Abstract
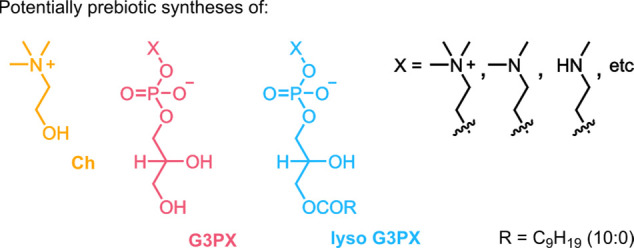
Phospholipids are the primary constituents of cell membranes across all domains of life, but how and when phospholipids appeared on early Earth remains unknown. Pressingly, most prebiotic syntheses of complex phospholipids rely upon substrates not yet shown to have been available on early Earth. Here, we describe potentially prebiotic syntheses of a diverse array of complex phospholipids and their building blocks. First, we show that choline could have been produced on early Earth by stepwise N-methylation of ethanolamine. Second, taking a systems chemistry approach, we demonstrate that the intrinsically activated glycerol-2,3-cyclic phosphate undergoes ring opening with combinations of prebiotic amino alcohols to yield complex phospholipid headgroups. Importantly, this pathway selects for the formation of 2-amino alcohol-bearing phospholipid headgroups and enables the accumulation of their natural regioisomers. Finally, we show that the dry-state ring opening of cyclic lysophosphatidic acids leads to a range of self-assembling lysophospholipids. Our results provide new prebiotic routes to key intermediates on the way toward modern phospholipids and illuminate the potential origin and evolution of cell membranes.
Introduction
Cell membranes comprise a chemically diverse set of phospholipids.1 In bacteria and eukaryotes, cell membranes are made predominantly of complex phospholipids bearing 2-amino alcohol motifs (e.g., choline, ethanolamine, and serine), the compositional and functional diversity of which influence their properties, including protein binding, substrate transport, and cell signaling.2 Due to their ubiquitous role in compartmentalization, phospholipids are thought to have appeared on Earth before the advent of cells capable of Darwinian evolution.3−5 Still, prebiotic synthetic pathways that could have led to the diverse array of phospholipids present in modern membranes remain largely unexplored.
Mixtures of single-chain amphiphiles (e.g., fatty acids, alcohols, and aldehydes) form membrane-bound compartments under prebiotically plausible conditions.6−8 However, their sensitivity to metal ions9 restricts their ability to host Mg2+-driven RNA polymerization10 and Fe-driven protometabolic processes,11,12 and suggests that complex phospholipids were likely needed for the emergence of robust primitive cells.3,4 Notably, phospholipids enhance the stability of fatty acid–based membranes to pH and divalent cations,13−15 but such blended membranes do not host the compositional diversity of phospholipids nor mirror the functional capabilities exhibited by modern membranes.
Only a few routes have been described for the prebiotic synthesis of phospholipids.16 For instance, activation of mixtures of fatty acids and glycerol phosphates gives rise to self-assembling (lyso)phosphatidic acids, the simplest of phospholipids.3,7,17−19 However, pathways leading to more complex phospholipids (e.g., phosphatidylcholines) remain scarce.
Cyanamide-driven dry-state condensation of phosphatidic acids with ethanolamine23 and choline (Figure 1a),20 or diacylglycerols with phosphoethanolamine,24 have been shown to provide phosphatidylethanolamines and phosphatidylcholines. It has also been demonstrated that solution-phase transacylation of N-acyl imidazoles and glycero-3-phosphocholine (Figure 1b),21 or acyl thioesters and lysophosphatidylcholines,22 results in the formation of phosphatidylcholines (Figure 1c). Nonetheless, these processes rely on substrates not yet shown to have been prebiotically available (choline, glycerol-3-phosphocholine, and lysophosphatidylcholine) and require activating agents and conditions that inevitably lead to byproduct formation. Moreover, to date, no bottom-up prebiotic study has addressed the predominance of 2-amino alcohol motifs, nor the universal occurrence of sn-glycerol-3-phosphodiesters (or their enantiomers, sn-glycerol-1-phosphodiesters), in complex (glycero)phospholipids.
Figure 1.

Previous syntheses of phosphatidylcholine (diacyl G3PC)20−22 under prebiotic conditions relied upon substrates (marked*) not known to have been available on early Earth.
Here, we describe potentially prebiotic pathways that could have led to the formation of natural phospholipid headgroups and lysophospholipids, key intermediates in the synthesis of complex phospholipids. We begin by showing that choline could have been produced on early Earth by the stepwise N-methylation of ethanolamine. We then take a systems chemistry approach and illustrate that the intrinsically activated glycerol-2,3-cyclic phosphate undergoes ring opening with combinations of 2-amino alcohols to furnish mixtures of phosphodiester headgroups. Finally, we demonstrate that dry-state ring opening of cyclic phosphatidic acids leads to a diverse array of self-assembling lysophospholipids. Importantly, our findings offer a possible explanation for the natural selection of 2-amino alcohol-based glycerol-3-phospholipids in bacterial and eukaryotic cell membranes. Overall, our results suggest that biologically relevant phospholipids could have been generated on early Earth before the emergence of cellular life.
