This study reports long-term safety and outcomes of topical atropine for childhood myopia control.
Key Points
Question
What long-term outcomes were noted in adulthood among individuals receiving atropine during childhood for myopia?
Findings
Among approximately one-quarter of the original cohort who received childhood atropine treatment (0.01% to 1.0%) for myopia control (2 to 4 years duration), there were no identified differences in final refractive errors. There was no association with increased incidence of treatment or myopia-related ocular complications in 1% atropine-treated vs placebo groups.
Meaning
Long-term follow-up of a minority of participants who received atropine for a limited duration during childhood did not affect final refractive errors or incidence of ocular complications in adulthood..
Abstract
Importance
Clinical trial results of topical atropine eye drops for childhood myopia control have shown inconsistent outcomes across short-term studies, with little long-term safety or other outcomes reported.
Objective
To report the long-term safety and outcomes of topical atropine for childhood myopia control.
Design, Setting, and Participants
This prospective, double-masked observational study of the Atropine for the Treatment of Myopia (ATOM) 1 and ATOM2 randomized clinical trials took place at 2 single centers and included adults reviewed in 2021 through 2022 from the ATOM1 study (atropine 1% vs placebo; 1999 through 2003) and the ATOM2 study (atropine 0.01% vs 0.1% vs 0.5%; 2006 through 2012).
Main Outcome Measures
Change in cycloplegic spherical equivalent (SE) with axial length (AL); incidence of ocular complications.
Results
Among the original 400 participants in each original cohort, the study team evaluated 71 of 400 ATOM1 adult participants (17.8% of original cohort; study age, mean [SD] 30.5 [1.2] years; 40.6% female) and 158 of 400 ATOM2 adult participants (39.5% of original cohort; study age, mean [SD], 24.5 [1.5] years; 42.9% female) whose baseline characteristics (SE and AL) were representative of the original cohort. In this study, evaluating ATOM1 participants, the mean (SD) SE and AL were −5.20 (2.46) diopters (D), 25.87 (1.23) mm and –6.00 (1.63) D, 25.90 (1.21) mm in the 1% atropine-treated and placebo groups, respectively (difference of SE, 0.80 D; 95% CI, −0.25 to 1.85 D; P = .13; difference of AL, −0.03 mm; 95% CI, −0.65 to 0.58 mm; P = .92). In ATOM2 participants, the mean (SD) SE and AL was −6.40 (2.21) D; 26.25 (1.34) mm; −6.81 (1.92) D, 26.28 (0.99) mm; and −7.19 (2.87) D, 26.31 (1.31) mm in the 0.01%, 0.1%, and 0.5% atropine groups, respectively. There was no difference in the 20-year incidence of cataract/lens opacities, myopic macular degeneration, or parapapillary atrophy (β/γ zone) comparing the 1% atropine-treated group vs the placebo group.
Conclusions and Relevance
Among approximately one-quarter of the original participants, use of short-term topical atropine eye drops ranging from 0.01% to 1.0% for a duration of 2 to 4 years during childhood was not associated with differences in final refractive errors 10 to 20 years after treatment. There was no increased incidence of treatment or myopia-related ocular complications in the 1% atropine-treated group vs the placebo group. These findings may affect the design of future clinical trials, as further studies are required to investigate the duration and concentration of atropine for childhood myopia control.
Introduction
Myopia currently affects about 2 billion individuals, or one-third of the world’s population, with almost 300 million individuals having high myopia.1,2 This is estimated to increase by the year 2050 to approximately 5 billion and 1 billion individuals with myopia and high myopia, respectively.3 High myopia is associated with an increased risk of sight-threatening complications, such as myopic macular degeneration (MMD) and myopia-associated optic neuropathy.4,5 Although it has not been definitively shown whether childhood myopia leads to an increased risk of developing pathologic myopia in adulthood, delaying the onset of myopia and reducing myopia progression in children have been key strategies used to reduce the potential risk of myopia-associated complications in adulthood.6,7
Over the past decades, topical administration of atropine eye drops has been demonstrated to be a safe and effective intervention in slowing the onset of myopia and reducing myopia progression in children.8,9,10,11,12,13 However, a recent clinical trial of 0.01% low-dose atropine eye drops did not slow myopia progression or axial elongation in US children.14 Additionally, a recent meta-analysis reported that low-dose (less than 0.1%) atropine met the equivalence criterion (less than 0.25 diopters [D]) showing a mean treatment difference of 0.24 D.15 Higher concentrations of atropine, eg, 0.5% and 1%, have been shown to reduce myopia progression but were also associated with a higher rate of adverse effects, such as photophobia.16,17,18 A rebound effect after cessation of the atropine therapy has been reported, particularly when higher concentrations of atropine had been used.16,17,18
As clinical trial results of topical atropine eye drops for myopia control in childhood have shown inconsistent outcomes across different short-term trials, establishing its long-term safety and effects remains crucial, although not well studied.19 To date, reports on clinical outcomes of atropine therapy have been limited to 5 years,17,20 including the Atropine for the Treatment of Myopia (ATOM) clinical trials.8,9,10,11,21 Therefore, we carried out the Atropine Treatment Long-term Assessment Study (ATLAS) to assess the 20-year follow-up outcomes and safety for the patients who participated in the ATOM1 study and the 10-year follow-up outcomes for the patients who took part in the ATOM2 study.
