Abstract
Auxin is a versatile plant growth regulator that triggers multiple signalling pathways at different spatial and temporal resolutions. A plant cell is surrounded by the cell wall, a complex and dynamic network of polysaccharides. The cell wall needs to be rigid to provide mechanical support and protection and highly flexible to allow cell growth and shape acquisition. The modification of the pectin components, among other processes, is a mechanism by which auxin activity alters the mechanical properties of the cell wall. Auxin signalling precisely controls the transcriptional output of several genes encoding pectin remodelling enzymes, their local activity, pectin deposition, and modulation in different developmental contexts. This review examines the mechanism of auxin activity in regulating pectin chemistry at organ, cellular, and subcellular levels across diverse plant species. Moreover, we ask questions that remain to be addressed to fully understand the interplay between auxin and pectin in plant growth and development.
Keywords: Auxin, calcium (Ca2+), cell wall, microdomains, pectin, pectin methylesterase, pH
The phytohormone auxin regulates most aspects of plant development. Auxin activity leads to intense modifications of the primary cell wall’s pectin matrix, which we will focus on in this review.
Introduction
Auxin plays a crucial role in plant growth and morphogenesis, controlling the balance between cell division and elongation, and integrating environmental cues (Leyser, 2018). The primary cell wall, which restricts plant cell expansion, is composed mainly of polysaccharides, which are long chains of sugars, such as cellulose, hemicellulose, and pectin, as well as proteins. The interactions between different polysaccharides in the cell wall are complex and dynamic, and changes in their composition or arrangement can alter the mechanical properties of the cell wall (Sarkar et al., 2009). Cellulose is a linear polymer of glucose molecules that form long chains held together by hydrogen bonds to form a rigid, crystalline structure (Wohlert et al., 2022). Pectin, a negatively charged polysaccharide, modulates cell wall mechanical properties by forming hydrogels and through interactions with other cell wall components (Voragen et al., 2009; Cosgrove, 2022). A plethora of enzymes play key roles in reorganizing the pectin backbone, allowing cell expansion through turgor-driven pressure from the vacuole (Mohnen, 2008). Auxin regulates the expression of numerous genes responsible for cell wall reorganization, and its effect on cell rigidity and growth is dependent on the developmental stage, tissue type, and organ (Majda and Robert, 2018).
The ‘acid growth theory’ posits that auxin induces cell elongation by acidifying the apoplast and thereby activating cell wall loosening enzymes in shoots (Rayle and Cleland, 1970; Hager et al., 1971), but the effect of auxin on apoplastic pH in roots is less clear and auxin may actually inhibit root cell expansion. Recent discoveries have shed light on the molecular mechanisms by which auxin can modulate the pH of cell wall compartments in root, but it is still uncertain how this affects the structure and mechanics of cell wall pectin (Fendrych et al., 2016, 2018; Barbez et al., 2017; Li et al., 2021; Lin et al., 2021). This review is an update on the recent advances concerning the connection between auxin and pectin modulation in the cell wall during plant growth and development. We first provide an overview of cell wall pectin and discuss recent discoveries related to auxin, before delving into the role of auxin in regulating pH and calcium (Ca2+) levels, and how this influences the formation of pectin ‘microdomains’ with varying compositions (Dauphin et al., 2022). Finally, we discuss auxin-mediated transcriptional regulation of genes responsible for pectin modification and how this process may involve feedback mechanisms that help to maintain a delicate balance in plant growth.
Cell wall pectin at a glance
Pectins are complex heteropolysaccharides making up roughly 30% of the primary cell wall in plants (Cosgrove, 2022). Pectin structure is diverse and based on four kinds of domains. Linear homogalacturonans (HG) are the most abundant and consist of α-(1-4)-linked d-galacturonic acid (GalA) linear chains where carboxyl groups can be methylesterified or acetylesterified (Caffall and Mohnen, 2009). The branched rhamnogalacturonan-I (RG-I) backbone is composed of repeating disaccharide units of GalA and α-(1-2)-l-rhamnose with side chains containing galactans, arabinans, and/or arabinogalactans. RG-II is the smallest but most complex domain. It is composed of a GalA backbone substituted by four chains containing more than 10 different sugars. Finally, xylogalacturonans are less common and are based on a β-1,4-linked glucan backbone that is decorated with a GalA chain and d-xylose residues.
Several carbohydrate-active enzyme families are involved in the biosynthesis, modification, and degradation of pectin. Polymerization and branching of the different pectin components are catalysed by glycosyltransferases while their degradation is mediated by glycosylhydrolases (Minic and Jouanin, 2006; Atmodjo et al., 2013). The HG backbone is secreted into the wall in a highly methylesterified form (Zhang and Staehelin, 1992). Modification of pectins can occur in muro through enzymes containing a broad spectrum of pH-dependent activities, making the pectin network dynamic. The stability of pectins is affected by the presence or absence of Ca2+ and borate, which can form ‘egg-box’ structures between demethylesterified HG and borate diester crosslinks in the case of RG-II polysaccharides (Cosgrove, 2022). Variations in apoplastic pH and ion movements across membranes inevitably impact the pectin network by altering the strength of intermolecular interactions and modifying enzyme activity (Haas et al., 2021). Pectin distribution, which can be heterogeneous along and across the wall of individual cells, plays a crucial role in regulating cell wall mechanical properties, and such heterogeneity has been linked to epidermal cell shape acquisition (Majda et al., 2017), xylem fibre elongation (Majda et al., 2021), pollen tube and root hair cell elongation (Dardelle et al., 2010; Chebli et al., 2012), and stomatal aperture (Amsbury et al., 2016). Thus, the precise distribution and composition of pectin within the cell wall is crucial for proper plant morphogenesis.
A quick response: revisiting the acid growth theory
The acid growth theory was first proposed by Rayle, Cleland, Hager, and colleagues (Rayle and Cleland, 1970; Hager et al., 1971) and was based on the observation of rapid elongation of Avena hypocotyls and Helianthus coleoptiles when grown in acidic buffers. The theory proposes that the presence of auxin in a plant cell stimulates the activity of membrane-bound H+-ATPase proton pumps, leading to an influx of protons (H+) into the cell wall. This leads to a decrease in apoplastic pH, which generates an acidic environment that activates enzymes to loosen the wall, thus allowing cell elongation. Two mechanisms by which auxin can activate plasma membrane H+-ATPase have now been uncovered (Fig. 1A, B). The first requires the canonical nuclear auxin signalling pathway (Fig. 1A) (Salehin et al., 2015). Upon binding of auxin to TRANSPORT INHIBITOR RESPONSE1/AUXIN-SIGNALING F-BOX (TIR1/AFB) receptor proteins, TIR1/AFB undergoes a conformational change that enables it to interact with AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) transcriptional repressor proteins. Recognition of AUX/IAA proteins by the SCFTIR1/AFB complex leads to the polyubiquitination of AUX/IAA and its subsequent degradation via the 26S proteasome, alleviating suppression of class A AUXIN RESPONSE FACTOR (ARF) transcription factors, which can now modulate the expression of downstream genes (Chapman and Estelle, 2009; Cancé et al., 2022). ARFs bind to specific DNA sequences called auxin response elements (AuxREs) located in the promoter regions of target genes, thereby regulating their expression (Leyser, 2018). Auxin induces the transcription of SMALL AUXIN UP RNA19 (SAUR19), leading to the activation of plasma membrane localized H+-ATPase by promoting phosphorylation of its C-terminal autoinhibitory domain. Furthermore, PROTEIN PHOSPHATASE 2C.D (PP2C.D) interacts with SAUR19 and negatively regulates H+-ATPase activity (Takahashi et al., 2012; Spartz et al., 2014).
Fig. 1.
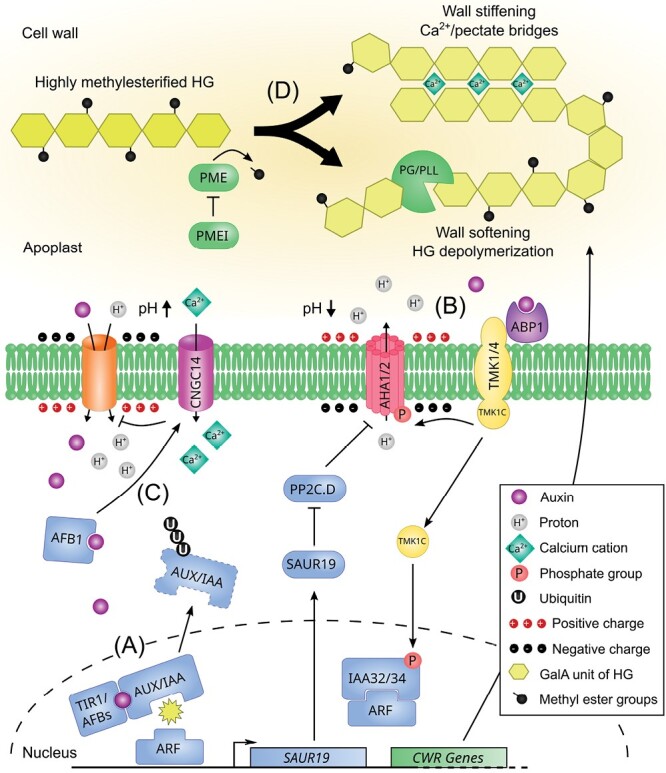
An updated view of the auxin-triggered signalling cascades. (A) Nuclear auxin signalling pathway. Auxin is perceived intracellularly by the TIR1/AFB–AUX/IAA co-receptor complex, resulting in the polyubiquitinylation and degradation of AUX/IAAs, which releases the inhibition of the ARF transcription factors in the nucleus. This triggers the expression of auxin responsive genes, among which SAUR19 whose protein inhibits the phosphatase PP2C.D, preventing the deactivation of the plasma membrane H+-ATPase AHA1/2 through dephosphorylation of the penultimate residue. This cascade results in gradual apoplast acidification and hyperpolarization of the plasma membrane. In the meantime, auxin regulates the expression of cell wall remodelling enzymes modulating pectin properties (see (D)). (B) TMK-based auxin extracellular signalling pathway. Auxin is perceived in the apoplast by the secreted receptor ABP1 and its plasma membrane partners from the TMK family. This activates the TMKs, which in turn phosphorylate AHA1/2, allowing a net efflux of H+ and the rapid hyperpolarization of the plasma membrane. In the context of apical hook growth cessation, the TMK1 C-terminal domain is processed by an unknown molecular actor and translocates to the nucleus where it phosphorylates and stabilizes the non-canonical IAA32/34 repressors. (C) TIR1/AFB auxin extranuclear signalling pathway. Auxin enters the cell via AUX1-based IAA–H+ symport, triggering rapid plasma membrane alkalinization and depolarization. IAA perception by the cytoplasmic AFB1 complex elicits Ca2+ influx dependent on CNGC14. The high [Ca2+]cyt inhibits AUX1-mediated IAA influx in a negative feedback loop through an unknown mechanism. (D) The main pectin component, homogalacturonan, is secreted into the cell wall in a highly methylesterified form. Demethylesterification is catalysed by PME enzymes, which are actively regulated by PME proteinaceous inhibitors. Depending on the PME processivity, blockwise demethylesterified HG galacturonic acid chains can form Ca2+/pectate bridges and organize into an ‘egg-box’-like structure thought to stiffen the cell wall. In contrast, non-blockwise or random demethylesterification results in HG depolymerization through pectinolytic enzyme activity (PG/PLL) and softening of the cell wall.