Note: Despite having used racemic G>P and 10:0 lyso G>P, for simplicity, we adopted the term G3P(X). The stereospecific numbering (sn) scheme for lipids is used only where appropriate.
Results and Discussion
Prebiotic Synthesis of Choline
Prebiotic syntheses of amino-alcohol-containing phospholipid headgroups would have been contingent on the availability of free amino alcohols. On early Earth, ethanolamine (EA) and N-methylethanolamine (MEA) could have been formed on reaction of glycolaldehyde with ammonia.25 Reductive methylation of EA with formaldehyde could also have produced MEA and N,N-dimethylethanolamine (DEA).26,27 However, no prebiotic synthesis of choline (Ch) has been reported to date.28
A biosynthetic route leading to Ch entails sequential N-methylation of EA.29 In each step, the methyl group is provided by S-adenosyl methionine, and the reactions are catalyzed by N-methyl transferases. Recently, the reaction of methylamine with nitroprusside in alkaline solution was shown to afford a transient, prebiotically plausible methylating agent.30,31 We thus wondered whether Ch could be produced by methylation of DEA using the methylamine-nitroprusside system.
To probe this possibility, we incubated a solution of DEA, methylamine, nitroprusside, and cyanide at pH 10. After 24 h, Ch was formed in 20% yield (1H NMR; Figures 2 and S1). Analogously, we detected DEA (7% yield) and traces of Ch (<1% yield) upon methylation of MEA and observed that MEA is produced on methylation of EA (10% yield) alongside ethylene glycol (3% yield, Figures S2 and S3). The methylation of DEA occurs most efficiently due to the higher nucleophilicity of DEA compared to that of MEA or EA. It is also possible that MEA is produced in a slightly higher yield than DEA (formed upon methylation of MEA) because it may be generated by an additional mechanism, namely, ring opening of oxirane by methylamine. Oxirane, which hydrolyzes to ethylene glycol, is likely produced as an intermediate following the diazotization of EA by nitroprusside. This pathway is not available for MEA and DEA.
Figure 2.
Stepwise prebiotic methylation of ethanolamine (EA) in a methylamine-nitroprusside system affords choline (Ch). Yields were determined by 1H NMR analysis based on conversion of the respective ethanolamine precursor. Each yield refers to the yield of a single step. See the Supporting Information for details.
This potentially prebiotic synthesis of Ch via methylated ethanolamines set the stage for our investigation of the emergence of complex phospholipid headgroups on early Earth.
Prebiotic Synthesis of Phospholipid Headgroups
Prebiotic phosphorylation of glycerol delivers glycerol-2,3-cyclic phosphate (G>P) alongside glycerol-3-phosphate (G3P) and glycerol-2-phosphate (G2P).18,32−35 While the hydrolysis of the intrinsically activated G>P has been thoroughly characterized,36 ring opening of G>P by nucleophiles other than water remains unexplored. We therefore investigated whether the reaction of G>P with Ch in alkaline solution could generate glycerophosphocholines (GPCs). The latter are among the most abundant phospholipid headgroups in eukaryotic membranes,1 and a potentially prebiotic precursor to phosphatidylcholines. Accordingly, an aqueous solution of G>P and Ch at pH 9.5 was heated at 40 °C, and the progress of the reaction was followed by 31P NMR. Over the course of several weeks, four products were detected: glycerol-3-phosphocholine, G3PC, and glycerol-2-phosphocholine, G2PC, resulting from ring opening of G>P by Ch (−0.23 and −0.92 ppm, respectively), and G3P and G2P, resulting from hydrolysis of the starting material (4.10 and 3.75 ppm, respectively) (Figure S4a,b). The product distribution varied over time, but the highest cumulative yield of GPCs (0.6%) was obtained after 7 weeks (Figure S4c). No GPCs were formed when the reaction was carried out at pH 8.5 or 7.5 (Figure S5). In contrast, at pH 10.5 GPCs appeared after just 1 day (0.7% total yield) (Figure S5). These results show that the reaction between G>P and Ch is subject to a specific base catalysis.
Next, as the potentially prebiotic synthesis of Ch described above does not entirely consume any of its precursors, we adopted a systems chemistry approach and investigated the ring opening of G>P by Ch in the presence of EA, MEA, or DEA. In the event, when an aqueous solution of G>P, Ch, and EA at pH 9.5 was heated at 40 °C, glycerol-3-phosphoethanolamine, G3PE (0.61 ppm) and glycerol-2-phosphoethanolamine, G2PE (0.06 ppm) were detected alongside G3PC and G2PC (Figures 3 and S6). The total yield of GPCs formed in the presence of EA was higher than the total yield of GPCs generated without EA (5 vs 0.5% total yields, respectively, after 10 weeks). Similar results were obtained when MEA or DEA was used in place of EA (Figures 3, S7 and S8).