Methods
Study Population
The study included the participants from the ATOM1 and ATOM2 clinical trials who were now evaluated almost 10 years and 20 years, respectively, after their final trial visit (Figure).8,9 The ATOM1 study commenced in 1999. It was a single-center, randomized, double-masked, placebo-controlled trial.8,9 The study included 400 children aged 6 to 12 years with a myopic refractive error (spherical equivalent [SE] ranging between −1.0 and −6.0 D and an astigmatism of −1.5 D or less). The children were assigned to receive either 1% atropine eye drops or vehicle (placebo) eye drops once nightly for 2 years (phase 1). They were followed up with for another year after stopping the atropine therapy (phase 2). In the atropine-treated group, only 1 randomly selected eye started treatment, while the other received vehicle. The ATOM2 study commenced in 2006 as a single-center, double-masked, randomized clinical trial.10,11 The participants were 400 children aged 6 to 12 years with myopia of at least −2.0 D and an astigmatism of −1.50 D or less. They were randomly assigned in a 2:2:1 ratio to 0.5%, 0.1%, and 0.01% atropine eye drops to be administered once nightly to both eyes for 2 years (treatment phase), after which medication was stopped for 1 year (washout phase). Children who had myopia progression by −0.50 D or more in at least 1 eye during the washout phase restarted atropine 0.01% therapy for a further 2 years (retreatment phase). Detailed results from these studies have been previously described.8,9,10,11
Figure. Timeline of the Atropine Treatment Long-Term Assessment Study.
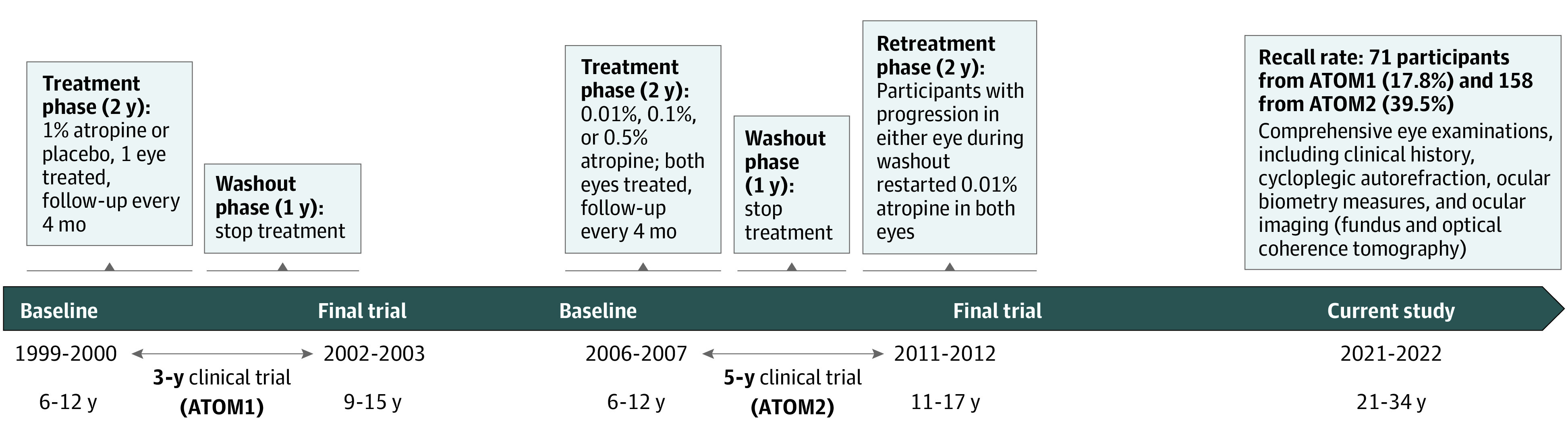
ATOM indicates Atropine for the Treatment of Myopia.
Written informed consent was obtained for each individual after screening for their eligibility. Participants received a $50 Singapore dollars ($36.47 US dollars) stipend per completed visit. The study was conducted according to the tenets of the Declaration of Helsinki. Ethics approval was obtained from the Singapore Eye Research institute review board (No. 2020/2249). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study Design
In the ATOM1 study, participants underwent cycloplegia with 1 drop of proparacaine hydrochloride followed by 3 drops of 1% cyclopentolate hydrochloride. Cycloplegic autorefraction was performed using the Canon RK5 autorefractor-autokeratometer and ocular biometry was measured by A-scan ultrasonography with the Nidek US-800 EchoScan. In the ATOM2 study, cycloplegia was performed with 3 drops of cyclopentolate 1%, 5 minutes apart. Cycloplegic autorefraction was performed using the Canon RK-F1 autorefractor and ocular biometry was measured using the Zeiss IOLMaster (Carl Zeiss Meditec). For the current study, all study participants underwent a comprehensive clinical history, including questions on previous ocular and medical conditions, surgical history, eye complaints, family history, and whether they had received any myopia control treatment since their final trial visits. The participants additionally had a comprehensive examination of the anterior and posterior segments of their eyes. Cycloplegia was achieved using 1 drop of tropicamide 1% and cyclopentolate 1%, followed by a second round of drops if the pupils were not sufficiently dilated 15 minutes after the first application. Cycloplegic autorefraction was performed using the Topcon Auto Kerato-Refractometer to obtain 3 readings with a maximal range of 0.50 D. The mean values of the 3 readings were taken. The SE of the refractive error was calculated as the spherical refractive error plus half of the cylindrical refractive error. Ocular biometry for measurement of axial length (AL), anterior chamber depth, and corneal curvature radius was performed using the Carl Zeiss IOLMaster. Five readings were taken and averaged with the repeated AL readings within a range of 0.05 mm. Ocular imaging, including fundus photography and optical coherence tomography, was conducted using the Topcon DRI OCT Triton device. Fundus photographs of the posterior pole were graded by 2 graders (Y.L. and J.C.) to identify the presence of MMD, parapapillary atrophy (β/γ zone), and optic disc changes. MMD was graded based on the International Meta-Analysis of Pathologic Myopia classification.22 Parapapillary β zone was defined by the presence of Bruch’s membrane and absence of retinal pigment epithelium, while γ zone was characterized by the absence of Bruch’s membrane and retinal pigment epithelium.23 The optic nerve head was suspicious for glaucoma if cup-disc diameter ratio (CDR) was more than 0.7 or the difference of the CDRs between the 2 eyes was more than 0.2.24 The physiologic dependence of the CDRs on the optic disc was taken into account.
