Abstract
OBJECTIVE
Mycobacterium avium subspecies (subsp.) paratuberculosis (MAP) is the causative agent of Johne's disease in ruminants and has been associated with Crohn's disease in humans. We sought to test growth rates and susceptibilities of various strains of MAP in two available growth media.
DESIGN
Paired comparison design.
METHODS
Using the BACTEC macrobroth radiometric growth system and Congo Red-staining agar media, we determined inherent differences in growth characteristics of three bovine and two human strains of MAP and compared susceptibility results obtained in each growth system.
RESULTS
Significant differences were observed in growth rate as well as mycobactin J dependence between strains and between a laboratory-adapted isolate of the same strain in the macrobroth system. Similarly, colonial morphology and Congo Red staining on agar media were observed. Two strains, one human and one bovine, demonstrated a 100% rough transparent colony with white coloration on Congo Red agar, while one bovine isolate exclusively grew as a smooth opaque colony with red coloration on Congo Red agar. The remaining strains exhibited mixtures of these two colonial morphotypes on agar media. Comparative susceptibility results between the BACTEC radiometric macrobroth method and the agar proportionality method showed good correlation for most antibiotics/inhibitors tested. However, erratic or poor growth in the macrobroth system prevented minimal inhibitory concentration determinations for two bovine strains by this method.
CONCLUSION
This study demonstrates the variability in the colonial morphology of MAP on Congo Red agar as well as the correlation of antibiotic susceptibility results between the BACTEC macro broth method and the agar proportionality method. This study also emphasizes the need for the development of improved, standardized culture and susceptibility test methods for MAP.
Keywords: Mycobacterium paratuberculosis, Antibiotic susceptibility, Paratuberculosis, Crohn disease, Microbiological techniques, Inflammatory bowel disease
INTRODUCTION
Johne's disease is a fatal disease of domestic and wild ruminants. Mycobacterium avium subspecies (subsp.) paratuberculosis (MAP) is the causative agent of this disease. Although the pathogenesis of MAP is poorly understood, it is believed that the organism infects the terminal ileum of most ruminants resulting in chronic inflammation, which leads to malabsorption of nutrients. As a result, animals lose weight and eventually die or must be destroyed.1–5
Transmission of this pathogen between animals is through the fecal-oral route, since the organism is excreted during later stages of the disease in high concentrations in the feces. Genetically, MAP is closely related to M. avium subsp. avium, sharing >99% genomic DNA homology and 100% identity with the M. avium subsp. avium 16S rRNA nucleotide sequence.6–8 Although recent work has demonstrated the presence of 21 unique genes in MAP, the IS900 insertion sequence and the hspX sequence, have been used most frequently to separate these two mycobacterial species. These sequences are unique to MAP.9–11 The prevalence of MAP has reached a staggering 34% in some cattle herds within the United States.12 This equates to annual economic losses of 1.5 billion dollars.13
The involvement of MAP in human disease has been controversial. M. avium subsp. paratuberculosis has been recovered from intestinal tissue of Crohn's disease patients.14 These findings have led to speculation regarding the role of this organism in human disease.14 The majority of evidence is based on detection of the organism in Crohn's disease patients by immunologic and molecular methods.15–18 For example, the IS900 insertion sequence has been detected by polymerase chain reaction (PCR) in intestinal biopsy specimens of Crohn's disease patients.14,16 However, many of these studies are conflicting and utilize a variety of laboratory techniques without culture confirmation.19–21 Similarly, empiric clinical trials with antimycobacterial therapy have not conclusively demonstrated evidence for MAP as a causal agent in this disease.16,22 However, the first culture-confirmed case of disseminated MAP infection in a patient with human immunodeficiency virus (HIV) has recently been reported.23
Currently there are no antibiotics approved for the treatment of Johne's disease. In instances where antibiotics have been used to prolong the life of a valuable animal, the disease remains nearly always fatal.24,25 The use of chemotherapy is further confounded by the lack of a standardized susceptibility method and the empiric use of antituberculous agents without in vitro susceptibility testing, which is hampered by the slow growth of the organism and its fastidious nutritional requirements.25,26
Two colonial morphotypes of MAP have been observed27 and have been reported to correlate with drug resistance. Rough variants were generally more drug resistant than smooth variants.27 This morphological difference has also been observed in M. avium subsp. avium, which are also referred to as transparent or opaque. Transparent variants of M. avium subsp. avium predominate in clinical samples,28 whereas opaque variants in general have been shown to be better adapted for growth in the laboratory and tend to predominate after multiple passages.29–33 In addition, M. avium subsp. avium has been shown to undergo red-white morphotypic switching when grown on agar media containing Congo Red.28,34 White opaque variants were found to be more resistant to multiple antibiotics than red opaque variants.
While attempting to evaluate the in vitro activity of an investigational antimycobacterial compound, we observed significant variation in growth characteristics between strains of MAP. In the following study, we examined strain variation in MAP relating to growth in broth and agar, colony morphology, and Congo Red staining characteristics. Additionally, the relationship between growth characteristics and in vitro susceptibility testing was examined using two susceptibility methods, the agar proportionality assay and the radiometric macrobroth dilution method.
