Abstract
This narrative review summarises “alternative” or “natural” over-the-counter (OTC) mouthwashes not covered elsewhere in this supplement and newly emerging products, as potential mouthwashes of the future. The “natural” mouthwashes reviewed include saltwater, baking soda, coconut oil, charcoal, propolis, seaweeds, and probiotics. Other than essential oils, it is apparent that their clinical effectiveness is still under debate, but there is some evidence to suggest that propolis reduces plaque and gingivitis. This review also covers the host immune response, via novel anti-inmmunomodulant mouthwashes, such as erythropoietin to reduce inflammation with oral mucositis (OM) after radiotherapy. The emerging concept of nanoparticle-containing mouthwashes, such as iron oxide, is further discussed for OM, this agent having the potential for more targeted delivery of chemical antimicrobials. Unfortunately, there are impacts on the environment of widening mouthwash use with more new products, including increased use of packaging, antimicrobial resistance, and possible detrimental effects on marine life. Further, there are roadblocks, relating to regularly approvals and side effects, that still need to be overcome for any OTC deivered immunomodulant or nanoformulation mouthwashes. Despite these caveats, there are many new mouthwashes under development, which could help manage major oral diseases such as caries, gingivitis, and periodontal disease.
Key words: Mouthwash, Naturopathic, Novel, Bacteria, Caries, Gingivitis
Introduction
Contemporary mouthwashes come in a variety of forms, shapes and sizes. Much like the well-known antimicrobial mouthwashes, there are also “alternative” or “natural” mouthwashes that are prferred by some and gaining popularity, with millions using them to self-manage oral disease. Hence, it is important for dental clinicians to be aware of the evidence for the antimicrobial basis and the clinical effectiveness of all mouthwashes that are available to their patients. New products that are so emerging, include immunomodulant mouthwashes that may alter the immune response to oral microbes as well as nanoparticles mouthwashes that may offer better targeted antimicrobial actions. However, any antimicrobial mouthwash, either natural or synthetic, may also affect the eco-system beyond the human host, including the soil and marine environments, and the ecological impact of mouthwash use must therefore be earnestly evaluated. Hence, this article aims to discuss some of the more controversial issues related to mouthwash use, and provide future directions for research and development. Often the hierarchy of evidence used here is lower, as these are typically newly emerging areas, often supported by case-control studies and sometimes only by expert opinion and websites.
Naturopathic mouthwashes
Naturopathic medicine is defined as using the body's natural healing abilities and incorporating natural therapies, such as herbal medicine and other approaches to healing. Natural mouthwashes can also be traditional home remedies , such as coconut oil, saltwater, and baking soda, and should therefore be evaluated. Probiotic bacteria and propolis mouthwashes are also available in health food stores; hence, there are a large number of emerging natural competitors influencing the future direction of mouthwash use.
Natural mouthwashes in dentistry (other than the traditional mouthwash essential oils previously mentioned) are increasingly popular, as people consider them “organic” options and include extracts from plants such as red ginseng, aloe vera, propolis, fennel, thyme, Ratanhia roots, ginger, Malva sylvestris, xylitol, activated charcoal made from sustainable bamboo, lemon extract, and curcumin. Essential oils may also be combined with cleansing agents like tea tree, grapefruit seed extract, and Icelandic moss and claim to have anti-inflammatory, antioxidant, and antimicrobial properties. Many claim to be organic and without alcohol, sugar, fluoride, or glycerine, and all of these can be found in alternative mouthwash products.
However, there is a dearth of long-term data and clinical studies comparing the efficiency of natural mouthwashes to conventional mouthwashes.1,2 Additionally, the technique-sensitive nature of plant extraction and volatile compounds confer some barriers to the development of polyherbal mouthwashes. Some studies use ethanol solvents to better dissolve the active ingredient.3,4 The efficacy of natural mouthwashes in controlling dental plaque, gingival inflammation, and enamel demineralisation compared to conventional approaches or placebo is still inconclusive.5 A recent meta-analysis reported the potential benefits of herbal mouthwashes as supplements to daily oral hygiene practices. Nonetheless, significant methodologic limitations, small sample sizes, and heterogeneity weaken the strength of the evidence and preclude making valid conclusions, thus calling for further high-quality randomised controlled trials (RCTs).5 Potential benefits of natural mouthwashes in wound healing as an alternative to conventional mouthwashes to reduce the corrosive effects on titanium brackets have been recorded. However, more evidence is needed to support these claims.
Interestingly, the perception by the user may be that natural mouthwashes are considered a “safe” alternative to conventional mouthwashes. The perception is they are not reported to stain teeth, affect oral tissues adversely, or alter taste; rather they can exhibit remedial benefits, but is this true? Recently, Gomaa and Abdel-Wadood reviewed the literature reporting the beneficial effects of Chinese medicine formulas like licorice and glycyrrhizin in combating COVID-196 However, these were primarily in vitro studies. Nonetheless, continuous exposure to high doses of liquorice, particularly glycyrrhizin, can cause pseudohypoaldosteronism, hypokalaemia, hypertension, metabolic alkalosis, and oedema due to its hyper mineralocorticoid-like effect.7
Much like traditional antimicrobial mouthwashes, natural mouthwashes can supplement mechanical plaque control as a disinfectant, given their antiseptic, antibacterial, antifungal, and anticaries properties. There are also claims of anticancer potential and mucosal wound-healing effects with low toxicity and minimal adverse effects. Further studies are necessary to facilitate informed decisions regarding using natural mouthwashes. Also, the evidence base regarding their efficacy is primarily laboratory-based, and disparity exists in the reported findings. Therefore, well-conducted randomised clinical trials are warranted to account for the various confounding variables and avoid potential biases.
Alternative mouthwashes
Saltwater
Saltwater or saline mouth rinses, using water containing sodium chloride (NaCl), reduce plaque scores and the colony counts in saliva of bacteria such as S mutans, L acidophilus, A actinomycetemcomitans, and P gingivalis.8 Saline reduces the pH within the oral cavity and may cause bacteria to lose water due to osmosis; thus, it makes sense that saltwater could reduce the growth of unwanted oral bacteria. Dental practitioners often suggest saltwater rinses for postoperative care after oral surgery, but there is little evidence to support this recommendation. One RCT suggested that warm saline rinses used following extraction reduced the risk of alveolar osteitis (dry socket) postoperatively.9 A further RCT suggested that saltwater rinses may reduce gingival inflammation following periodontal surgery,10 whilst in vitro there are suggestions that saltwater may improve wound healing via its actions on fibroblasts.11 To our knowledge, no systematic reviews are available.