A second auxin response, occurring within a minute, potentially involves extracellular auxin perception by secreted AUXIN BINDING PROTEIN 1 (ABP1)/ABP-LIKE1 and 2 (ABL1/2) and its interaction with the co-receptor TRANSMEMBRANE KINASE1/4 (TMK1/4) (Fig. 1B) (Friml et al., 2022; Yu et al., 2022, Preprint). TMK then phosphorylates ARABIDOPSIS H+-ATPase 1 (AHA1) and initiates its activation, leading to apoplast acidification and rapid cell expansion in shoot tissue (Lin et al., 2021). TMKs are part of a signalling hub, possibly involving other kinases, where the auxin-induced phospho-response of thousands of proteins occurs after 30 s of IAA treatment (Roosjen et al., 2022, Preprint). Among the most notable targets are the polar auxin efflux transporters PIN-FORMED 1 (PIN1) and PIN2, whose phosphorylation status regulates their cellular localization (Zhang et al., 2010; Rodriguez et al., 2022, Preprint; Wang et al., 2022, Preprint). In addition, auxin promotes the cleavage of the TMK1 C-terminus in a TIR1/AFB-independent manner. The TMK1 C-terminal domain translocates to the nucleus where it interacts with and stabilizes IAA32/34, affecting the auxin transcriptional machinery (Cao et al., 2019). To conclude, we can say that auxin triggers significant acidification of the cell wall in the hypocotyl and possibly in other parts of the shoot. However, in the root, the initial response to auxin is actually an increase in alkalinity, implicating the existence of diverse and intricate mechanisms in various parts of the plant. Despite activating AHA1 through TMK-mediated phosphorylation, auxin arrests primary root growth within minutes (Monshausen et al., 2011; Li et al., 2021). This physiological effect is preceded by apoplast alkalinization and relies on the TIR1/AFB module, with the AFB1 paralogue playing a crucial role, but not on nuclear auxin signalling components (Barbez et al., 2017; Fendrych et al., 2018; Serre et al., 2021; Dubey et al., 2023, Preprint) (Fig. 1C). The TIR1/AFB downstream molecular mechanisms fully explaining apoplast alkalinization are still unclear. Auxin influx transporter AUXIN RESISTANT 1 (AUX1) can partially mediate IAA–H+ symport and proton uptake, as the aux1 mutant remains slightly sensitive to auxin-mediated alkalinization (Monshausen et al., 2011; Dindas et al., 2018). In addition, AUX1 plays a key role in the establishment of an alkaline surface domain in the root transition zone (Serre et al., 2022, Preprint). The receptor-like kinase FERONIA (FER) triggers apoplastic alkalinization upon binding of its ligand RAPID ALKALINIZATION FACTOR 1 (RALF1) (Haruta et al., 2014). During the gravitropic root response, FER modulates cell expansion while auxin controls the onset of cellular elongation through the regulation of apoplastic acidification (Barbez et al., 2017). Activation of plasma membrane H+-ATPases in roots occurs through phosphorylation of cell surface TMK1-based auxin signalling, resulting in apoplast acidification, which acts in opposition to the dominant TIR1/AFB-mediated alkalization (Li et al., 2021). Further, it is proposed that TIR1/AFB-mediated apoplastic alkalization does not require FER, as fer-4 mutants display normal auxin-induced rapid growth inhibition (Li et al., 2021). So, it remains to be clarified how FER contributes to apoplast acidification.
Interestingly, AFB1-mediated inhibition of root growth is also preceded by membrane depolarization and cellular Ca2+ intake (Serre et al., 2021, 2022, Preprint; Li et al., 2021). The activation of the CYCLIC NUCLEOTIDE GATED CHANNEL 14 (CNGC14) Ca2+ channel requires operational TIR1/AFB auxin-based perception (Monshausen et al., 2011; Shih et al., 2015; Dindas et al., 2018). Strikingly, high cytosolic Ca2+ concentration or blocking CNGC14 inhibits AUX1 activity, suggesting the existence of a Ca2+-dependent negative feedback loop in the AUX1–TIR1/AFB–CNGC14 module (Dindas et al., 2018). Regardless of the speed of the response, it appears clear that both nuclear and extranuclear auxin signalling pathways play roles in cell wall modulation (Hocq et al., 2017a; Majda and Robert, 2018).
Auxin-induced apoplast pH variations regulate pectin chemistry or structure
The incubation of plant organs in acidic buffers quickly triggers cell elongation (Barbez et al., 2017; Li et al., 2021). Auxin induces striking cell wall acidification in the shoot, but in the root the first effect of auxin observed is actually alkalinization, suggesting the existence of complex and varied mechanisms in different parts of the plant. While plant cells have a wide range of pH values ranging from 3.5 to 8.3 (Yu et al., 2000; Moreau et al., 2021; Tsai and Schmidt, 2021), the apoplast is generally acidic, with pH values ranging from 4 to 6.3 and decreasing towards the plasma membrane (Martinière et al., 2018). Among several other factors, the apoplastic pH plays a major role in determining the optimal activity of cell wall and pectin remodelling enzymes. The degree of methylesterification is one of the most studied pectin modifications and can be controlled by both PECTIN METHYLESTERASES (PMEs) and their inhibitors (PMEIs) (Fig. 1D; Sénéchal et al., 2014). While plant PMEs usually show increased activity in alkaline buffers (Dixit et al., 2013; Del Corpo et al., 2020; Hocq et al., 2021, Preprint), their inhibition by PMEIs is more effective under acidic conditions (Sénéchal et al., 2015; Bonavita et al., 2016; Hocq et al., 2017b; Xu et al., 2022a). This can be explained by the formation of a pH-dependent reversible protein complex comprising both a PME and PMEI. For example, the Arabidopsis PME3 and PMEI7 complex is stable at a low pH, depending on the protonation state of amino acid residues at the binding interface (Sénéchal et al., 2017). Changes in pH can regulate blockwise PME processivity, with full processivity occurring in slightly alkaline conditions and partial processivity in acidic conditions (Hocq et al., 2021, Preprint). The pH-dependent fine-tuning of PME processivity can affect the mechanical properties of the cell wall by forming stiffening pectate–Ca2+ structures or triggers substrate availability for pectin degrading enzyme (Fig. 1D). In addition to the pH microenvironment, the degree of pectin methylesterification can also be fine-tuned by the stoichiometry of PMEs and PMEIs, and specific residues in their docking sites (Sénéchal et al., 2017; Hocq et al., 2017b). These findings highlight the complexity of the mechanisms involved in pectin modification and underscore the importance of understanding the role of pH in regulating these processes.
PME enzymes catalyse the de-esterification of methylesterified HG, resulting in the release of demethylesterified HG, methanol, and protons. The released protons potentially contribute to apoplast acidification in the direct microenvironment (Hocq et al., 2017a). According to their pH optimum, this could set up a negative feedback loop wherein a PME is locally inactivated by a PMEI in acidic conditions. Interestingly, pectin degrading enzymes, such as polygalacturonases (PGs), have been reported to act preferentially on demethylesterified substrates at a slightly acidic pH (<5.5) (Hocq et al., 2020; Ohashi et al., 2022), typically in the range of the apoplastic pH observed during auxin-induced acidification.
Despite recent advances in measuring wall mechanical properties using atomic force microscopy and Brillouin microscopy (Bacete et al., 2022), the consequences of PME processing for pectin stiffness remain unclear. For example, the induction of PME5 expression in Arabidopsis resulted in HG demethylesterification, which caused an increase or decrease in wall stiffness in dark-grown hypocotyl, depending on the study (Peaucelle et al., 2015; Bou-Daher et al., 2018). In the shoot apical meristem, the expression of PME5 led to a softer cell wall (Peaucelle et al., 2011). Gallemí et al. (2022, Preprint) proposed that the discrepancies observed between experiments might be attributed to differences in the inducible line used or the duration of transgene induction, which might influence the accumulation of the induced protein. PME1 and PME5 induction for a short time generated a stiffer cell wall, while a sustained induction resulted in a softer cell wall. Periodic and/or moderate PME activity likely produces low methylesterified HG (blockwise demethylesterification), which is able to form Ca2+ cross-links, strengthening the pectin structural network. On the other hand, continuous and/or high PME activity generates long stretches of demethylesterified HG that could be degraded by hydrolysing enzymes (Gallemí et al., 2022, Preprint). This prolonged action of PME could release a substantial amount of H+ and that could further acidify the apoplast, activating other cell wall actors such as expansins to induce cell wall loosening (Cosgrove, 2022).
When Ca2+ enters the pectin matrix
The ability of demethylesterified HG to interact with divalent cations to form ‘egg-box’ structures affects the cell wall mechanical properties. Additionally, Ca2+ is also an important second messenger in many cellular signalling cascades and Ca2+ waves are an important feature of the rapid auxin response (Vanneste and Friml, 2013). In roots, cytosolic Ca2+ concentration ([Ca2+]cyt) increases within seconds after auxin treatment (Monshausen et al., 2011), an effect mediated by the AUX1–TIR1/AFB–CNGC14 module (Fig. 1, Shih et al., 2015; Dindas et al., 2018; Serre et al., 2022, Preprint). Apoplastic Ca2+ concentration ([Ca2+]apo) usually ranges between 10 µM and 10 mM while [Ca2+]cyt is much lower, at around 100 nM (Hepler and Winship, 2010). For auxin-induced Ca2+ influx to occur in the cytosol, the presence of available Ca2+ for transport is a prerequisite, and this process also requires the activity of CNGC14. It has been shown that auxin-mediated rapid root growth inhibition in maize requires Ca2+ ions and their availability limits the auxin effect (Hasenstein and Evans, 1986). Ca2+ is substantially stored in vacuoles where the CATION EXCHANGER 1/3 (CAX1/3) Ca2+/H+ exchangers finely regulate pH gradients and Ca2+ transients during stomatal closure (Conn et al., 2011; Cho et al., 2012). The cax1/3 double mutant displays constitutive plasma membrane hypopolarization and auxin insensitivity during abscisic acid-induced stomatal closure, which can be rescued by lowering the apoplastic pH (Cho et al., 2012). Curiously, AUX1 pharmacological inhibition phenocopied cax1/3, and cax1 root phenotypes resemble that of the aux1 mutant (Cheng et al., 2003; Cho et al., 2012). These results suggest a requirement for vacuolar Ca2+/H+ exchangers in the proper regulation of ion and pH homeostasis between the different cell compartments.
The cell wall also acts as an important source of Ca2+ for the plant cell. It can be nicely depicted in the growing pollen tube tip region, where demethylesterification through PME leads to the release of Ca2+ in the apoplastic region of the cell wall. PME activity can facilitate the formation of Ca2+ pectate bridges between blockwise demethylesterified HG galacturonic acid chains, resulting in the arrangement of these chains into an ‘egg-box’-like structure to stiffen the cell wall (Hepler et al., 2012). Interestingly, guard cell walls are depleted in Ca2+-crosslinked HG, and PME activity is needed for stomatal closure (Amsbury et al., 2016). The addition of EGTA is known to stimulate expansin-mediated cell growth by depleting the [Ca2+]apo (Zhao et al., 2008). Auxin-mediated cell wall softening and hypocotyl elongation are limited by the addition of Ca2+ to the growth medium (Gallemí et al., 2022, Preprint). Interestingly, the addition of Ca2+ enhances the stiffening of onion epidermal walls by treatment with PME (Wang et al., 2020). This synergistic effect with PME is also seen in Arabidopsis overexpressing PME1 for a short period of time, where the addition of the Ca2+ chelator EGTA suppresses the stiff cell wall phenotype (Gallemí et al., 2022, Preprint). Overall, these results question the notion that the structure of the pectin matrix is the sole determinant of any growth effects mediated by auxin.
