Abstract
BACKGROUND
Women with obesity have higher rates of complications following cesarean delivery, such as wound infection and endometritis, with risks being the highest if a cesarean delivery is performed after labor. Previous efforts at predicting whether a patient's labor course would ultimately result in cesarean delivery have been intermediate with area under the curve in the 0.75 to 78 range.
OBJECTIVE
This study aimed to assess whether machine learning algorithms would outperform traditional modeling in developing a cesarean delivery prediction model among gravidas with morbid obesity (body mass index of ≥40 kg/m2) to determine whether a primary cesarean delivery may be beneficial.
STUDY DESIGN
This was a secondary analysis of a retrospective cohort of 1298 patients with morbid obesity presenting for vaginal delivery at ≥37 weeks of gestation between 2011 and 2016 at a single institution. Data available at the time of admission and delivery were modeled using logistic regression, decision tree, random forest, and support vector modeling with evaluation of area under the curve, accuracy, sensitivity, and specificity.
RESULTS
Logistic regression demonstrated an area under the curve of 0.816 (95% confidence interval, 0.810–0.817), which was superior to machine learning models when evaluating data at the time of delivery (demographic data, initial cervical examinations, comorbidities, and obstetrical interventions) (P<.001). However, there was no significant difference between most machine learning models and logistic regression area under the curve of 0.799 (95% confidence interval, 0.795–0.804) when evaluating parameters available at the time of admission (demographic data, initial cervical examinations, and comorbidities). Race was noted to be a significant predictor in both models (P<.001).
CONCLUSION
Machine learning and traditional modeling techniques are likely equivalent concerning cesarean delivery prediction in this population. The models developed showed good discrimination and may be used to guide clinical decision-making concerning the optimal mode of delivery.
Key words: cesarean delivery, delivery, labor, machine learning, morbid obesity, obesity, obstetrics, prediction, primary cesarean
AJOG Global Reports at a Glance.
Why was this study conducted?
This study aimed to determine whether primary cesarean delivery among gravidas with morbid obesity could be predicted using machine learning (ML) to avoid morbidity associated with cesarean delivery after labor.
Key findings
Both ML and traditional statistical methods were equivalent to the development of a good model with an area under the curve of 0.802. However, ML was not superior to traditional statistical techniques.
What does this add to what is known?
Previous models do not focus on gravidas with morbid obesity where the postcesarean delivery morbidity is the highest. The model developed in our study has good predictive ability and may help reduce maternal morbidity.
Introduction
The Centers for Disease Control and Prevention reports that 41.9% of the American population is obese (body mass index [BMI], ≥30 kg/m2). Obesity is an established risk factor for various adverse pregnancy outcomes,1 including the need for cesarean delivery.2,3 In addition, women with obesity have higher rates of complications after cesarean delivery, such as wound infection and endometritis, with risks being the highest if a cesarean delivery is performed after labor.4,5 In this high-risk group, knowing a woman's risk of cesarean delivery could help guide management decisions.
Previous efforts at identifying factors associated with unplanned primary cesarean delivery demonstrated that maternal sociodemographic characteristics, along with pregnancy and labor management variables (eg, attempted labor induction, gestational and pregestational diabetes mellitus, macrosomia, the timing of hospital admission, labor augmentation with oxytocin, and hypertension) significantly modified this risk profile.6,7 These factors have been combined into several different prediction models to help counsel patients as to the best course of action regarding their labor and delivery.8, 9, 10, 11 Hamm et al12 demonstrated that there is an association between cesarean delivery after labor induction with increased length of labor, risk of cesarean, and maternal and neonatal morbidity using a modest predictive model with area under the curve (AUC) of 0.73. Subramaniam et al5 demonstrated a 45% risk of maternal morbidity with cesarean delivery after labor induction among gravidas with morbid obesity (BMI, ≥40 kg/m2). With the associated risks, it may be preferable for some patients to consider a primary cesarean delivery before labor induction to reduce the morbidity associated with a cesarean delivery during labor.