Coconut oil
Oil pulling is a traditional method of oral hygiene in Ayurvedic medicine that has recently become popular in Western medicine. It involves swishing around 1 tablespoon of oil in the mouth for 10 to 20 minutes to reduce the populations of bacteria in the mouth and improve oral health.12 Two small preliminary studies have shown some clinical effectiveness, as rinsing twice a day with coconut oil for 7 days can reduce plaque scores, plaque regrowth, and gingival inflammation.13,14 However, recent RCTs have demonstrated no effect on populations of aerobic or anaerobic bacteria after 28 days of coconut oil pulling.15 Therefore, further research is needed to determine the mechanisms and effectiveness of this approach.
Baking soda
Baking soda has been used in dental products for centuries, as it has bactericidal and virucidal properties, an alkaline pH, and a gentle abrasive nature that can remove plaque from tooth surfaces when used in brushing.16 A solution of half a teaspoon (2.5 g) of sodium bicarbonate in 250 mL water, making a 1% (W/V) solution, can be used as a mouth rinse for 1 minute, 3 or 4 times a day, as recommended in oral health care websites, for its bactericidal effects.17 This homemade baking soda rinse neutralises the salivary pH and reduces the bcterial counts, such as viridans group streptococci.18
However, there is limited evidence regarding the clinical effectiveness of using sodium bicarbonate as a mouthwash. Some studies suggest that it may be beneficial in managing oral mucositis (OM) following chemotherapy, but there is not enough evidence to make recommendations at present.19,20 More research is needed to determine whether sodium bicarbonate could be harmful due to hypernatraemia (high sodium in the bloodstream) following its absorption from the oral cavity.21
Propolis
Propolis is a resinous substance produced by honeybees which is rich in flavonoids and phenolic compounds.22,23 It has shown strong antimicrobial and anti-inflammatory properties, making it a good option for the management of oral disease.24,25 Following this, several studies have investigated the effectiveness of propolis on plaque and gingivitis compared to chlorhexidine. Some workers have reported the superior efficacy of propolis,26, 27, 28 whilst others reported better efficacy with chlorhexidine.29, 30, 31 Two systematic reviews concluded that propolis is safe to use and has potential benefits in reducing plaque and gingival inflammation.27 However, given some methodologic limitations as well as small sample sizes of the reviewed studies, the strength of the evidence is still weak and further studies are needed. Of note, the composition of propolis can substantially change due to botanical origin, honeybee characteristics, and environmental factors. Whilst some studies have shown the antibacterial effect of propolis against some oral pathogens,32 its effect on other coonstituents of the oral microbiota is unclear. Further research is also required to investigate the effectiveness of propolis in the management of gingivitis and periodontitis.
Essential oils
Eucalyptol, menthol, peppermint, methyl salicylate, clove oil, and thymol are some of the essential oils most commonly associated with well known over-the-counter (OTC) mouthwashes. In addition, tea tree oil and aloe vera are also natural products with antiseptic properties, making them useful as mouthwashes. They are considered safe when used correctly and can reduce plaque and gingivitis. For example, tea tree oil may increase salivary pH and reduce bleeding indices. Aloe vera and tea tree mouthwashes can also reduce plaque, gingivitis, and S mutans in children.33, 34, 35 However, the potential for tea tree oil–containing mouthwashes to establish eubiosis or reverse dysbiosis in oral microbial communities remains to be established.36
Charcoal
Mouthwashes containing activated charcoal are widely available online and in health food stores, often in combination with other “natural” antimicrobial agents such as peppermint, coconut oil, and tea tree oil37 or with antimicrobial mouthwash constituents, such as chlorhexidine and cetylpyridinium chloride.38 There is some evidence that charcoal in toothpaste may enhance the whitening of enamel,39 but to our knowledge there are no studies determining the effects of charcoal mouthwash on plaque, gingivitis, or oral bacteria and viruses in vivo associated with oral disease; hence, at present, dental practitioners should be cautious on the efficacy of charcoal mouthwashes.
Seaweed
Seaweed is commonly found in seawater as well as in freshwater, and they are divided into 3 groups according to their colour.40 They are rich in bioactive molecules such as polysaccharides, polyphenols, and peptides and omega rich oils with potential application for oral health.40 Several studies have shown that extract from different type of seaweeds had antibacterial activity against S mutans41, 42, 43 and P gingivalis.44 Red algae was shown to be effective in vitro to enhance dental enamel mineralisation.45 Despite these promising findings, further studies, especially clinical studies, are required to investigate their effect in mouthwash formulations for managing oral disease.
Evidence for probiotics in mouthwashes
Probiotics and periodontal disease
Dental probiotics show promise for gum disease management. Probiotic mouthwashes contain living microbes (Lactobacillus, Bifidobacterium, Bacillus, Saccharomyces) that can rebalance the oral microbiome, promote beneficial bacteria, and compete with pathogenic bacteria.46 Studies show that probiotic mouthwashes reduce S mutans and plaque in children.1, 2, 3,47 In adults, mouthwash with L salivarius reduces A actinomycetemcomitans, associated with periodontal disease, and decreases bleeding on probing scores.48 Mouthwash with Lactobacillus, Bifidobacterium, and Saccharomyces reduces plaque index and pocket depth and increases salivary pH and IgA in patients with stage II periodontitis.5 Probiotic mouthwash with 14 strains of Lactobacillus, Bifidobacterium, Bacillus, and Streptococcus has been shown to reduce plaque and bleeding in diabetic patients.49 An important limitation from all these studies was the lack of next-generation sequencing (NGS) analyses of the oral microbiome, which would uncover detail on the effect of probiotics on the composition of the whole oral bacterial ecosystem, including different oral niches.