The visualization of the Ca2+–pectate structure has been inferred widely by using the antibody 2F4, but this method requires high [Ca2+]. Mravec et al. (2017) demonstrated real time imaging of calcium mediated crosslinking of HG in muro using the fluorescently tagged long oligogalacturonides probe OG7-13. Usage of OG7-13 might better help us to understand the importance of auxin in the formation of pectin ‘egg-box’ structures in planta. Despite the availability of tools such as the genetically encoded Förster resonance energy transfer-based Ca2+ sensor Yellow Cameleon 3.60 (Nagai et al., 2004; Monshausen et al., 2011) or the single fluorophore R-GECO1 (Zhao et al., 2011; Keinath et al., 2015), it remains challenging to observe and measure changes in [Ca2+] at the subcellular resolution in the context of rapid auxin growth responses, owing to its highly dynamic fluctuations. The sensor family from the CamelliA toolbox and its plasma membrane-anchored version might help to uncover Ca2+ dynamics at the apoplast–cytosol interface (Guo et al., 2022). While it is known that high [Ca2+]cyt can induce the expression of pectin remodelling enzyme genes (Conn et al., 2011; Wang et al., 2015), the mechanism underlying the connection between this phenomenon and auxin-mediated Ca2+ entry remains unclear. Downstream signalling components might be identified in the future that with the help of pharmacological inhibitors of auxin-induced Ca2+ signalling may improve our understanding (De Vriese et al., 2019).
The spatial control of pectin remodelling
The feedback loop between chemical instructions and mechanical changes for pattern formation in development has recently gained significant interest. Auxin reduces tissue rigidity in the shoot apex of Arabidopsis before organ outgrowth by demethylesterifying pectin, and the development of functional organs requires auxin signalling (Braybrook and Peaucelle, 2013). Also, cell wall rigidity affects the localization of PIN1 in the shoot apex, suggesting a feedback loop between auxin and cell wall mechanics in phyllotactic patterning (Braybrook and Peaucelle, 2013). The pleiotropic effects of auxin are highly dependent on organ sensitivity and the developmental stage under investigation. As we previously mentioned, auxin tends to rapidly promote cell elongation in the shoot whereas it quickly represses cell expansion in the root (Fendrych et al., 2016, 2018; Barbez et al., 2017; Li et al., 2021; Lin et al., 2021).
Many insights have been gained from studying organs that exhibit differential growth, where cells present on one side elongate more than those on the other side. One such example of this is apical hook formation, which involves the bending of the hypocotyl upon germination to protect the shoot apical meristem from mechanical damage. Auxin signalling plays a key role in the regulation of this process (Béziat and Kleine-Vehn, 2018). Asymmetric distribution of auxin, via polar localization of auxin transporters, controls the differential degree of methylesterification of pectins between the inner and outer side of the apical hook (Jonsson et al., 2021). Indeed, cells located on the inner side of the hook display a strong auxin response and a higher degree of methylesterification than cells located on the outer side, which is correlated with a reduction in cell deformation and elongation (Jonsson et al., 2021; Du et al., 2022). It is noteworthy that plants overexpressing PMEI5, with a uniformly high degree of pectin methylesterification, fail to establish the proper auxin response gradient, which results in the failure of apical hook formation. These observations suggest the existence of a positive feedback loop between pectin demethylesterification and auxin signalling (Jonsson et al., 2021). Gravistimulation induces differential cell growth in the root elongation zone, and auxin plays a dual role in promoting and inhibiting cell elongation on the upper and lower sides of the bending root, respectively. Despite our deep understanding of the auxin role in this model (reviewed in Konstantinova et al., 2021), the dynamics of the cell wall and, in particular, pectin, remain poorly understood. Further research is needed to understand how the complex interactions between cell wall components and other cellular components lay the foundation for differential cell growth.
Auxin and pectin remodelling are also tightly intertwined in other physiological processes such as cell adhesion, where temporal and spatial regulations are critical for the maintenance of cell-to-cell contacts, which are essential for the integrity and function of tissues and organs. During lateral root emergence, the cells located in front of the growing lateral root primordium undergo extensive cell wall modifications. Auxin leaks from the primordium tip and is funnelled into the overlying cells through the activation of the auxin influx carrier LIKE AUX1 3 (LAX3) in a positive feedback loop (Swarup et al., 2008; Péret et al., 2013). Auxin induces the local expression of INFLORESCENCE DEFICIENT IN ABSCISSION (IDA), which encodes a peptide that then binds to its receptor, HAESA/HAESA-LIKE2 (HAE/HSL2), to trigger a signalling cascade that activates a transcriptional amplification mechanism for pectin remodelling genes. This in turn affects cell wall properties and ultimately influences cell growth and tissue morphogenesis (Kumpf et al., 2013). Intriguingly, low methylesterified pectins were found to be reduced at the site of lateral root emergence, at the pericycle–endodermis and endodermis–cortex junctions, as revealed via immunolabelling using the LM19 antibody (Wachsman et al., 2020). This result seems counterintuitive with the prevailing model, which suggests that demethylesterification of pectins serves to prime the substrate for pectin-degrading enzyme-encoding genes such as POLYGALACTURONASE INVOLVED IN LATERAL ROOT (PGLR), which are expressed in those cells (Kumpf et al., 2013; Hocq et al., 2020). Instead, the authors propose that the loss of demethylesterified pectins may lead to a reduction in cell adhesion (Wachsman et al., 2020). Auxin’s positive effect on secondary root emergence seems to be largely conserved in plants. A recent study using white lupin (Lupinus albus) as an alternative model showed that auxin triggers pectin demethylesterification in the rootlet emergence zone (Jobert et al., 2022b). Similarly to Arabidopsis primordium overlying cells, auxin induces the expression of LaPG3 in the rootlet emergence zone of white lupin, which is a close homologue of PGLR and predicted to act on demethylesterified pectins (Jobert et al., 2022b).
Cell separation occurs in floral organs and fruits, where the balance between auxin and ethylene coordinates abscission (Basu et al., 2013; Gao et al., 2019; Dong et al., 2021). Auxin transport plays a pivotal role in determining the abscission zone in Arabidopsis mature siliques, yellow lupin flowers, and tomato fruits (Sorefan et al., 2009; Kućko et al., 2020; Dong et al., 2021). The IDA–HAE/HSL2 module plays a conserved role in controlling organ shedding in many species by upregulating PG genes (Aalen et al., 2013). Furthermore, a recent study using Arabidopsis floral cell sorting combined with single cell RNAseq identified the expression of pectin remodelling genes regulated by HAE/HSL2 in the abscission zone (Taylor et al., 2022, Preprint). While it is unclear whether auxin is also involved in IDA–HAE/HSL2 signalling cascade activation during lateral root emergence, the expression of POLYGALACTURONASE ABSCISSION ZONE A. THALIANA (PGAZAT) and PGLR in cells overlying lateral root primordia and in abscission zones imply the presence of a shared regulatory module (Kumpf et al., 2013). Moreover, this suggests that different tissues can have similar HG composition that could represent a possible hallmark for the breakdown of the middle lamella and, ultimately, cell separation. In the oil palm, immunolocalization and Fourier-transform infrared spectroscopy experiments revealed highly demethylesterified pectin content in the separated abscission zone cell layer surface (Roongsattham et al., 2016). The acquisition of cell polarity for local pectin modification and degradation implies the formation of wall regional territories. This exciting research field has been extensively reviewed recently (Raggi et al., 2020; Dauphin et al., 2022). Thus, the interplay between auxin and pectin dynamics is a fundamental aspect of plant development and physiology, with broad implications for plant adaptation and survival.
How auxin defines cell wall microdomains
Cell wall microdomains refer to specific regions of the plant cell wall that possess distinct compositions and organizations of wall polymers, enabling the plant to undergo localized processes of wall softening or stiffening, which are essential for its growth and development (Dauphin et al., 2022). The clustering of PIN proteins and its impact on polar auxin transport is influenced not only by the connections between the plasma membrane and the cell wall, but also by the composition of the cell wall itself (Feraru et al., 2011; Kleine-Vehn et al., 2011; Ke et al., 2021; Marhava, 2022). Specifically, the composition of pectin and cellulose within the cell wall affects the size, density, and diffusion rate of PIN clusters (McKenna et al., 2019; Ke et al., 2021). This suggests that dynamic changes in the cell wall could provide feedback cues to alter auxin gradient formation by changing PIN polarity in the plasma membrane.
Intracellular wall heterogeneity in regards to pectin chemistry has been observed in several developmental contexts including hypocotyl anisotropic growth (Bou Daher et al., 2018), pavement cell lobe initiation and growth (Majda et al., 2017; Altartouri et al., 2019), pollen tube and root hair bulging/elongation (Chebli et al., 2012; Mravec et al., 2017; Schoenaers et al., 2018), stomatal closure (Amsbury et al., 2016), seed mucilage secretory cell differentiation (Francoz et al., 2019), and phloem sieve element maturation (Kalmbach et al., 2023). Distinct pectin polymer signatures and pectin modifying enzymes are also found in the vicinity of plasmodesmata. Plasmodesmata cell wall microdomains display specific RG-I, enriched in α-(1-5)-arabinans and depleted in β-(1-4)-galactans, as well as low and highly methylesterified HG and RG-II, respectively (Orfila and Knox, 2000; Giannoutsou et al., 2013; Paterlini et al., 2022). PME subcellular localization around plasmodesmata, with additional cell wall remodelling enzymes, could cause the modification of pectin in muro (Morvan et al., 1998; Fernandez-Calvino et al., 2011). Such specific pectin patterns are suggested to regulate channel structure and symplastic cell–cell communication and might be critical for systemic virus infection (Chen et al., 2000; Andika et al., 2013). Further research is needed to fully understand the mechanisms involved in the formation and function of these pectin-specific microdomains.
The case of epidermal pavement cell interdigitated growth
Arabidopsis leaf pavement cells possess a complex jigsaw puzzle-like shape that makes them an outstanding model for investigating cell polarity. The development of stomatal spiral cell complexes is regulated by a fluctuating auxin response gradient that is controlled by the precise localization of auxin transporters in young pavement cells (Grones et al., 2020; Liu et al., 2021). As these cells grow and form lobes, the pectin heterogeneity across their anticlinal curved cell walls becomes apparent. Specifically, RG-I side chain components galactan and arabinan are enriched on the convex side (neck or indentation region; Majda et al., 2017) while HGs are less methylesterified in this region, where atomic force microscopy measurements show a softer wall. Conversely, highly methylesterified pectins are found on the concave side (lobed region), where the stiffness values are the highest (Majda et al., 2017). Using a combination of super-resolution microscopy (3D-dSTORM) and cryo-scanning electron microscopy, Haas and colleagues revealed organized pectin nanofilament structures in the anticlinal wall that are able to swell at a low HG methylesterification degree, a conformation not observed in the periclinal wall (Haas et al., 2020). This enrichment in low methylesterified HG and galactan/arabinan is observed in straight cell wall regions of young cells where lobes will likely appear, indicating a finely-tuned local regulation and the creation of microdomains prior to any observable growth asymmetry (Majda et al., 2017).
Pavement cell interdigitated growth is regulated by the Rho of plants (ROP) guanosine triphosphatase (GTPase) ROP2/4 and ROP6 signalling pathways (Fig. 2A). ROP2/4 and ROP6 have opposite effects on cell growth and interact with different proteins to control growth in different regions of the cell contour. At the neck region, ROP6 interacts with the ROP-interactive CRIB motif-containing (RIC) protein RIC1 to restrict growth and promote microtubule rearrangement, while at the lobe ROP2/4 interacts with RIC4 to promote cortical F-actin assembly and cell expansion (Fu et al., 2005, 2009). Accumulating at the neck region and colocalizing with microtubules, the PLECKSTRIN HOMOLOGY GTPase-ACTIVATING proteins PHGAP1/2 inactivate ROP2. However, high brassinosteroid signalling can activate ROP2 in the lobe by suppressing BRASSINOSTEROID INSENTIVE 2-triggered phosphostabilization of PHGAPs (Fig. 2A) (Lauster et al., 2022; Zhang et al., 2022b). The demethylesterification of pectins in the cell wall at the neck region activates the transmembrane receptor-like kinase FER, which phosphorylates ROP guanine exchange factor 14 (RopGEF14) and activates ROP6 signalling (Fig. 2A) (Lin et al., 2022; Tang et al., 2022). The modification of pectins occurs prior to the initiation of lobes, indicating that the activation of FER could be one of the first elements to trigger the ROP/RIC signalling cascade. The activation of ROP2/4 and ROP6 antagonistic pathways in their respective lobe and neck locations is mediated by TMK-based auxin signalling at the cell surface, a mechanism that may involve ABP1 and/or ABL auxin receptors (Xu et al., 2010, 2014; Yu et al., 2022, Preprint). Pan et al. (2020) provide a comprehensive description of how auxin contributes to the formation of sterol and TMK nanoclusters at the plasma membrane leading to the local activation of ROP6 and the subsequent cortical microtubule reorganization that restrict the diffusive movement of TMK1 and ordered lipids. This process creates a self-amplifying ‘hot-spot’ for lobe outgrowth (Pan et al., 2020). Auxin-mediated ROP6 nanoclustering was also observed in root cells, where it is dependent on an anionic phospholipid microenvironment (Platre et al., 2019). It is unclear whether auxin–TMK-mediated ROP2/4 local accumulation also depends on distinct lipid nanodomains and membrane dynamics, and this requires further investigation.