MATERIALS AND METHODS
Mycobacterial strains and maintenance conditions
M. avium subsp. paratuberculosis strains ATCC 43544 (Ben-A)1 and M. avium subsp. avium ATCC 25291 were recently purchased prior to this study from the American Type Culture Collection (Rockville, Maryland). MAP ATCC 43015 (Linda), MAP (Kay), MAP (#47 ileum), MAP (#44 fecal), MAP ATCC 19698 (type strain) and MAP ATCC 43544 (Ben-B) were obtained from Jay Ellingson while residing in Ames, Iowa. MAP ATCC 43544 (Ben-A and Ben-B) and MAP ATCC 43015 (Linda) were of human origin. M. avium subsp. avium ATCC 25291 was of chicken origin, while the remaining strains included in the study were of bovine origin. The identity of all MAP strains was confirmed by 16S rRNA gene sequencing and the presence of IS900 and hspX by PCR.35 Similarly, the M. avium subsp. avium ATCC 25291 was identified on the basis of 16S rRNA gene sequencing and the presence of IS901 and IS1245 by PCR. Strains were maintained on Lowenstein-Jensen agar slants or Middlebrook 7H10 agar plates (Difco, Detroit, Michigan) supplemented with 1 µg/ml mycobactin J (Allied Monitor, Fayette, Missouri). MAP (#47 ileum), and MAP (#44 fecal) were primary isolates originating from ileum and fecal samples, respectively, of two infected animals. Passage history of the mycobacteria is unknown with the exception of MAP ATCC 43544 (Ben-A and Ben-B). Ben-A had a passage history of less than five subcultures following reconstitution from the lyophilized state, while Ben-B was maintained in culture for greater than 7 years and a minimum of 30 subcultures.
Characterization of growth in broth culture and mycobactin J dependence
Broth culture studies were performed in triplicate using commercially prepared BACTEC #12B media (12B) and the BACTEC radiometric growth system (Becton Dickinson, Sparks, Maryland). For mycobactin J studies, mycobactin J stock solutions (2 mg/ml) were brought up in 95% ethanol and diluted in sterile distilled water to a concentration of 40 µg/ml. Mycobactin J (0.1 ml) was then added to each BACTEC culture vial resulting in a final concentration of mycobactin J and ethanol of 1 µg/ml and 0.05%, respectively. Inocula for MAP strains were prepared from 10–20 day old cultures grown at 37°C in 10% CO2 on Middlebrook 7H10 agar supplemented with 1 µg/ml mycobactin J. Organisms were suspended in commercially prepared diluting fluid (Becton Dickinson), vortexed slightly with glass beads, and allowed to settle for 30 minutes. The supernatant was adjusted to a 1.0 McFarland standard (A600=1.0) and inoculated (0.1 ml) into each BACTEC vial, unsupplemented and supplemented with mycobactin J. Inoculated vials were read on the BACTEC 460 instrument daily at 24-hour intervals for up to 1 week or until the growth index (GI) readings reached 999 and growth curves were plotted. For quantitative comparison, growth rates were estimated by calculation of the mean change in growth index (ΔGI) over a 24-hour period for each of the test strains prior to the GI reaching its maximum reading, 999. The resulting ΔGI/day provided a quantitative comparison of growth between strains permitting evaluation of the effect of mycobactin J on growth.
An alternative method for standardizing inocula to examine differences in growth rates was also used. A separate set of 12B vials were inoculated with each strain as above and allowed to incubate until a GI of 999 was reached. At that time, 0.1 ml from these “seed vials” was used to inoculate a new 12B bottle and to make three 10-fold dilutions which were also added to individual 12B vials with and without mycobactin J. All vials were subsequently incubated at 37°C and the GI readings monitored daily at 24-hour intervals for several days.
Characterization of colony morphology and Congo Red staining on solid media
All isolates were prepared as described above for agar proportionality assays and plated in triplicate onto M7H10 agar containing 100 µg/ml Congo Red (Sigma, St. Louis, Missouri). Plates were subsequently incubated an average of 15 to 24 days and observed for colony morphology. Morphology was defined according to the following characteristics: rough or smooth, opaque or transparent, red or white.
Radiometric macrobroth susceptibility testing
Susceptibility testing and minimal inhibitory concentration (MIC) determination for each compound against M. avium subsp. avium and MAP strains were done taking into consideration differences in inherent growth rates between strains. Susceptibility testing of the M. avium subsp. avium type strain (25291) and MAP strains Ben-B and Kay was done in triplicate using a modification of the standard BACTEC radiometric protocol (Becton Dickinson) adopted by The National Jewish Center for Immunology and Respiratory Medicine (Denver, Colorado) for MIC determinations of the M. avium complex.36 Inocula for MAP strains ATCC 43544 (Ben-A), #47 ileum, and strain Linda were prepared using the BACTEC standard radiometric protocol. Briefly, suspensions were made from 2-week old cultures, vortexed slightly with glass beads, and allowed to settle for 30 minutes. The supernatant was adjusted to a 1.0 McFarland standard and inoculated (0.1 ml) into each BACTEC vial (a 0.5 McFarland standard was used for strain Linda). A 1:100 dilution was done as a GI control. To minimize exogenous carbon or other potential growth substrates in the media, carbon-free, commercially prepared diluting fluid (Becton Dickinson) was used.
For all MAP strains commercially prepared 12B media was supplemented with 1.0 µg/ml mycobactin J. Initial mycobactin J stock solutions (2 mg/ml) were brought up in 95% ethanol and diluted in sterile distilled water to a concentration of 40 µg/ml. Mycobactin J (0.1 ml) was then added to each BACTEC vial (final ethanol concentration per vial = 0.05%). Antibiotics and final concentrations tested included amikacin (Sigma), 8 µg/ml, 4 µg/ml, 2 µg/ml; ciprofloxacin (Bayer Corporation, Pittsburgh, Pennsylvania), 4 µg/ml, 2 µg/ml, and 1 µg/ml; gatifloxacin (Bristol Meyers Squibb, New York, New York), 4 µg/ml, 2 µg/ml, and 1 µg/ml; rifampin (Aventis Pharmaceuticals, Bridgewater, New Jersey), 8 µg/ml, 2 µg/ml, and 0.5 µg/ml; streptomycin (Sigma); and n-decanesulfonylacetamide (DSA) (Craig Townsend, Johns Hopkins University, Baltimore, Maryland), 25 µg/ml, 12.5 µg/ml, and 6.25 µg/ml. Antibiotic/inhibitor concentrations were validated using the known MICs of M. tuberculosis strain H37Rv (ATCC 27294) and M. avium subsp. avium type strain 25291 (ATCC) as positive controls.