Probiotics and halitosis
In addition to the management of gum disease, probiotic mouthwashes may manage halitosis by suppressing bacteria that produce sulphur compounds. A meta-analysis of 7 studies found that probiotics may reduce volatile sulphur compounds, relieving halitosis for less than 4 weeks. However, these studies did not use NGS analysis to explore the impact on oral microbes or potential harm to beneficial bacteria.7
Probiotics and dental caries
Although probiotics may have the potential to modulate the composition of oral biofilms and mitigate caries, the evidence remains weak. Whilst there is some evidence to show that probiotics, particularly L rhamnosus, can prevent caries in preschool children by reducing high levels of S mutans in the saliva, Bifidobacterium, another probiotic strain, was found to be ineffective in preventing dental caries. It is noteworthy that in vitro studies reported that L rhamnosus GG increased mineral loss in dentin caries lesions and did not inhibit S mutans, whilst it contributed to the caries process.50, 51, 52
Taken together, studies on the effect of probiotics on the oral microbiome and oral health are not well researched. More comprehensive laboratory methods, for example, NGS, are needed to understand their effectiveness.
Biological and immunostimulant agents
Biologic agents
Mouthwashes may be used as vehicles of targeted therapy. Targeting may be aimed at components of the host inflammatory response or against specific pathogens. This is important because it is the host immune response to bacteria that causes inflammation and bone loss especially in periodontal disease. Targeting pro-inflammatory pathways or associated pathogenic bacteria could in turn reduce inflammation and symptoms of oral disease. Biologic agents, also known as biologics, are therapeutics derived from, or are products of, living organisms or cells and include monoclonal antibodies and interleukins. They are highly specific and considered to have fewer side effects. Biologic agents are currently unavailable and may prove to be an area worthy of further exploration as useful mouthwashes.
In the early 1990s, pipetted application with S mutans–specific monoclonal antibodies showed sustained effects via passive immunity in a primate model, demonstrating the possibility of topical delivery as mouthwash23 and effectiveness related to dental caries (S mutans). Little is known about their effectiveness on the oral soft tissues in relation to periodontal disease. However, the early 2000s, a biologic mouthwash with ONYX-015 adenovirus caused resolution of oral dysplasia in one-third of patients, but responses were mostly transient.53
Recent exploration has focused on delivery of antibody or interleukin treatments by mouthwash. IVIG, a biologic agent usually given intravenously, used in children with oral candidiasis led to a significant decrease in Candida colony–forming units. Recombinant human interleukin-11 (rhIL-11) mouthwash also demonstrated some clinical effectiveness against chemotherapy-associated OM when a 0.3% solution mouthwash was gargled 4 times a day for 2 to 3 minutes.54 Whilst the use of biologic agents as mouthwashes is still in it's early stages, these experimental studies have shown promising results delivering targeted therapy and, so far, they have not demonstrated any side effects on surrounding tissue and organ systems.
Immunostimulants
Immunostimulants are entities that stimulate the immune system. They can be natural or synthetic. Use of immunostimulants as mouthwashes began in the 2000s. Colony-stimulating factors are the most explored class of immunostimulants in mouthwash for the prevention and treatment of OM caused by cancer treatments, but their use for other oral diseases like periodontitis and dental caries has not been extensively studied.
OM is inflammation of the oral mucosa involving injured mucosa, soft tissue, and white blood cells, leading to cell death, mucosal atrophy, and ulceration.55 It is common in patients with head and neck cancer undergoing radiotherapy or chemotherapy. Colony-stimulating factors like G-CSF and GM-CSF used to mobilise bone marrow progenitor cells have generally failed to effectively prevent or treat OM when used topically.56, 57 Growth factors like TGF-β3 have also been ineffective in large studies.58,59 However, topical rhEGF spray and swallow treatment have shown promise for managing OM and could be translated to mouthwash delivery.60 Likewise, an erythropoietin anti-inflammatory mouthwash significantly reduced OM intensity and duration in patients undergoing high-dose chemotherapy.61 However, the use of immunostimulant mouthwashes, successful in an OM model, need to be explored for their use for other oral diseases.
Nanotechnology
Nanotechnology delivers drugs for various purposes, including antimicrobial, immunomodulatory, analgesic/anti-inflammatory, or tissue engineering, and may extend to the chemicals in mouthwashes. Nanotechnology encompasses functional systems of at least 1 dimension at the nanometer scale (1–100 nm). Any of the “cargo” described in the above sections may be delivered through nanoparticles, and this extends to the antimicrobial chemicals found in mouthwashes. Chemicals can be loaded inside or tethered on the nanoparticle surface to protect the cargo from degradation, improve retention, and reduce the drug concentration required for therapeutic effects.62 Developing antimicrobial nanoparticle mouthwashes requires rapid activity within 1 to 2 minutes, no toxicity, and modulation of biofilm without broad-spectrum killing, either by selectively killing pathogenic species or suppressing virulence.63,64 These challenges make commercialising nanoparticle-containing mouthwashes difficult, but promising nanoparticles have been developed as antimicrobials or carriers of antimicrobial agents.
Metal nanoparticles
Metal and metal oxide nanoparticles, such as silver, zinc oxide, titanium dioxide, copper, and iron oxide, have demonstrated effective antimicrobial properties against various oral bacteria and fungi. However, the potential toxicity and accumulation of these particles within cells or organs are a significant concern.65 Silver nitrate is toxic to micro-organisms and imparts different antibacterial effects depending on its concentration. At lower concentrations, it induces synthesis of silver nanoparticles (directly toxic). At higher concentrations, it induces cell death (apoptosis) via inactivation of thiol group containing proteins (eg, NADH dehydrogenase II) and direct binding of silver to DNA; this stops replication causing apoptosis.66
Although the efficacy of metal nanoparticles against oral biofilms in realistic conditions remains underexplored, iron oxide nanoparticles, specifically ferumoxytol, have shown promising results in degrading oral biofilms in vitro, due to selectively binding and killing cariogenic bacteria, with no significant effects on commensal species.67 Nanoparticle-based delivery can also be useful for delivering antimicrobial compounds that show extremely limited water solubility and hence poor bioavailability. Mesoporous nanoparticles that were used to carry chlorhexidine showed potent antibacterial and antibiofilm activity in a concentration- and particle morphology–dependent manner.68 It may be possible to apply nanostructures on hard tissues for caries prevention by directly inhibiting microbial adhesion and biofilm development or promoting remineralisation,69 but little is known about whether this translates to clinical effectiveness against soft tissue diseases such as periodontitis.