Fig. 2.
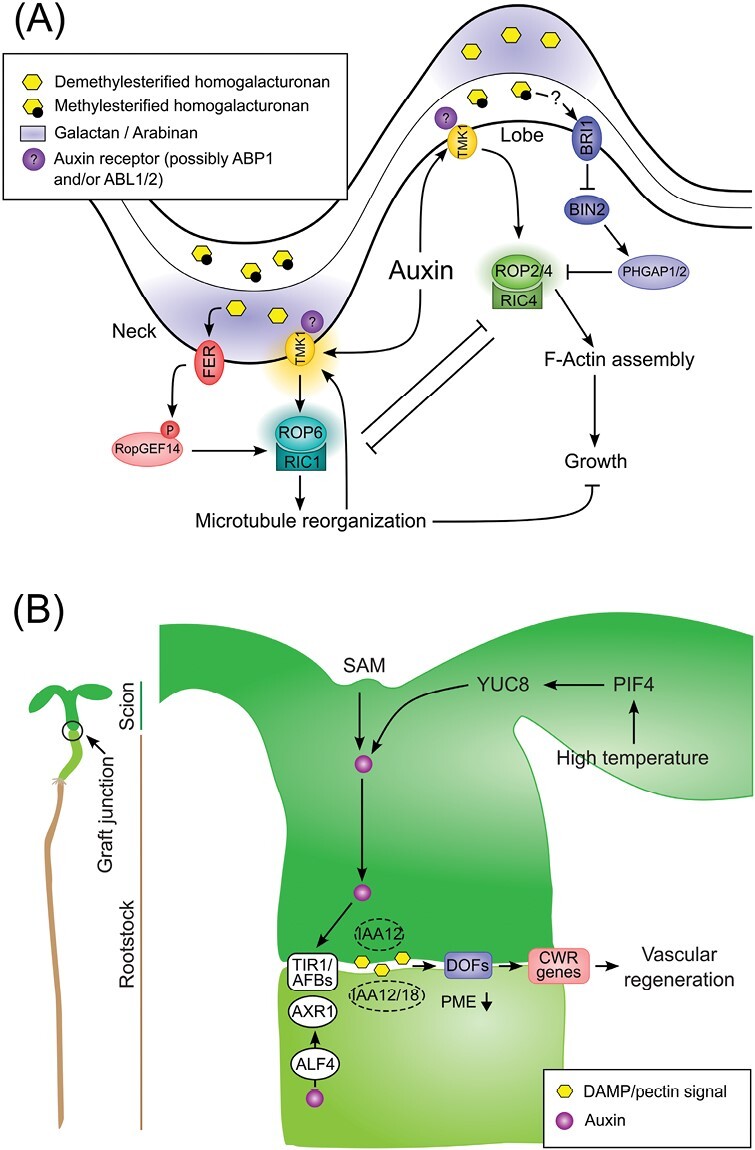
Overview of the auxin and pectin interplay in Arabidopsis epidermal pavement cell shape acquisition and hypocotyl graft interface. (A) Epidermal pavement cell shape working model. Cell surface auxin sensitive TMK1-based module, possibly involving the ABP1 extracellular auxin receptor or its close paralogue ABL, activates the ROP2/4-RIC4 module to trigger F-Actin filament assembly and lobe expansion. At the neck location, auxin promotes TMK1 nanoclusters activating the ROP6–RIC1 module to induce cortical microtubule reorganization and growth restriction. The convex side of the cell wall is enriched in galactan and arabinan pectin components and in demethylesterified homogalacturonans. Demethylesterified pectin are sensed by the extracellular domain of FER triggering phosphorylation of RopGEF14, in turn activating ROP6 signalling. At the neck location, high BRI1-mediated brassinosteroid signalling supresses BIN2-triggered phosphostabilization of PHGAP resulting in the activation of ROP2 signalling. On the concave side of the cell wall, the highly methylesterifified pectin signal is possibly transduced to BRI1 by an unknown mechanism. (B) Hypocotyl graft interface working model. Auxin is synthesized in the shoot apical meristem (SAM) or in the leaves under high temperature through a PIF4/YUC8 module, moves across the grafting interface, and elicits the TIR1/AFBs cascade degrading IAA12/18 and activating unknown ARFs to promote vascular regeneration. AXR1 and ALF4 are required in the root stock for vascular reconnection but not in the scion. Wounding at the graft interface produces a damage associated molecular pattern (DAMP), possibly resulting from pectin degradation products, and activates DOF transcription factors that regulate the expression of cell wall remodeling (CWR) genes to allow vascular regeneration. Reduced PME activity below the graft junction is a prerequisite for plant healing.
Auxin-mediated proteolipid cluster formation plays a critical role in the formation of microdomains along the plasma membrane. Modification of the cell wall composition, particularly the pectin methylesterification status, affects protein dynamics, including PIN2 and PIN3 lateral mobility at the plasma membrane (Martinière et al., 2012; McKenna et al., 2019; Daněk et al., 2020). Interestingly, loss of function of FER perturbs nanoscale dynamics of the immune receptor FLAGELLIN SENSITIVE 2/BRI1-ASSOCIATED RECEPTOR KINASE 1 (FLS2/BAK1) and perception of its small peptide ligand RALF is important for the nanodomain organization of BAK1 (Gronnier et al., 2022). Besides the RALF peptide, locally demethylesterified pectins can bind to and activate FER (Feng et al., 2018; Lin et al., 2022), which, among other cell wall integrity sensor candidates, could potentially represent a link between the pectin matrix and protein clustering at the plasma membrane. Moreover, the relationship between FER and TMK1 in the light of auxin and pectin modification is worth investigating as they typically have opposite roles in plant growth and development. TMKs and FER could establish acidic or alkaline pH microdomains, respectively, by phosphorylating the common target, AHA2 (Haruta et al., 2014; Lin et al., 2021). Eventually, apoplast pH modification could alter the activity of pectin modifying enzymes, thereby introducing a feedback regulation mechanism to the model.
How to sustain and pull the brakes on auxin action? The transcriptional control of auxin
Auxin exerts a critical transcriptional control to regulate cell growth by fine-tuning the expression dynamics of many key cell wall remodelling enzymes across diverse plant species (Laskowski et al., 2006; Swarup et al., 2008; Wei et al., 2021; Zhang et al., 2022c). MicroRNA167 (MIR167) targets ARF6 and ARF8 orthologues in diverse plant species (Barik et al., 2015). In tomato, RNAseq analysis in MIR167 overexpression lines revealed down-regulation of many cell wall metabolism genes including pectin methyl transferase inhibitors, gluco-, manno- and galactosidases, UDP-glucosyl and -glucoronyl transferases, invertases, and β-1,3-glucan hydrolases (Liu et al., 2014). Promoter analysis showed the presence of AuxREs in the promoter region of 109 out of a total of 686 mis-expressed genes including Solyc06g060170.2 (pectin lyase-like superfamily protein) (Liu et al., 2014).
Pectin remodelling is a prerequisite for fruit ripening in both climacteric and non-climacteric fleshy fruits (Wang et al., 2018). Reducing pectin degradation through silencing of pectate lyase or polygalacturonase genes delayed fruit ripening in tomato and strawberry (Santiago-Doménech et al., 2008; Posé et al., 2013; Uluisik et al., 2016; Yang et al., 2017; Wang et al., 2019). The demethylesterification of HG and depletion in RG-I galactan side chains usually occurs during the ripening process (Wang et al., 2018). Interestingly, the down-regulation of Solanum lycopersicum ARF4 resulted in enhanced fruit firmness at the late ripening stage, an effect linked to differences in pectin fine structure and tissue architectural changes (Guillon et al., 2008). In Japanese plum (Prunus salicina L.), auxin regulates pectin remodelling gene expression and accelerates ripening-related events such as fruit softening (El-Sharkawy et al., 2016). In contrast, auxin delays ripening in pre-veraison grape berries (Vitis vinifera) and inhibits the transcription of several pectin-related genes including PG, PECTIN LYASE (PL), and PME (Dal Santo et al., 2020). Accordingly, auxin treatment seems to delay pectin demethylesterification in this case, causing the berries to have stiffer wall and be less prone to cell expansion (Dal Santo et al., 2020). Long non-coding RNA 7 (lncRNA7) and lncRNA2 play key role in modulating the cell wall defence response in cotton chromosome segment substitution lines when infected with the fungus Verticillium dahliae (Zhang et al., 2022c). It was discovered that a lncRNA7–GbPMEI13 module plays a positive role, while a lncRNA2–GbPG12 module plays a negative role, in regulating disease resistance. The upregulation of lncRNA7 by pectin-derived oligogalacturonide promotes IAA accumulation and activates GbPMEI13 expression, which inhibits V. dahliae mycelial growth and spore germination. ARF5 has been shown to induce GbPMEI13 transcription. These findings suggest that GbPMEI13 has the potential to be a useful tool for improving disease resistance in plants (Zhang et al., 2022c).
The transcriptional cascade initiated by ARF7 results in the accumulation of auxin in cells overlying the lateral root primordium (Stoeckle et al., 2018). At this specific location, auxin activates the transcription of pectin modifying genes to accommodate the growth of the underlying primordium (Swarup et al. 2008; Kumpf et al., 2013; Lewis et al., 2013). Lewis and co-workers identified many pectin-modifying genes under the influence of auxin over a detailed and physiologically relevant time course during lateral root development (Lewis et al., 2013). Equally, auxin controlled transcriptional responses are cell type dependent and fluctuate along the longitudinal axis of the primary root. Individual cells within four different root tissues in Arabidopsis exhibit specific response to auxin, with the epidermis being more susceptible to auxin-induced cell wall gene regulation (Bargmann et al., 2013). Recently, it has been shown that in maize, there is a significant enrichment of transcripts associated with cell wall biogenesis in the root elongation zone following auxin treatment (McReynolds et al., 2022). Interestingly, a pectin biosynthetic gene, GALACTURONOSYLTRANSFERASE 15 (GAUT15), is transcriptionally regulated by auxin and required for root gravitropism (Lewis et al., 2013). Quantitative root proteomics have identified another galacturonosyltansferase, GAUT10, that is post-transcriptionally regulated by auxin (Pu et al., 2019). The ubiquitous role of GAUT10 in plant development implies a broad role for auxin-regulated pectin biosynthesis in many developmental contexts (Caffall et al., 2009; Voiniciuc et al., 2018; Guo et al., 2021). A recent study on the role of GAUT10 in root apical meristem activity has identified a possible negative feedback loop on auxin signalling and metabolism (Dash et al., 2023, Preprint). Pectin demethylesterification also appears to modulate auxin content during seedling development (Jobert et al., 2022a, Preprint). Exogenously expressed fungal polygalacturonase increased pectin degradation and reduced auxin sensitivity in tobacco (Ferrari et al., 2008). Furthermore, treatment with oligogalacturonide fragments, which typically result from polygalacturonase activity, produced a similar effect in Arabidopsis (Savatin et al., 2011). Likewise, the down-regulation of a polygalacturonase involved in rootlet emergence in white lupin increased the expression of early auxin responsive GRETCHEN-HAGEN 3 (GH3) genes (Jobert et al., 2022b). Altogether, these results suggest that pectin backbone integrity affects auxin responses.
