Abstract
Background
In the beginning of the SARS-CoV-2 pandemic, health care professionals dealing with COVID-19 had to rely exclusively on general supportive measures since specific treatments were unknown. The subsequent waves could be faced with new diagnostic and therapeutic tools (e.g., anti-viral medications and vaccines). We performed a meta-analysis and systematic review to compare clinical endpoints between the first and subsequent waves.
Methods
Three databases were assessed. The primary outcome was in-hospital mortality. The secondary outcomes were intensive care unit (ICU) mortality, ICU length of stay (LOS), acute renal failure, extracorporeal membrane oxygenation (ECMO) implantation, mechanical ventilation time, hospital LOS, systemic thromboembolism, myocarditis and ventilator associated pneumonia.
Results
A total of 25 studies with 126,153 patients were included. There was no significant difference for the primary endpoint (OR=0.94, 95% CI 0.83-1.07, p=0.35). The first wave group presented higher rates of ICU LOS (SMD= 0.23, 95% CI 0.11-0.35, p<0.01), acute renal failure (OR=1.71, 95% CI 1.36-2.15, p<0.01) and ECMO implantation (OR=1.64, 95% CI 1.06-2.52, p=0.03). The other endpoints did not show significant differences.
Conclusions
The analysis suggests that the first wave group, when compared with the subsequent waves group, presented higher rates of ICU LOS, acute renal failure and ECMO implantation, without significant difference in in-hospital or ICU mortality, mechanical ventilation time, hospital LOS, systemic thromboembolism, myocarditis or ventilator- associated pneumonia.
Key words: COVID-19, SARS-CoV-2, critical care, extracorporeal membrane oxygenation
Introduction
In the beginning of the SARS-CoV-2 pandemic, health care professionals dealing with COVID-19 had to rely exclusively in general supportive measures since specific treatments were unknown [1]. National and societies guidelines released initially advised against the administration of systemic corticosteroids and limited the use of non-invasive ventilation to specific populations or clinical scenarios [1,2]. Subsequently, the knowledge about the care of these patients has increased progressively as results of clinical studies became available. While initial observational studies have pointed out high proportions of intensive care unit [3] (ICU) admissions, frequent need of mechanical ventilation (MV) and high mortality in the critically ill patients [1,4], several subsequent randomized controlled trials (RCT) have shown clinical benefit of pharmacological and noninvasive respiratory interventions [3,5-10]. These studies found reduced mortality with administration of systemic corticosteroids and interleukin-6 receptor antagonists [5,6,10]. Although remdesivir was not found to reduce mortality in hospitalized patients its administration led to faster recovery time and reduced intubation rates [7]. Non-invasive respiratory interventions (high flow nasal oxygen - HFNO and non-invasive ventilation - NIV) were shown to reduce the need of intubation and invasive MV [8,9]. As clinical experience increased and evidence from clinical studies became available, it is expected that the clinical profile, employed treatments and outcomes of COVID-19 critically ill patients have also changed. The objective of this systematic review and meta-analysis was to assess and describe differences in the clinical and demographic features, treatments and outcomes of COVID-19 adult patients admitted in subsequent waves of the pandemic.
Methods
Ethical approval of this analysis was not required as no human or animal subjects were involved. This review was registered with the National Institute for Health Research International Registry of Systematic Reviews (PROSPERO, CRD42023405088).
Search strategy
We performed a comprehensive literature search to identify contemporary studies reporting short- and long-term outcomes between patients who had SARS-CoV-2 in the first and subsequent waves. Searches were run on July, 2022, in the following databases: Ovid MEDLINE; Web of Science; and The Cochrane Library (Wiley). The search strategy for Ovid MEDLINE is available in Supplementary Table 1.
Study selection and data extraction
The study selection followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) strategy. After de-duplication, records were screened by two independent reviewers (TC and FV). Any discrepancies and disagreements were resolved by a third author (JJ). Titles and abstracts were reviewed against pre-defined inclusion and exclusion criteria. Studies were considered for inclusion if they were written in English and reported direct comparison between patients who had SARS-CoV-2 in the first versus in the subsequent waves. Animal studies, abstracts, case reports, commentaries, editorials, expert opinions, conference presentations, and studies not reporting the outcomes of interest were excluded. The full text was pulled for the selected studies for a second round of eligibility screening. References for articles selected were also reviewed for relevant studies not captured by the original search. The quality of the included studies was assessed using the “Tool to Assess Risk of Bias in Cohort Studies” developed by the CLARITY Group at McMaster University (Supplementary Table 2).