Radiometric MICs were defined as the lowest concentration of drug required to inhibit >99% of the bacterial population for both the modified and standard BACTEC protocols. Interpretation of MICs in terms of the susceptible, moderately susceptible, and resistant categories were based on criteria established at the National Jewish Center for Immunology and Respiratory Medicine.36
Agar proportionality assays
In addition to the radiometric broth dilution method, isolates of MAP and the control M. avium subsp. avium (ATCC 25291) strain were evaluated in triplicate by the agar proportion method for susceptibility testing of slowly growing mycobacteria.29,37,38 Inocula were prepared using a culture suspension in M7H9 broth, which was vortexed with glass beads and allowed to settle for 30 minutes. The supernatant was adjusted to a 1.0 McFarland standard (A600=1.0) and a 10-fold dilution series made in additional media.
Subsequently, 0.1 ml of each appropriate dilution (usually 10-2 and 10-4) was plated onto M7H10 agar and M7H10 agar containing the antibiotics and concentrations described above. Additionally, MICs for isoniazid (0.4 µg/ml) (Sigma) and ethambutol (8 µg/ml) (Sigma) were determined by the agar proportionality method for all isolates. Plates were permitted to dry following inoculation, placed in individual CO2-permeable polyethylene bags, and incubated at 37°C in 10% CO2. All inocula were subcultured to trypticase soy agar plates with 5% sheep blood and M7H10 agar plates to check for purity. Once the control plate containing no antibiotic demonstrated 50 to 150 colonies, the number of colonies observed at each drug concentration was expressed as a percentage of the number of colonies on the control plate. Resistance was defined as ≥1% growth of the strain on antibiotic containing media compared to the control plate.
RESULTS
Growth characteristics
In this study, initial attempts to use the BACTEC radiometric growth system for susceptibility testing of MAP revealed inherent differences in growth rates between strains in vitro. Thus, in order to adjust inoculum sizes to reflect these differences, growth curves plotted over the course of several days were done in the presence and absence of 1.0 µg/ml mycobactin J. Figure 1 (A and B) shows the growth rates of five of the MAP strains used in this study. Growth curves were plotted over the course of a 6-day period using a modification of the standard BACTEC method. This method employed the use of seed vials (GI=999) to standardize inoculum size into new 12B bottles. As shown in figure 1, MAP strains Ben-B and Kay exhibited the fastest growth rates (≥792 ΔGI/day and 483 ΔGI/day, respectively) in the BACTEC system using this protocol as compared with the ATCC strain 43544 (Ben-A), which demonstrated an intermediate growth rate of 206 ΔGI/day. Although these two isolates, Ben-A and Ben-B, represent the same strain differing only in number of subcultures, they differed significantly in their growth rate. These data suggest the occurrence of laboratory growth adaptation of MAP with continued subculture. Strain Linda and #47 ileum demonstrated the slowest growth (111 ΔGI/day and 103 ΔGI/day, respectively). The growth rates of all strains were reduced in the absence of mycobactin J (figure 1B), although there was variance in the degree of mycobactin J dependence between strains. Quantitatively, MAP strains Ben-B, Kay and Ben-A continued to exhibit the highest growth rates (≥775 ΔGI/day, 393 ΔGI/day, and 141 ΔGI/day, respectively). The remaining strains, Linda (29 ΔGI/day) and #47 ileum (21 ΔGI/day), showed a marked reduction in growth rate in the absence of mycobactin J. The average change in growth rate between the presence and absence of mycobactin J in terms of ΔGI/day for strains Ben-B, Kay, ATCC 43544 (Ben-A), Linda, and #47 ileum were +17.5 ΔGI/day, +90.5 ΔGI/day, +65 ΔGI/day, +82.4 ΔGI/day, and +81.6 ΔGI/day, respectively. Strain differences in growth rate and mycobactin J dependence were less apparent on solid media (data not shown).
Figure 1.
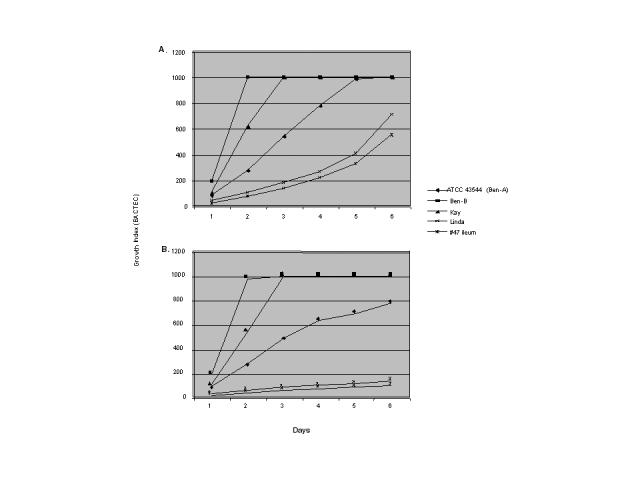
Growth of various strains of Mycobacterium avium subsp. paratuberculosis in the presence (A) and the absence (B) of mycobactin J (1.0 µg/ml). Standard BACTEC radiometric media was used supplemented with or without mycobactin J. Growth curves were plotted over several days at 24-hour intervals.