Figure 3.

Prebiotic synthesis of phospholipid headgroups. (a) Reactions of G>P (50 mM) with amino alcohols (500 mM) in H2O, pH 9.5, 40 °C, give glycerol phosphates (GPs) and phospholipid headgroups (GPXs). (b) Yields of GPs and GPXs obtained in reactions of G>P with amino alcohols. (c) 31P{1H}-NMR spectra showing the signals for GPXs formed in the reactions in entries 1–4 of Table in (b). See the Supporting Information for details.
Focusing on the reaction of G>P with Ch in the presence of EA, we found that the initial rate of formation of GPCs is proportional to the concentration of EA (Figure S9). Collectively, these data suggest that the ring opening of G>P by Ch is also general base-catalyzed, and EA, MEA, and DEA all facilitate the process. Consistent with this notion, when the reaction of G>P with Ch in the presence of EA was performed at pH 10.5, GPCs were formed in 6% cumulative yield after 7 days (Figure S10). In all cases, the cumulative yields of glycerophosphoethanolamines (GPEs) and their methylated derivatives (GPMs and GPDs) were consistently higher than those of glycerophosphocholines (GPCs) (Figures 3 and S6–S8). Ring opening of G>P with EA, MEA, and DEA is evidently more efficient than that with Ch. Thus, while the ring opening of G>P would have led to a prevalence of GPEs, GPMs, and GPDs, GPCs could have been selected through acylation and self-assembly of the ensuing lipids.21,22
To gain deeper insight, we investigated the reactions of G>P with EA and each of its methylated derivatives in the absence of Ch. When an aqueous solution of G>P and EA at pH 9.5 was heated at 40 °C, we observed the formation of GPEs after 1 day (31P NMR; Figure S11). Similar results were obtained for transformations involving MEA and DEA (Figures S12 and S13). At higher pH values (10.5) and temperatures (60 and 80 °C), the reaction of G>P with EA resulted in the hydrolysis of the starting material, whereas at lower pH values (8.5) and temperatures (22 °C) GPEs were generated with increasing efficiency over longer periods of time (Figures S14 and S15).
We subsequently established that while the rate of consumption of G>P is independent of its concentration, it is linearly dependent on the concentration of EA (Figures S16–S18). These findings imply that phospholipid headgroups could have accumulated under prebiotic conditions if sufficient concentrations of amino alcohols were available in the environment.
Finally, because Ch, EA, and its derivatives all add to G>P, we evaluated the reactivity of other amino alcohols found in biological phospholipid headgroups,2 namely, serine (Ser) and threonine (Thr). The nucleophilic attack of the two amino acids on G>P resulted in the formation of glycerophosphoserines (GPSs) and glycerophosphothreonines (GPTs) in both the presence and absence of EA (31P NMR; Figures S19–S22).
Taken together, our results demonstrate that the ring opening of G>P with amino alcohols offers a general, potentially prebiotic pathway to a diverse array of biological phospholipid headgroups.
Selection of Amino Alcohols
To probe the structural requirements of amino alcohols that can ring-open G>P, we investigated the importance of the proximity of the hydroxyl group (pronucleophile) and the amino group (general base). Accordingly, we prepared solutions of G>P and propanolamine (PrA), butanolamine (BuA), or pentanolamine (PeA) at pH 9.5. Upon heating at 40 °C for 1 week, the cumulative yields of glycerophosphodiesters (GPXs) derived from PrA and BuA were 4 and 8 times lower, respectively, than those obtained with EA (Figures 4 and S23–S25). No phosphodiesters resulted from the ring opening of G>P by PeA (31P NMR). These results suggest that amino alcohols catalyze their own addition to G>P (i.e., their amine acts as an internal general base). Thus, as the amino and hydroxyl groups are placed further apart, expanding the size of the cyclic transition state for general base catalysis from a five- to eight-membered ring, addition to G>P becomes progressively less favored (Figure 4).
Figure 4.