The construction of the aforementioned models used various methods, including univariate and multivariate analyses. However, the accuracy of the available prediction models remains in an intermediate zone, with AUCs of 0.75 and 0.78.10,13 Recently, there has been an increasing interest in using machine learning (ML) methods to improve the accuracy of prediction models in healthcare settings, including obstetrics and gynecology.14 In general, ML models use different algorithms, such as those noted in the Glossary to identify patterns in data in a training dataset. Subsequently, the pattern is applied to a test dataset to determine the accuracy. The advantages of ML compared with traditional model building include the ability to analyze large multidimensional data, with continuous revision of pattern from 1 patient to the next and the adjustment of the importance of any given variable in the model in an iterative fashion. In large datasets, such as thousands of patient medical records, there are a large number of variables with differing distributions, types of information, and significant amounts of missing data that may be skewed, which can be challenging to handle with traditional modeling. The analytical methodology of ML allows for the analysis of such datasets without significant data manipulation. This approach has been used to improve the prediction of maternal intensive care unit admission and postpartum maternal readmission rates among others with good discrimination.14 As such, we hypothesized that ML algorithms would outperform traditional modeling in developing a cesarean delivery prediction model among gravidas with morbid obesity (BMI, ≥40 kg/m2) who presented for delivery at ≥37 weeks of gestation.
Materials and Methods
This was a secondary analysis of a retrospective cohort study of 1298 patients presenting to a large tertiary care referral center for delivery at ≥37 weeks of gestation with a BMI of ≥40 kg/m2 between 2011 and 2016, regardless of gravidity. Obstetrical data included maternal demographic variables and features (eg, age, weight, height, prepregnancy BMI, race, payor status [eg, self, government, or commercial]) and maternal and fetal clinical variables (eg, parity, chronic hypertension, antihypertensive medication, gestational hypertension, preeclampsia, preeclampsia with severe features, gestational diabetes mellitus, pregestational diabetes mellitus, fetal growth restriction, macrosomia, group B streptococcus [GBS] colonization status, previous cesarean delivery, antepartum hospitalization, stillbirth, date of delivery admission, gestational age at delivery, in labor on admission, BMI at delivery, cervical dilation, cervical effacement, station of the presenting part, use of ripening agent, ripening agent specification, oxytocin induction, oxytocin augmentation, oxytocin maximum dose, labor epidural, intrapartum antibiotics, GBS prophylaxis, chorioamnionitis, purulent discharge, urinary tract infection or pyelonephritis, number of hours in labor and delivery, and neonatal sex). Both demographic and clinical variables were abstracted from the medical record by trained clinicians. Women who were not eligible for vaginal delivery were excluded. We considered the mode of delivery (cesarean delivery vs noncesarean delivery) as an outcome variable of interest. We developed 2 models. The first was to explore the ability to predict cesarean delivery with all variables available until the time of delivery, the second was to use only features potentially available at admission. Institutional ethical review board approval was obtained for the original study (n=7179; August 25, 2016).
Before learning models, categorical variables were converted into binary values by performing one-hot encoding, which produces a vector with a length equal to the number of categories of a variable. This allows for the translation of categorical variables into a format that can be inputted into ML algorithms. Continuous variables were normalized using a MinMaxScaler, which scales the lowest and highest values of any numeric variable to 0 and 1. This ensures that all variables contribute equally to the analysis without any bias created toward a specific range. Missing values within the cervix dilation, effacement, and fetal station parameters were imputed using an iterative imputation technique, called multivariate imputation by chained equations (MICE).15 To measure the significance of MICE in our data, we further compared our ML models with the models where no missing data imputation is used and data with missing values are removed. The data preprocessing scheme and overview of our approach are illustrated in Supplemental Figure. Statistical analysis was performed with Python 3.9.7 and scikit-learn library 1.0.2 on an Intel core i5, 2.42 GHz with 8 GB of RAM in a 64-bit OS platform.
To determine significant predictor variables, we used recursive feature elimination (RFE),16 which is a wrapper method that eliminates variables with less importance in a recursive manner. Random forest (RF) and support vector machine (SVM) algorithms are used, which are well-known base classifiers for the RFE method to determine significant variables. This resulted in 2 subsets of variables, one given by RF-RFE and the other by SVM-RFE. A correlation matrix was created, and variables with high degrees of positive or negative relationship were eliminated.
Here, we used several ML models, including decision tree (DT), RF, and SVM with linear kernel (SVM-LI) and SVM with radial basis kernel (SVM-RB) and compared with backward elimination logistic regression (LR) based on both the significant variables obtained from RF-RFE and SVM-RFE. Tuning hyperparameters is an important task in enhancing the performance of an ML model. Hyperparameter optimization was implemented on all classification algorithms to select the best-performing model. AUC, accuracy (ACC), sensitivity, and specificity were calculated as performance measures for classification models. For the sake of consistency and robustness of the results, the ACC and AUC values for all models were calculated over 100 iterations through the same train and test split. The models were trained on a training set (75% of data) and tested on the test set (25% of data). We further performed a pairwise Wilcoxon rank-sum test to compare these models.