Colonial morphology and Congo Red staining characteristics
Table 1 shows the predominant colony morphology (percent) observed for each strain and the Congo Red staining characteristic. The majority of MAP strains tested, with the exception of MAP Kay and MAP Linda strains, exhibited the rough colonial morphology. This morphology appeared white in the Congo Red assay. Other strains exhibited a smooth, opaque morphology. In some instances, such as the ATCC strain of M. avium subsp. avium (25291), the Congo Red phenotype was 100% red, whereas MAP strain #47 ileum had an evenly distributed mix of rough, transparent and smooth, opaque colonies. As with growth rate, MAP strain Ben-B demonstrated divergence from MAP strain Ben-A in colony morphology and Congo Red staining, reflecting potential laboratory in vitro adaptation. All smooth opaque morphologies observed were red in color by Congo Red staining. No white, smooth, or opaque colonies were observed.
Table 1.
Colony morphology (%) of each strain and Congo Red staining characteristic.
| Mycobacterial species/strains | Rough transparent morphology Congo Red stain* = white | Smooth opaque morphology Congo Red stain* = red |
| M. avium 25291 | 100 | |
| MAP 43544 (Ben-A) | 100 | |
| MAP Ben-B | 80 | 20 |
| MAP Kay | 20 | 80 |
| MAP Linda | 100 | |
| MAP 47 ileum | 47 | 53 |
| MAP fecal | 100 |
*Note: All rough transparent morphologies stained white by Congo Red; all smooth opaque morphologies stained red by Congo Red.
MAP, M. paratuberculosis
In vitro susceptibility testing
As shown in table 2, the correlation between the agar proportionality and broth BACTEC susceptibility methods was fairly consistent and in most cases fell within a 1- to 2-fold dilution for all drugs/compounds tested in this study.
Table 2.
Comparative minimal inhibitory concentrations (µg/ml) for M. avium and M. paratuberculosis by the agar proportionality and BACTEC broth methods.
| Antibiotic/Inhibitor | ||||||||||||||
| AMI | CIP | GAT | RIF | STR | CLR | DSA | ||||||||
| Mycobacterial species/strain | Agar Broth | Agar Broth | Agar Broth | Agar Broth | Agar Broth | Agar Broth | Agar Broth | |||||||
| M. avium 25291 | 8.0 | 2.0 | ≤1.0 | 1.0 | ≤1.0 | 1.0 | ≤0.5 | 0.5 | ≤2.0 | 2.0 | 1.0 | ≤0.5 | 6.25 | 25.0 |
| MAP 43544 (Ben-A) | 8.0 | 4.0 | >4.0 | >4.0 | 2.0 | 4.0 | 8.0 | 8.0 | ≤2.0 | 2.0 | 2.0 | ≤0.5 | 12.5 | 12.5 |
| MAP Ben-B | 8.0 | >8.0 | >4.0 | >4.0 | 2.0 | >4.0 | 8.0 | >8.0 | ≤2.0 | 8.0 | 4.0 | ≤0.5 | 12.5 | 25.0 |
| MAP Kay | 8.0 | 8.0 | >4.0 | >4.0 | >4.0 | >4.0 | >8.0 | 8.0 | 4.0 | 8.0 | 4.0 | ≤0.5 | ≤25.0 | 25.0 |
| MAP Linda | 8.0 | 4.0 | >4.0 | >4.0 | 2.0 | 4.0 | >8.0 | 8.0 | 2.0 | 2.0 | 4.0 | ≤0.5 | 12.5 | 25.0 |
| MAP 47 ileum | 8.0 | ND | 4.0 | ND | 2.0 | ND | >8.0 | ND | ≤2.0 | ND | 4.0 | ND | ≤25.0 | 25.0 |
| MAP fecal | 8.0 | ND | >4.0 | ND | 2.0 | ND | >8.0 | ND | ≤2.0 | ND | 2.0 | ND | 17.0 | ND |
MAP, M. paratuberculosis; ND, not done due to erratic growth; AMI, amikacin; CIP, ciprofloxacin; GAT, gatifloxacin; RIF, rifampin; STR, streptomycin; CLR, clarithromycin; DSA, n-decanesulfonylacetamide.
Exceptions included the MICs for amikacin and DSA in M. avium strain 25291 in agar and broth (8.0 µg/ml and 2.0 µg/ml, and 6.25 µg/ml and 25.0 µg/ml, respectively) and streptomycin in strain Ben (≤2.0 µg/ml and 8.0 µg/ml in agar and broth, respectively). MAP (ATCC 43544) Ben-A and the laboratory adapted Ben-B were significantly divergent (MIC ≥4 fold difference) by the broth method for amikacin and streptomycin. All isolates used in this study were resistant to isoniazid (MIC >0.4 µg/ml) and ethambutol (MIC >8 µg/ml) (data not shown). Two strains, #47 ileum and #44 fecal, could not be evaluated in the BACTEC system due to poor or erratic growth, irrespective of inoculum size or mycobactin J concentration, which prevented an accurate assessment of actual MICs to the various drugs/inhibitors tested in this study.
An attempt was made to rank order the resistance of each isolate in comparison with growth rate and predominant colony morphology characteristics (table 3). Ranking was determined on the basis of the overall degree of resistance (MICs) for all drugs tested using established breakpoints: amikacin ≥8.0 µg/ml, ciprofloxacin ≥4.0 µg/ml, gatifloxacin ≥4.0 vg/ml, rifampin ≥8.0 µg/ml, and streptomycin ≥8.0 µg/ml.36 Strains Ben-B and Kay were resistant to amikacin, ciprofloxacin, gatifloxacin, rifampin, and streptomycin. Strain Ben-A (ATCC strain 43544) and strain Linda showed similar MICs and were resistant to ciprofloxacin, gatifloxacin, rifampin, and streptomycin. Interestingly, the ATCC type strain (25291) of M. avium subsp. avium was susceptible to all drugs.
Table 3.