Two reviewers (TC and FV) independently performed data extraction. Accuracy was verified by a third author (JJ). The extracted variables included study characteristics (publication year, country, sample size, study design, mean follow up, presence or absence from population adjustment and outcome definitions) as well as patient demographics (age, sex, hypertension, diabetes, smoking status, prior cerebrovascular accident – CVA, prior myocardial infarction MI, prior PCI, renal failure, chronic obstructive pulmonary disease – COPD, Charlson Comorbidities Index, vaccination status, Simplified Acute Physiology Score – SAPS, Acute Physiology and Chronic Health Evaluation – APACHE, Sequential Organ Failure Assessment – SOFA, oxygenation index, corticosteroids, remdesivir and IL-6 use).
Outcomes
Primary outcome was in-hospital mortality. Secondary outcomes were ICU mortality, mechanical ventilation time, hospital length of stay (LOS), ICU LOS, systemic thromboembolism, myocarditis, acute renal failure, ventilator associated pneumonia and necessity of extracorporeal membrane oxygenation (ECMO) implantation.
Statistical analysis
We conducted meta-analyses to compare the outcomes during the first wave of SARS-CoV-2 versus the subsequent waves of SARS-CoV-2. Continuous variables were analyzed using standardized mean difference (SMD) and 95% confidence intervals (95% CI). A SMD greater than zero corresponded to larger values in the first wave of COVID-19. Categorical values were analyzed using odds ratio (OR) and 95% CI. An OR greater than 1 indicated that the outcome was more frequently present in the first wave of SARS-CoV-2. Inherent clinical heterogeneity between the studies was balanced via the implementation of random effects models (DerSimonian-Laird). Results were displayed in forest plots.
Between-study statistical heterogeneity was assessed with the Cochran Q statistic and by estimating I2. High heterogeneity was confirmed with a significance level of p<0.10 and I2 of at least 50% or more. Publication bias was assessed via funnel plots and Eggers’ test for each outcome of interest and p<0.10 was considered statistically significant. All analyses were performed using STATA IC17.0 (StataCorp LLC, College Station, TX, USA).
Results
Study characteristics
A total of 1,179 studies were retrieved from the systematic search, out of which 25 met the criteria for inclusion in the final analysis. Figure 1 shows the PRISMA flowchart for study selection. Included studies were published between 2021 and 2022, all studies were observational cohorts, and 13 were multicentric. 1 study was multinational, 1 study originated from Australia, 1 from Austria, 1 from Belgium, 1 from Brazil, 1 from Denmark, 4 from France, 1 from Germany, 1 from Greece, 1 from India, 1 from Japan, 1 from Mexico, 1 from Netherlands, 1 from Pakistan, 1 from South Africa, 1 from Spain, 1 from Sweden, 1 from Switzerland and 3 from the United Kingdom. Table 1 shows the details of the included studies. Thirteen studies were based on risk-adjusted populations. A total of 126,153 patients were included in the final analysis. The number of patients in each study ranged from 72 to 67,242.
Patient characteristics
Supplementary Table 3 summarizes the demographic data of the patient population in each study. The median age ranged from 49 to 72 years. Percentage of female patients ranged from 11% to 67%; percentage of hypertension ranged from 28% to 65%; percentage of diabetes ranged from 18% to 54%; percentage of positive smoking status ranged from 2.4% to 30%; percentage of prior CVA ranged from 6% to 14%; percentage of prior MI ranged from 5% to 29%; percentage of renal failure ranged from 3% to 63% and the percentage of COPD ranged from 1.5% to 28%.
Meta-analysis
Figure 2 and Table 2 outline the detailed results of the metaanalysis.
Figure 1.