Despite the profound benefits of ML models compared with traditional statistical models, their predictions are often difficult to interpret. Therefore, we used the Local Interpretable Model-Agnostic Explanations (LIME) method to interpret the behavior of the complex ML models for each patient.17 LIME is a common technique to explain a prediction based on a model concerning a similar patient and accounting for the differences in any factors.
Results
All the significant variables selected by SVM-RFE and RF-RFE and their order of importance without reference to the directionality of their effect (ranking) are shown in Figure 1, Figure 2. When each modeling technique was compared using variables selected by SVM-RFE and RF-RFE, all the models using SVM-RFE were noted to be superior to those using RF-RFE (P<.05) in both sets of analyses. The initial analysis examined all available variables to optimize model performance with Table 1 demonstrating the AUC, ACC, sensitivity, and specificity of each approach using SVM-RFE. We further simplified the model by dropping features that were highly correlated. In addition, the importance of iterative imputation was confirmed by evaluating the model with and without missing variables (cervix dilation, effacement, and fetal station), indicating a significant difference among all models (P<.001). Finally, each ML model was compared with the logistic regression model, with the LR model outperforming the rest (AUC, 0.813; 95% confidence interval [CI], 0.810–0.817; P<.001) concerning AUC, ACC, sensitivity, and specificity. The second analysis employing similar techniques examined the predictive ability concerning cesarean delivery isolating variables available at the time of admission and noted reduced model performance across each modeling type (P<.0001); however, SVM-LI, SVM-RB, and RF were noted to be equivalent to LR concerning AUC and ACC (P=.45, P=.06, and P=.14) (Table 2). Furthermore, the importance of race as a predictor was explored by excluding this characteristic in each prediction model across both analyses with a significant change in the model performance characteristics (P<.001).
Figure 1.
Cesarean associated variables ranked non-directionally by SVM-RFE using all data
The ranking criterion used in SVM-RFE is W2 where W is the weight vector computed by SVM-LI when trained on the set of all features.
BMI, body mass index; GBS, group B streptococcus; SVM-LI, support vector machine with linear function; SVM-RFE, support vector machine with recursive feature elimination function.
Kolli. Predicting cesarean with machine learning. Am J Obstet Gynecol Glob Rep 2023.
Figure 2.
Cesarean associated variables ranked non-directionally by SVM-RFE - only admission data
The ranking criterion used in SVM-RFE is W2 where W is the weight vector computed by SVM-LI when trained on the set of all features.
BMI, body mass index; GBS, group B streptococcus; SVM-LI, support vector machine with linear function; SVM-RFE, support vector machine with recursive feature elimination function.
Kolli. Predicting cesarean with machine learning. Am J Obstet Gynecol Glob Rep 2023.
Table 1.
Computational results for machine learning algorithms using significant variables selected by SVM-RFE after dropping colinear variables
| Model | AUC | ACC | Sensitivity | Specificity |
|---|---|---|---|---|
| LR | 0.813a | 0.830a | 0.757 | 0.981 |
| DT | 0.743 | 0.765 | 0.550 | 0.972 |
| RF | 0.793 | 0.807 | 0.739 | 0.931 |
| SVM-LI | 0.797 | 0.816 | 0.715 | 0.963 |
| SVM-RB | 0.802 | 0.820 | 0.721 | 0.977 |
ACC, accuracy; AUC, area under the curve; DT, decision tree; LR, logistic regression; RF, random forest; SVM-LI, support vector machine with linear function; SVM-RB, support vector machine with radial basis function; SVM-RFE, support vector machine with recursive feature elimination function.
P<.001: Wilcoxon rank-sum test LR compared with DT, RF, SVM-LI, and SVM-RB.
Kolli. Predicting cesarean with machine learning. Am J Obstet Gynecol Glob Rep 2023.
Table 2.