Statistical Analysis
The mean and SD were summarized for continuous variables. t Test for independent samples or analysis of variance were performed to compare the groups with different atropine concentrations. For the categorical variables, the number and proportion grouped by atropine concentration, and the χ2 test and Fisher exact test were applied to assess the statistical significance of differences between the groups. The mean difference or odds ratios (ORs) with corresponding 95% CIs were calculated. Multivariate binary logistic-regression analysis was performed with both direction stepwise manner with the myopia progression after stopping treatment or the incidence of MMD at study visit as the dependent variable and other parameters such as age, gender, race, therapeutic concentration of atropine, retreatment, SE/AL at baseline, SE progression during treatment, and axial elongation during treatment as independent variables. The variance inflation factor was calculated and variables with multicollinearity were dropped from the list of independent variables. When comparing the effect between groups with different atropine concentrations in ATOM2, the measurements from both eyes were pooled in a combined analysis using the Huber-White robust standard errors to allow for the correlation between 2 eyes within a person.25 For the ATOM2 participants, eyes were classified according to myopia progression based on the refractive error changes which had occurred between the final trial visit and the current study visit. The cutoff value for the presence of myopia progression was an increase in myopic refractive error by at least 1.0 D. P values were reported for the global null hypothesis of no differences among atropine groups. P values were 2-sided but not adjusted for multiple analyses. All analyses were performed using the statistical software R, version 4.1.0 (The R Foundation for Statistical Computing).
Results
Among the original participants, 71 of 400 from ATOM1 (17.8%) and 158 of 400 from ATOM2 (39.5%) participated in our ATLAS study (eTable 1 in Supplement 1). For the individuals of the ATOM1 study, the participants of the current study and the nonparticipants were found without difference in age, gender, race, baseline SE or AL, or change of SE or AL. For the individuals of the ATOM2 study, the mean (SD) age of the participants of the current study and the nonparticipants was 9.5 (1.5) and 9.9 (1.6) years (P = .01) (eTable 2 in Supplement 1).
Long-Term Efficacy of Childhood Atropine Treatment—ATOM1 Study Participants
Of 71 ATOM1 study participants who returned for the current follow-up evaluation, 63 individuals who had completed 2 years of atropine treatment in childhood were included in the statistical analysis. The demographic characteristics, SE and AL at the baseline visit, at the 2-year and 3-year visit (final trial visit), and at the current study visit are presented in Table 1. At the 20-year study visit, there were no differences in final SE or AL between 1% atropine-treated eyes vs untreated eyes and 1% atropine-treated eyes vs placebo. The eyes in all groups exhibited progression in myopic refractive error and axial elongation following the examination that took place at the final trial visit 20 years ago (eFigure 1 in Supplement 1).
Table 1. Characteristics of Study Participants With Baseline and Current Study Visit.
| ATOM1 | ATOM2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1% Atropine-treated eyes (n = 32) | 1% Atropine- untreated fellow eyes (n = 32) | Mean difference (95% CI)a | Placebo eyes (n = 31) | Mean difference (95% CI)b | 0.01% Atropine (n = 56) | 0.1% Atropine (n = 122) | 0.5% Atropine (n = 118) | Mean difference (95% CI)c | Mean difference (95% CI)d | |
| Baseline age, mean (SD), y | 9.2 (1.4) | 9.2 (1.4) | NA | 8.8 (1.3) | NA | 9.6 (1.4) | 9.7 (1.4) | 9.5 (1.4) | NA | NA |
| Study age, mean (SD), y | 30.5 (1.2) | 30.5 (1.2) | NA | 30.0 (1.5) | NA | 24.5 (1.4) | 24.4 (1.5) | 24.1 (1.4) | NA | NA |
| Sex, No. (%) | ||||||||||
| Female | 13 (40.6) | 13 (40.6) | NA | 18 (58.1) | NA | 24 (42.9) | 54 (44.3) | 64 (54.2) | NA | NA |
| Male | 19 (59.4) | 19 (59.4) | 13 (41.9) | 32 (57.1) | 68 (55.7) | 54 (45.8) | ||||
| Race,e No. (%) | ||||||||||
| Chinese | 32 (100.0) | 32 (100.0) | NA | 31 (100) | NA | 54 (96.4) | 108 (88.5) | 106 (89.8) | NA | NA |