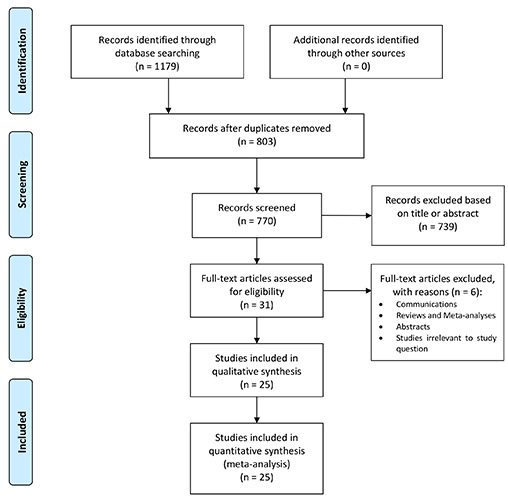
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Figure 2.
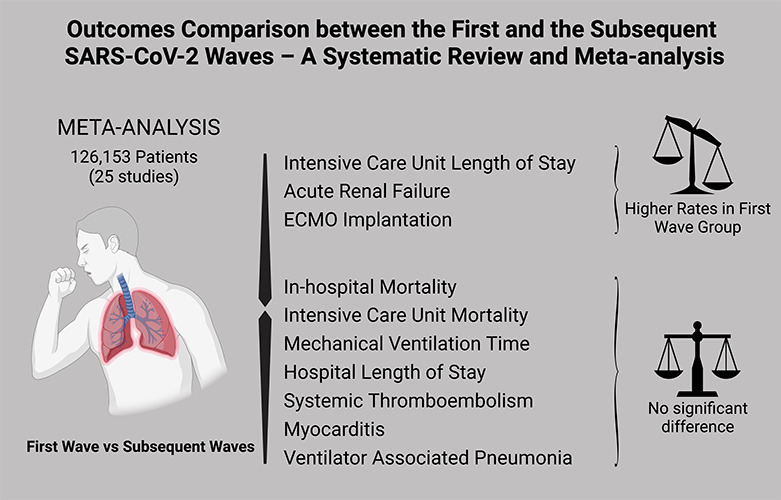
Graphical abstract showing the main findings of the analysis.
Table 1.
Summary of included studies. References are reported in the Supplementary Material.
| Author | Year of publication | Country | N of patients | Study design | Selected outcomes |
|---|---|---|---|---|---|
| Aries | 2022 | France (Mayotte Island) | 156 | Single center, retrospective | Mortality, MVT, hospital LOS, ICU LOS, S T, myocarditis, VAP and ECMO implantation |
| Asghar | 2021 | Pakistan | 160 | Single center, retrospective | Mortality, ST, myocarditis and ARF |
| Begum | 2022 | Australia | 2493 | Multicenter, prospective | Mortality, MVT, hospital LOS, ICU LOS, S T, myocarditis, ARF and ECMO implantation |
| Carbonell | 2021 | Spain, Andorra and Ireland | 3795 | Multicenter, retrospective | Mortality, MVT, hospital LOS, ICU LOS, myocarditis, ARF, VAP and ECMO implantation |
| Countou | 2021 | France | 132 | Single center, retrospective | Mortality, MVT, ICU LOS, ST, ARF and VAP |
| Demoule | 2022 | France | 1166 | Multicenter, retrospective | Mortality, MVT, hospital LOS, ICU LOS, ARF, VAP and ECMO implantation |
| Dongelmans | 2022 | Netherlands | 12,218 | Multicenter, prospective | Mortality, MVT, hospital LOS, ICU LOS, S T, myocarditis, VAP and ECMO implantation |
| Haase | 2022 | Denmark | 1374 | Multicenter, retrospective | Mortality, MVT, hospital LOS, ICU LOS, ARF and ECMO implantation |
| Hosoda | 2022 | Japan | 128 | Multicenter, retrospective | Mortality, MVT, ICU LOS, ARF and ECMO implantation |
| Kerai | 2021 | India | 220 | Multicenter, retrospective | Mortality, MVT, ICU LOS and ARF |
| Kieninger | 2022 | Germany | 157 | Single center, retrospective | Mortality, ICU LOS, ARF and ECMO implantation |
| Lalla | 2021 | South Africa | 490 | Single center, prospective | Mortality, MVT, hospital LOS, ICU LOS, S T, myocarditis, VAP and ECMO implantation |
| Lazaro | 2022 | Brazil | 767 | Single center, prospective | Mortality, ICU LOS and ARF |
| Le Terrier | 2022 | Switzerland | 223 | Single center, prospective | Mortality, MVT, hospital LOS, ICU LOS, ST, ARF, VAP and ECMO implantation |
| Lopez | 2021 | France | 111 | Single center, retrospective | Mortality, hospital LOS, ICU LOS, ST, ARF, VAP and ECMO implantation |
| Mayerhofer | 2021 | Austria | 508 | Multicenter, prospective | Mortality, MVT, hospital LOS, ICU LOS, ARF and ECMO implantation |
| Namendis-Silva | 2021 | Mexico | 67,242 | Multicenter, retrospective | Mortality |
| Perez-Acosta | 2022 | Spain (Canary Islands) | 72 | Single center, prospective | Mortality, MVT, hospital LOS, ICU LOS, ST, myocarditis and ARF |
| Piagnerelli | 2021 | Belgium | 174 | Single center, retrospective | Mortality, MVT, hospital LOS, ICU LOS, ST and ARF |