Rank order comparison of growth rate of selected M. paratuberculosis strains with drug susceptibility and colony morphology.
| Growth rate + mycobactin J (1.0 mg/ml) | Drug resistance | Predominant colony morphology | Congo Red staining | |
| MAP Ben-B | >792 ΔGI/day | AMI, CIP, GAT, RIF, STR | Rough | White |
| MAP Kay | >483 ΔGI/day | AMI, CIP, GAT, RIF, STR | Smooth opaque | Red |
| MAP 43544 (Ben-A) | >206 ΔGI/day | CIP, GAT, RIF, STR | Rough | White |
| MAP Linda | >103 ΔGI/day | CIP, GAT, RIF, STR | Smooth opaque | Red |
| MAP 47 ileum | >111 ΔGI/day | Not done* | Smooth opaque/rough | White/red |
*Not done due to erratic growth rate of this strain in the BACTEC 12B system. Differences in growth rates based on BACTEC 12B system. Published guidelines36,51 used to establish breakpoints for susceptible, moderately susceptible and resistant strains for the following drugs: AMI, amikacin; CIP, ciprofloxacin; GAT, gatifloxacin; RIF, rifampin; STR, streptomycin; and CLR, clarithromycin.
MAP, M. paratuberculosis
DISCUSSION
The cultivation and growth of MAP continues to be a challenge, despite a relatively intense study. Unlike most other mycobacterial species, MAP does not produce detectable levels of mycobactin J, an important growth-requiring siderophore. The initial requirement for mycobactin J by MAP reportedly diminishes with repeated subculture in vitro. This was initially postulated to be potentially due to mycobactin J carryover from primary media.37–40 The MAP strains evaluated in this study demonstrated varying degrees of dependence on mycobactin J in a consistently reproducible manner. This suggests that inherent strain differences exist for mycobactin J dependence for growth rather than mycobactin J carryover from media during transfer. Early work by Matthews et al.41 previously demonstrated varying degrees of mycobactin J dependence among strains of M. avium subsp. avium and MAP. However, despite improved growth by all strains in liquid media containing an adequate concentration of mycobactin J, growth rate variation between strains remained significant. These growth rate differences could represent genetic strain variation, the variable expression of an alternative iron-acquisition system, such as exochelins38 or potentially the requirement for another undescribed growth factor of MAP. Accumulating data suggests additional nutritional requirements may be required for recovery and optimum growth of MAP.42 Cultivation of MAP has been suggested to be both host-specific and localespecific.42 Whittington et al.,42 in evaluating primary culture media for the recovery of MAP from sheep, demonstrated that the reliable primary recovery of MAP required both mycobactin J and egg yolk supplementation of BACTEC 12B radiometric broth medium or Middlebrook 7H10 agar. These studies point out the variability of growth requirements among strains of MAP and the need for development of a media that will reproducibly grow all strains.
To date, few reports adequately describe the growth rate, antibiotic susceptibility, and colonial morphology of MAP.27 However, previous investigators have demonstrated three primary morphologies for the closely related M. avium subsp. avium. They are smooth opaque, smooth transparent, and rough.34 Smooth variants have a dome-shaped appearance, whereas rough variants are characterized by flat, transparent colonies with irregular edges. M. avium subsp. avium undergoes a reversible, phenotypic switch between the smooth and transparent morphologies. Transparent variants predominate in fresh clinical isolates, whereas opaque variants appear with repeated passage in vitro.34 Conversion to the rough phenotype is irreversible and involves a chromosomal deletion.34 This change has been observed in M. avium subsp. avium following passage of the isolate through animals43 in which these variants grew at a faster rate in vivo and were more virulent resulting in 60% to 70% mortality versus 10% for their smooth counterparts. The authors claimed that these rough variants apparently had altered cell-wall components and permeability characteristics because they failed to reduce alamar blue or neutral red.
In the present study, only two morphologies, rough and smooth opaque, were observed for the MAP strains evaluated. No smooth transparent variants were observed. This may be due to the fact that these strains had been maintained for several generations in vitro, which would favor conversion to the opaque phenotype. Rough variants would presumably remain the same since the change is irreversible.
Additional differences in strain morphologies were demonstrated by growth on Congo Red containing agar plates. Congo Red is a planar, hydrophobic compound that binds to lipids and lipoproteins,34 and has been used by a number of investigators to examine staining characteristics of various strains of M. avium subsp. avium. Differences in Congo Red staining between strains has been interpreted to indicate differences in cell wall components, which in turn may lead to changes in virulence and or drug susceptibility. Previous investigators have shown that M. avium subsp. avium rough/white clones were significantly more resistant to various antimicrobials than their smooth opaque/white counterparts. Thus, drug susceptibility appeared to coincide with colony morphology and Congo Red staining characteristics. In the present study, the laboratory-adapted MAP strain Ben-B, an 80% rough/white variant, had consistently higher MICs to most of the drugs/compounds tested as compared with the other strains. However, MAP strain ATCC 43544 (Ben-A), also a rough/white variant, was much less resistant than either the laboratory-adapted strain Ben-B or Kay, a predominantly smooth/red strain (80%). Thus, unlike M. avium subsp. avium, drug susceptibility in this study did not correlate with either colony morphology or Congo Red staining characteristics.
In the United States, the generally accepted method for susceptibility testing of M. avium subsp. avium is a radiometric broth macrodilution method (BACTEC), which is regarded to be more reliable and rapid than an agar-based method.31,44,45 A controversial aspect of this method involves careful preparation of the inoculum.46,47 An optimal inoculum size for broth macrodilution is 104 to 105 CFU/ml.47 Additional difficulties in susceptibility testing of M. avium subsp. avium strains include variations in growth rate associated with particular colony morphologies. Although such reports are rare, Heifets et al.36 observed that some rough opaque strains of M. avium subsp. avium exhibit slower growth than other colony types and initiated the use of “seed vials” to standardize inoculum sizes. The MAP strains evaluated in this study exhibited reproducible differences in growth rates relative to each other, which were independent of mycobactin J concentration. These differences did not segregate with any particular colony morphology. Susceptibility testing of these strains in the BACTEC system required careful adjustment of inoculum size for individual strains, which compensated for differences in growth rates. Thus, despite fairly good correlation in MICs between the BACTEC radiometric and agar proportionality assays observed in this study, the BACTEC macrobroth method had drawbacks in our hands in testing different strains of MAP.