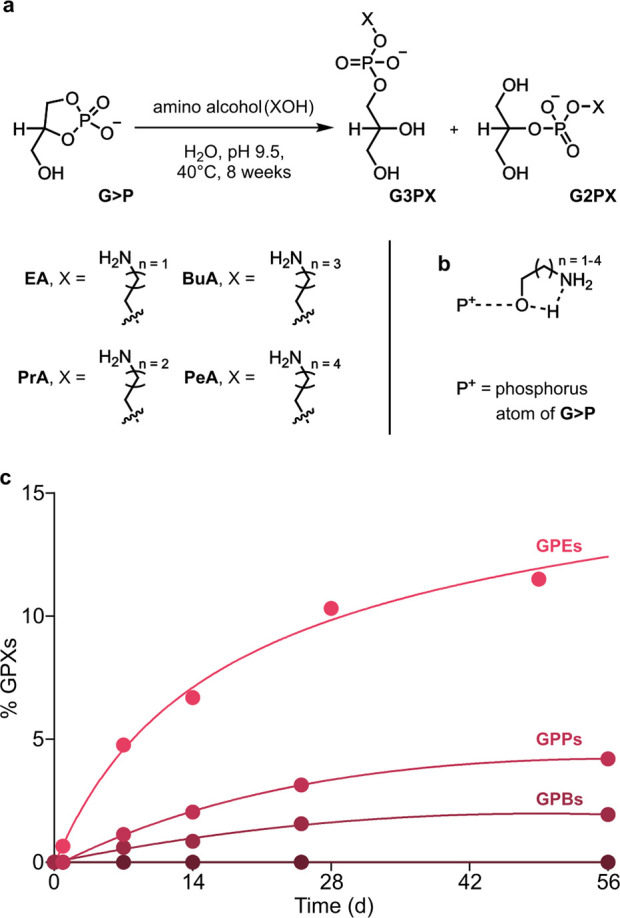
Selection of 2-amino alcohols in the ring opening of G>P. (a) Reactions of G>P (50 mM) with amino alcohols (500 mM, with different spacers) in H2O, pH 9.5, 40 °C, provide GPXs. (b) Proposed pathway by which an amino alcohol catalyzes its addition to G>P, with the amine group serving as the general base. (c) Graph showing the % yields of GPXs formed vs time in reactions described in (a). GPEs (n = 2) form more efficiently than GPPs (n = 3) and GPBs (n = 4). No phosphodiester products were formed on reaction of G>P with PeA (n = 4).
Implicit in these data is that the occurrence in modern phospholipid headgroups of the 2-amino alcohol motif present in Ch, EA and its methylated derivatives, Ser and Thr, might not have been coincidental but was rather predicated by the intrinsic reactivity of these nucleophiles.
Selection of Phospholipid Headgroup Isomers
Though the ring opening of G>P with amino alcohols produces both phosphodiester regioisomers (G3PXs and G2PXs), the product distribution changed over time. For example, in the reaction of G>P with EA, the non-natural phospholipid headgroup isomer, G2PE, initially formed more readily than its natural counterpart, G3PE (Figures 5 and S26). However, after 3 weeks 31P NMR analysis indicated that the G2PE peak had markedly decreased in intensity, relative to that of the G3PE peak, and continued doing so. By contrast, the natural regioisomer G3PE accumulated for 10 weeks. Importantly, the same trend was observed for all other GPXs (Figures S27–S31).
Figure 5.

Preferential hydrolysis of non-natural G2PE over natural G3PE. (a) Mechanistic proposal for the hydrolysis of GPXs. XOH refers to any 2-amino alcohol. (b) 31P{1H} NMR spectra showing the extended time-evolution of the reaction of G>P with EA in H2O, pH 9.5, 40 °C.
As hydrolysis of G3PC has been proposed to proceed via a cyclic intermediate,37 we wondered if an analogous pathway might provide a rationale for the selective hydrolysis of G2PXs over G3PXs (Figures 5 and S26–S31). We suggest that hydrolysis of the phosphodiester occurs by intramolecular attack of the glycerol hydroxyl groups to generate G>P, and that subsequent hydrolysis affords G3P and G2P (Figure S32a). For a G2PX, two proximal primary hydroxyl groups are available to attack the phosphorus atom to form G > P, while in the case of G3PX, only one proximal secondary hydroxyl group is available. This mechanistic proposal would account for the preferential hydrolysis of G2PE over G3PE, and of G2PX over a G3PX.
To probe the mechanism of hydrolysis of GPXs, we studied the reaction of G3PE in the presence and absence of EA at pH 9.5. Upon heating at 40 °C, G>P was formed in both instances (Figure S32b,c), confirming its intermediacy. On the other hand, the rate of hydrolysis of G3PE was found to depend on the concentration of EA (Figure S32). As expected, in the presence of EA, G2PE was formed following the reaction of transient G>P with EA (Figures S32 and S33). Finally, we observed that, in the absence of EA, the rate of hydrolysis of G3PE is proportional to the pH of the solution (Figure S34). These outcomes reveal that the hydrolysis of G3PE is subject to general and, more importantly, specific base catalysis.
The preferential hydrolysis of a G2PX over a G3PX could have occurred in any alkaline body of water, such as a carbonate-rich lake,38 and the selection of any natural glycerophospholipid headgroup (G3PX) could have originated from its greater hydrolytic stability relative to that of its non-natural analogue (G2PX).