| Ritchie | 2022 | United Kingdom | 330 | Multicenter, retrospective | Mortality and ICU LOS |
| Routsie | 2021 | Greece | 262 | Single center, retrospective | Mortality, MVT, Hospital LOS, ICU LOS and ARF |
| Szakmany | 2021 | United Kingdom | 178 | Single center, retrospective | Mortality and ICU LOS |
| Taxbro | 2021 | Sweden | 264 | Multicenter, retrospective | Mortality, MVT, hospital LOS, ICU LOS, S T, myocarditis, ARF and ECMO implantation |
| Wilcox | 2022 | United Kingdom | 30,035 | Multicenter, retrospective | Mortality and ICU LOS |
| Zirpe | 2021 | India | 3498 | Multicenter, prospective | Mortality |
ARF, acute renal failure; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LOS, length of stay; MVT, mechanical ventilation time; ST, systemic thromboembolism; VAP, ventilator associated pneumonia.
Table 2.
Summary of included studies. References are reported in the Supplementary Material.
| Outcome | Number of studies | Number of patients | Effect estimate (95%CI, p) |
|---|---|---|---|
| In-hospital mortality | 18 | 123,923 | OR= 0.94 (0.83-1.07, p=0.35) |
| ICU mortality | 16 | 56,408 | OR= 0.94 (0.79-1.13, p=0.51) |
| ICU LOS | 22 | 48,222 | SMD= 0.23 (0.11-0.35, p<0.01) |
| ECMO implantation | 11 | 10,019 | OR= 1.64 (1.06-2.52, p=0.03) |
| Mechanical ventilation time | 14 | 8,870 | SMD= 0.10 (-0.01-0.21, p=0.09) |
| Hospital LOS | 14 | 16,343 | SMD= 0.10 (-0.04-0.24, p=0.17) |
| Systemic thromboembolism | 9 | 3,135 | OR= 1.25 (0.82-1.91, p=0.29) |
| Myocarditis | 6 | 6,330 | OR= 1.49 (0.72-3.07, p=0.28) |
| Ventilator associated pneumonia | 6 | 5,583 | OR= 0.78 (0.51-1.18, p=0.24) |
CI, confidence interval; ECMO, extracorporeal membrane oxygenation; LOS, length of stay; SMD, standard mean difference; OR, odds ratio.
Primary outcome
Figure 3 shows the forest plot for in-hospital mortality. There was no significant difference between the two groups (OR= 0.94, 95% CI 0.83-1.07, p=0.35). Supplementary Figure 1 shows the leave-one-out analysis showing that most of the studies confirm the robustness of the analysis, with minimal variations of the confidence interval. Supplementary Figure 2 provides the funnel plot for the publication bias assessment.
Secondary outcomes
Figure 4 shows the forest plot for ICU mortality. There was no significant difference between the two groups (OR=0.94, 95% CI 0.79-1.13, p=0.51). Figure 5 shows the forest plot for ICU length of stay. The first wave group presented higher ICU length of stay in comparison with the subsequent waves (SMD=0.23, 95% CI 0.11-0.35, p<0.01). Figure 6 shows the forest plot for acute renal failure. The first wave group presented higher acute renal failure rates in comparison with the subsequent waves (OR=1.71, 95% CI 1.36-2.15, p<0.01). Figure 7 shows the forest plot for ECMO implantation. The first wave group presented higher ECMO implantation rates in comparison with the subsequent waves (OR=1.64, 95% CI 1.06-2.52, p=0.03).