| Indian | NA | NA | NA | NA | NA | NA | 2 (1.6) | 2 (1.7) | NA | NA |
| Malay | NA | NA | NA | NA | NA | NA | 10 (8.2) | 8 (6.8) | NA | NA |
| Otherf | NA | NA | NA | NA | NA | 2 (3.6) | 2 (1.7) | NA | NA | |
| Retreatment, No. % | NA | NA | NA | NA | NA | 14 (25.0) | 74 (60.7) | 86 (72.9) | NA | NA |
| SE, mean (SD), D | ||||||||||
| Baseline | −2.82 (1.23) | −3.25 (1.21) | 0.43 (−0.18 to 1.04) | −3.39 (1.05) | 0.57 (0-1.14) | −4.51 (1.41) | −4.49 (1.37) | −4.62 (1.98) | −0.02 (−0.47 to 0.43) | 0.11 (−0.41 to 0.63) |
| 2 y | −3.26 (1.50) | −4.51 (1.59) | 1.25 (0.48-2.03) | −4.63 (1.19) | 1.37 (0.69-2.06) | −5.11 (1.53) | −4.82 (1.30) | −4.84 (1.85) | −0.30 (−0.76 to 0.17) | −0.27 (−0.80 to 0.25) |
| 3 y | −4.31 (1.73) | −4.89 (1.83) | 0.58 (−0.31 to 1.47) | −5.07 (1.27) | 0.76 (0-1.52) | NA | NA | NA | NA | NA |
| 5 y | NA | NA | NA | NA | NA | −5.97 (1.83) | −6.25 (1.64) | −6.63 (2.40) | 0.28 (−0.29 to 0.85) | 0.66 (0.01-1.31) |
| Current | −5.20 (2.46) | −5.42 (2.37) | 0.22 (−0.99 to 1.43) | −6.00 (1.63) | 0.80 (−0.25 to 1.85) | −6.40 (2.21) | −6.81 (1.92) | −7.19 (2.87) | 0.40 (−0.28 to 1.08) | 0.79 (0-1.57) |
| Change of SE, mean (SD), D | ||||||||||
| Baseline to 2 y | −0.44 (0.78) | −1.26 (0.74) | 0.82 (0.44-1.20) | −1.25 (0.81) | 0.81 (0.40-1.21) | −0.60 (0.66) | −0.33 (0.61) | −0.22 (0.56) | −0.28 (−0.48 to −0.07) | −0.39 (−0.59 to −0.18) |
| Baseline to 3 y | −1.49 (0.93) | −1.64 (0.99) | 0.15 (−0.33 to 0.63) | −1.68 (1.01) | 0.19 (−0.29 to 0.68) | NA | NA | NA | NA | NA |
| 3 y to current | −0.90 (1.21) | −0.54 (1.19) | −0.36 (−0.96 to 0.24) | −0.94 (1.08) | 0.04 (−0.54 to 0.62) | NA | NA | NA | NA | NA |
| Baseline to 5 y | NA | NA | NA | NA | NA | −1.47 (1.10) | −1.77 (1.15) | −2.01 (1.17) | 0.30 (−0.06 to 0.66) | 0.55 (0.19-0.91) |
| 5 y to current | NA | NA | NA | NA | NA | −0.43 (0.82) | −0.55 (0.85) | −0.56 (0.98) | 0.12 (−0.14 to 0.39) | 0.13 (−0.15 to 0.41) |
| Baseline to current | −2.38 (1.69) | −2.17 (1.59) | −0.21 (−1.03 to 0.61) | −2.62 (1.71) | 0.23 (−0.62 to 1.09) | −1.90 (1.62) | −2.32 (1.58) | −2.57 (1.71) | 0.42 (−0.09 to 0.94) | 0.68 (0.14-1.21) |
| AL, mean (SD), mm | ||||||||||
| Baseline | 24.74 (0.76) | 24.80 (0.79) | −0.06 (−0.45 to 0.33) | 24.75 (0.87) | −0.01 (−0.42 to 0.41) | 25.20 (1.01) | 25.06 (0.79) | 25.04 (0.92) | 0.14 (−0.16 to 0.44) | 0.16 (−0.16 to 0.47) |
| 2 y | 24.81 (0.87) | 25.19 (0.83) | −0.38 (−0.80 to 0.05) | 25.15 (0.88) | −0.34 (−0.78 to 0.10) | 25.61 (1.08) | 25.32 (0.76) | 25.28 (0.96) | 0.28 (−0.03 to 0.60) | 0.33 (−0.01 to 0.67) |
| 3 y | 25.11 (0.92) | 25.26 (0.91) | −0.15 (−0.61 to 0.31) | 25.34 (0.95) | −0.23 (−0.70 to 0.24) | NA | NA | NA | NA | NA |
| 5 y | NA | NA | NA | NA | NA | 25.98 (1.13) | 25.89 (0.82) | 25.95 (1.12) | 0.09 (−0.24 to 0.43) | 0.03 (−0.33 to 0.39) |
| Current | 25.87 (1.23) | 25.80 (1.18) | 0.07 (−0.53 to 0.67) | 25.90 (1.21) | −0.03 (−0.65 to 0.58) | 26.25 (1.34) | 26.28 (0.99) | 26.31 (1.31) | −0.03 (−0.42 to 0.37) | −0.06 (−0.48 to 0.37) |
| Change of AL, mean (SD), mm | ||||||||||
| Baseline to 2 y | 0.07 (0.29) | 0.39 (0.37) | −0.32 (−0.49 to −0.15) | 0.40 (0.34) | −0.34 (−0.49 to −0.18) | 0.41 (0.34) | 0.27 (0.27) | 0.24 (0.25) | 0.15 (0.04-0.25) | 0.17 (0.07-0.27) |
| Baseline to 3 y | 0.37 (0.31) | 0.46 (0.52) | −0.09 (−0.31 to 0.12) | 0.60 (0.50) | −0.23 (−0.44 to −0.01) | NA | NA | NA | NA | NA |
| 3 y to current | 0.78 (0.64) | 0.54 (0.75) | 0.24 (−0.11 to 0.59) | 0.56 (0.66) | 0.22 (−0.11 to 0.55) | NA | NA | NA | NA | NA |
| Baseline to 5 y | NA | NA | NA | NA | NA | 0.78 (0.54) | 0.83 (0.50) | 0.91 (0.52) | −0.05 (−0.21 to 0.12) | −0.12 (−0.29 to 0.05) |
| 5 y to current | NA | NA | NA | NA | NA | 0.28 (0.45) | 0.39 (0.41) | 0.36 (0.40) | −0.12 (−0.26 to 0.02) | −0.09 (−0.23 to 0.05) |
| Baseline to current | 1.13 (0.73) | 1.00 (0.84) | 0.13 (−0.27 to 0.52) | 1.16 (0.92) | −0.02 (−0.45 to 0.39) | 1.06 (0.87) | 1.22 (0.73) | 1.27 (0.73) | −0.16 (−0.43 to 0.10) | −0.21 (−0.48 to 0.06) |
Abbreviations: AL, axial length; ATOM, Atropine for the Treatment of Myopia; D, diopters; NA, not applicable; SE, spherical equivalent.
1% Atropine-treated eyes vs 1% atropine-untreated fellow eyes.
1% Atropine-treated eyes vs placebo eyes.
0.1% Atropine group vs 0.01% atropine group.
0.5% Atropine group vs 0.01% atropine group.
Race was identified by each individual’s National Registration Identity Card during registration.
Includes Eurasian and Sikh.