Mineral nanoparticles
Casein phosphopeptide (CPP)–amorphous calcium phosphate (ACP) nanocomplexes prevent demineralisation and promote remineralisation by maintaining a state of supersaturation and buffering free calcium and phosphate ions. When used for 5 days, CPP-ACP mouthwash increased calcium and inorganic phosphate levels in supragingival plaque.70 In a randomised double-blind clinical trial, a commercial nanosilver- and xylitol-containing mouthwash (CORAL) was more effective in reducing white spot lesions compared to 0.05% chlorhexidine and fluoride mouthwashes.71 In other studies, chitosan nanoparticles, a natural polycationic linear polysaccharide, demonstrated antimicrobial, antibiofilm, and mineralisation properties,72,73 whilst nanodiamonds demonstrated potent biofilm inhibitory properties against bacteria and fungi.74 However, it is unknown whether these nanoparticle formulations can demonstrate their effects within a few minutes when used as a mouthwash and whether this translates into clinical effectiveness against oral diseases in vivo.
Natural nanoparticle compounds
As previously described, natural compounds, such as curcumin, trans-cinnamaldehyde, and essential oils, possess antimicrobial and antibiofilm properties in controlling plaque. Delivering these compounds through porous silicone nanoparticles and other delivery systems effectively inhibits biofilms of various pathogens.75 Biosurfactants approved by the US Food and Drug Administration like sophorolipids have been used to make nanocomplexes with curcumin, which can thwart biofilm assembly and filamentation of Candida albicans. These natural compounds can therefore potentially alleviate the onset of radiation-induced OM and oral candidiasis in patients. However, the effectiveness of these compounds in a mouthwash-like application has not been demonstrated yet.76
Synbiotic nanoparticles
Prebiotics and probiotics mouthwashes have also been introduced, and combinations of the 2, called synbiotics, are being investigated. However, the harsh oral environment, with low oxygen concentrations, temperature, and pH, can be detrimental to probiotic strains, leading to bacterial death.77,78 To overcome this, nanoprebiotics and nanoprobiotics have been developed and studied, but their efficacy as mouthwashes or potential to shape oral ecology to prevent disease require further investigation. Nonetheless, they present a promising direction for future mouthwash research.
Environmental perspectives
This supplement has describes the antimicrobial effects of a range of chemical, natural, immunological, and metallic and synthetic compounds included in mouthwashes and their clinical effectiveness. The immune response of the human host is also described where possible. However, going forwards it is also important to evaluate the environmental impact of mouthwash use as well.
In this context, one should appreciate how much carbon dioxide–equivalent emissions are generated through mouthwash use or consider their use in more humanistic terms, such as disability-adjusted life years (DALYs), that is, how much the health of a person is affected by the environmental impact of a product. Environmentally aware dental practitioners must therefore consider the whole life cycle of the mouthwash: where it is produced, how it is packaged, the material elements of the product, how it is used, and its disposal. There is an increasingly large number of life cycle assessments (LCAs) conducted on differing products both within dentistry and the wider health care field.79,80 As an example, a recent textbook on sustainable dentistry included an LCA of a non-antimicrobial mouthwash.81 In this analysis, a 10-mL 0.05% daily fluoride mouthwash was compared with a 10-mL 0.2% weekly mouthwash, calculated for 5 years. Both mouthwashes were packaged and transported in a half-litre plastic bottle and included water, glycerine, propylene glycol, sorbitol, peppermint oil, sodium fluoride, and sodium saccharine.
The 2021 results from the latter study showed that 5 years of a daily mouthwash produced 148 kg of carbon, approximately 7 times greater than the 21.1 kg produced for the weekly mouthwash use, 300 times more than receiving water fluoridation, and 30 to 150 times that of a fluoride varnish programme (see Table 1). Interestingly, many discussions of environmental factors are focussed on fluoride-based mouthwashes, but the same principles probably applys to all mouthwashes covered throughout this supplement.
Table 1.
The carbon footprint of different oral health prevention methods, 5 years of prevention (calculated in 2021).
| F | BTb | PTb | ETb | Wf | Fv | TbS | MwD | MwW |
|---|---|---|---|---|---|---|---|---|
| 3.07 | 4.26 | 25.6 | 47.9 | 0.443 | 3.31 | 1.95 | 148 | 21.1 |
F, floss; B, bamboo; Tb, toothbrush; P, plastic; E, electric; Wf, water fluoridation; Fv, fluoride varnish in schools; TbS, Tb in schools; MwD, mouthwash daily; MwW, mouthwash weekly.
The breakdown of the carbon footprint (CF) of the daily mouthwash over 5 years can also be seen in Table 2. The obvious benefits of weekly mouthwash use, compared with daily, are less product manufacturing, less plastic bottle production, as well as less transportation from factory to the consumer. Recalculation of mouthwash CF using an updated 2022 Ecoinvent database (and with changes in international renewable energy and product profiles) demonstrates that daily mouthwash has a CF of 84.20 kg.82 Within this modelling, electricity accounted for around one-third of the CF, with the sweeteners and glycol/glycerine being around one-third and the remaining one-third from transportation as well as the plastic product (Table 2).
Table 2.
The carbon footprint of mouthwash (recalculated in 2022 using 2022 Ecoinvent database).
| Once-daily mouthwash for 5 years for 1 person | kg | % |
|---|---|---|
| Electricity | 29.13 | 34.6% |
| Polyol (sorbitol sweetener) | 12.08 | 14.3% |
| Glycol | 9.88 | 11.7% |
| Transportation | 9.81 | 11.7% |
| Glycerine | 9.15 | 10.9% |
| Plastic product (polypropylene) | 7.18 | 8.5% |
| Shaping plastic product | 2.72 | 3.2% |
| Plastic product (polyethylene) | 1.26 | 1.5% |
| Other | 3.00 | 3.6% |
| Total | 84.20 | 100% |
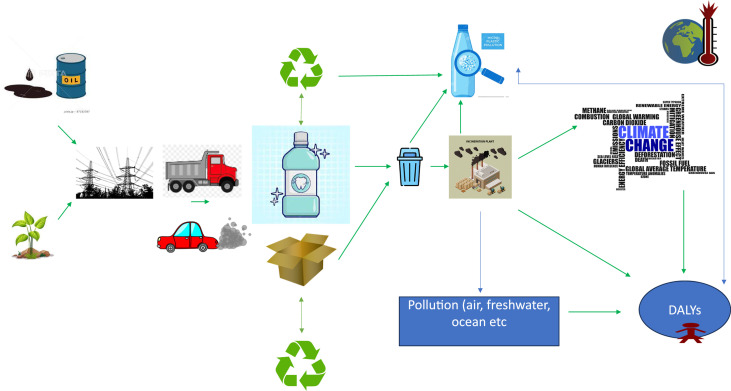
The Figure clearly illustrates the impact of mouthwash and associated products on the environment. There is pollution from production of mouthwashes, usually through energy use and water use. The transportation of the product via truck produces carbon emissions but also particulate matter and other forms of air pollution.83 Although the container bottles can be recycled, which has in some respects a comparative negative footprint the process can cause significant levels of microplastic release.84 If the bottle or cardboard waste is simply managed via incineration, this also has a high CF, as well as impact on fresh/sea water.82 All of the processes inevitably contribute to climate change and impacts DALYs (Figure).