Comparison of machine learning algorithms with variables selected by SVM-RFE only considering data available at the time of admission
| Model | AUC | ACC | Sensitivity | Specificity |
|---|---|---|---|---|
| LR | 0.799a | 0.809 | 0.775 | 0.844 |
| DT | 0.746 | 0.767 | 0.757 | 0.739 |
| RF | 0.797a | 0.810 | 0.763 | 0.867 |
| SVM-LI | 0.800a | 0.811 | 0.786 | 0.863 |
| SVM-RB | 0.802a | 0.818 | 0.751 | 0.908 |
ACC, accuracy; AUC, area under the curve; DT, decision tree; LR, logistic regression; RF, random forest; SVM-LI, support vector machine with linear function; SVM-RB, support vector machine with radial basis function; SVM-RFE, support vector machine with recursive feature elimination function.
P<.05: Wilcoxon rank-sum test LR, SVM-LI, SVM-RB, and RF compared with DT.
Kolli. Predicting cesarean with machine learning. Am J Obstet Gynecol Glob Rep 2023.
Discussion
Principal findings
ML approaches have become popular and have provided significant insights into various obstetrics and gynecology applications. Compared with traditional regression analysis, ML algorithms are more robust in their ability to handle various data types and seamlessly integrate both parametric and nonparametric data and skewed data to optimize prediction models. The goal of this article was to compare traditional modeling techniques and ML approaches to predict the risk of cesarean delivery in a large cohort of patients with morbid obesity with a large array of clinically available variables with different distributions, skew, sparsity, and categorizations. Contrary to our expectation, logistic regression and ML approaches seem to perform similarly to predict the risk of cesarean delivery in our population. However, the model did demonstrate good discriminatory ability to predict cesarean delivery after labor with an AUC of 0.802. In addition, we observed that the inclusion of race, as a variable, did improve the performance of our models (P<.001).
Results
Our study represents outcomes of a large cohort of gravidas with morbid obesity that have been studied to create an optimal model to inform clinical decision-making. Previous studies have evaluated the chances of failed labor induction in both populations with and without obesity, some using only demographic data and others using patient-specific physical examination data. However, our work includes a combination of a large cohort, with both demographic and physical examination data and only examines outcomes in patients with morbid obesity. The AUC of 0.802 seems to be superior to previously published models with AUCs of 0.73 to 0.789,10,12 and may improve patient-centered care.
Clinical implications
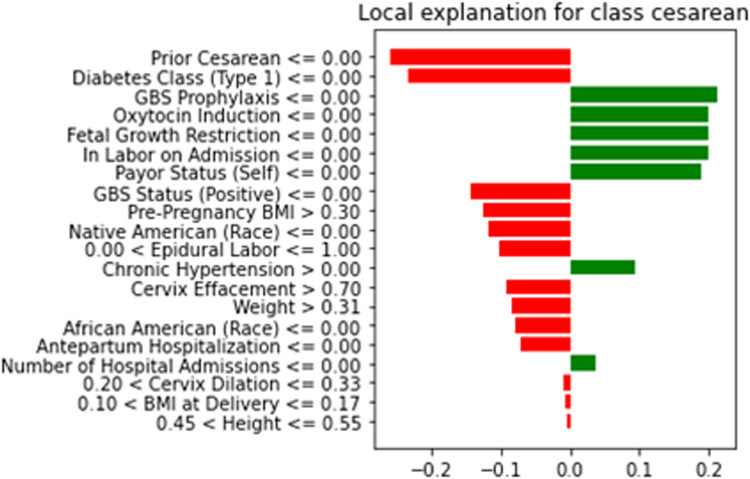
Gravidas with morbid obesity are more likely to undergo labor induction and cesarean delivery and are at high risk of surgical site infections.18 Furthermore, cesarean delivery after failed labor induction in this group has been demonstrated to almost double maternal morbidities, triple infection risks, and double wound complications.5 Here, the model may help select patients who would benefit from primary cesarean delivery, as opposed to those undergoing cesarean delivery after labor to reduce morbidity. Visually intuitive explanations using algorithms, such as LIME (Figure 3), can help explain individual patient factors that could increase the risk of cesarean delivery and facilitate open and deliberate conversations surrounding delivery approaches, including methods of labor induction. However, before implementation, this model must be externally validated. Interestingly, the inclusion of parity did not improve the AUC of our model, and the model remains robust among both parous and nonparous gravidas. We will plan on studying this further with a cohort that includes all classes of obesity.
Figure 3.
Using LIME to illustrate reasons for cesarean in a given patient
The graph explains the behavior of the model in relation to classifying a test instance in the cesarean delivery prediction problem. All the positive correlations toward cesarean delivery are shown in red, and the negative correlations are shown in green. From this figure, we can explain which features are contributing to cesarean delivery prediction for a patient.