Long-Term Efficacy of Childhood Atropine Treatment—ATOM2 Study Participants
Data from 148 participants who had completed the initial 2 years of atropine treatment of the 158 ATOM2 participants who returned for follow-up evaluation were included in the statistical analysis. The demographic characteristics, SE and AL at the baseline visit, 2-year visit, 5-year visit (final trial visit), and the current study visit of the participants in atropine 0.01%, 0.1%, and 0.5% treatment groups are presented in Table 1. At the current study visit, the 3 atropine groups did not differ in SE or AL. Participants in all groups showed SE progression and axial elongation since the final trial visit 10 years prior to the current study visit (eFigure 2 in Supplement 1).
Higher myopia progression from the final trial visit to the current study visit was identified with younger age (mean difference = 0.6 years; 95% CI, 0.2-0.9 years; P = .002), randomization to higher concentrations of atropine (0.1% and 0.5% vs 0.01%) (88.3% vs 77.7%; P = .03), and in those requiring retreatment (68.1% vs 54.5%; P = .03) (eTable 3 in Supplement 1). Eyes that progressed 1.0 D or more showed greater SE progression and axial elongation during the clinical trial period (year 0 to year 5) and following the cessation of atropine treatment (year 5 to year 15), leading to greater SE and longer AL at both the final trial visit and the current study visit (eFigure 3 in the Supplement). The study team found that 10-year myopia progression following the cessation of atropine treatment was associated with a younger age at treatment initiation (OR, 0.82; 95% CI, 0.67-0.99; P = .04) and higher concentration of atropine treatment (0.1% vs 0.01%: OR, 2.57; 95% CI, 1.16-5.69; P = .02; 0.5% vs 0.01%: OR, 2.48; 95% CI, 1.09-5.62; P = .03) after adjusting for gender (P = .06) and SE progression during treatment (P = .15) (Table 2).
Table 2. Multivariate Logistic Regression Analysis of Factors Associated With 10-Year Myopia Progression Following the Cessation of Childhood Atropine Treatment in Atropine for the Treatment of Myopia Study Participants.
| Variable | Coefficient | Standard error | P value | OR (95% CI) |
|---|---|---|---|---|
| Age, y | −0.20 | 0.10 | .04 | 0.82 (0.67-0.99) |
| Gender (male vs female) | 0.49 | 0.26 | .06 | 1.63 (0.98-2.73) |
| SE progression during treatment, D | −0.34 | 0.23 | .15 | 0.71 (.45-1.12) |
| Atropine treatment | ||||
| 0.1% vs 0.01% | 0.94 | 0.41 | .02 | 2.57 (1.16-5.69) |
| 0.5% vs 0.01% | 0.91 | 0.42 | .03 | 2.48 (1.09-5.62) |
Abbreviations: SE, spherical equivalent; D, diopters; OR, odds ratio.
Long-Term Safety Incidence of Potential Ocular Complications in ATOM1 and ATOM2 Study Participants
The incidence of potential treatment or myopia-related ocular complications in these study participants following 10 or 20 years after childhood atropine treatment are summarized in Table 3. In ATOM1 participants, the incidence of cataract/lens opacity, glaucoma suspect, MMD, parapapillary atrophy (β/γ zone), and optic disc tilt was not different comparing 1% atropine-treated eyes with untreated or placebo eyes. In ATOM2 participants, the incidence of MMD was 19.6%, 28.7%, and 38.1% in 0.01%, 0.1%, and 0.5% atropine groups, respectively (0.1% vs 0.01%: OR, 1.64; 95% CI, 0.73-3.93; 0.5% vs 0.01%: OR, 2.51; 95% CI, 1.13-5.95; P = .04). The 10-year incidence of MMD was associated with female gender (OR, 0.52; 95% CI, 0.29-0.92; P = .03), longer AL at baseline (OR, 1.92; 95% CI, 1.38-2.68; P < .001), greater axial elongation during treatment (OR, 4.03; 95% CI, 2.33-6.97; P < .001), and higher concentration of atropine treatment (0.5% vs 0.01%: OR, 2.60; 95% CI, 1.15-5.89; P = .02) (Table 4).
Table 3. Safety of Childhood Atropine Treatment: 10- and 20-Year Incidence of Ocular Complications in Study Participants.
| ATOM1 | ATOM2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1% Atropine-treated eyes (n = 32) | 1% Atropine- untreated fellow eyes (n = 32) | OR (95% CI)a | P valuea | Placebo eyes (n = 62) | OR (95% CI)b | P valueb | 0.01% Atropine (n = 56) | 0.1% Atropine (n = 122) | 0.5% Atropine (n = 118) | OR (95% CI)c | OR (95% CI)d | P value | |
| MMD, No. (%) | 6 (18.8) | 5 (15.6) | 0.81 (0.17-3.61) | 1.00 | 16 (25.8) | 1.50 (0.48-5.28) | .61 | 11 (19.6) | 35 (28.7) | 45 (38.1) | 1.64 (0.73-3.93) | 2.51 (1.13-5.95) | .04 |
| Parapapillary atrophy, No. (%)e | |||||||||||||
| Mildf | 17 (53.1) | 18 (56.3) | 1.31 (0.42-4.24) | .79 | 38 (61.3) | 1.58 (0.57-4.30) | .35 | 32 (57.1) | 77 (63.1) | 69 (58.5) | 1.42 (0.63-3.13) | 0.94 (0.43-2.02) | .70 |
| Moderateg | 2 (6.3) | 3 (9.4) | 5 (8.1) | 9 (16.1) | 17 (13.9) | 13 (11.0) | |||||||
| Severeh | 1 (3.1) | 1 (3.1) | 2 (3.2) | 0 (0) | 3 (2.5) | 3 (2.5) | |||||||
| Optic disc tilt, No. (%) | 13 (40.6) | 15 (46.9) | 1.28 (0.43-3.89) | .80 | 38 (61.3) | 2.29 (0.89-6.08) | .08 | 31 (55.4) | 71 (58.2) | 58 (49.2) | 1.12 (0.56-2.23) | 0.78 (0.39-1.55) | .37 |
| Posterior staphyloma, No. (%) | 0 (0) | 0 (0) | NA | NA | 0 (0) | NA | NA | 0 (0) | 0 (0) | 4 (3.4) | NA | NA | .05 |
| Cataract/lens opacity, No. (%) | 0 (0) | 0 (0) | NA | NA | 4 (6.5) | NA | NA | 0 (0) | 0 (0) | 0 (0) | NA | NA | 1.00 |
| Glaucoma suspect, No. (%) | 1 (3.1) | 1 (3.1) | 1 (0.01-80.95) | 1.00 | 3 (4.8) | 1.57 (0.12-85.35) | 1.00 | 2 (3.6) | 2 (1.6) | 4 (3.4) | 0.45 (0.03-6.39) | 0.95 (0.13-10.78) | .64 |
| WWP, No. (%) | 0 (0) | 0 (0) | NA | NA | 1 (1.6) | NA) | NA | 3 (5.4) | 6 (4.9) | 11 (9.3) | 0.91 (0.19-5.86) | 1.81 (0.45-10.53) | .40 |
| Lattice degeneration, No. (%) | 1 (3.1) | 0 (0) | NA | NA | 0 (0) | NA | NA | 0 (0) | 0 (0) | 2 (1.7) | NA | NA | .50 |
| Retinal holes/tears/breaks, No. (%) | 0 (0) | 1 (3.1) | NA | NA | 0 (0) | NA | NA | 0 (0) | 0 (0) | 1 (0.8) | NA | NA | .59 |
Abbreviations: ATOM, Atropine for the Treatment of Myopia; MMD, NA, not applicable; myopic macular degeneration; OR, odds ratio; WWP, white with pressure.