The majority of these problems were related to significant strain variation in growth rate, which confounded appropriate inoculum standardization. This resulted in frequently repeating susceptibility tests to meet established guidelines for this method.36 Additionally, two of the seven strains evaluated in this study demonstrated negligible or erratic growth in the BACTEC system, despite the inclusion of mycobactin J, resulting in an inability to determine in vitro susceptibility using this method. In our hands, agar proportion was a viable alternative for determination of antibiotic susceptibility with poorly growing strains. On the basis of our difficulties, we would recommend the standard agar proportionality method for antibiotic susceptibility testing of MAP.
The in vitro activity of the antibiotics evaluated in this study correlate in large part with results reported by other investigators for MAP.36,48 In vivo and in vitro intrinsic resistance has been reported in M. avium subsp. avium and MAP isolates to isoniazid and ethambutol, antibiotics active against most strains of M. tuberculosis and M. bovis. In this study, all strains tested were resistant to isoniazid (MIC >0.4 µg/ml) and ethambutol (MIC >8.0 µg/ml) (data not shown). Among the compounds tested, clarithromycin, streptomycin, amikacin, and DSA demonstrated the most consistent in vitro activity. The combination of clarithromycin and amikacin, in addition to rifabutin and ethambutol are currently used for the treatment of human infections by the M. avium complex. The in vitro activity of clarithromycin and amikacin against MAP has been demonstrated previously in broth-based systems49 and a luciferase-based assay.26
Recently, Rastogi et al.48 have compared the related ketolides, telithromycin and HMR 3004, to clarithromycin against a variety of mycobacteria, including MAP, and found clarithromycin to be superior in an in vitro radiometric broth assay. The novel compound DSA demonstrated consistent activity against all strains tested and was more active than the closely related n-octanesulfonylacetamide.50
Our study illustrates the significant strain variability in MAP. This variability is reflected in growth rate, nutritional requirements, colonial morphology, and staining characteristics, as well as within strain changes during subculture in vitro. Similarly, the susceptibility methods and results obtained in this study point out the need for a uniform, standardized susceptibility test method for MAP. An acceptable method will require the development of a nutritionally adequate media, which will support the timely growth of all strains of MAP. In the absence of a standardized susceptibility method, the ability to reproducibly evaluate the utility of new antibiotics in vitro is tenuous and makes in vitro-in vivo correlations improbable.
Acknowledgments
We appreciate the invaluable advice and discussions with Dr. Craig Townsend. We also acknowledge the technical support of Annie Hedgepeth. We thank Marshfield Clinic Research Foundation for its support through the assistance of Sally Sloan, Dr. Roy P. Radcliff, Alice Stargardt and Graig Eldred.
Contributor Information
Nicole M. Parrish, Department of Pathology, School of Medicine, Johns Hopkins University, Baltimore, Maryland.
Chiew G. Ko, Department of Pathology, School of Medicine, Johns Hopkins University, Baltimore, Maryland.
James D. Dick, Department of Pathology, School of Medicine, Johns Hopkins University, Baltimore, Maryland.
Paul B. Jones, Department of Chemistry, Wake Forest University, Winston-Salem, North Carolina.
Jay L.E. Ellingson, Marshfield Clinic Laboratories - Food Safety Services, Marshfield, Wisconsin.
References
- 1.Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 2.Doyle TM. Johne's Disease. Vet Rec. 1956;68:869–886. [Google Scholar]
- 3.Lepper AW. The aetiology and pathogenesis of Johne's disease. In: Milner AR, Wood PR, editors. Johne's disease. Current trends in research, diagnosis and management. Melbourne, Australia: CSIRO; 1989. pp. 74–86. [Google Scholar]
- 4.Reimann HP, Abbas B. Diagnosis and control of bovine paratuberculosis (Johne's disease) Adv Vet Sci Comp Med. 1983;27:481–506. [PubMed] [Google Scholar]
- 5.Thoen CO, Muscoplat CC. Recent developments in diagnosis of paratuberculosis (Johne's disease) J Am Vet Med Assoc. 1979;174:838–840. [PubMed] [Google Scholar]
- 6.Shapiro HS, Splitter GA, Welch RA. Deoxyribonucleic acid relatedness of Mycobacterium paratuberculosis to other members of the family Mycobacteriaceae. Int J Syst Bacteriol. 1988;38:143–146. [Google Scholar]
- 7.Saxegaard F, Baess I, Jantzen E. Characterization of clinical isolates of Mycobacterium paratuberculosis by DNA-DNA hybridization and cellular fatty acid analysis. APMIS. 1988;96:497–502. [PubMed] [Google Scholar]
- 8.Whipple DL, Le Febvre RB, Andrews RE, Jr, Thiermann AB. Isolation and analysis of restriction endonuclease digestive patterns of chromosomal DNA from Mycobacterium paratuberculosis and other Mycobacterium species. J Clin Microbiol. 1987;25:1511–1515. doi: 10.1128/jcm.25.8.1511-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins DM, Stephens DM, de Lisle GW. Comparison of polymerase chain reaction tests and faecal culture for detecting Mycobacterium paratuberculosis in bovine faeces. Vet Microbiol. 1993;36:289–299. doi: 10.1016/0378-1135(93)90095-o. [DOI] [PubMed] [Google Scholar]