Prebiotic Synthesis of Complex Lysophospholipids
Cyclic lysophosphatidic acids, considered as precursors to complex phospholipids,22 have been proposed as components of primitive membranes due to their stability to a broad range of pH values and divalent metal ions.13 Bolstered by the findings described above, we wondered if cyclic lysophosphatidic acids could be converted into lysophosphatidylethanolamines (10:0 lyso GPEs), lysophosphatidylcholines (10:0 lyso GPCs), and lysophosphatidylserines (10:0 lyso GPSs), all known substrates for the nonenzymatic synthesis of diacyl glycerophospholipids.22 We thus investigated the ring opening of 1-decanoyl glycerol-2,3-cyclic phosphate (10:0 lyso G>P) with 2-amino alcohols, starting with the reaction of EA.
In an aqueous medium at pH 9.5, ester hydrolysis predominated over ring opening, but traces (<1%) of 10:0 lyso GPEs were formed after 24 h (31P NMR; Figures 6 and S35). To minimize ester hydrolysis, the same process was performed in the dry state: 10:0 lyso G>P and EA were dissolved at pH 9.5, and the solvent was allowed to evaporate at room temperature in an open Petri dish. The dried mixture was heated at 60 °C for 24 h and dissolved in 0.1 M Triton X-100 in H2O:D2O (9:1, v/v) to preclude the formation of any supramolecular aggregates that would hinder analysis by NMR.40 Formation of 10:0 lyso GPEs was indicated by a pair of peaks at 0.05 ppm (10:0 lyso G3PE, 14%) and −0.74 ppm (10:0 lyso G2PE, 10%), alongside their ester-hydrolyzed derivatives G3PE and G2PE (25% cumulative yield) (31P NMR; Figures 6 and S35). Spectroscopic (1H-coupled 31P NMR) studies, spiking experiments, and LC-MS analyses supported these assignments. Yields of 10:0 lyso GPEs increased with temperature (Figure S36), and only traces of lysophosphatidic acids were detected. Notably, independent of the heating temperature, the natural regioisomer 10:0 lyso G3PE was generated preferentially (Figure S36). By comparison, the reaction of G>P with EA in the dry state (24 h, 60 °C) gave a mixture of GPEs and GPs in 49 and 6% cumulative yields, respectively (Figure S37). When the transformation of 10:0 lyso G>P was repeated in the presence of MEA, DEA, and Ch, the corresponding lysophospholipids were detected by 31P NMR and LC-MS (Figure S38). However, when 10:0 lyso G>P was reacted with Ser, 10:0 lyso GPSs were not observed (Figure S38).
Figure 6.

Prebiotic synthesis of lysophospholipids. (a) Dry-state ring opening reactions of 10:0 lyso G>P (50 mM) with amino alcohols (500 mM) afford phospholipid headgroups (GPXs) and lysophospholipids (10:0 lyso GPXs). (b) Yields of GPXs and 10:0 lyso GPXs obtained in reactions of 10:0 lyso G>P with amino alcohols at 80°C. (c) 31P{1H} NMR spectra showing the signals for G3PE, G2PE, 10:0 lyso G3PE, and 10:0 lyso G2PE formed in reactions in the dry state (top) and in solution (bottom) at 60°C, 24 h.
Taking the systems chemistry approach described above for the combined synthesis of GPCs and GPEs, the dry-state reaction of 10:0 lyso G>P was performed with EA and Ch, or EA and Ser. In these experiments, 10:0 lyso GPCs or 10:0 lyso GPSs were obtained in higher yields than before, alongside 10:0 lyso GPEs (1P NMR; Figures S39 and S40). The presence of each 10:0 lyso G3PX was established by 31P NMR, and was supported by spiking studies with spectroscopic standards. LC-MS analyses confirmed the presence of both lysophospholipid isomers in the various mixtures of products. The mixtures of 10:0 lyso GPEs formed were further characterized by dynamic light scattering for their ability to self-assemble or integrate into pre-existing aggregates present in the solution (see the Supporting Text).
The prebiotic synthesis of lysophospholipids with diverse headgroups could potentially have been beneficial for the emergence of functional primitive cells given the compositional diversity found in biological membranes. It is plausible to suggest that a diverse array of prebiotic lipids would give rise to liposomes with an equally diverse range of capabilities, able to cope or better adapt to changing environmental conditions and undergo chemical evolution. Taken together, our results define a general and potentially prebiotic pathway that leads from cyclic lysophosphatidic acids to a diverse range of biological lysophospholipids.