Fig.
Graphic illustration of some of the environmental impacts of mouthwashes.
It may also be considered that widespread use of mouthwashes end up entering the sewage system following use, and this disposal could ultimately affect marine life and other living species within the water ecosystem. This includes antimicrobial resistance of bacteria within the ecological microenvironment. However, interestingly, and in line with our other LCAs, within our decontamination products there was less than expected environmental impact from the actual fluoride mouthwash or active chemical. Each chemical or active ingredient had a different environmental impact, depending on the elements and the concentrations evalauted. Such environmental impacts for a single chemical ingredient has been reviewed by Dhama.85
Conclusions
Natural and alternative approaches are demonstrating exciting potential as antimicrobial mouthwashes, and most appear to do no harm, but clinical effectiveness is not yet fully supported by high-quality evidence, other than for essential oils. There also exist critical roadblocks in the synthesis and development of efficient nanoformulations and immunostimulants delivered by mouthwash vehicles, for the use in oral mucosal and other oral diseases, relating to the accompanying regulatory approvals.
There is further a lack of research into the effects of chemical and antimicrobial mouthwashes on the environment, but clinicians should consider that the impact of a daily mouthwash is much higher than a weekly rinse, with the increased packaging needed for mouthwashes also considerably more harmful to the planet than that for toothpastes and similar alternatives.
With the currently available databse the clinicians may not be able to confidently advise their patients which OTC natural or alternative mouthwash is both clinically effective and safe for management of oral disease. As patients are using these natural mouthwashes regardless, urgent research is needed in this area. The future for mouthwash use is bright, with many interesting new approaches on the horizon; it may be that future mouthwashes will be able to target delivery at specific pathogenic bacteria, modulate the host immune response, “balance” the oral microbiome, or even reduce the extent of oral hygiene measures required to maintain good oral health. Nevertheless, investment in research, as well as overcoming regulatory barriers, are road blocks for these new approaches to reach fruition.
Funding
The authors have not received any commercial sponsorship directly or indirectly for this review. The narrative review reflects the authors opinions based on evidence considered of the active ingredients of the more widely available mouthwashes. The authors views should not necessarily be interpreted as the views of their faculties, universities or associated organisations.
Conflict of interest
None disclosed.
Footnotes
This article is published as part of a supplement sponsored by FDI World Dental Federation.
References
- 1.Krupa NC, Thippeswamy HM, Chandrashekar BR. Antimicrobial efficacy of xylitol, probiotic and chlorhexidine mouth rinses among children and elderly population at high risk for dental caries - a randomized controlled trial. J Prev Med Hyg. 2022;63(2) doi: 10.15167/2421-4248/jpmh2022.63.2.1772. E282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhallak E, Kouchaje C, Hasan A, Makieh R. Evaluation of the effectiveness of probiotic mouthwashes in reducing dental plaque in primary and permanent teeth: a randomized clinical trial. Cureus. 2022;14(8):e28125. doi: 10.7759/cureus.28125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gedam KY, Katre AN. Efficacy of probiotic, chlorhexidine, and sodium fluoride mouthrinses on mutans streptococci in 8- to 12-year-old children: a crossover randomized trial. Lifestyle Genomics. 2022;15(1):35–44. doi: 10.1159/000519916. [DOI] [PubMed] [Google Scholar]
- 4.Sajedinejad N, Paknejad M, Houshmand B, et al. Lactobacillus salivarius NK02: a potent probiotic for clinical application in mouthwash. Probiotics Antimicrob Proteins. 2018;10(3):485–495. doi: 10.1007/s12602-017-9296-4. [DOI] [PubMed] [Google Scholar]
- 5.Ranjith A, Nazimudeen NB, Baiju KV. Probiotic mouthwash as an adjunct to mechanical therapy in the treatment of stage II periodontitis: a randomized controlled clinical trial. Int J Dent Hyg. 2022;20(2):415–421. doi: 10.1111/idh.12589. [DOI] [PubMed] [Google Scholar]
- 6.Gomaa AA, Abdel-Wadood YA. The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomed Plus. 2021;1:100043. doi: 10.1016/j.phyplu.2021.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang N, Li J, Qiao X, et al. Efficacy of probiotics in the management of halitosis: a systematic review and meta-analysis. BMJ Open. 2022;12(12) doi: 10.1136/bmjopen-2022-060753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aravinth V, Aswath Narayanan MB, Ramesh Kumar SG, Selvamary AL, Sujatha A. Comparative evaluation of salt water rinse with chlorhexidine against oral microbes: a school-based randomized controlled trial. J Indian Soc Pedod Prev Dent. 2017;35(4):319–326. doi: 10.4103/JISPPD.JISPPD_299_16. [DOI] [PubMed] [Google Scholar]
- 9.Stewart M, Levey E, Nayyer N. Salt water mouthwash post extraction reduced post operative complications. Evid Based Dent. 2015;16(1):27–28. doi: 10.1038/sj.ebd.6401084. [DOI] [PubMed] [Google Scholar]
- 10.Collins JR, Veras K, Hernández M, Hou W, Hong H, Romanos GE. Anti-inflammatory effect of salt water and chlorhexidine 0.12% mouthrinse after periodontal surgery: a randomized prospective clinical study. Clin Oral Investig. 2021;25(7):4349–4357. doi: 10.1007/s00784-020-03748-w. [DOI] [PubMed] [Google Scholar]
- 11.Huynh NC, Everts V, Leethanakul C, Pavasant P, Ampornaramveth RS. Rinsing with saline promotes human gingival fibroblast wound healing in vitro. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanbhag VK. Oil pulling for maintaining oral hygiene - a review. J Tradit Complement Med. 2016;7(1):106–109. doi: 10.1016/j.jtcme.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peedikayil FC, Sreenivasan P, Narayanan A. Effect of coconut oil in plaque related gingivitis - a preliminary report. Niger Med J. 2015;56(2):143–147. doi: 10.4103/0300-1652.153406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sezgin Y, Memis Ozgul B, Alptekin NO. Efficacy of oil pulling therapy with coconut oil on four-day supragingival plaque growth: a randomized crossover clinical trial. Complement Ther Med. 2019;47 doi: 10.1016/j.ctim.2019.102193. [DOI] [PubMed] [Google Scholar]
- 15.Siripaiboonpong N, Matangkasombut O, Pengcharoen H, Boonchaiyapluk B, Rujiraprasert P, Srithanyarat SS. Microbiological effects of virgin coconut oil pulling in comparison with palm oil pulling as an adjunctive oral hygiene care for patients with gingival inflammation: a randomized controlled clinical trial. J Indian Soc Periodontol. 2022;26(1):58–63. doi: 10.4103/jisp.jisp_768_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciancio SG. Baking soda dentifrices and oral health. J Am Dent Assoc. 2017;148(11S):S1–S3. doi: 10.1016/j.adaj.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Is baking powder mouthrinse safe and effective? Colgate Global Scientific Communications. Available from: https://www.colgate.com/en-us/oral-health/selecting-dental-products/is-baking-soda-mouth-rinse-safe-and-effective. Accessed 10 June 2023.