Supplementary Figure 3 shows the forest plot for mechanical ventilation time. There was no significant difference between the two groups (SMD=0.10, 95% CI -0.01-0.21, p=0.09). Supplementary Figure 4 shows the forest plot for hospital length of stay. There was no significant difference between the two groups (SMD=0.10, 95% CI -0.04-0.24, p=0.17). Supplementary Figure 5 shows the forest plot for systemic thromboembolism. There was no significant difference between the two groups (OR=1.25, 95% CI 0.82-1.91, p=0.29). Supplementary Figure 6 shows the forest plot for myocarditis. There was no significant difference between the two groups (OR=1.49, 95% CI 0.72-3.07, p=0.28). Supplementary Figure 7 shows the forest plot for ventilator associated pneumonia. There was no significant difference between the two groups (OR=0.78, 95% CI 0.51-1.18, p=0.24).
Discussion
The analysis suggests that the first wave group when compared with the subsequent waves group presented higher rates of ICU LOS, acute renal failure and ECMO implantation, without significant difference in in-hospital or ICU mortality, mechanical ventilation time, hospital LOS, systemic thromboembolism, myocarditis or ventilator associated pneumonia.
Although appointing the reasons of the differences found is beyond the scope of the current study, some aspects are worth considering. First, to study subsequent waves of patients requires comparing data from patients presenting over a different time frame. Second, the fluctuations in ICU bed strain during the pandemic might have changed the ICU admission criteria in each wave. These two factors inherently lead to significant differences of the populations analyzed, which might have driven outcome. For example, in four of the studies included the subsequent waves had less severe patients as shown by the scores of SAPS, APACHE or SOFA, while in two studies the subsequent waves had more severe patients.
Figure 3.
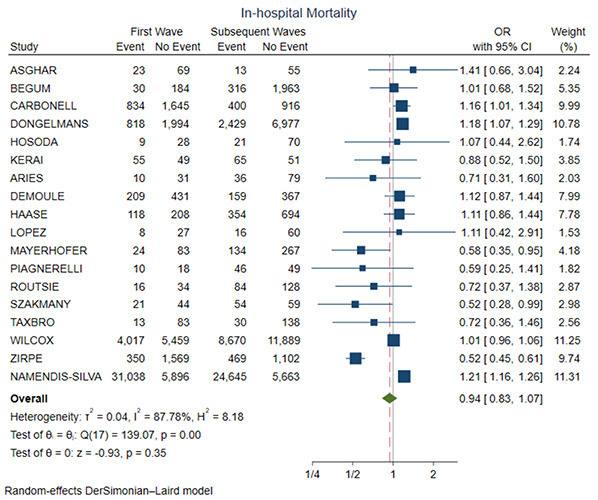
Forest plot for in-hospital mortality. CI, confidence interval; OR, odds ratio.
It is worth mentioning the organizational aspect of the pandemic in relation to its waves. The reaction to the second and subsequent waves was shaped by the initial reaction to the onset of the disease, so that the disease in this second moment had a relatively more predictable character. In other words, the medical community had no specific weapon for facing the pandemic at that time.
During the first wave of the COVID-19 pandemic, mechanical ventilation played a critical role in the management of severely ill patients [11-14]. As the virus primarily affected the respiratory system, many patients experienced severe respiratory distress and required immediate respiratory support. Invasive ventilation was the primary method used to deliver oxygen to these patients. Ventilation strategies like low tidal volume and higher positive end-expiratory pressure (PEEP) were frequently employed to manage the compromised lung function in these patients [11-14]. Additionally, healthcare professionals faced the daunting task of managing the increased risk of ventilator-associated complications, such as ventilator-associated pneumonia and lack of equipment [15,16].