- 10.Moss MT, Green EP, Tizard ML, Malik ZP, Hermon-Taylor J. Specific detection of Mycobacterium paratuberculosis by DNA hybridisation with a fragment of the insertion element IS900. Gut. 1991;32:395–398. doi: 10.1136/gut.32.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thoresen OF, Olsaker I. Distribution and hybridization patterns of the insertion element IS900 in clinical isolates of Mycobacterium paratuberculosis. Vet Microbiol. 1994;40:293–303. doi: 10.1016/0378-1135(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Collins MT, Sockett DC, Goodger WJ, Conrad TA, Thomas CB, Carr DJ. Herd prevalence and geographic distribution of, and risk factors for, bovine paratuberculosis in Wisconsin. J Am Vet Med Assoc. 1994;204:636–641. [PubMed] [Google Scholar]
- 13.Jones RL. Review of the economic impact of Johne's disease in the United States. In: Milner AR, Wood PR, editors. Johne's disease. Current trends in research, diagnosis and management. Melbourne, Australia: CSIRO; 1989. pp. 46–50. [Google Scholar]
- 14.Mishina D, Katsel P, Brown ST, Gilberts EC, Greenstein RJ. On the etiology of Crohn disease. Proc Natl Acad Sci USA. 1996;93:9816–9820. doi: 10.1073/pnas.93.18.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarkston WK, Presti ME, Petersen PF, Zachary PE, Jr, Fan WX, Leonardi CL, Vernava AM, 3rd, Longo WE, Kreeger JM. Role of Mycobacterium paratuberculosis in Crohn's disease: a prospective, controlled study using polymerase chain reaction. Dis Colon Rectum. 1998;41:195–199. doi: 10.1007/BF02238248. [DOI] [PubMed] [Google Scholar]
- 16.Cocito C, Gilot P, Coene M, De Kesel M, Poupart P, Vannuffel P. Paratuberculosis. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidler HM, Thurrell W, Johnson NM, Rook GA, McFadden JJ. Specific detection of Mycobacterium paratuberculosis DNA associated with granulomatous tissue in Crohn's disease. Gut. 1994;35:506–510. doi: 10.1136/gut.35.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanderson JD, Moss MT, Tizard ML, Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut. 1992;33:890–896. doi: 10.1136/gut.33.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cellier C, De Beenhouwer H, Berger A, Penna C, Carbonnel F, Parc R, Cugnenc PH, Le Quintrec Y, Gendre JP, Barbier JP, Portaels F. Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum DNA cannot be detected by PCR in Crohn's disease tissue. Gastroenterol Clin Biol. 1998;22:675–678. [PubMed] [Google Scholar]
- 20.Chiba M, Fukushima T, Horie Y, Iizuka M, Masamune O. No Mycobacterium paratuberculosis detected in intestinal tissue, including Peyer's patches and lymph follicles, of Crohn's disease. J Gastroenterol. 1998;33:482–487. doi: 10.1007/s005350050119. [DOI] [PubMed] [Google Scholar]
- 21.Kanazawa K, Haga Y, Funakoshi O, Nakajima H, Munakata A, Yoshida Y. Absence of Mycobacterium paratuberculosis DNA in intestinal tissues from Crohn's disease by nested polymerase chain reaction. J Gastroenterol. 1999;34:200–206. doi: 10.1007/s005350050244. [DOI] [PubMed] [Google Scholar]
- 22.Hermon-Taylor J, Bull TJ, Sheridan JM, Cheng J, Stellakis ML, Sumar N. Causation of Crohn's disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol. 2000;14:521–539. doi: 10.1155/2000/798305. [DOI] [PubMed] [Google Scholar]
- 23.Richter E, Wessling J, Lugering N, Domschke W, Rusch-Gerdes S. Mycobacterium avium subsp. paratuberculosis infection in a patient with HIV, Germany. Emerg Infect Dis. 2002;8:729–731. doi: 10.3201/eid0807.010388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris NB, Feng Z, Liu X, Cirillo SL, Cirillo JD, Barletta RG. Development of a transposon mutagenesis system for Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol Lett. 1999;175:21–26. doi: 10.1111/j.1574-6968.1999.tb13597.x. [DOI] [PubMed] [Google Scholar]
- 25.St Jean G. Treatment of clinical paratuberculosis in cattle. Vet Clin North Am Food Anim Pract. 1996;12:417–430. doi: 10.1016/s0749-0720(15)30414-x. [DOI] [PubMed] [Google Scholar]
- 26.Williams SL, Harris NB, Barletta RG. Development of a firefly luciferase-based assay for determining antimicrobial susceptibility of Mycobacterium avium subsp. paratuberculosis. J Clin Microbiol. 1999;37:304–309. doi: 10.1128/jcm.37.2.304-309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Boxtel RM, Lambrecht RS, Collins MT. Effects of colonial morphology and tween 80 on antimicrobial susceptibility of Mycobacterium paratuberculosis. Antimicrob Agents Chemother. 1990;34:2300–2303. doi: 10.1128/aac.34.12.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cangelosi GA, Palermo CO, Bermudez LE. Phenotypic consequences of red-white colony type variation in Mycobacterium avium. Microbiology. 2001;147((Pt 3)):527–533. doi: 10.1099/00221287-147-3-527. [DOI] [PubMed] [Google Scholar]
- 29.Belisle JT, Brennan PJ. Molecular basis of colony morphology in Mycobacterium avium. Res Microbiol. 1994;145:237–242. doi: 10.1016/0923-2508(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 30.Belisle JT, McNeil MR, Chatterjee D, Inamine JM, Brennan PJ. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J Biol Chem. 1993;268:10510–10516. [PubMed] [Google Scholar]
- 31.Inderlied CB, Nash KA. Antimycobacterial agents: in vitro susceptibility testing, spectra of activity, mechanisms of action and resistance, and assays for activity in biologic fluids. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, MD: Williams & Wilkins; 1996. pp. 127–175. [Google Scholar]