Conclusions
Previous reports on the synthesis of potentially prebiotic phospholipids used substrates (e.g., choline, glycerophosphocholine, and lysophosphatidylcholine)20−23 for which no prebiotic syntheses had been described (Figure 1). Moreover, no bottom-up prebiotic investigation has yet addressed the predominance of 2-amino alcohol motifs or the ubiquity of sn-glycerol-3-phosphodiesters (or their enantiomers, sn-glycerol-1-phosphodiesters) in modern phospholipids. Our work addresses these key issues.
First, we put forth a potentially prebiotic synthesis of choline by biomimetic stepwise methylation of ethanolamine. Second, embracing a systems chemistry approach, we show that ring opening of the intrinsically activated glycerol-2,3-cyclic phosphate with combinations of prebiotic amino alcohols leads to a diverse array of phospholipid headgroups. The reactivity of biologically relevant 2-amino alcohols dictates their selection in the ring-opening reaction. Furthermore, our investigations suggest that the greater hydrolytic stability of the natural G3PXs compared to the non-natural G2PXs may have resulted in their preferential accumulation in the prebiotic milieu. Taken together, our results offer a plausible explanation for the predominance of 2-amino alcohol-bearing G3PXs in biology. Finally, we demonstrate that dry-state ring opening of 10:0 lyso G>P with amino alcohols, a process that could have occurred in evaporating hydrothermal springs,39 results in the formation of a variety of complex lysophospholipids (precursors to diacyl phospholipids), which integrate into pre-existing supramolecular assemblies.
In brief, our study outlines possible prebiotic pathways that could have led to many complex phospholipids and their building blocks on early Earth. Whether such reactions, for example, the synthesis of Ch from EA, could have occurred with greater efficiency under different conditions remains to be determined. Similarly, a general acylation pathway for all headgroups and lysophospholipids reported herein, and the effect that such a diverse array of phospholipids could have had on the biophysical properties of primitive membranes, await further investigations. Nevertheless, by enhancing the prebiotic plausibility of previously reported syntheses,20−23 we challenge the hypothesis that such phospholipids appeared at a later stage in the molecular evolutionary timeline (i.e., after the emergence of ribozymes).14 We instead suggest that they could have been prebiotically available on early Earth and played a key role in the emergence of primitive cells that were able to support fusion, fission, and replication through cycles of growth and division.
Acknowledgments
The authors gratefully acknowledge support from Foundation Jean-Marie Lehn, the University of Strasbourg, and the Agence Nationale de la Recherche. The authors thank Wahnyalo Kazone for help with LC-MS analyses, and Profs. Amir Hoveyda, Pawel Dydio, Filippo Romiti, colleagues at ISIS and lab members for fruitful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c07319.
Experimental procedures, spectroscopic data (1H and 31P NMR spectra); supporting text; stack of 1H-NMR spectra, stack of 31P{1H}-NMR spectra, expansions of the +2.2 to −2.2 ppm regions of 31P{1H}-NMR spectra, graphs showing % yields, scheme showing the course of hydrolysis, and size profiles obtained by dynamic light scattering (PDF)
The project was funded by the Foundation Jean-Marie Lehn, the University of Strasbourg and the Agence Nationale de la Recherche (ResponCell, AAPG2022-JCJC, ITI SysChem within the Investissement d’Avenir program ANR-10-IDEX-0002, and AAP IdEx 2022 Attractivité to C.B.), and the CSC Graduate School, funded by the Agence Nationale de la Recherche (CSC-IGS ANR-17-EURE-0016 to M.A. and F.R.).
The authors declare no competing financial interest.
Supplementary Material
References
- Alberts B.; Johnson A.; Lewis J.; Raff M.; Roberts K.; Walter P.. Mol. Biol. Cell; Garland Science: New York, 2014; p 1464. [Google Scholar]
- Harayama T.; Riezman H. Understanding the Diversity of Membrane Lipid Composition. Nat. Rev. Mol. Cell Biol. 2018, 19 (5), 281–296. 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- Hargreaves W. R.; Mulvihill S. J.; Deamer D. W. Synthesis of Phospholipids and Membranes in Prebiotic Conditions. Nature 1977, 266 (5597), 78–80. 10.1038/266078a0. [DOI] [PubMed] [Google Scholar]
