Abstract
Aims/Introduction
To evaluate the efficacy of sensor‐augmented pump (SAP) for improving obstetric and neonatal outcomes among pregnant women with type 1 diabetes mellitus by comparing it with continuous subcutaneous insulin infusion plus self‐monitoring of blood glucose (continuous subcutaneous insulin infusion [CSII]/SMBG).
Materials and Methods
This retrospective cohort study included 40 cases of pregnancy complicated by type 1 diabetes mellitus treated with SAP (SAP group), and 29 cases of pregnancy complicated by type 1 diabetes mellitus treated with CSII/SMBG (CSII/SMBG group). The obstetric and neonatal outcomes were compared between the two groups.
Results
The median of the glycoalbumin levels in the first (18.8% vs 20.9%; P < 0.05) and second (15.4% vs 18.0%; P < 0.05) trimesters, the hemoglobin A1c levels in the peripartum period (6.1% vs 6.5%; P < 0.05) and the standard deviation score of birthweights (0.36 vs 1.52; P < 0.05) were significantly lower in the SAP group than in the CSII/SMBG group. The incidence rate of large for gestational age newborns was significantly lower in the SAP group than in the CSII/SMBG group (27.5% vs 65.5%; P < 0.05). No significant differences in the incidence rates of hypertensive disorders of pregnancy, small for gestational age, respiratory distress syndrome, neonatal hypoglycemia, hypervolemia and hyperbilirubinemia were observed between the groups.
Conclusion
The present study showed that SAP therapy is more effective in preventing large for gestational age newborns in pregnant women with type 1 diabetes mellitus than CSII/SMBG.
Keywords: Pregnant women, Sensor‐augmented pump, Type 1 diabetes mellitus
INTRODUCTION
Pregnant women with type 1 diabetes mellitus are at risk of adverse obstetric and neonatal outcomes, including hypertensive disorders of pregnancy (HDP), pre‐eclampsia, cesarean delivery, preterm delivery, large for gestational age (LGA), small for gestational age (SGA) and respiratory distress syndrome (RDS) 1 . Maternal hyperglycemia that occurs during the second and third trimesters is associated with the incidence of pre‐eclampsia, preterm delivery, LGA, and neonatal hypoglycemia and hyperbilirubinemia 2 , 3 . Therefore, optimal glycemic control during pregnancy is necessary for pregnant women with type 1 diabetes mellitus to avoid such adverse outcomes.
The currently available treatments for type 1 diabetes mellitus are insulin therapy (two modes: multiple daily injections [MDI] and continuous subcutaneous insulin infusion [CSII]) and glucose monitoring (two modes: self‐monitoring of blood glucose [SMBG] and continuous glucose monitoring [CGM]). In the Continuous Glucose Monitoring in Pregnant Women with Type 1 Diabetes (CONCEPTT) study, which was a randomized controlled trial of MDI versus CSII in pregnant women with type 1 diabetes mellitus, MDI users had better glycemic control, and lower incidence of HDP and neonatal hypoglycemia than CSII users, regardless of whether CGM or SMBG was used 4 . Whereas, in comparisons of CGM versus SMBG, the incident rates of LGA and neonatal hypoglycemia were lower in CGM users than in SMBG users, regardless of whether MDI or CSII was used 5 . However, there are no recommendations for a combination of insulin therapy and glucose monitoring in pregnant women with type 1 diabetes mellitus.
Sensor‐augmented pump (SAP) therapy involves the integration of an insulin pump with real‐time CGM. It was found to be associated with better glycemic control than MDI in non‐pregnant women with type 1 diabetes mellitus 6 , 7 . In Japan, SAPs for patients with type 1 diabetes mellitus have been covered by insurance since February 2015, and the number of SAP users has been increasing along with the increase in the number of CSII users.
To the best of our knowledge, there are no studies comparing the obstetric and neonatal outcomes of pregnant women with type 1 diabetes mellitus between SAP users and CSII plus SMBG (CSII/SMBG) users. The present retrospective cohort study aimed to evaluate the efficacy of SAP therapy for improving the obstetric and neonatal outcomes of pregnant women with type 1 diabetes mellitus by comparing it with CSII/SMBG.
MATERIALS AND METHODS
The present retrospective cohort study was carried out according to the principles of the Declaration of Helsinki and approved by the research ethics committee of Kobe University Graduate School of Medicine (reference number: B220081).
This study enrolled pregnant women with type 1 diabetes mellitus who used SAP (MiniMed 620G or MiniMed 640G; Medtronic, Hertfordshire, UK) or CSII (Paradigm 712 or Paradigm 722; Medtronic) plus SMBG and who delivered after 22 gestational weeks (GW) between April 2008 and August 2022 at Kobe University Hospital, Kobe, Japan. The patients were managed by diabetologists and obstetricians at the university hospital. They were re‐educated about carbohydrate counting during early pregnancy, and were recommended the six‐divided diet with tripartition energy (total energy intake: body mass index <25 kg/m2, standard bodyweight × 30 + 250 [+50 in the first trimester] kcal; body mass index ≥25 kg/m2, standard bodyweight × 30 kcal according to the guidelines for the management of diabetes). Insulin titration was determined according to the attending physician's instruction or the patients' own judgment. The target blood glucose (BG) levels were <100 mg/dL preprandially, and <120 mg/dL postprandially according to the guidelines of Japan Society of Obstetrics and Gynecology for diabetes management in pregnancy throughout the study period 8 , 9 , 10 .
Patients who changed the methods of insulin therapy or glucose monitoring after 22 GW were excluded from the study analyses. Patients who used intermittently scanned CGM (isCGM), which became available in Japan in September 2017, were also excluded, because the number of patients who used isCGM in this study period (n = 4) was too small to compare.
