health, dental care and mouthwash associated with upper aerodigestive tract cancer risk in Europe: the ARCAGE study. Oral Oncol. 2014;50(6):616–625. doi: 10.1016/j.oraloncology.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Calderón-Montaño JM, Jiménez-Alonso JJ, Guillén-Mancina E, Burgos-Morón E, López-Lázaro M. A 30-s exposure to ethanol 20% is cytotoxic to human keratinocytes: possible mechanistic link between alcohol-containing mouthwashes and oral cancer. Clin Oral Investig. 2018;22:2943–2946. doi: 10.1007/s00784-018-2602-z. [DOI] [PubMed] [Google Scholar]
- 37.Stornetta A, Guidolin V, Balbo S. Alcohol-derived acetaldehyde exposure in the oral cavity. Cancers. 2018;10(1):20. doi: 10.3390/cancers10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Himemiya-Hakucho A, Tanaka T, Liu J, Fujimiya T. Effect of alcohol sensitivity in healthy young adults on breath pharmacokinetics of acetaldehyde after mouth washing with alcohol. Alcoholism. 2018;42(11):2100–2106. doi: 10.1111/acer.13878. [DOI] [PubMed] [Google Scholar]
- 39.Moritani K, Takeshita T, Shibata Y, Ninomiya T, Kiyohara Y, Yamashita Y. Acetaldehyde production by major oral microbes. Oral Dis. 2015;21(6):748–754. doi: 10.1111/odi.12341. [DOI] [PubMed] [Google Scholar]
- 40.Salaspuro M, ed. Interrelationship between alcohol, smoking, acetaldehyde and cancer. Acetaldehyde-Related Pathology: Bridging the Trans-Disciplinary Divide: Novartis Foundation Symposium, 285; 2006: Wiley Online Library. [DOI] [PubMed]
- 41.Carr E, Aslam-Pervez B. Does the use of alcohol mouthwash increase the risk of developing oral cancer? Evidence-Based Dentistry. 2022;23(1):28–29. doi: 10.1038/s41432-022-0236-0. [DOI] [PubMed] [Google Scholar]
- 42.Wilson G, Conway DI. Mouthwash use and associated head and neck cancer risk. Evid Based Dent. 2016;17(1):8–9. doi: 10.1038/sj.ebd.6401146. [DOI] [PubMed] [Google Scholar]
- 43.Wirth T, Kawecki MM, Reeve J, Cunningham C, Bovaird I, Macfarlane TV. Can alcohol intake from mouthwash be measured in epidemiological studies? Development and validation of mouthwash use questionnaire with particular attention to measuring alcohol intake from mouthwash. J Oral Maxillofac Res. 2012;3(3) doi: 10.5037/jomr.2012.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 45.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa MJF, de Araújo IDT, da Rocha Alves L, et al. Relationship of Porphyromonas gingivalis and Alzheimer's disease: a systematic review of pre-clinical studies. Clin Oral Investig. 2021;25:797–806. doi: 10.1007/s00784-020-03764-w. [DOI] [PubMed] [Google Scholar]
- 47.Ide M, Harris M, Stevens A, et al. Periodontitis and cognitive decline in Alzheimer's disease. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer's disease. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dioguardi M, Crincoli V, Laino L, et al. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer's disease: a systematic review. J Clin Med. 2020;9(2):495. doi: 10.3390/jcm9020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo D-O, O'Donnell D, Jain N, et al. ApoE isoform–and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science. 2023;379(6628):eadd1236.. doi: 10.1126/science.add1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borsa L, Dubois M, Sacco G, Lupi L. Analysis the link between periodontal diseases and Alzheimer's disease: a systematic review. Int J Environ Res Public Health. 2021;18(17):9312. doi: 10.3390/ijerph18179312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamer AR, Craig RG, Niederman R, Fortea J, de Leon MJ. Periodontal disease as a possible cause for Alzheimer's disease. Periodontology 2000. 2020;83(1):242–271. doi: 10.1111/prd.12327. [DOI] [PubMed] [Google Scholar]
- 53.Kuznetsov IA, Kuznetsov AV. A numerical study of sensitivity coefficients for a model of amyloid precursor protein and tubulin-associated unit protein transport and agglomeration in neurons at the onset of Alzheimer's disease. J Biomech Eng. 2019;141(3) doi: 10.1115/1.4041905. [DOI] [PubMed] [Google Scholar]
- 54.Dimitriadis E, Rolnik DL, Zhou W, et al. Pre-eclampsia. Nat Rev Dis Prim. 2023;9(1):8. doi: 10.1038/s41572-023-00417-6. [DOI] [PubMed] [Google Scholar]
- 55.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366 doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 56.Le QA, Akhter R, Coulton KM, et al. Periodontitis and preeclampsia in pregnancy: a systematic review and meta-analysis. Matern Child Health J. 2022;26(12):2419–2443. doi: 10.1007/s10995-022-03556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merchant AT, Gupta RD, Akonde M, et al. Association of chlorhexidine use and scaling and root planing with birth outcomes in pregnant individuals with periodontitis: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(12) doi: 10.1001/jamanetworkopen.2022.47632. [DOI] [PMC free article] [PubMed] [Google Scholar]