- Szostak J. W.; Bartel D. P.; Luisi P. L. Synthesizing Life. Nature 2001, 409 (6818), 387–390. 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- Aleksandrova M.; Bonfio C. Bioenergetics of Early Life. EMBO Rep. 2022, 23 (8), e55679–e55679. 10.15252/embr.202255679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves W. R.; Deamer D. W. Liposomes from Ionic, Single-Chain Amphiphiles. Biochemistry 1978, 17 (18), 3759–3768. 10.1021/bi00611a014. [DOI] [PubMed] [Google Scholar]
- Bonfio C.; Caumes C.; Duffy C. D.; Patel B. H.; Percivalle C.; Tsanakopoulou M.; Sutherland J. D. Length-Selective Synthesis of Acylglycerol-Phosphates through Energy-Dissipative Cycling. J. Am. Chem. Soc. 2019, 141 (9), 3934–3939. 10.1021/jacs.8b12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfio C.; Russell D. A.; Green N. J.; Mariani A.; Sutherland J. D. Activation Chemistry Drives the Emergence of Functionalised Protocells. Chem. Sci. 2020, 11 (39), 10688–10697. 10.1039/D0SC04506C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer S. E.; Nguyen G. Prebiotic Vesicle Formation and the Necessity of Salts. Origins Life Evol. Biospheres 2016, 46 (2–3), 215–222. 10.1007/s11084-015-9476-8. [DOI] [PubMed] [Google Scholar]
- Mansy S. S.; Schrum J. P.; Krishnamurthy M.; Tobé S.; Treco D. A.; Szostak J. W. Template-Directed Synthesis of a Genetic Polymer in a Model Protocell. Nature 2008, 454, 122–125. 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfio C.; Valer L.; Scintilla S.; Shah S.; Evans D. J.; Jin L.; Szostak J. W.; Sasselov D. D.; Sutherland J. D.; Mansy S. S. UV-Light-Driven Prebiotic Synthesis of Iron–Sulfur Clusters. Nat. Chem. 2017, 9, 1229–1234. 10.1038/nchem.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfio C.; Godino E.; Corsini M.; Fabrizi de Biani F.; Guella G.; Mansy S. S. Prebiotic Iron–Sulfur Peptide Catalysts Generate a pH Gradient across Model Membranes of Late Protocells. Nat. Catal. 2018, 1, 616–623. 10.1038/s41929-018-0116-3. [DOI] [Google Scholar]
- Toparlak Ö. D.; Karki M.; Egas Ortuno V.; Krishnamurthy R.; Mansy S. S. Cyclophospholipids Increase Protocellular Stability to Metal Ions. Small 2019, 1903381, 1903381–1903381. 10.1002/smll.201903381. [DOI] [PubMed] [Google Scholar]
- Jin L.; Kamat N. P.; Jena S.; Szostak J. W. Fatty Acid/Phospholipid Blended Membranes: A Potential Intermediate State in Protocellular Evolution. Small 2018, 14, 1–9. 10.1002/smll.201704077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budin I.; Szostak J. W. Physical Effects Underlying the Transition from Primitive to Modern Cell Membranes. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 5249–5254. 10.1073/pnas.1100498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M.; Chieffo C.; Lopez A.; Fayolle D.; Ruiz J.; Soulère L.; Oger P.; Altamura E.; Popowycz F.; Buchet R. Synthesis of Phospholipids Under Plausible Prebiotic Conditions and Analogies with Phospholipid Biochemistry for Origin of Life Studies. Astrobiology 2022, 22 (5), 598–627. 10.1089/ast.2021.0059. [DOI] [PubMed] [Google Scholar]
- Epps D. E.; Sherwood E.; Eichberg J.; Oró J. Cyanamide Mediated Syntheses under Plausible Primitive Earth Conditions. J. Mol. Evol. 1978, 11 (4), 279–292. 10.1007/BF01733838. [DOI] [PubMed] [Google Scholar]
- Gibard C.; Bhowmik S.; Karki M.; Kim E.-K.; Krishnamurthy R. Phosphorylation, Oligomerization and Self-Assembly in Water under Potential Prebiotic Conditions. Nat. Chem. 2018, 10 (2), 212–217. 10.1038/nchem.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Wu L.-F.; Xu J.; Bonfio C.; Russell D. A.; Sutherland J. D. Harnessing Chemical Energy for the Activation and Joining of Prebiotic Building Blocks. Nat. Chem. 2020, 12 (11), 1023–1028. 10.1038/s41557-020-00564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M.; Eichberg J.; Oró J. Synthesis of Phosphatidylcholine under Possible Primitive Earth Conditions. J. Mol. Evol. 1982, 18 (3), 196–202. 10.1007/BF01733046. [DOI] [PubMed] [Google Scholar]
- Fernández-García C.; Matthew W. P. Selective Acylation of Nucleosides, Nucleotides, and Glycerol-3-Phosphocholine in Water. Synlett 2017, 28 (01), 78–83. 10.1055/s-0036-1588626. [DOI] [Google Scholar]