- 32.Inderlied CB, Kemper CA, Bermudez LE. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prinzis S, Rivoire B, Brennan PJ. Search for the molecular basis of morphological variation in Mycobacterium avium. Infect Immun. 1994;62:1946–1951. doi: 10.1128/iai.62.5.1946-1951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cangelosi GA, Palermo CO, Laurent JP, Hamlin AM, Brabant WH. Colony morphotypes on Congo Red agar segregate along species and drug susceptibility lines in the Mycobacterium avium-intracellulare complex. Microbiology. 1999;145((Pt 6)):1317–1324. doi: 10.1099/13500872-145-6-1317. [DOI] [PubMed] [Google Scholar]
- 35.Ellingson JL, Stabel JR, Bishai WR, Frothingham R, Miller JM. Evaluation of the accuracy and reproducibility of a practical PCR panel assay for rapid detection and differentiation of Mycobacterium avium species. Mol Cell Probes. 2000;14:153–161. doi: 10.1006/mcpr.2000.0299. [DOI] [PubMed] [Google Scholar]
- 36.Heifets H, Lindholm-Levy P, Libonati J, et al. Radiometric broth macrodilution method for determination of minimal inhibitory concentrations (MICs) with Mycobacterium avium complex isolates. Denver, CO: National Jewish Center for Immunology and Respiratory Medicine; 1993. [Google Scholar]
- 37.Aduriz JJ, Juste RA, Cortabarria N. Lack of mycobactin dependence of mycobacteria isolated on Middlebrook 7H11 from clinical cases of ovine paratuberculosis. Vet Microbiol. 1995;45:211–217. doi: 10.1016/0378-1135(95)00037-b. [DOI] [PubMed] [Google Scholar]
- 38.Barclay R, Ratledge C. Iron-binding compounds of Mycobacterium avium, M. intracellulare, M. scrofulaceum, and mycobactin-dependent M. paratuberculosis and M. avium. J Bacteriol. 1983;153:1138–1146. doi: 10.1128/jb.153.3.1138-1146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambrecht RS, ollins MT. Mycobacterium paratuberculosis. Factors that influence mycobactin dependence. Diagn Microbiol Infect Dis. 1992;15:239–246. doi: 10.1016/0732-8893(92)90119-e. [DOI] [PubMed] [Google Scholar]
- 40.Snow GA. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970;34:99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews PR, McDiarmid A, Collins P, Brown A. The dependence of some strains of Mycobacterium avium on mycobactin for initial and subsequent growth. J Med Microbiol. 1978;11:53–57. doi: 10.1099/00222615-11-1-53. [DOI] [PubMed] [Google Scholar]
- 42.Whittington RJ, Marsh I, McAllister S, Turner MJ, Marshall DJ, Fraser CA. Evaluation of modified BACTEC 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. J Clin Microbiol. 1999;37:1077–1083. doi: 10.1128/jcm.37.4.1077-1083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kansal RG, Gomez-Flores R, Mehta RT. Change in colony morphology influences the virulence as well as the biochemical properties of the Mycobacterium avium complex. Microb Pathog. 1998;25:203–214. doi: 10.1006/mpat.1998.0227. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqi SH, Heifets LB, Cynamon MH, Hooper NM, Laszlo A, Libonati JP, Lindholm-Levy PJ, Pearson N. Rapid broth macrodilution method for determination of MICs for Mycobacterium avium isolates. J Clin Microbiol. 1993;31:2332–2338. doi: 10.1128/jcm.31.9.2332-2338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yajko DM, Nassos PS, Hadley WK. Broth microdilution testing of susceptibilities to 30 antimicrobial agents of Mycobacterium avium strains from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1987;31:1579–1584. doi: 10.1128/aac.31.10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hacek D. Modified proportion agar dilution test for slowly growing mycobacteria. In: Isenberg HD, editor. Clinical microbiology procedures handbook. Vol. 1. Washington, DC: American Society for Microbiology; 1992. pp. 5.13.1–5.13.15. [Google Scholar]
- 47.Inderlied CB, Salfinger M. Antimycobacterial agents and susceptibility tests. In: Murray P, editor. Manual of clinical microbiology. ASM Press, Washington, DC: ASM Press; 1999. pp. 1601–1623. [Google Scholar]
- 48.Rastogi N, Goh KS, Berchel M, Bryskier A. In vitro activities of the ketolides telithromycin (HMR 3647) and HMR 3004 compared to those of clarithromycin against slowly growing mycobacteria at pHs 6.8 and 7.4. Antimicrob Agents Chemother. 2000;44:2848–2852. doi: 10.1128/aac.44.10.2848-2852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rastogi N, Goh KS, Labrousse V. Activity of clarithromycin compared with those of other drugs against Mycobacterium paratuberculosis and further enhancement of its extracellular and intracellular activities by ethambutol. Antimicrob Agents Chemother. 1992;36:2843–2846. doi: 10.1128/aac.36.12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrish NM, Houston T, Jones PB, Townsend C, Dick JD. In vitro activity of a novel antimycobacterial compound, N-octanesulfonylacetamide, and its effects on lipid and mycolic acid synthesis. Antimicrob Agents Chemother. 2001;45:1143–1150. doi: 10.1128/AAC.45.4.1143-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Committee for Clinical Laboratory Standards, author. Proposed standard M24-T. Villanova, PA: National Committee for Clinical Laboratory Standards, Villanova; 1995. Antimycobacterial Susceptibility Testing. [Google Scholar]