With the arrival of the second wave of COVID-19, lessons learned from the initial surge guided improvements in the management of mechanically ventilated patients [11-14]. Healthcare systems were better prepared with increased ventilator capacity and improved allocation strategies. Furthermore, advancements in knowledge and experience allowed for refinements in ventilation protocols. Ventilatory management strategies, such as prone positioning and lung-protective ventilation, became more widely utilized during the second wave to optimize patient outcomes [17,18].
The second wave also emphasized the importance of a multidisciplinary approach in mechanical ventilation. Collaborative efforts among pulmonologists, intensivists, respiratory therapists, and nurses played a crucial role in providing comprehensive care [19,20]. Knowledge exchange and shared experiences among healthcare professionals helped develop effective ventilation strategies and mitigate complications. Additionally, advancements in technology and remote monitoring allowed for more efficient and accurate ventilator management, reducing the burden on healthcare providers and improving patient care.
We have found a reduced incidence of acute renal failure (ARF) during the second and subsequent pandemic waves. Acute renal failure is an important risk factor for mortality in these patients [21] and a meta-analysis of the first wave including 142 studies and 49,048 hospitalized patients reported an incidence of ARF of 5.5% in China and of 28.6% in USA and Europe [22]. In patients admitted to the ICU the incidence of ARF and renal replacement therapy was respectively 29.2% and 20.6% [22]. A study evaluating kidney biopsies taken from 47 patients with COVID-19 related ARF showed acute tubular injury in 42.6% of the patients, while glomerular injury was reported in 36.2% of the patients [23].
Figure 4.
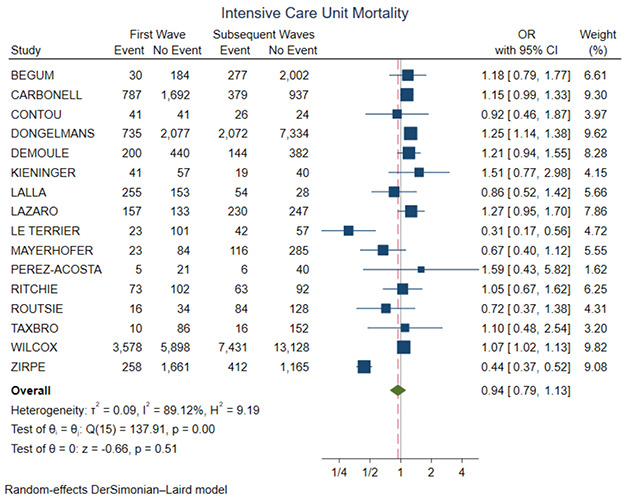
Forest plot for ICU mortality. CI, confidence interval; OR, odds ratio.
Mechanical ventilation and acute renal failure may be just surrogates of the overall clinical severity of critically ill patients, however in COVID-19 there is a temporal relation between the two, as acute renal failure develops 1 to 2 days after the beginning of mechanical ventilation [24,25]. Mechanical ventilation and acute renal failure in these cases may potentially be explained through lung-kidney cross talking or aggressive fluid restrictive therapy leading to hypovolemia [26]. In the initial COVID-19 cases, ECMO implantation was not so common, mainly because of the limited availability of ECMO machines and the lack of experience in managing COVID-19 patients with ECMO. However, after approaching more inside the pathophysiological mechanisms of the disease, ECMO implantation has become more common due to a number of factors. Firstly, as the pandemic has progressed, hospitals have gained more experience in managing critically ill COVID-19 patients with ECMO. Secondly, the availability of ECMO machines has increased, as more hospitals have invested in them. Thirdly, the emergence of new COVID-19 variants, which are more virulent and transmissible, has led to an increase in the number of patients requiring intensive care, including ECMO. The reduction of the ECMO implantation may be associated with the vaccination campaigns, which may have reduced the number of severe cases.