BMI, body mass index; GBS, group B streptococcus.
Kolli. Predicting cesarean with machine learning. Am J Obstet Gynecol Glob Rep 2023.
Research implications
Several studies have examined the role of conventional statistical modeling and compared it to ML approaches, with occasional studies demonstrating equality between the techniques. Although this study evaluated a large cohort of patients, many datasets for which ML analysis is used include tens of thousands of patients with hundreds of variables. Perhaps the relative size of the cohort is 1 reason for the disparity in the expected outcome. To this end, recent studies have investigated the concept of synthetic data generation and transfer learning to improve the accuracy of predictive models built with ML for small datasets.19 Future studies should work to identify an optimal cohort size at which ML may outperform conventional modeling. Furthermore, it remains a challenge to design fair ML algorithms, where sensitive features, such as race, gender, age, geographic location, or any specific information about minority groups, do not contribute to biased predictions.20 Unlike in the recent retooling of the vaginal birth after cesarean calculator,21 we did note a significant difference in the AUC of our model when excluding race and ethnicity. Understandably, there is concern that the inclusion of such parameters may represent historical and ongoing implicit bias within our population. Presumably, the association with cesarean delivery among Native American and African American patients is a consequence of prejudice rather than underlying biology. However, the main goal of our study was to evaluate the ability of ML compared with logistic regression. Certainly, we are already planning further external validation studies to ensure we are not perpetuating such bias. Furthermore, as recognition and training to minimize implicit bias becomes more widespread, new data can be easily added or compared with such datasets to determine whether these features become less associated with cesarean delivery.
Strengths and limitations
The retrospective nature of this study certainly limits the assessment of unrecorded confounders and missing data that may explain the outcome. Furthermore, this study would be enhanced with prospective or external validation. However, the large size of the cohort that included (1287 patients) the lack of a prescriptive delivery management strategy and the diverse population that included a large number of self-identified Native American or Alaska Natives are significant strengths.
Conclusions
Our work demonstrates that ML algorithms did not outperform conventional logistic regression modeling in predicting cesarean delivery in a cohort of gravidas with morbid obesity. Future investigations should include external validation and work toward a model with excellent discriminatory ability to minimize maternal morbidity.
Glossary
Recursive feature elimination (RFE): Recursive feature elimination (RFE) is a feature selection technique used in selecting the subset of features or variables that are significant in predicting the outcome of a target variable. RFE works by iteratively discarding the least important features at every step based on the ranking criterion of the individual ML model, which fits the data. This process is repeated multiple times by refitting the model until it obtains a specified number of features.
Support vector machine (SVM): A support vector machine (SVM) is a supervised machine learning algorithm used for classification, regression, and outlier identification problems. The SVM iteratively generates hyperplanes to partition the datasets into a certain number of categories until the maximum marginal hyperplane that best separates all the categories is identified. The SVM further employs a different set of algorithms called kernel methods to analyze the data pattern and transform the data into desirable output. Different kernel functions include linear, polynomial, radial basis function kernel, and sigmoid.
Random forest (RF): A random forest (RF) is a supervised machine learning algorithm used for classification and regression problems. It is an ensemble learning method made up of multiple decision trees called estimators, each of which makes its predictions. A more accurate forecast is made by integrating all the estimator's predictions.
Decision tree (DT): A decision tree is a supervised machine learning algorithm that can handle both numerical and categorical data. It represents a sequence of decisions and their possible outcomes and consequences in the form of a tree.
Logistic regression (LR): A logistic regression is a commonly used machine learning algorithm for binary classification problems. It is a statistical method used to estimate the probability of an event occurring by measuring the relationship between a binary-dependent variable and one or more independent variables.
Local Interpretable Model-Agnostic Explanations (LIME): Local Interpretable Model Explanations are used to explain the predictions of any given model. Although machine learning models are considered a black box, these techniques interpret the math behind the algorithms. We built these frameworks on our best-performed logistic regression algorithm by evaluating their ability to define distinct groups of observations, employing the weights assigned to features through their local interpretability algorithm.
Footnotes
Patient consent is not required because no personal information or detail is included. The original study was institutional review board approved.
The authors report no conflict of interest to report.
This study did not receive funding.
This study was presented at the Society for Maternal-Fetal Medicine Annual Clinical Virtual Meeting held on January 31, 2022, to February 5, 2022.