- 18.Chandel S, Khan MA, Singh N, Agrawal A, Khare V. The effect of sodium bicarbonate oral rinse on salivary pH and oral microflora: a prospective cohort study. Natl J Maxillofac Surg. 2017;8(2):106–109. doi: 10.4103/njms.NJMS_36_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire DB, Fulton JS, Park J, et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21(11):3165–3177. doi: 10.1007/s00520-013-1942-0. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zeng L, Feng X, Zhao N, Feng N, Du X. Did you choose appropriate mouthwash for managing chemoradiotherapy-induced oral mucositis? The therapeutic effect compared by a Bayesian network meta-analysis. Front Oral Health. 2023;3 doi: 10.3389/froh.2022.977830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madeswaran S, Jayachandran S. Sodium bicarbonate: a review and its uses in dentistry. Indian J Dent Res. 2018. doi: 10.4103/ijdr.IJDR_30_17. https://www.ijdr.in/text.asp?2018/29/5/672/244935 Available from: Accessed 10 June 2023. [DOI] [PubMed] [Google Scholar]
- 22.Peña M, Barallat L, Vilarrasa J, Vicario M, Violant D, Nart J. Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: a triple-blind randomized clinical trial. Clin Oral Investig. 2019;23(4):1673–1683. doi: 10.1007/s00784-018-2578-8. [DOI] [PubMed] [Google Scholar]
- 23.Ma JK, Lehner T. Prevention of colonization of Streptococcus mutans by topical application of monoclonal antibodies in human subjects. Arch Oral Biol. 1990;35(Suppl)):115s–122s. doi: 10.1016/0003-9969(90)90140-6. [DOI] [PubMed] [Google Scholar]
- 24.Zulhendri F, Felitti R, Fearnley J, Ravalia M. The use of propolis in dentistry, oral health, and medicine: a review. J Oral Biosci. 2021;63(1):23–34.. doi: 10.1016/j.job.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Więckiewicz W, Miernik M, Więckiewicz M, Morawiec T. Does propolis help to maintain oral health? Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/351062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dehghani M, Abtahi M, Hasanzadeh N, Farahzad Z, Noori M, Noori M. Effect of propolis mouthwash on plaque and gingival indices over fixed orthodontic patients. J Clin Exp Dent. 2019;11(3):e244–e249. doi: 10.4317/jced.55026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halboub E, Al-Maweri SA, Al-Wesabi M, et al. Efficacy of propolis-based mouthwashes on dental plaque and gingival inflammation: a systematic review. BMC Oral Health. 2020;20:198. doi: 10.1186/s12903-020-01185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anauate-Netto C, Anido-Anido A, Leegoy HR, et al. Randomized, double-blind, placebo-controlled clinical trial on the effects of propolis and chlorhexidine mouthrinses on gingivitis. Braz Dent Sci. 2014;17(1):11–15. doi: 10.14295/bds.2014.v17i1.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray MC, Worthington HV, Blinkhorn AS. A study to investigate the effect of a propolis-containing mouthrinse on the inhibition of de novo plaque formation. J Clin Periodontol. 1997;24(11):796–798. doi: 10.1111/j.1600-051x.1997.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 30.Dodwad V, Kukreja BJ. Propolis mouthwash: a new beginning. J Indian Soc Periodontol. 2011;15(2):121–125. doi: 10.4103/0972-124X.84379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santiago KB, Piana GM, Conti BJ, et al. Microbiological control and antibacterial action of a propolis-containing mouthwash and control of dental plaque in humans. Nat Prod Res. 2018;32(12):1441–1445. doi: 10.1080/14786419.2017.1344664. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira JMDS, Cavalcanti TFS, Leite IF, et al. Propolis in oral healthcare: antibacterial activity of a composite resin enriched with brazilian red propolis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.787633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manikandan S, Bhambal AM, Ratchambiga KS, Nithiela M, Swatheka JK, Sridarshini B. Comparative evaluation of the effect of 0.2% chlorhexidine, 2% lemongrass oil, and 2% tea tree oil mouth rinse on salivary pH: an in vivo study. J Pharm Bioallied Sci. 2021;13:S757–S760. doi: 10.4103/jpbs.JPBS_667_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ripari F, Cera A, Freda M, Zumbo G, Zara F, Vozza I. Tea tree oil versus chlorhexidine mouthwash in treatment of gingivitis: a pilot randomized, double blinded clinical trial. Eur J Dent. 2020;14(1):55–62. doi: 10.1055/s-0040-1703999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvatori C, Barchi L, Guzzo F, Gargari M. A comparative study of antibacterial and anti-inflammatory effects of mouthrinse containing tea tree oil. Oral Implantol (Rome) 2017;10(1):59–70. doi: 10.11138/orl/2017.10.1.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uncovering the benefits and risks of tea tree oil mouthwash. Tina Holloway. 2023. Available from: https://dentalehub.com/uncovering-the-benefits-and-risks-of-tea-tree-oil-mouthwash/. Accessed 10 June 2023.