1% Atropine-treated eyes vs 1% atropine-untreated fellow eyes.
1% Atropine-treated eyes vs placebo eyes.
0.1% Atropine group vs 0.01% atropine group.
0.5% Atropine group vs 0.01% atropine group.
OR comparing the total positive cases of parapapillary atrophy between groups.
1/3 or Less longest diameter of visible nerve in width.
In between mild and severe.
Greater in width than 1 longest diameter of visible nerve.
Table 4. Multivariate Regression Analysis of 10-Year Incidence of Myopic Macular Degeneration at Study Visit in Atropine for the Treatment of Myopia 2 Study Participants.
| Variable | Coefficient | Standard error | P value | OR (95% CI) |
|---|---|---|---|---|
| Gender (male vs female) | −0.66 | 0.30 | .03 | 0.52 (0.29-0.92) |
| AL at baseline, mm | 0.65 | 0.17 | <.001 | 1.92 (1.38-2.68) |
| Axial elongation during treatment, mm | 1.39 | 0.28 | <.001 | 4.03 (2.33-6.97) |
| Atropine treatment | ||||
| 0.1% vs 0.01% | 0.65 | 0.42 | .12 | 1.92 (0.85-4.36) |
| 0.5% vs 0.01% | 0.95 | 0.42 | .02 | 2.60 (1.15-5.89) |
Abbreviations: AL, axial length; OR, odds ratio.
Discussion
In this long-term follow-up study in adults who had previous childhood atropine treatment for myopia, we reviewed 71 of 400 ATOM1 (17.8%) and 158 of 400 ATOM2 participants (39.5%). Since only a minority of the originally randomized participants were included in this 10- to 20-year follow-up study, this observational investigation primarily provides hypothesis generation from associations, as opposed to the original randomized clinical trials which were designed to establish causality. We found that 10 to 20 years after the short-term use of atropine in childhood, there were no differences in final SE and AL between atropine treated and untreated fellow/placebo eyes in the ATOM1 participants and between groups of different atropine concentrations in the ATOM2 participants (Table 1). Greater myopia progression postcessation of treatment was associated with eyes that were treated at a younger age and randomized to higher concentrations of atropine in the ATOM2 participants (Table 2). In addition, we observed no differences in treatment or myopia-associated ocular complications between atropine treated and untreated fellow/placebo eyes in ATOM1 participants, though we noted a higher incidence of MMD associated with the 0.5% atropine group compared with the 0.01% atropine group in ATOM2 (Table 3).
This study suggests an atropine treatment quandary. The original ATOM1 and ATOM2 clinical trials supported the hypothesis that atropine has a dose-related effect on myopia progression with higher concentrations potentially being more effective in slowing progression, albeit with more adverse effects (near vision blur and glare).8,11 After stopping atropine for 2 years, there was a reverse concentration-related effect suggested with greater rebound noted, especially in higher concentrations and younger children, which negated the initial benefits of atropine use.21,26 Zadnik et al13 also recently reported reduction in myopia progression in their secondary outcome of 0.01% atropine compared with placebo but not for the primary outcome of 0.02% atropine over 3 years of treatment. However, other similar randomized clinical trials by the National Eye Institute-sponsored Pediatric Eye Disease Investigator Group did not show a benefit of 0.01% atropine compared with placebo and suggested evaluation of the use of higher concentrations.14 While a Cochrane meta-analysis further supports that low dose atropine (less than 0.1%) had a mean treatment difference of 0.24 D with equivalence criteria being less than 0.25 D,13,14,15 these studies do not incorporate the recent lack of an effect for the primary 0.02% atropine trial by Zadnik et al nor the lack of an effect of the 0.01% atropine dose in the Pediatric Eye Disease Investigator Group.