Concerning pharmacological treatment, 12 out of 15 studies that reported data on corticosteroid use have shown significant increase. As has been shown previously in the RECOVERY Trial that dexamethasone use leads not only to reduced mortality but also to reduced ICU length of stay and lower use of renal replacement therapy [6], it is possible that the increase in the administration of corticosteroids may have influenced the outcomes. By December 2020 several countries started vaccination against SARS-CoV-2. In 15 out of 25 of the studies analyzed there was an overlap between the time frames of the vaccination and the second and subsequent waves (Supplementary Table 4). In these studies, the subsequent waves have probably included vaccinated patients. As only one study reported data on vaccination status its influence on outcomes is unknown, although it is expected that vaccination might reduce morbidity even when considering only severe cases [27]. One interesting possibility is that the increase in expertise acquired during the pandemic lead progressively to better indication of supportive and pharmacological measures, which ultimately reduced morbidity, but was not enough to reduce mortality. The reduced morbidity observed may have allowed more patients to be cared in ICUs during a time of unprecedented strain over hospital beds.
Study strength and limitations
This is the first meta-analysis data to address this important topic with a wide systematic approach. We analyzed 9 different outcomes besides in-hospital mortality. However, this work has the intrinsic limitations of observational series, including the risk of methodological heterogeneity of the included studies and residual confounders. Additionally, it was not known the fraction of infected patients being admitted to the hospital in each wave, so a possible selection bias of the sickest patients is possible, and the possible improvements in therapy and through vaccination may not be as evident.
Figure 5.
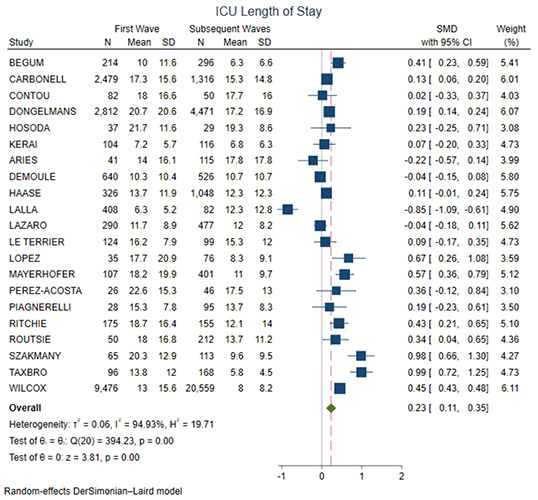
Forest plot for ICU length of stay. CI, confidence interval; SMD, standard mean difference.
Figure 6.
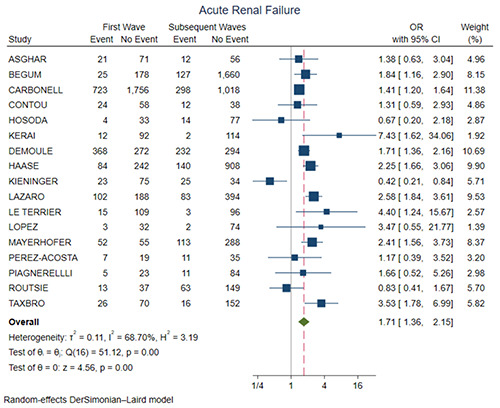
Forest plot for acute renal failure. CI, confidence interval; OR, odds ratio.
Figure 7.
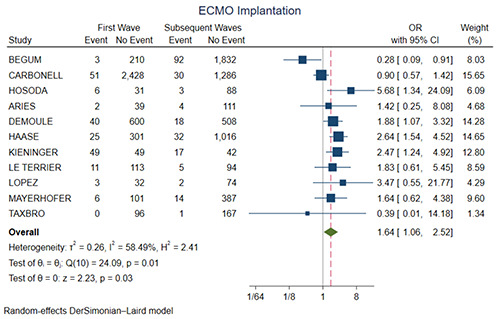
Forest plot for ECMO implantation. CI, confidence interval; OR, odds ratio.
Moreover, most of the studies involved patients who were diagnosed with SARS-CoV-2 infection based on PCR analysis. However, it is not possible to exclude cases of patients with viral or bacterial co-infections in the initial phase.
Conclusions
The analysis suggests that the first wave group when compared with the subsequent waves group presented higher rates of ICU LOS, acute renal failure and ECMO implantation, without significant difference in in-hospital or ICU mortality, mechanical ventilation time, hospital LOS, systemic thromboembolism, myocarditis or ventilator associated pneumonia.
Acknowledgements
TC and RET are supported by the Clinical Scientist Program (Jena University Hospital).