Cite this article as: Kolli R, Razzaghi T, Pierce S, et al. Predicting cesarean delivery among morbidly obese gravidas—a machine learning approach. Am J Obstet Gynecol Glob Rep 2023;XX:x.ex–x.ex.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xagr.2023.100276.
Appendix. Supplementary materials
References
- 1.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate–a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser PS, Kirby RS. Obesity as a risk factor for cesarean in a low-risk population. Obstet Gynecol. 2001;97:39–43. doi: 10.1016/s0029-7844(00)01078-4. [DOI] [PubMed] [Google Scholar]
- 3.Chu SY, Kim SY, Schmid CH, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8:385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 4.Khalifa E, El-Sateh A, Zeeneldin M, et al. Effect of maternal BMI on labor outcomes in primigravida pregnant women. BMC Pregnancy Childbirth. 2021;21:753. doi: 10.1186/s12884-021-04236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramaniam A, Jauk VC, Goss AR, Alvarez MD, Reese C, Edwards RK. Mode of delivery in women with class III obesity: planned cesarean compared with induction of labor. Am J Obstet Gynecol. 2014;211:700. doi: 10.1016/j.ajog.2014.06.045. e1–9. [DOI] [PubMed] [Google Scholar]
- 6.Sakala C, Belanoff C, Declercq ER. Factors associated with unplanned primary Cesarean birth: secondary analysis of the listening to mothers in California survey. BMC Pregnancy Childbirth. 2020;20:462. doi: 10.1186/s12884-020-03095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol. 2004;191:969–974. doi: 10.1016/j.ajog.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 8.Bertossa P, Novakov Mikic A, Stupar ZT, et al. Validity of clinical and ultrasound variables to predict the risk of cesarean delivery after induction of labor. Obstet Gynecol. 2012;120:53–59. doi: 10.1097/AOG.0b013e31825b9adb. [DOI] [PubMed] [Google Scholar]
- 9.Levine LD, Downes KL, Parry S, Elovitz MA, Sammel MD, Srinivas SK. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol. 2018;218:254. doi: 10.1016/j.ajog.2017.11.603. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi RM, Requarth EW, Warshak CR, Dufendach K, Hall ES, DeFranco EA. Predictive model for failed induction of labor among obese women. Obstet Gynecol. 2019;134:485–493. doi: 10.1097/AOG.0000000000003377. [DOI] [PubMed] [Google Scholar]
- 11.Tolcher MC, Holbert MR, Weaver AL, et al. Predicting Cesarean delivery after induction of labor among nulliparous women at term. Obstet Gynecol. 2015;126:1059–1068. doi: 10.1097/AOG.0000000000001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamm RF, Downes KL, Srinivas SK, Levine LD. Using the probability of Cesarean from a validated Cesarean prediction calculator to predict labor length and morbidity. Am J Perinatol. 2019;36:561–566. doi: 10.1055/s-0038-1675625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamm RF, Teefey CP, Dolin CD, Durnwald CP, Srinivas SK, Levine LD. Risk of Cesarean delivery for women with obesity using a standardized labor induction protocol. Am J Perinatol. 2021;38:1453–1458. doi: 10.1055/s-0041-1732459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shazly SA, Trabuco EC, Ngufor CG, Famuyide AO. Introduction to machine learning in obstetrics and gynecology. Obstet Gynecol. 2022;139:669–679. doi: 10.1097/AOG.0000000000004706. [DOI] [PubMed] [Google Scholar]
- 15.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 16.Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46:389–422. [Google Scholar]
- 17.Ribeiro MT. Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. Association for Computing Machinery; New York, NY: 2016. Why should I trust you? Explaining the predictions of any classifier; pp. 1135–1144. [Google Scholar]
- 18.Robinson HE, O'Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357–1364. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- 19.Douzas G, Lechleitner M, Bacao F. Improving the quality of predictive models in small data GSDOT: a new algorithm for generating synthetic data. PLoS One. 2022;17 doi: 10.1371/journal.pone.0265626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyon I, Weston J, Barnhill S, et al. Gene Selection for Cancer Classification using Support Vector Machines. Machine Learning. 2002;46:389–422. doi: 10.1023/A:1012487302797. [DOI] [Google Scholar]
- 21.Grobman WA, Sandoval G, Rice MM, et al. Prediction of vaginal birth after cesarean delivery in term gestations: a calculator without race and ethnicity. Am J Obstet Gynecol. 2021;225:664. doi: 10.1016/j.ajog.2021.05.021. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.