In this ATLAS cohort, at their 20-year and 10-year follow-up visits, we did not observe any differences in SE, AL, or the prevalence of high myopia when comparing different groups in either the ATOM1 or ATOM2 study participants. This study may support existing literature that atropine may be ineffective at reducing myopia progression, with this study specifically examining progress into adulthood through observation of outcomes over a 10- to 20-year period. Reasons for these results could be that there is no benefit to short-term atropine treatment or attributed to rebound effects following abrupt cessation and the possibility of long-term rebound effects or other potentially confounding factors. These observations raise several research questions that warrant further study—the duration of atropine treatment required to provide a sustained outcome, when treatment can be stopped, and whether tapering dosage or continuing treatment into the mid-teenage years.6
This observational study does provide some information regarding concerns of complications with childhood atropine usage, including long-term effects from prolonged pupil dilation (eg, cataract formation or macular photo-toxicity) and paralysis of accommodation (eg, early onset of presbyopia),24 although again, the results should be viewed from the perspective that only a minority of participants were included in this observational study; it is possible that the participants with adverse effects were less likely to return for follow-up.
A potential reported adverse effect of topical atropine is increased intraocular pressure.27,28 In this study, we found no differences in the incidence of cataract, macular degeneration, or glaucoma between groups (Table 3). Clinical studies also have reported that atropine had no effect on intraocular pressure in children,20,29 and that the risk of atropine-induced glaucoma was low (1 in 20 000).30
We found that a longer AL at baseline in childhood and greater axial elongation during treatment over the clinical trial period were associated with a higher incidence of early MMD in our adult participants. It has to be taken into account, however, that most eyes had grade 1 MMD (ie, fundus tessellation), which has not been considered to be pathological and which can but does not need to progress to pathological grades of MMD.22,31 Nonetheless, AL elongation could be an important parameter in assessing the response to atropine treatment during childhood, which could be associated with the risk of MMD development in adulthood. Our observations are consistent with another 19-year follow-up study on myopia progression from childhood to adulthood, as adolescent AL was reported as an important factor in predicting early MMD in young adults.32,33 Although this study showed there was no difference in incidence of MMD between atropine treated and untreated eyes in ATOM1 participants, we observed in the follow-up of ATOM2 participants that treatment with a higher concentration of atropine (0.5%) compared with a lower concentration (0.01%) was independently associated with a higher 10-year incidence of MMD. Our observational study supports the importance of monitoring axial elongation and MMD development in all patients receiving atropine treatment.28
Limitations
Despite the potentially valuable insights from the long-term follow-up of 2 landmark clinical trials, we recognize the limitations of our study in evaluating adults who had previous childhood treatment for myopia. First, due to the long interval from the final clinical trial visit, many individuals were not observed for this longer term follow-up; we evaluated only 71 of the 400 ATOM1 (17.8%) and only 158 of the 400 ATOM2 participants (39.5%). Attaining a follow-up rate of approximately one-quarter of the original participants may introduce selection bias, potentially affecting the interpretation of the results. Furthermore, differences in the instrumentation used for autorefraction and ocular biometry, as well as variations in the cycloplegic drop regimen between the current study and the original studies, potentially could have affected the comparisons of myopia progression and axial elongation following the cessation of atropine treatment. Our observational study also may have overlooked other peripheral retinal changes that are challenging to assess through fundus photographs only of the posterior pole.
Conclusion
In conclusion, among approximately one-quarter of the original participants, our long-term observational study after a short duration of childhood atropine treatment for myopia control demonstrates that use of topical atropine eye drops ranging from 0.01% to 1.0% for a duration of 2 to 4 years during childhood for myopia control was not associated with the final refractive errors 10 to 20 years after treatment. There was no increased incidence of treatment or myopia-related ocular complications in 1% atropine-treated vs placebo groups. These findings may affect the design of future clinical trials for childhood myopia control as further research is required in terms of the duration and concentration of atropine treatment needed to produce a sustained effect on myopia progression and reduction of myopia-related complications in adulthood.
eTable 1. Follow-up Rates of the ATOM Studies
eTable 2. Comparison between the Study Participants and the Residual Participants
eTable 3. Demographics and Characteristics of ATOM2 Study Participants Classified by Myopia Progression from the Final Trial Visit to the Current Study Visit
eFigure 1. Box plots showing the change of mean spherical equivalent (SE) and axial length (AL) from baseline of ATOM1 study participants
eFigure 2. Box plots showing the change of mean spherical equivalent (SE) and axial length (AL) from baseline of ATOM2 study participants
eFigure 3. Line chart showing the change of mean spherical equivalent (SE) and axial length (AL) of ATOM2 study participants over time. Error bars show the standard errors of the means
Data sharing statement
References
- 1.Baird PN, Saw S-M, Lanca C, et al. Myopia. Nat Rev Dis Primers. 2020;6(1):99. doi: 10.1038/s41572-020-00231-4 [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Foo L-L, Wong CW, et al. Pathologic myopia: advances in imaging and the potential role of artificial intelligence. Br J Ophthalmol. 2023;107:(5):600-606. doi: 10.1136/bjophthalmol-2021-320926 [DOI] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The risks and benefits of myopia control. Ophthalmology. 2021;128(11):1561-1579. doi: 10.1016/j.ophtha.2021.04.032 [DOI] [PubMed] [Google Scholar]