Funding Statement
Funding: TC and RET was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Clinician Scientist Program OrganAge funding number 413668513 and by the Interdisciplinary Center of Clinical Research of the Medical Faculty, Jena University. TC was also funded by the Deutsche Herzstiftung (DHS, German Heart Foundation) funding number S/03/23. The authors acknowledge support by the German Research Foundation Projekt-Nr. 512648189 and the Open Access Publication Fund of the Thueringer Universitaets- und Landesbibliothek Jena.
References
- 1.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cinesi Gómez C, Peñuelas Rodríguez Ó, Luján Torné M, Egea Santaolalla C, Masa Jiménez JF, García Fernández J, et al. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection. Med Intensiva (Engl Ed) 2020;44:429-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ospina-Tascón GA, Calderón-Tapia LE, García AF, Zarama V, Gómez-Álvarez F, Álvarez-Saa T, et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA 2021;326:2161-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, Shu J, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid- 19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT randomized clinical trial. JAMA 2021;325:1731-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, et al. An adaptive randomized controlled trial of non-invasive respiratory strategies in acute respiratory failure patients with COVID-19. medRxiv 2021:2021.08.02.21261379. [Google Scholar]
- 10.Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. Interleukin-6 Receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021;384:1491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res 2010;1:274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estenssoro E, Loudet CI, Ríos FG, Kanoore Edul VS, Plotnikow G, Andrian M, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med 2021;9:989-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hol L, Van Oosten P, Nijbroek S, Tsonas A, Botta M, Neto AS, et al. The effect of age on ventilation management and clinical outcomes in critically ill COVID-19 patients—insights from the PRoVENT-COVID study. Aging (Albany NY) 2022;14:1087-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grasselli G, Cattaneo E, Florio G, Ippolito M, Zanella A, Cortegiani A, et al. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care 2021;25:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maes M, Higginson E, Pereira-Dias J, Curran MD, Parmar S, Khokhar F, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care 2021;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baskin RG, Bartlett R. Healthcare worker resilience during the COVID-19 pandemic: An integrative review. J Nurs Manag 2021;29:2329-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fossali T, Pavlovsky B, Ottolina D, Colombo R, Basile MC, Castelli A, et al. Effects of prone position on lung recruitment and ventilation-perfusion matching in patients with COVID-19 Acute respiratory distress syndrome: a combined CT scan/electrical impedance tomography study. Crit Care Med 2022;50:723-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrone P, Brathwaite CEM, Joseph DK. Prone ventilation as treatment of acute respiratory distress syndrome related to COVID-19. Eur J Trauma Emerg Surg 2021;47:1017-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gediz Erturk A, Sahin A, Bati Ay E, Pelit E, Bagdatli E, Kulu I, et al. A multidisciplinary approach to coronavirus disease (COVID-19). Molecules 2021;26:3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Almeida SMV, Santos Soares JC, Dos Santos KL, Alves JEF, Ribeiro AG, Jacob ITT, et al. COVID-19 therapy: What weapons do we bring into battle? Bioorg Med Chem 2020;28:115757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Jin Y, Li R, Zhang Z, Sun R, Chen D. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care 2020;24:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu EL, Janse RJ, de Jong Y, van der Endt VHW, Milders J, van der Willik EM, et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and metaanalysis. Clin Kidney J 2020;13:550-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferlicot S, Jamme M, Gaillard F, Oniszczuk J, Couturier A, May O, et al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant 2021;36:1253-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arrestier R, Gendreau S, Mokrani D, Bastard JP, Fellahi S, Bagate F, et al. Acute kidney injury in critically-ill COVID-19 patients. J Clin Med 2022;11:2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doher MP, Torres de Carvalho FR, Scherer PF, Matsui TN, Ammirati AL, Caldin da Silva B, et al. Acute kidney injury and renal replacement therapy in critically ill COVID-19 patients: risk factors and outcomes: a single-center experience in Brazil. Blood Purif 2021;50:520-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med 2016;194:402-14. [DOI] [PubMed] [Google Scholar]
- 27.Begum H, Neto AS, Alliegro P, Broadley T, Trapani T, Campbell LT, et al. People in intensive care with COVID-19: demographic and clinical features during the first, second, and third pandemic waves in Australia. Med J Aust 2022;217:352-60. [DOI] [PMC free article] [PubMed] [Google Scholar]