Despite the identification of various genes associated with pectin biosynthesis, the mechanisms that regulate their transcriptional activities remain mostly unclear. Recently, Xu et al. (2022b) showed that DE1 BINDING FACTOR 1 (DF1) is a key regulator of mucilage RG-I biosynthesis. DF1 and GLABRA2 (GL2) transcriptionally regulate the expression of MUCILAGE MODIFIED 4 (MUM4) and GALACTURONOSYLTRANSFERASE-LIKE5 (GATL5). Furthermore, it was shown that the expression of DF1 and GL2 is directly regulated by TRANSPARENT TESTA GLABRA2 (TTG2) (Xu et al., 2022b). Interestingly, the gl2 mutant displays hypersensitivity to auxin, and ARF1 and ARF2 interact with GL2 at the protein level (Llavata-Peris, 2013). Indeed, ARFs interact with many key transcription factors at the protein level (Cancé et al., 2022). The BZR1–ARF6–PIF4/DELLA–ERF (BAP/DE) module has been shown to synergistically induce the expression of genes involved in cell wall biogenesis and organization, thereby integrating multiple hormonal and environmental signals during seedling morphogenesis (Bai et al., 2012; Oh et al., 2014; Liu et al., 2018).
The case of plant tissue regeneration during hypocotyl grafting
Plant grafting is a widely used technique in horticulture and scientific research, but the molecular mechanisms of graft formation and vascular reconnection remain poorly understood. Auxin gradient formation by the PINs plays a key role in regulating vascular development (Scarpella et al., 2006). The scion and root stock tissues lose their asymmetric cell division, cell differentiation and gene expression patterns upon contact and develop vascular connections (Melnyk et al., 2015). Auxin-mediated ABERRANT LATERAL ROOT FORMATION 4 (ALF4) activity promotes vascular connection by an inter-tissue communication process at the graft junction (Fig. 2B) (Melnyk et al., 2015). Transcriptome analysis of the grafting interface revealed several genes associated with auxin signalling and cell wall remodelling during the early wound recognition and regeneration steps, respectively (Melnyk et al., 2018). Recently, it has been shown that the perception of high temperatures in Arabidopsis leaves facilitates the regeneration of vascular tissues and formation of grafts in remote parts of the plant (Serivichyaswat et al., 2022). Mutations in auxin biosynthetic genes (YUCCA2/5/8/9) or PHYTOCHROME INTERACTING FACTOR 4 (PIF4) negate the regenerative capability at high temperature (27 °C) (Fig. 2B) (Serivichyaswat et al., 2022). Furthermore, auxin activity and cell wall damage activate four DNA BINDING WITH ONE FINGER (DOF) transcription factor family genes, namely HIGH CAMBIAL ACTIVITY2 (HCA2), TARGET OF MONOPTEROS6 (TMO6), DOF2.1, and DOF6, to promote wound healing and tissue regeneration in Arabidopsis (Fig. 2B) (Zhang et al., 2022a). The dof quadruple mutant exhibits changes in pectin composition and failed to trigger wound-induced pectin modification, suggesting a critical role for HCA2, TMO6, DOF2.1, and DOF6 in transducing cell wall signals in the early grafting events. A PMEI5 overexpression line and the endo1,4-β-glucanase-deficient mutant korrigan1 exhibit changes in the pectin and cellulose matrix, respectively, and display altered HCA2 and TMO6 expression (Zhang et al., 2022a). Furthermore, alterations in the pectin and cellulose matrix can activate the wound-associated ETHYLENE RESPONSE FACTOR 115 (ERF115) and ANAC096 transcription factors, suggesting that cell wall damage induces a shared mechanism for wound perception and promotion of tissue regeneration (Zhang et al., 2022a).
Conclusion
Regulation of the pectin matrix by auxin occurs at the organ, tissue, cellular and subcellular levels and has crucial implications for how a plant responds to its environment. Auxin induces rapid variations in apoplastic microenvironment properties such as the pH value and concentration of important ions. This in turn locally affects the pectin backbone of the cell wall through the modulation of enzyme activity but is also dependent on the native pectin composition. Such cell wall microdomains should be seen as a dynamic continuum that links the cell wall, the plasma membrane, and cytosolic actors. Recent progress made in cell wall imaging using fluorescent probes and super resolution microscopy could help to paint a better picture of the pectin matrix structure in the near future. Simultaneously, advanced biophysical and biochemical analyses are required to establish a connection between wall mechanical properties and pectin chemistry. This could be facilitated by technologies such as atomic force microscopy and Brillouin microscopy coupled with high resolution LC-MS pectin dosage or Fourier-transform infrared spectroscopy. The precise mechanisms by which various PME enzymes operate with regards to de-esterification pattern and resulting mechanical outcomes are not yet fully comprehended. The regulation of different PMEs by auxin in different tissues may result in varying degrees of de-esterification and consequential effects. Additionally, pH and calcium sensors coupled to the expanding auxin molecular and genetic toolbox are allowing us to understand the very fast auxin cellular response. It will be interesting to investigate rapid modifications of pectin during these early auxin responses in a dynamic fashion. Transcriptional control of pectin remodelling by auxin activity has been extensively studied, but much less is known about the feedback exerted by pectin to the auxin machinery. Since cell wall structure is critical for plant adaptation to biotic and abiotic stresses, a balance between auxin and pectin remodelling needs to be reached. Understanding of feedback loops existing in this system and how they are connected to other molecular actors such as other phytohormones opens an exciting new area for future research (Box 1).
Box 1. Outstanding questions.
How can the generation of a structural interactome map and conducting a biochemical characterization aid in understanding the pH-dependent control of PME and PMEI, which belong to a large multigenic family, and shed light on the specific function of PME and PMEI in pH-dependent cell expansion?
How much of the Ca2+ fraction required for rapid auxin-mediated growth inhibition originates from the pectin matrix? Can those pectin conformations be considered as a reservoir for Ca2+ transients observed during rapid auxin-induced growth inhibition in roots?
Auxin rapid response signatures are too quick for transcriptional regulation, and therefore the cell wall must be predisposed to respond to changes in apoplast pH, whether it becomes more acidic or alkaline. Can the pectin matrix structure including the presence of readily active and pH sensitive remodelling enzymes determine the extent of auxin rapid response based on the cellular context?
FERONIA and TMKs signalling outcomes on plant growth and stress response are well-documented but their direct interaction is still poorly described. Do these two receptors directly interact and to what extent do they share phosphorylation targets?
How does local pectin heterogeneity occur along the straight wall before the initiation of a lobe in epidermal pavement cells? Does auxin play a role in establishing those pectin microdomains?
How do pectin microdomains influence plasma membrane dynamics and the phospholipid environment?
Auxin regulation of pectin remodelling genes is well described but much less is known regarding the impact of pectin integrity on auxin signalling and metabolism. A clearer picture that includes molecular actors is needed to better understand the feedback from the pectin matrix on auxin.
Acknowledgements
We are grateful to Siamsa M. Doyle, Kristoffer Jonsson, Mateusz Majda, and Stéphane Verger for their helpful comments on the manuscript. We apologize to all colleagues whose work was not referenced due to space constraints.
Glossary
Abbreviations
- GalA
d-galacturonic acid
- HG
homogalacturonan
- PME
pectin methylesterase
- PMEI
pectin methylesterase inhibitor
- RG-I
rhamnogalacturonan-I
- RG-II
rhamnogalacturonan-II
Contributor Information
François Jobert, Umeå Plant Science Centre, Department of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences (SLU), 90183, Umeå, Sweden; CRRBM, Université de Picardie Jules Verne, 80000, Amiens, France.
Sandeep Yadav, Umeå Plant Science Centre, Department of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences (SLU), 90183, Umeå, Sweden.
Stéphanie Robert, Umeå Plant Science Centre, Department of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences (SLU), 90183, Umeå, Sweden.
John Lunn, MPI of Molecular Plant Physiology, Germany.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by a Carl Tryggers Stiftelse scholarship CTS 21:1344 (SY), the Swedish Research Council Vetenskapsrådet grant VR-2020-03420 (FJ), the Knut and Alice Wallenberg Foundation, and VINNOVA (Verket för Innovationssystem) (FJ, SY, SR).