- 37.Hello™TM Activated Charcoal Mouthwash. Available from: https://www.hello-products.co.uk/products/charcoal-mouthwash. Accessed 10 June 2023.
- 38.Brooks JK, Bashirelahi N, Hsia RC, Reynolds MA. Charcoal-based mouthwashes: a literature review. Br Dent J. 2020;228(4):290–294. doi: 10.1038/s41415-020-1265-8. [DOI] [PubMed] [Google Scholar]
- 39.Dionysopoulos D, Papageorgiou S, Malletzidou L, Gerasimidou O, Tolidis K. Effect of novel charcoal-containing whitening toothpaste and mouthwash on color change and surface morphology of enamel. J Conserv Dent. 2020;23(6):624–631. doi: 10.4103/JCD.JCD_570_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lomartire S, Gonçalves AMM. An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications. Mar Drugs. 2022;20(2):141. doi: 10.3390/md20020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH, Kim CY. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int J Antimicrob Agents. 2001;18(6):553–557. doi: 10.1016/s0924-8579(01)00434-4. [DOI] [PubMed] [Google Scholar]
- 42.Davoodbasha M, Edachery B, Nooruddin T, Lee SY, Kim JW. An evidence of C16 fatty acid methyl esters extracted from microalga for effective antimicrobial and antioxidant property. Microb Pathog. 2018;115:233–238. doi: 10.1016/j.micpath.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 43.Hodnik Ž, Łoś JM, Žula A, et al. Inhibition of biofilm formation by conformationally constrained indole-based analogues of the marine alkaloid oroidin. Bioorg Med Chem Lett. 2014;24(11):2530–2534. doi: 10.1016/j.bmcl.2014.03.094. [DOI] [PubMed] [Google Scholar]
- 44.Kim YH, Kim JH, Jin HJ, Lee SY. Antimicrobial activity of ethanol extracts of Laminaria japonica against oral microorganisms. Anaerobe. 2013;21:34–38. doi: 10.1016/j.anaerobe.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Carrilho M, Bretz W. Red marine algae Lithothamnion calcareum supports dental enamel mineralization. Mar Drugs. 2023;21(2):109. doi: 10.3390/md21020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Stefano M, Santonocito S, Polizzi A, et al. A reciprocal link between oral, gut microbiota during periodontitis: the potential role of probiotics in reducing dysbiosis-induced inflammation. Int J Mol Sci. 2023;24(2) doi: 10.3390/ijms24021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harini PM, Anegundi RT. Efficacy of a probiotic and chlorhexidine mouth rinses: a short-term clinical study. J Indian Soc Pedod Prev Dent. 2010;28(3):179–182. doi: 10.4103/0970-4388.73799. [DOI] [PubMed] [Google Scholar]
- 48.Sajedinejad N, Paknejad M, Houshmand B, et al. Lactobacillus salivarius NK02: a potent probiotic for clinical application in mouthwash. Probiotics Antimicrob Proteins. 2018;10(3):485–495. doi: 10.1007/s12602-017-9296-4. [DOI] [PubMed] [Google Scholar]
- 49.Bollero P, Di Renzo L, Franco R, et al. Effects of new probiotic mouthwash in patients with diabetes mellitus and cardiovascular diseases. Eur Rev Med Pharmacol Sci. 2017;21(24):5827–5836. doi: 10.26355/eurrev_201712_14031. [DOI] [PubMed] [Google Scholar]
- 50.Meng N, Liu Q, Dong Q, Gu J, Yang Y. Effects of probiotics on preventing caries in preschool children: a systematic review and meta-analysis. J Clin Pediatr Dent. 2023;47(2):85–100. doi: 10.22514/jocpd.2023.014. [DOI] [PubMed] [Google Scholar]
- 51.Schwendicke F, Dörfer C, Kneist S, Meyer-Lueckel H, Paris S. Cariogenic effects of probiotic Lactobacillus rhamnosus GG in a dental biofilm model. Caries Res. 2014;48(3):186–192. doi: 10.1159/000355907. [DOI] [PubMed] [Google Scholar]
- 52.Hao S, Wang J, Wang Y. Effectiveness and safety of Bifidobacterium in preventing dental caries: a systematic review and meta-analysis. Acta Odontol Scand. 2021;79(8):613–622. doi: 10.1080/00016357.2021.1921259. [DOI] [PubMed] [Google Scholar]
- 53.Rudin CM, Cohen EE, Papadimitrakopoulou VA, et al. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J Clin Oncol. 2003;21(24):4546–4552. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei H, Wei J, Dong X. A prospective interventional study of recombinant human interleukin-11 mouthwash in chemotherapy-induced oral mucositis. BMC Oral Health. 2022;22(1):313. doi: 10.1186/s12903-022-02348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, et al. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol. 2017;8:354. doi: 10.3389/fphar.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karthaus M, Rosenthal C, Huebner G, et al. Effect of topical oral G-CSF on oral mucositis: a randomised placebo-controlled trial. Bone Marrow Transplant. 1998;22(8):781–785. doi: 10.1038/sj.bmt.1701434. [DOI] [PubMed] [Google Scholar]
- 57.Dodd MJ, Cho MH, Cooper BA, MacPhail L, Miaskowski C. A randomized clinical trial of granulocyte macrophage colony stimulating factor mouthwash for oral mucositis in head and neck cancer. Eur J Oncol Nurs. 2022;56 doi: 10.1016/j.ejon.2022.102093. [DOI] [PubMed] [Google Scholar]
- 58.Wymenga ANM, van der Graaf WTA, Hofstra LS, et al. Phase I study of transforming growth factor-β3 mouthwashes for prevention of chemotherapy-induced mucositis. Clin Cancer Res. 1999;5(6):1363–1368. [PubMed] [Google Scholar]
- 59.Foncuberta MC, Cagnoni PJ, Brandts CH, et al. Topical transforming growth factor-β3 in the prevention or alleviation of chemotherapy-induced oral mucositis in patients with lymphomas or solid tumors. J Immunother. 2001;24(4) doi: 10.1097/00002371-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Wu HG, Song SY, Kim YS, et al. Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: a double-blind placebo-controlled prospective phase 2 multi-institutional clinical trial. Cancer. 2009;115(16):3699–3708. doi: 10.1002/cncr.24414. [DOI] [PubMed] [Google Scholar]