- Liu L.; Zou Y.; Bhattacharya A.; Zhang D.; Lang S. Q.; Houk K. N.; Devaraj N. K. Enzyme-Free Synthesis of Natural Phospholipids in Water. Nat. Chem. 2020, 12 (11), 1029–1034. 10.1038/s41557-020-00559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M.; Eichberg J.; Oró J. Synthesis of Phosphatidylethanolamine under Possible Primitive Earth Conditions. J. Mol. Evol. 1987, 25 (1), 1–6. 10.1007/BF02100033. [DOI] [PubMed] [Google Scholar]
- Fayolle D.; Altamura E.; D’Onofrio A.; Madanamothoo W.; Fenet B.; Mavelli F.; Buchet R.; Stano P.; Fiore M.; Strazewski P. Crude Phosphorylation Mixtures Containing Racemic Lipid Amphiphiles Self-Assemble to Give Stable Primitive Compartments. Sci. Rep. 2017, 7, 18106. 10.1038/s41598-017-18053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S. M.; Waddell T. G. Prebiotic Synthesis of Vitamin B6-Type Compounds. Origins Life Evol. Biospheres 1999, 29 (3), 287–296. 10.1023/A:1006532518221. [DOI] [PubMed] [Google Scholar]
- Waddell T. G.; Eilders L. L.; Patel B. P.; Sims M. Prebiotic Methylation and the Evolution of Methyl Transfer Reactions in Living Cells. Origins Life Evol. Biospheres 2000, 30 (6), 539–548. 10.1023/A:1026523222285. [DOI] [PubMed] [Google Scholar]
- Ritson D. J.; Sutherland J. D. Thiophosphate Photochemistry Enables Prebiotic Access to Sugars and Terpenoid Precursors. Nat. Chem. 2023, 15 (10), 1470–1477. 10.1038/s41557-023-01251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. L.; Schlesinger G. Prebiotic Syntheses of Vitamin Coenzymes: I. Cysteamine and 2-Mercaptoethanesulfonic Acid (Coenzyme M). J. Mol. Evol. 1993, 36 (4), 302–307. 10.1007/BF00182177. [DOI] [PubMed] [Google Scholar]
- Kewitz H.; Pleul O. Synthesis of Choline from Ethanolamine in Rat Brain. Proc. Natl. Acad. Sci. U. S. A. 1976, 73 (7), 2181–2185. 10.1073/pnas.73.7.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer P. B.; Thompson S. J.; Xu J.; Russell D. A.; Green N. J.; Ritson D. J.; Sutherland J. D.; Queloz D. P. Timescales for Prebiotic Photochemistry Under Realistic Surface Ultraviolet Conditions. Astrobiology 2021, 21 (9), 1099–1120. 10.1089/ast.2020.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani A.; Russell D. A.; Javelle T.; Sutherland J. D. A Light-Releasable Potentially Prebiotic Nucleotide Activating Agent. J. Am. Chem. Soc. 2018, 140, 8657–8661. 10.1021/jacs.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B. H.; Percivalle C.; Ritson D. J.; Duffy C. D.; Sutherland J. D. Common Origins of RNA, Protein and Lipid Precursors in a Cyanosulfidic Protometabolism. Nat. Chem. 2015, 7, 301–307. 10.1038/nchem.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire O. R.; Smokers I. B. A.; Huck W. T. S. A Physicochemical Orthophosphate Cycle via a Kinetically Stable Thermodynamically Activated Intermediate Enables Mild Prebiotic Phosphorylations. Nat. Commun. 2021, 12 (1), 5517–5517. 10.1038/s41467-021-25555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcar B.; Pasek M.; Gull M.; Cafferty B. J.; Velasco F.; Hud N. V.; Menor-Salv C. Darwin’s Warm Little Pond: A One-Pot Reaction for Prebiotic Phosphorylation and the Mobilization of Phosphate from Minerals in a Urea-Based Solvent. Angew. Chem., Int. Ed. 2016, 55, 13249–13253. 10.1002/anie.201606239. [DOI] [PubMed] [Google Scholar]
- Epps D. E.; Nooner D. W.; Eichberg J.; Sherwood E.; Oró J. Cyanamide Mediated Synthesis under Plausible Primitive Earth Conditions. J. Mol. Evol. 1979, 14 (4), 235–241. 10.1007/BF01732490. [DOI] [PubMed] [Google Scholar]
- Kugel L.; Halmann M.. Hydrolysis of glycero-1,2-cyclic phosphate; ACS Publications, 10.1021/ja00992a030. [DOI] [Google Scholar]
- Baer E.; Kates M. Migration During Hydrolysis of Esters of Glycerophosphoric Acid: I. The Chemical Hydrolysis of Lα-Glycerylphosphorylcholine. J. Biol. Chem. 1948, 175 (1), 79–88. 10.1016/S0021-9258(18)57236-7. [DOI] [PubMed] [Google Scholar]
- Toner J. D.; Catling D. C. A Carbonate-Rich Lake Solution to the Phosphate Problem of the Origin of Life. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (2), 883–888. 10.1073/pnas.1916109117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damer B.; Deamer D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20 (4), 429–452. 10.1089/ast.2019.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E.; Feigenson G. W. Phosphorus NMR Analysis of Phospholipids in Detergents. J. Lipid Res. 1979, 20 (3), 408–412. 10.1016/S0022-2275(20)40624-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