References
- Aalen RB, Wildhagen M, Stø IM, Butenko MA.. 2013. IDA: a peptide ligand regulating cell separation processes in Arabidopsis. Journal of Experimental Botany 64, 5253–5261. 10.1093/jxb/ert338. [DOI] [PubMed] [Google Scholar]
- Altartouri B, Bidhendi AJ, Tani T, Suzuki J, Conrad C, Chebli Y, Liu N, Karunakaran C, Scarcelli G, Geitmann A.. 2019. Pectin chemistry and cellulose crystallinity govern pavement cell morphogenesis in a multi-step mechanism. Plant Physiology 181, 127–141. 10.1104/pp.19.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsbury S, Hunt L, Elhaddad N, Baillie A, Lundgren M, Verhertbruggen Y, Scheller HV, Knox JP, Fleming AJ, Gray JE.. 2016. Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Current Biology 26, 2899–2906. 10.1016/j.cub.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andika IB, Sun L, Xiang R, Li J, Chen J.. 2013. Root-specific role for Nicotiana benthamiana RDR6 in the inhibition of Chinese wheat mosaic virus accumulation at higher temperatures. Molecular Plant-Microbe Interactions 26, 1165–1175. 10.1094/MPMI-05-13-0137-R. [DOI] [PubMed] [Google Scholar]
- Atmodjo MA, Hao Z, Mohnen D.. 2013. Evolving views of pectin biosynthesis. Annual Review of Plant Biology 64, 747–779. 10.1146/annurev-arplant-042811-105534. [DOI] [PubMed] [Google Scholar]
- Bacete L, Schulz J, Engelsdorf T, et al. 2022. THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 119, e2119258119. 10.1073/pnas.2119258119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M-Y, Shang J-X, Oh E, Fan M, Bai Y, Zentella R, Sun T, Wang Z-Y.. 2012. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nature Cell Biology 14, 810–817. 10.1038/ncb2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbez E, Dünser K, Gaidora A, Lendl T, Busch W.. 2017. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 114, E4884–E4893. 10.1073/pnas.1613499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann BOR, Vanneste S, Krouk G, et al. 2013. A map of cell type-specific auxin responses. Molecular Systems Biology 9, 688. 10.1038/msb.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S, Kumar A, Sarkar Das S, Yadav S, Gautam V, Singh A, Singh S, Sarkar AK.. 2015. Coevolution pattern and functional conservation or divergence of miR167s and their targets across diverse plant species. Scientific Reports 5, 14611. 10.1038/srep14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu MM, González-Carranza ZH, Azam-Ali S, Tang S, Shahid AA, Roberts JA.. 2013. The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signaling is a prerequisite for organ shedding. Plant Physiology 162, 96–106. 10.1104/pp.113.216234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béziat C, Kleine-Vehn J.. 2018. The road to auxin-dependent growth repression and promotion in apical hooks. Current Biology 28, R519–R525. 10.1016/j.cub.2018.01.069. [DOI] [PubMed] [Google Scholar]
- Bonavita A, Carratore V, Ciardiello MA, Giovane A, Servillo L, D’Avino R.. 2016. Influence of pH on the structure and function of Kiwi pectin methylesterase inhibitor. Journal of Agricultural and Food Chemistry 64, 5866–5876. 10.1021/acs.jafc.6b01718. [DOI] [PubMed] [Google Scholar]
- Bou Daher F, Chen Y, Bozorg B, Clough J, Jönsson H, Braybrook SA.. 2018. Anisotropic growth is achieved through the additive mechanical effect of material anisotropy and elastic asymmetry. eLife 7, e38161. 10.7554/eLife.38161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrook SA, Peaucelle A.. 2013. Mechano-chemical aspects of organ formation in Arabidopsis thaliana: The relationship between auxin and pectin. PLoS One 8, e57813. 10.1371/journal.pone.0057813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D.. 2009. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research 344, 1879–1900. 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Caffall KH, Pattathil S, Phillips SE, Hahn MG, Mohnen D.. 2009. Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Molecular Plant 2, 1000–1014. 10.1093/mp/ssp062. [DOI] [PubMed] [Google Scholar]
- Cancé C, Martin-Arevalillo R, Boubekeur K, Dumas R.. 2022. Auxin response factors are keys to the many auxin doors. New Phytologist 235, 402–419. 10.1111/nph.18159. [DOI] [PubMed] [Google Scholar]
- Cao M, Chen R, Li P, et al. 2019. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568, 240–243. 10.1038/s41586-019-1069-7. [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M.. 2009. Mechanism of auxin-regulated gene expression in plants. Annual Review of Genetics 43, 265–285. 10.1146/annurev-genet-102108-134148. [DOI] [PubMed] [Google Scholar]
- Chebli Y, Kaneda M, Zerzour R, Geitmann A.. 2012. The cell wall of the Arabidopsis pollen tube—spatial distribution, recycling, and network formation of polysaccharides. Plant Physiology 160, 1940–1955. 10.1104/pp.112.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-H, Sheng J, Hind G, Handa AK, Citovsky V.. 2000. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. The EMBO Journal 19, 913–920. 10.1093/emboj/19.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N-H, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD.. 2003. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. The Plant Cell 15, 347–364. 10.1105/tpc.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Villiers F, Kroniewicz L, Lee S, Seo YJ, Hirschi KD, Leonhardt N, Kwak JM.. 2012. Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiology 160, 1293–1302. 10.1104/pp.112.201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, et al. 2011. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. The Plant Cell 23, 240–257. 10.1105/tpc.109.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. 2022. Building an extensible cell wall. Plant Physiology 189, 1246–1277. 10.1093/plphys/kiac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Santo S, Tucker MR, Tan H-T, Burbidge CA, Fasoli M, Böttcher C, Boss PK, Pezzotti M, Davies C.. 2020. Auxin treatment of grapevine (Vitis vinifera L.) berries delays ripening onset by inhibiting cell expansion. Plant Molecular Biology 103, 91–111. 10.1007/s11103-020-00977-1. [DOI] [PubMed] [Google Scholar]
- Daněk M, Angelini J, Malínská K, Andrejch J, Amlerová Z, Kocourková D, Brouzdová J, Valentová O, Martinec J, Petrášek J.. 2020. Cell wall contributes to the stability of plasma membrane nanodomain organization of Arabidopsis thaliana FLOTILLIN2 and HYPERSENSITIVE INDUCED REACTION1 proteins. The Plant Journal 101, 619–636. 10.1111/tpj.14566. [DOI] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, Bardor M, Lerouge P, Driouich A, Mollet J-C.. 2010. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiology 153, 1563–1576. 10.1104/pp.110.158881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash L, Swaminathan S, Šimura J, Montes C, Solanki N, Mejia L, Ljung K, Zabotina OA, Kelley DR, 2023. GAUT10 is required for Arabidopsis root cell differentiation and elongation. bioRxiv 527497. [Preprint]. 10.1101/2023.02.07.527497 [DOI]
- Dauphin BG, Ranocha P, Dunand C, Burlat V.. 2022. Cell-wall microdomain remodeling controls crucial developmental processes. Trends in Plant Science 27, 1033–1048. 10.1016/j.tplants.2022.05.010. [DOI] [PubMed] [Google Scholar]
- Del Corpo D, Fullone MR, Miele R, Lafond M, Pontiggia D, Grisel S, Kieffer-Jaquinod S, Giardina T, Bellincampi D, Lionetti V.. 2020. AtPME17 is a functional Arabidopsis thaliana pectin methylesterase regulated by its PRO region that triggers PME activity in the resistance to Botrytis cinerea. Molecular Plant Pathology 21, 1620–1633. 10.1111/mpp.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriese K, Himschoot E, Dünser K, et al. 2019. Identification of novel inhibitors of auxin-induced Ca2+ signaling via a plant-based chemical screen. Plant Physiology 180, 480–496. 10.1104/pp.18.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindas J, Scherzer S, Roelfsema MRG, et al. 2018. AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nature Communications 9, 1174. 10.1038/s41467-018-03582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit S, Upadhyay SK, Singh H, Pandey B, Chandrashekar K, Verma PC.. 2013. Pectin Methylesterase of Datura species, purification, and characterization from Datura stramoniumand its application. Plant Signaling & Behavior 8, e25681. 10.4161/psb.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Ma C, Xu T, Reid MS, Jiang C-Z, Li T.. 2021. Auxin response and transport during induction of pedicel abscission in tomato. Horticulture Research 8, 192. 10.1038/s41438-021-00626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Bou Daher F, Liu Y, et al. 2022. Biphasic control of cell expansion by auxin coordinates etiolated seedling development. Science Advances 8, eabj1570. 10.1126/sciadv.abj1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey SM, Han S, Stutzman N, Prigge MJ, Medvecká E, Platre MP, Busch W, Fendrych M, Estelle M, 2023. The AFB1 auxin receptor controls the cytoplasmic auxin response pathway in Arabidopsis thaliana. bioRxiv 522696. [Preprint]. 10.1101/2023.01.04.522696 [DOI] [PMC free article] [PubMed]
- El-Sharkawy I, Sherif S, Qubbaj T, Sullivan AJ, Jayasankar S.. 2016. Stimulated auxin levels enhance plum fruit ripening, but limit shelf-life characteristics. Postharvest Biology and Technology 112, 215–223. 10.1016/j.postharvbio.2015.09.012. [DOI] [Google Scholar]
- Fendrych M, Akhmanova M, Merrin J, Glanc M, Hagihara S, Takahashi K, Uchida N, Torii KU, Friml J.. 2018. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nature Plants 4, 453–459. 10.1038/s41477-018-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M, Leung J, Friml J.. 2016. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife 5, e19048. 10.7554/eLife.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Kita D, Peaucelle A, et al. 2018. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Current Biology 28, 666–675.e5. 10.1016/j.cub.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Feraru MI, Kleine-Vehn J, Martinière A, Mouille G, Vanneste S, Vernhettes S, Runions J, Friml J.. 2011. PIN polarity maintenance by the cell wall in Arabidopsis. Current Biology 21, 338–343. 10.1016/j.cub.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Fernandez-Calvino L, Faulkner C, Walshaw J, Saalbach G, Bayer E, Benitez-Alfonso Y, Maule A.. 2011. Arabidopsis plasmodesmal proteome. PLoS One 6, e18880. 10.1371/journal.pone.0018880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Pontiggia D, Manfredini C, Lionetti V, Bellincampi D, Cervone F, De Lorenzo G.. 2008. Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiology 146, 669–681. 10.1104/pp.107.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoz E, Ranocha P, Le Ru A, Martinez Y, Fourquaux I, Jauneau A, Dunand C, Burlat V.. 2019. Pectin demethylesterification generates platforms that anchor peroxidases to remodel plant cell wall domains. Developmental Cell 48, 261–276.e8. 10.1016/j.devcel.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Friml J, Gallei M, Gelová Z, et al. 2022. ABP1–TMK auxin perception for global phosphorylation and auxin canalization. Nature 609, 575–581. 10.1038/s41586-022-05187-x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z.. 2005. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120, 687–700. 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Fu Y, Xu T, Zhu L, Wen M, Yang Z.. 2009. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Current Biology 19, 1827–1832. 10.1016/j.cub.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallemí M, Montesinos JC, Zarevski N, Pribyl J, Skládal P, Hannezo E, Benková E, 2022. Dual role of Pectin Methyl Esterase activity in the regulation of plant cell wall biophysical properties. bioRxiv 495617. [Preprint]. 10.1101/2022.06.14.495617 [DOI]
- Gao Y, Liu Y, Liang Y, Lu J, Jiang C, Fei Z, Jiang C-Z, Ma C, Gao J.. 2019. Rosa hybrida RhERF1 and RhERF4 mediate ethylene- and auxin-regulated petal abscission by influencing pectin degradation. The Plant Journal 99, 1159–1171. 10.1111/tpj.14412. [DOI] [PubMed] [Google Scholar]
- Giannoutsou E, Sotiriou P, Apostolakos P, Galatis B.. 2013. Early local differentiation of the cell wall matrix defines the contact sites in lobed mesophyll cells of Zea mays. Annals of Botany 112, 1067–1081. 10.1093/aob/mct175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grones P, Majda M, Doyle SM, Van Damme D, Robert S.. 2020. Fluctuating auxin response gradients determine pavement cell-shape acquisition. Proceedings of the National Academy of Sciences, USA 117, 16027–16034. 10.1073/pnas.2007400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronnier J, Franck CM, Stegmann M, et al. 2022. Regulation of immune receptor kinase plasma membrane nanoscale organization by a plant peptide hormone and its receptors. eLife 11, e74162. 10.7554/eLife.74162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon F, Philippe S, Bouchet B, Devaux M-F, Frasse P, Jones B, Bouzayen M, Lahaye M.. 2008. Down-regulation of an Auxin Response Factor in the tomato induces modification of fine pectin structure and tissue architecture. Journal of Experimental Botany 59, 273–288. 10.1093/jxb/erm323. [DOI] [PubMed] [Google Scholar]