- 61.Hosseinjani H, Hadjibabaie M, Gholami K, et al. The efficacy of erythropoietin mouthwash in prevention of oral mucositis in patients undergoing autologous hematopoietic SCT: a double-blind, randomized, placebo-controlled trial. Hematol Oncol. 2017;35(1):106–112. doi: 10.1002/hon.2250. [DOI] [PubMed] [Google Scholar]
- 62.Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: a review. J Endod. 2016;42(10):1417–1426. doi: 10.1016/j.joen.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Micro. 2018;16(12):745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shanmugam K, Sarveswari HB, Udayashankar A, et al. Guardian genes ensuring subsistence of oral Streptococcus mutans. Crit Rev Microbiol. 2020;46(4):475–491. doi: 10.1080/1040841X.2020.1796579. [DOI] [PubMed] [Google Scholar]
- 65.Padovani GC, Feitosa VP, Sauro S, et al Advances in dental materials through nanotechnology: facts, perspectives and toxicological aspects. Trends Biotechnol. 2015;33(11):621–636. doi: 10.1016/j.tibtech.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Kumar Pandian SR, Deepak V, Kalishwaralal K, Viswanathan P, Gurunathan S. Mechanism of bactericidal activity of silver nitrate - a concentration dependent bi-functional molecule. Braz J Microbiol. 2010;41(3):805–809. doi: 10.1590/S1517-83822010000300033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Huang Y, Kim D, et al. Ferumoxytol nanoparticles target biofilms causing tooth decay in the human mouth. Nano Lett. 2021;21(22):9442–9449. doi: 10.1021/acs.nanolett.1c02702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Wong CH, Ng TW, Zhang CF, Leung KC, Jin L. The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. Int J Nanomedicine. 2016;11:2471–2480. doi: 10.2147/IJN.S105681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nat Nanotechnol. 2010;5(8):565–569. doi: 10.1038/nnano.2010.83. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds EC, Cai F, Shen P, Walker GD. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res. 2003;82(3):206–211. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 71.Ali A, Ismail H, Amin K. Effect of nanosilver mouthwash on prevention of white spot lesions in patients undergoing fixed orthodontic treatment - a randomized double-blind clinical trial. J Dent Sci 17(1):249–55. [DOI] [PMC free article] [PubMed]
- 72.Landzberg G, Hussein H, Kishen A. A novel self-mineralizing antibacterial tissue repair varnish to condition root-end dentin in endodontic microsurgery. J Endod. 2021;47(6):939–946. doi: 10.1016/j.joen.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Poornima P, Krithikadatta J, Ponraj RR, Velmurugan N, Kishen A. Biofilm formation following chitosan-based varnish or chlorhexidine-fluoride varnish application in patients undergoing fixed orthodontic treatment: a double blinded randomised controlled trial. BMC Oral Health. 2021;21(1):465. doi: 10.1186/s12903-021-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang T, Kalimuthu S, Rajasekar V, et al. Biofilm inhibition in oral pathogens by nanodiamonds. Biomater Sci. 2021;9(15):5127–5135. doi: 10.1039/d1bm00608h. [DOI] [PubMed] [Google Scholar]
- 75.Jailani A, Kalimuthu S, Rajasekar V, et al. Trans-Cinnamaldehyde eluting porous silicon microparticles mitigate cariogenic biofilms. Pharmaceutics. 2022;14(7):1428. doi: 10.3390/pharmaceutics14071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajasekar V, Darne P, Prabhune A, et al. A curcumin-sophorolipid nanocomplex inhibits Candida albicans filamentation and biofilm development. Colloids Surf B Biointerfaces. 2021;200 doi: 10.1016/j.colsurfb.2021.111617. [DOI] [PubMed] [Google Scholar]
- 77.Kawasaki S, Mimura T, Satoh T, Takeda K, Niimura Y. Response of the microaerophilic Bifidobacterium species, B. boum and B. thermophilum, to oxygen. Appl Environ Microb. 2006;72:6854–6858. doi: 10.1128/AEM.01216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dangi P, Chaudhary N, Chaudhary V, et al. Nanotechnology impacting probiotics and prebiotics: a paradigm shift in nutraceuticals technology. Int J Food Microbiol. 2023;388 doi: 10.1016/j.ijfoodmicro.2022.110083. [DOI] [PubMed] [Google Scholar]
- 79.Duane B, Pilling J, Saget S, Ashley P, Pinhas AR, Lyne A. Hand hygiene with hand sanitizer versus handwashing: what are the planetary health consequences? Environ Sci Pollut Res Int. 2022;29(32):48736–48747. doi: 10.1007/s11356-022-18918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borglin L, Pekarski S, Saget S, Duane B. The life cycle analysis of a dental examination: quantifying the environmental burden of an examination in a hypothetical dental practice. Community Dent Oral Epidemiol. 2021;49(6):581–593. doi: 10.1111/cdoe.12630. [DOI] [PubMed] [Google Scholar]
- 81.Duane B. Sustainable dentistry 2022. Available from: https://link.springer.com/book/10.1007/978-3-031-07999-3. Accessed 10 June 2023.
- 82.Ecoinvent. Available from: https://ecoinvent.org/. Accessed 28 July 2023.
- 83.Wu Y, Song P, Lin S, et al. Global burden of respiratory diseases attributable to ambient particulate matter pollution: findings from the global burden of disease study 2019. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.740800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carbon waste and resources metric. Available from: https://wrap.org.uk/resources/report/carbon-waste-and-resources-metric. Accessed 28 July 2023.
- 85.Dhama K, Patel SK, Kumar R, et al. The role of disinfectants and sanitizers during COVID-19 pandemic: advantages and deleterious effects on humans and the environment. Environ Sci Pollut Res. 2021;28:34211–34228. doi: 10.1007/s11356-021-14429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]