- 5.Modjtahedi BS, Abbott RL, Fong DS, Lum F, Tan D; Task Force on Myopia . Reducing the global burden of myopia by delaying the onset of myopia and reducing myopic progression in children: the academy’s task force on myopia. Ophthalmology. 2021;128(6):816-826. doi: 10.1016/j.ophtha.2020.10.040 [DOI] [PubMed] [Google Scholar]
- 6.Ang M, Flanagan JL, Wong CW, et al. Review: Myopia control strategies recommendations from the 2018 WHO/IAPB/BHVI Meeting on Myopia. Br J Ophthalmol. 2020;104(11):1482-1487. doi: 10.1136/bjophthalmol-2019-315575 [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Ang M, Cho P, et al. IMI Prevention of Myopia and Its Progression. Invest Ophthalmol Vis Sci. 2021;62(5):6. doi: 10.1167/iovs.62.5.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua W-H, Balakrishnan V, Chan Y-H, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-2291. doi: 10.1016/j.ophtha.2006.05.062 [DOI] [PubMed] [Google Scholar]
- 9.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua W-H. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572-579. doi: 10.1016/j.ophtha.2008.10.020 [DOI] [PubMed] [Google Scholar]
- 10.Chia A, Chua W-H, Cheung Y-B, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. doi: 10.1016/j.ophtha.2011.07.031 [DOI] [PubMed] [Google Scholar]
- 11.Chia A, Lu Q-S, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391-399. doi: 10.1016/j.ophtha.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Yam JC, Zhang XJ, Zhang Y, et al. Effect of Low-Concentration Atropine Eyedrops vs Placebo on Myopia Incidence in Children: The LAMP2 Randomized Clinical Trial. JAMA. 2023;329(6):472-481. doi: 10.1001/jama.2022.24162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zadnik K, Schulman E, Flitcroft I, et al. ; CHAMP Trial Group Investigators . Efficacy and Safety of 0.01% and 0.02% Atropine for the Treatment of Pediatric Myopia Progression Over 3 Years: A Randomized Clinical Trial. JAMA Ophthalmol. 2023;e232097. doi: 10.1001/jamaophthalmol.2023.2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repka MX, Weise KK, Chandler DL, et al. ; Pediatric Eye Disease Investigator Group . Low-dose 0.01% atropine eye drops vs placebo for myopia control: a randomized clinical trial. JAMA Ophthalmol. 2023;141(8):756-765. doi: 10.1001/jamaophthalmol.2023.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrenson JG, Shah R, Huntjens B, et al. Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database Syst Rev. 2023;2(2):CD014758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pineles SL, Kraker RT, VanderVeen DK, et al. Atropine for the Prevention of Myopia Progression in Children: A Report by the American Academy of Ophthalmology. Ophthalmology. 2017;124(12):1857-1866. doi: 10.1016/j.ophtha.2017.05.032 [DOI] [PubMed] [Google Scholar]
- 17.Gong Q, Janowski M, Luo M, et al. Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-analysis. JAMA Ophthalmol. 2017;135(6):624-630. doi: 10.1001/jamaophthalmol.2017.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and Safety of 8 Atropine Concentrations for Myopia Control in Children: A Network Meta-Analysis. Ophthalmology. 2022;129(3):322-333. doi: 10.1016/j.ophtha.2021.10.016 [DOI] [PubMed] [Google Scholar]
- 19.Wong CW, Foo LL, Morjaria P, et al. Highlights from the 2019 International Myopia Summit on ‘controversies in myopia’. Br J Ophthalmol. 2021;105(9):1196-1202. doi: 10.1136/bjophthalmol-2020-316475 [DOI] [PubMed] [Google Scholar]
- 20.Wu T-EJ, Yang C-C, Chen H-S. Does atropine use increase intraocular pressure in myopic children? Optom Vis Sci. 2012;89(2):E161-E167. doi: 10.1097/OPX.0b013e31823ac4c1 [DOI] [PubMed] [Google Scholar]
- 21.Chia A, Chua W-H, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014; 157(2): 451-7. e1. doi: 10.1016/j.ajo.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 22.Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015; 159(5): 877-83. e7. doi: 10.1016/j.ajo.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Panda-Jonas S, Jonas JB. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: Histology and clinical features. Prog Retin Eye Res. 2021;83:100933. doi: 10.1016/j.preteyeres.2020.100933 [DOI] [PubMed] [Google Scholar]
- 24.Ahmad SS. Glaucoma suspects: A practical approach. Taiwan J Ophthalmol. 2018;8(2):74-81. doi: 10.4103/tjo.tjo_106_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645-646. doi: 10.1111/j.0006-341X.2000.00645.x [DOI] [PubMed] [Google Scholar]
- 26.Loh KL, Lu Q, Tan D, Chia A. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol. 2015;159(5):945-949. doi: 10.1016/j.ajo.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 27.Upadhyay A, Beuerman RW. Biological mechanisms of atropine control of myopia. Eye Contact Lens. 2020;46(3):129-135. doi: 10.1097/ICL.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu PC, Chuang MN, Choi J, et al. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond). 2019;33(1):3-13. doi: 10.1038/s41433-018-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C-Y, Sun C-C, Lin Y-F, Lin K-K. Effects of topical atropine on intraocular pressure and myopia progression: a prospective comparative study. BMC Ophthalmol. 2016;16(1):114. doi: 10.1186/s12886-016-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandit RJ, Taylor R. Mydriasis and glaucoma: exploding the myth. A systematic review. Diabet Med. 2000;17(10):693-699. doi: 10.1046/j.1464-5491.2000.00368.x [DOI] [PubMed] [Google Scholar]
- 31.Kassam I, Foo L-L, Lanca C, et al. The Potential of Current Polygenic Risk Scores to Predict High Myopia and Myopic Macular Degeneration in Multiethnic Singapore Adults. Ophthalmology. 2022;129(8):890-902. doi: 10.1016/j.ophtha.2022.03.022 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Lanca C, Htoon HM, et al. High Myopes in Singapore: 19-Year Progression from Childhood to Adulthood. Ophthalmology. 2020;127(12):1768-1770. doi: 10.1016/j.ophtha.2020.05.031 [DOI] [PubMed] [Google Scholar]
- 33.Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology. 2018;125(6):863-877. doi: 10.1016/j.ophtha.2017.12.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Follow-up Rates of the ATOM Studies
eTable 2. Comparison between the Study Participants and the Residual Participants
eTable 3. Demographics and Characteristics of ATOM2 Study Participants Classified by Myopia Progression from the Final Trial Visit to the Current Study Visit
eFigure 1. Box plots showing the change of mean spherical equivalent (SE) and axial length (AL) from baseline of ATOM1 study participants
eFigure 2. Box plots showing the change of mean spherical equivalent (SE) and axial length (AL) from baseline of ATOM2 study participants
eFigure 3. Line chart showing the change of mean spherical equivalent (SE) and axial length (AL) of ATOM2 study participants over time. Error bars show the standard errors of the means
Data sharing statement