- Guo H, Xiao C, Liu Q, Li R, Yan Z, Yao X, Hu H.. 2021. Two galacturonosyltransferases function in plant growth, stomatal development, and dynamics. Plant Physiology 187, 2820–2836. 10.1093/plphys/kiab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, He J, Dehesh K, Cui X, Yang Z.. 2022. CamelliA-based simultaneous imaging of Ca2+ dynamics in subcellular compartments. Plant Physiology 188, 2253–2271. 10.1093/plphys/kiac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KT, Wightman R, Meyerowitz EM, Peaucelle A.. 2020. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 367, 1003–1007. 10.1126/science.aaz5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KT, Wightman R, Peaucelle A, Höfte H.. 2021. The role of pectin phase separation in plant cell wall assembly and growth. The Cell Surface 7, 100054. 10.1016/j.tcsw.2021.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Menzel H, Krauss A.. 1971. Versuche und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum. Planta 100, 47–75. [DOI] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR.. 2014. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343, 408–411. 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K-H, Evans ML.. 1986. Calcium dependence of rapid auxin action in maize roots. Plant Physiology 81, 439–443. 10.1104/pp.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Kunkel JG, Rounds CM, Winship LJ.. 2012. Calcium entry into pollen tubes. Trends in Plant Science 17, 32–38. 10.1016/j.tplants.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Hepler PK, Winship LJ.. 2010. Calcium at the cell wall-cytoplast interface. Journal of Integrative Plant Biology 52, 147–160. 10.1111/j.1744-7909.2010.00923.x. [DOI] [PubMed] [Google Scholar]
- Hocq L, Guinand S, Habrylo O, et al. 2020. The exogenous application of AtPGLR, an endo-polygalacturonase, triggers pollen tube burst and repair. The Plant Journal 103, 617–633. 10.1111/tpj.14753. [DOI] [PubMed] [Google Scholar]
- Hocq L, Habrylo O, Voxeur A, et al. 2021. Arabidopsis AtPME2 has a pH-dependent processivity and control cell wall mechanical properties. bioRxiv 433777. [Preprint]. 10.1101/2021.03.03.433777 [DOI]
- Hocq L, Pelloux J, Lefebvre V.. 2017a. Connecting homogalacturonan-type pectin remodeling to acid growth. Trends in Plant Science 22, 20–29. 10.1016/j.tplants.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Hocq L, Sénéchal F, Lefebvre V, et al. 2017b. Combined experimental and computational approaches reveal distinct pH dependence of pectin methylesterase inhibitors. Plant Physiology 173, 1075–1093. 10.1104/pp.16.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobert F, Guénin S, Voxeur A, et al. 2022a. Pectin remodeling belongs to a homeostatic system and triggers transcriptomic and hormonal modulations. bioRxiv 453319. [Preprint]. 10.1101/2021.07.22.453319 [DOI]
- Jobert F, Soriano A, Brottier L, Casset C, Divol F, Safran J, Lefebvre V, Pelloux J, Robert S, Péret B.. 2022b. Auxin triggers pectin modification during rootlet emergence in white lupin. The Plant Journal 112, 1127–1140. 10.1111/tpj.15993. [DOI] [PubMed] [Google Scholar]
- Jonsson K, Lathe RS, Kierzkowski D, Routier-Kierzkowska A-L, Hamant O, Bhalerao RP.. 2021. Mechanochemical feedback mediates tissue bending required for seedling emergence. Current Biology 31, 1154–11164.e3. 10.1016/j.cub.2020.12.016. [DOI] [PubMed] [Google Scholar]
- Kalmbach L, Bourdon M, Belevich I, et al. 2023. Putative pectate lyase PLL12 and callose deposition through polar CALS7 are necessary for long-distance phloem transport in Arabidopsis. Current Biology 33, 926–939.e9. 10.1016/j.cub.2023.01.038. [DOI] [PubMed] [Google Scholar]
- Ke M, Ma Z, Wang D, et al. 2021. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytologist 229, 963–978. 10.1111/nph.16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath NF, Waadt R, Brugman R, Schroeder JI, Grossmann G, Schumacher K, Krebs M.. 2015. Live cell imaging with R-GECO1 sheds light on flg22- and chitin-induced transient [Ca2+]cyt patterns in Arabidopsis. Molecular Plant 8, 1188–1200. 10.1016/j.molp.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Wabnik K, Martinière A, et al. 2011. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Molecular Systems Biology 7, 540. 10.1038/msb.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova N, Korbei B, Luschnig C.. 2021. Auxin and root gravitropism: Addressing basic cellular processes by exploiting a defined growth response. International Journal of Molecular Sciences 22, 2749. 10.3390/ijms22052749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kućko A, Wilmowicz E, Pokora W, Alché JDD.. 2020. Disruption of the auxin gradient in the abscission zone area evokes asymmetrical changes leading to flower separation in yellow lupine. International Journal of Molecular Sciences 21, 3815. 10.3390/ijms21113815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpf RP, Shi C-L, Larrieu A, Stø IM, Butenko MA, Péret B, Riiser ES, Bennett MJ, Aalen RB.. 2013. Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proceedings of the National Academy of Sciences, USA 110, 5235–5240. 10.1073/pnas.1210835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R.. 2006. Expression profiling of auxin-treated Arabidopsis roots: Toward a molecular analysis of lateral root emergence. Plant and Cell Physiology 47, 788–792. 10.1093/pcp/pcj043. [DOI] [PubMed] [Google Scholar]
- Lauster T, Stöckle D, Gabor K, et al. 2022. Arabidopsis pavement cell shape formation involves spatially confined ROPGAP regulators. Current Biology 32, 532–544.e7. 10.1016/j.cub.2021.12.042. [DOI] [PubMed] [Google Scholar]
- Lewis DR, Olex AL, Lundy SR, Turkett WH, Fetrow JS, Muday GK.. 2013. A kinetic analysis of the auxin transcriptome reveals cell wall remodeling proteins that modulate lateral root development in Arabidopsis. The Plant Cell 25, 3329–3346. 10.1105/tpc.113.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. 2018. Auxin signaling. Plant Physiology 176, 465–479. 10.1104/pp.17.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Verstraeten I, Roosjen M, et al. 2021. Cell surface and intracellular auxin signalling for H+ fluxes in root growth. Nature 599, 273–277. 10.1038/s41586-021-04037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Tang W, Pan X, Huang A, Gao X, Anderson CT, Yang Z.. 2022. Arabidopsis pavement cell morphogenesis requires FERONIA binding to pectin for activation of ROP GTPase signaling. Current Biology 32, 497–507.e4. 10.1016/j.cub.2021.11.030. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhou X, Tang W, et al. 2021. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 599, 278–282. 10.1038/s41586-021-03976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Li Y, Chen X, Li L, Liu K, Zhao H, Wang Y, Han S.. 2018. ERF72 interacts with ARF6 and BZR1 to regulate hypocotyl elongation in Arabidopsis. Journal of Experimental Botany 69, 3933–3947. 10.1093/jxb/ery220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Wu S, Van Houten J, Wang Y, Ding B, Fei Z, Clarke TH, Reed JW, van der Knaap E.. 2014. Down-regulation of AUXIN RESPONSE FACTORS 6 and 8 by microRNA 167 leads to floral development defects and female sterility in tomato. Journal of Experimental Botany 65, 2507–2520. 10.1093/jxb/eru141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Jobert F, Rahneshan Z, Doyle SM, Robert S.. 2021. Solving the puzzle of shape regulation in plant epidermal pavement cells. Annual Review of Plant Biology 72, 525–550. 10.1146/annurev-arplant-080720-081920. [DOI] [PubMed] [Google Scholar]
- Llavata Peris CIL. 2013. Proteomic and mechanistic analysis of Auxin Response Factors in the Arabidopsis embryo. PhD Thesis, Wageningen University. [Google Scholar]
- Majda M, Grones P, Sintorn I-M, et al. 2017. Mechanochemical polarization of contiguous cell walls shapes plant pavement cells. Developmental Cell 43, 290–304.e4. 10.1016/j.devcel.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Majda M, Kozlova L, Banasiak A, Derba-Maceluch M, Iashchishyn IA, Morozova-Roche LA, Smith RS, Gorshkova T, Mellerowicz EJ.. 2021. Elongation of wood fibers combines features of diffuse and tip growth. New Phytologist 232, 673–691. 10.1111/nph.17468. [DOI] [PubMed] [Google Scholar]
- Majda M, Robert S.. 2018. The role of auxin in cell wall expansion. International Journal of Molecular Sciences 19, 951. 10.3390/ijms19040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhava P. 2022. Recent developments in the understanding of PIN polarity. New Phytologist 233, 624–630. 10.1111/nph.17867. [DOI] [PubMed] [Google Scholar]
- Martinière A, Gibrat R, Sentenac H, Dumont X, Gaillard I, Paris N.. 2018. Uncovering pH at both sides of the root plasma membrane interface using noninvasive imaging. Proceedings of the National Academy of Sciences, USA 115, 6488–6493. 10.1073/pnas.1721769115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Lavagi I, Nageswaran G, et al. 2012. Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proceedings of the National Academy of Sciences, USA 109, 12805–12810. 10.1073/pnas.1202040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JF, Rolfe DJ, Webb SED, Tolmie AF, Botchway SW, Martin-Fernandez ML, Hawes C, Runions J.. 2019. The cell wall regulates dynamics and size of plasma-membrane nanodomains in Arabidopsis. Proceedings of the National Academy of Sciences, USA 116, 12857–12862. 10.1073/pnas.1819077116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds MR, Dash L, Montes C, Draves MA, Lang MG, Walley JW, Kelley DR.. 2022. Temporal and spatial auxin responsive networks in maize primary roots. Quantitative Plant Biology 3, e21. 10.1017/qpb.2022.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk CW, Gabel A, Hardcastle TJ, Robinson S, Miyashima S, Grosse I, Meyerowitz EM.. 2018. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proceedings of the National Academy of Sciences, USA 115, E2447–E2456. 10.1073/pnas.1718263115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk CW, Schuster C, Leyser O, Meyerowitz EM.. 2015. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Current Biology 25, 1306–1318. 10.1016/j.cub.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minic Z, Jouanin L.. 2006. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiology and Biochemistry 44, 435–449. 10.1016/j.plaphy.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Mohnen D. 2008. Pectin structure and biosynthesis. Current Opinion in Plant Biology, Physiology and Metabolism 11, 266–277. 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S.. 2011. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. The Plant Journal 65, 309–318. 10.1111/j.1365-313X.2010.04423.x. [DOI] [PubMed] [Google Scholar]
- Moreau H, Zimmermann SD, Gaillard I, Paris N.. 2021. pH biosensing in the plant apoplast—a focus on root cell elongation. Plant Physiology 187, 504–514. 10.1093/plphys/kiab313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan O, Quentin M, Jauneau A, Mareck A, Morvan C.. 1998. Immunogold localization of pectin methylesterases in the cortical tissues of flax hypocotyl. Protoplasma 202, 175–184. 10.1007/BF01282545. [DOI] [Google Scholar]
- Mravec J, Kračun SK, Rydahl MG, Westereng B, Pontiggia D, De Lorenzo G, Domozych DS, Willats WGT.. 2017. An oligogalacturonide-derived molecular probe demonstrates the dynamics of calcium-mediated pectin complexation in cell walls of tip-growing structures. The Plant Journal 91, 534–546. 10.1111/tpj.13574. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A.. 2004. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proceedings of the National Academy of Sciences, USA 101, 10554–10559. 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu J-Y, Bai M-Y, Arenhart RA, Sun Y, Wang Z-Y.. 2014. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 3, e03031. 10.7554/eLife.03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Sari N, Misaki R, Fujiyama K.. 2022. Biochemical characterization of Arabidopsis clade F polygalacturonase shows a substrate preference toward oligogalacturonic acids. Journal of Bioscience and Bioengineering 133, 1–7. 10.1016/j.jbiosc.2021.08.007. [DOI] [PubMed] [Google Scholar]
- Orfila C, Knox JP.. 2000. Spatial regulation of pectic polysaccharides in relation to pit fields in cell walls of tomato fruit pericarp. Plant Physiology 122, 775–781. 10.1104/pp.122.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Fang L, Liu J, et al. 2020. Auxin-induced signaling protein nanoclustering contributes to cell polarity formation. Nature Communications 11, 3914. 10.1038/s41467-020-17602-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini A, Sechet J, Immel F, Grison MS, Pilard S, Pelloux J, Mouille G, Bayer EM, Voxeur A.. 2022. Enzymatic fingerprinting reveals specific xyloglucan and pectin signatures in the cell wall purified with primary plasmodesmata. Frontiers in Plant Science 13, 1020506. 10.3389/fpls.2022.1020506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H.. 2011. Pectin-Induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Current Biology 21, 1720–1726. 10.1016/j.cub.2011.08.057. [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Wightman R, Höfte H.. 2015. The control of growth symmetry breaking in the Arabidopsis hypocotyl. Current Biology 25, 1746–1752. 10.1016/j.cub.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Péret B, Middleton AM, French AP, et al. 2013. Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Molecular Systems Biology 9, 699. 10.1038/msb.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platre MP, Bayle V, Armengot L, et al. 2019. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 364, 57–62. 10.1126/science.aav9959. [DOI] [PubMed] [Google Scholar]
- Posé S, Paniagua C, Cifuentes M, Blanco-Portales R, Quesada MA, Mercado JA.. 2013. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. Journal of Experimental Botany 64, 3803–3815. 10.1093/jxb/ert210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Walley JW, Shen Z, Lang MG, Briggs SP, Estelle M, Kelley DR.. 2019. Quantitative early auxin root proteomics identifies GAUT10, a galacturonosyltransferase, as a novel regulator of root meristem maintenance. Molecular & Cellular Proteomics 18, 1157–1170. 10.1074/mcp.RA119.001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi S, Demes E, Liu S, Verger S, Robert S.. 2020. Polar expedition: mechanisms for protein polar localization. Current Opinion in Plant Biology, Growth and development 53, 134–140. 10.1016/j.pbi.2019.12.001. [DOI] [PubMed] [Google Scholar]
- Rayle DL, Cleland R.. 1970. Enhancement of wall loosening and elongation by acid solutions. Plant Physiology 46, 250–253. 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L, Fiedler L, Zou M, et al. 2022. Cell surface auxin signalling directly targets PIN-mediated auxin fluxes for adaptive plant development. bioRxiv 518503. [Preprint]. 10.1101/2022.11.30.518503 [DOI]
- Roongsattham P, Morcillo F, Fooyontphanich K, Jantasuriyarat C, Tragoonrung S, Amblard P, Collin M, Mouille G, Verdeil J-L, Tranbarger TJ.. 2016. Cellular and pectin dynamics during abscission zone development and ripe fruit abscission of the monocot oil palm. Frontiers in Plant Science 7, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosjen M, Kuhn A, Mutte SK, Boeren S, Krupar P, Koehorst J, Fendrych M, Friml J, Weijers D, 2022. An ultra-fast, proteome-wide response to the plant hormone auxin. bioRxiv 517949. [Preprint]. 10.1101/2022.11.25.517949 [DOI]
- Salehin M, Bagchi R, Estelle M.. 2015. SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. The Plant Cell 27, 9–19. 10.1105/tpc.114.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Doménech N, Jiménez-Bemúdez S, Matas AJ, Rose JKC, Muñoz-Blanco J, Mercado JA, Quesada MA.. 2008. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. Journal of Experimental Botany 59, 2769–2779. 10.1093/jxb/ern142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Bosneaga E, Auer M.. 2009. Plant cell walls throughout evolution: towards a molecular understanding of their design principles. Journal of Experimental Botany 60, 3615–3635. 10.1093/jxb/erp245. [DOI] [PubMed] [Google Scholar]
- Savatin DV, Ferrari S, Sicilia F, De Lorenzo G.. 2011. Oligogalacturonide-auxin antagonism does not require posttranscriptional gene silencing or stabilization of auxin response repressors in Arabidopsis. Plant Physiology 157, 1163–1174. 10.1104/pp.111.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T.. 2006. Control of leaf vascular patterning by polar auxin transport. Genes & Development 20, 1015–1027. 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenaers S, Balcerowicz D, Breen G, et al. 2018. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Current Biology 28, 722–732.e6. 10.1016/j.cub.2018.01.050. [DOI] [PubMed] [Google Scholar]
- Sénéchal F, Graff L, Surcouf O, et al. 2014. Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Annals of Botany 114, 1161–1175. 10.1093/aob/mcu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, Habrylo O, Hocq L, Domon J-M, Marcelo P, Lefebvre V, Pelloux J, Mercadante D.. 2017. Structural and dynamical characterization of the pH-dependence of the pectin methylesterase–pectin methylesterase inhibitor complex. Journal of Biological Chemistry 292, 21538–21547. 10.1074/jbc.RA117.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, Mareck A, Marcelo P, Lerouge P, Pelloux J.. 2015. Arabidopsis PME17 activity can be controlled by Pectin Methylesterase Inhibitor4. Plant Signaling & Behavior 10, e983351. 10.4161/15592324.2014.983351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serivichyaswat PT, Bartusch K, Leso M, Musseau C, Iwase A, Chen Y, Sugimoto K, Quint M, Melnyk CW.. 2022. High temperature perception in leaves promotes vascular regeneration and graft formation in distant tissues. Development 149, dev200079. 10.1242/dev.200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre NBC, Kralík D, Yun P, Slouka Z, Shabala S, Fendrych M.. 2021. AFB1 controls rapid auxin signalling through membrane depolarization in Arabidopsis thaliana root. Nature Plants 7, 1229–1238. 10.1038/s41477-021-00969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre NBC, Wernerová D, Vittal P, Dubey SM, Medvecká E, Jelínková A, Petrášek J, Grossmann G, Fendrych M, 2022. The AUX1-AFB1-CNGC14 module establishes longitudinal root surface pH profile. bioRxiv 517700. [Preprint]. https://doi.org/10.1101/2022.11.23.517700. [DOI] [PMC free article] [PubMed]
- Shih H-W, DePew CL, Miller ND, Monshausen GB.. 2015. The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Current Biology 25, 3119–3125. 10.1016/j.cub.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Sorefan K, Girin T, Liljegren SJ, Ljung K, Robles P, Galván-Ampudia CS, Offringa R, Friml J, Yanofsky MF, Østergaard L.. 2009. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459, 583–586. 10.1038/nature07875. [DOI] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM.. 2014. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. The Plant Cell 26, 2129–2142. 10.1105/tpc.114.126037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckle D, Thellmann M, Vermeer JE.. 2018. Breakout—lateral root emergence in Arabidopsis thaliana. Current Opinion in Plant Biology, Growth and development 41, 67–72. 10.1016/j.pbi.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, et al. 2008. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology 10, 946–954. 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hayashi K, Kinoshita T.. 2012. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiology 159, 632–641. 10.1104/pp.112.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Lin W, Zhou X, Guo J, Dang X, Li B, Lin D, Yang Z.. 2022. Mechano-transduction via the pectin-FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Current Biology 32, 508–517.e3. 10.1016/j.cub.2021.11.031. [DOI] [PubMed] [Google Scholar]
- Taylor IW, Patharkar OR, Hsu C-W, Baer J, Niederhuth CE, Ohler U, Benfey PN, Walker JC, 2022. Single-cell transcriptomics of the Arabidopsis floral abscission zone. bioRxiv 500021. [Preprint]. 10.1101/2022.07.14.500021 [DOI]
- Tsai H-H, Schmidt W.. 2021. The enigma of environmental pH sensing in plants. Nature Plants 7, 106–115. 10.1038/s41477-020-00831-8. [DOI] [PubMed] [Google Scholar]
- Uluisik S, Chapman NH, Smith R, et al. 2016. Genetic improvement of tomato by targeted control of fruit softening. Nature Biotechnology 34, 950–952. 10.1038/nbt.3602. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J.. 2013. Calcium: The missing link in auxin action. Plants 2, 650–675. 10.3390/plants2040650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Engle KA, Günl M, Dieluweit S, Schmidt MH-W, Yang J-Y, Moremen KW, Mohnen D, Usadel B.. 2018. Identification of key enzymes for pectin synthesis in seed mucilage. Plant Physiology 178, 1045–1064. 10.1104/pp.18.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voragen AGJ, Coenen G-J, Verhoef RP, Schols HA.. 2009. Pectin, a versatile polysaccharide present in plant cell walls. Structural Chemistry 20, 263–275. 10.1007/s11224-009-9442-z. [DOI] [Google Scholar]
- Wachsman G, Zhang J, Moreno-Risueno MA, Anderson CT, Benfey PN.. 2020. Cell wall remodeling and vesicle trafficking mediate the root clock in Arabidopsis. Science 370, 819–823. 10.1126/science.abb7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yeats TH, Uluisik S, Rose JKC, Seymour GB.. 2018. Fruit softening: Revisiting the role of pectin. Trends in Plant Science 23, 302–310. 10.1016/j.tplants.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Chang M, Huang R, Gallei M, Friml J, Yu Y, Wen M, Yang Z, Xu T, 2022. Self-regulation of PIN1-driven auxin transport by cell surface-based auxin signaling in Arabidopsis. bioRxiv 518523. [Preprint]. 10.1101/2022.11.30.518523 [DOI]
- Wang J, Tergel T, Chen J, Yang J, Kang Y, Qi Z.. 2015. Arabidopsis transcriptional response to extracellular Ca2+ depletion involves a transient rise in cytosolic Ca2+. Journal of Integrative Plant Biology 57, 138–150. 10.1111/jipb.12218. [DOI] [PubMed] [Google Scholar]
- Wang R, da Rocha Tavano EC, Lammers M, Martinelli AP, Angenent GC, de Maagd RA.. 2019. Re-evaluation of transcription factor function in tomato fruit development and ripening with CRISPR/Cas9-mutagenesis. Scientific Reports 9, 1696. 10.1038/s41598-018-38170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wilson L, Cosgrove DJ.. 2020. Pectin methylesterase selectively softens the onion epidermal wall yet reduces acid-induced creep. Journal of Experimental Botany 71, 2629–2640. 10.1093/jxb/eraa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Zhang L, Zhu R, Jiang X, Yue C, Su Y, Ren H, Wang M.. 2021. A gain-of-function mutant of IAA7 inhibits stem elongation by transcriptional repression of EXPA5 genes in Brassica napus. International Journal of Molecular Sciences 22, 9018. 10.3390/ijms22169018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlert M, Benselfelt T, Wågberg L, Furó I, Berglund LA, Wohlert J.. 2022. Cellulose and the role of hydrogen bonds: not in charge of everything. Cellulose 29, 1–23. 10.1007/s10570-021-04325-4. [DOI] [Google Scholar]
- Xu F, Gonneau M, Faucher E, et al. 2022a. Biochemical characterization of Pectin Methylesterase Inhibitor 3 from Arabidopsis thaliana. The Cell Surface 8, 100080. 10.1016/j.tcsw.2022.100080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, et al. 2014. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343, 1025–1028. 10.1126/science.1245125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen J-G, Wu M-J, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z.. 2010. Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143, 99–110. 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang Y, Du J, et al. 2022b. A DE1 BINDING FACTOR 1–GLABRA2 module regulates rhamnogalacturonan I biosynthesis in Arabidopsis seed coat mucilage. The Plant Cell 34, 1396–1414. 10.1093/plcell/koac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huang W, Xiong F, Xian Z, Su D, Ren M, Li Z.. 2017. Silencing of SlPL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnology Journal 15, 1544–1555. 10.1111/pbi.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Tang C, Kuo J.. 2000. A critical review on methods to measure apoplastic pH in plants. Plant and Soil 219, 29–40. 10.1023/A:1004724610550. [DOI] [Google Scholar]
- Yu Y, Tang W, Lin W, et al. 2022. ABLs and TMKs are co-receptors for extracellular auxin. bioRxiv 518138. [Preprint]. 10.1101/2022.11.28.518138 [DOI] [PMC free article] [PubMed]
- Zhang A, Matsuoka K, Kareem A, et al. 2022a. Cell-wall damage activates DOF transcription factors to promote wound healing and tissue regeneration in Arabidopsis thaliana. Current Biology 32, 1883–1894.e7. 10.1016/j.cub.2022.02.069. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lauster T, Tang W, et al. 2022b. ROPGAP-dependent interaction between brassinosteroid and ROP2-GTPase signaling controls pavement cell shape in Arabidopsis. Current Biology 32, 518–531.e6. 10.1016/j.cub.2021.12.043. [DOI] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA.. 1992. Functional compartmentation of the Golgi apparatus of plant cells: Immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiology 99, 1070–1083. 10.1104/pp.99.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Nodzyński T, Pěnčík A, Rolčík J, Friml J.. 2010. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proceedings of the National Academy of Sciences, USA 107, 918–922. 10.1073/pnas.0909460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu J, Cheng J, et al. 2022c. lncRNA7 and lncRNA2 modulate cell wall defense genes to regulate cotton resistance to Verticillium wilt. Plant Physiology 189, 264–284. 10.1093/plphys/kiac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Yuan S, Wang X, Zhang Y, Zhu H, Lu C.. 2008. Restoration of mature etiolated cucumber hypocotyl cell wall susceptibility to expansin by pretreatment with fungal pectinases and EGTA in vitro. Plant Physiology 147, 1874–1885. 10.1104/pp.108.116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Araki S, Wu J, et al. 2011. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891. 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]