The patients' and newborns' characteristics and clinical findings, including the methods of insulin therapy and glucose monitoring, dose of insulin, age at delivery, gravidity, parity, body mass index before pregnancy, hemoglobin A1c (HbA1c) and glycoalbumin (GA) in the first and second trimesters and the peripartum period, incidence of maternal severe hypoglycemia, GW at delivery, incidence of HDP, delivery mode, birthweight, incidence of RDS, neonatal serum BG levels and hematocrit at birth, use of phototherapy for neonatal hyperbilirubinemia, and morphological anomalies of newborns were collected from the medical records. Furthermore, the total basal insulin dose (TBD) and total daily insulin dose (TDD) were collected from the medical records. The time in range (TIR), time above range (TAR) and time below range (TBR) were analyzed from CGM data of patients who used SAP by using the CareLink software (Medtronic).
The HbA1c values of the Japan Diabetes Society (JDS) were converted to the National Glycohemoglobin Standardization Program (NGSP) values of HbA1c as the formula: NGSP (%) = 1.02 × JDS (%) ± 0.25% 11 . Maternal severe hypoglycemia was defined as an event requiring assistance of another person to treat due to low BG levels. HDP was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg during pregnancy 12 . The standard deviation score and percentiles of birthweights were calculated using normative birthweights for Japanese newborns 13 . LGA and SGA were defined as birthweights of more than the 90th percentile and less than the 10th percentile, respectively. RDS was diagnosed based on the presence of clinical symptoms and chest X‐ray findings. Hypoglycemia was defined as neonatal serum glucose levels at birth of <35 mg/dL. Polycythemia was defined as neonatal hematocrit of >65%. Hyperbilirubinemia was defined as the need for phototherapy according to the 1992 Kobe University treatment criterion 14 , 15 . The target range of sensor glucose levels for calculating TIR, TAR and TBR was defined as 63–140 mg/dL 16 .
Statistical analysis
Clinical characteristics were compared between the SAP and CSII/SMBG groups. The differences were analyzed using the Student's t‐test or the Mann–Whitney U‐test, and Fisher's exact test or the χ2‐test. P < 0.05 was considered to show statistical significance. The Bonferroni correction was used for adjustment in multiple comparisons. All statistical analyses were carried out using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A flow diagram of the study population is presented in Figure 1. During the study period, 94 pregnancies among 75 pregnant women with type 1 diabetes mellitus were managed at Kobe University Hospital. All patients diagnosed with type 1 diabetes mellitus received insulin treatment before pregnancy. Of the 15 patients who had two pregnancies, six used SAP in both pregnancies; five used CSII/SMBG in both pregnancies; one used MDI in both pregnancies; one used CSII/SMBG and SAP in the first and second pregnancy, respectively; one used CSII/SMBG and CSII plus isCGM in the first and second pregnancy, respectively; and one used SAP and MDI plus isCGM in the first and second pregnancy, respectively. Of the two patients who had three pregnancies, one used MDI in all pregnancies, and the other one used CSII/SMBG in the first pregnancy and SAP in the second and third pregnancies. In the present study, 25 pregnancies among 22 patients were excluded: 20 pregnancies among 17 patients who used MDI, two pregnancies among two patients who used CSII plus isCGM, one pregnancy of a patient who switched from MDI to CSII at 28 GW, and two pregnancies among two patients who switched from CSII to SAP at 23 GW and 27 GW, respectively.
Figure 1.
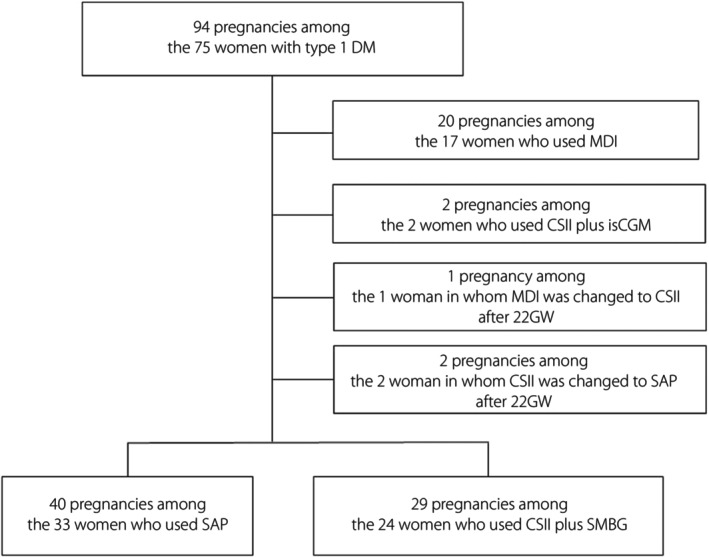
A flow diagram of the study population. CSII, continuous subcutaneous insulin infusion; DM, diabetes mellitus; GW, gestational weeks; isCGM, intermittently scanned continuous glucose monitoring; MDI, multiple daily injections; SAP, sensor‐augmented insulin pump; SMBG, self‐monitoring of blood glucose.
The final analysis included 40 pregnancies among 33 patients with type 1 diabetes mellitus in the SAP group and 29 pregnancies among 24 patients with type 1 diabetes mellitus in the CSII/SMBG group.
The clinical characteristics of pregnant women with type 1 diabetes mellitus and their newborns are presented in Table 1. The GA levels in the first trimester (P < 0.05), the second trimester (P < 0.01) and the peripartum period (P < 0.05), HbA1c levels in the second trimester (P < 0.05) and the peripartum period (P < 0.05), and standard deviation score of the birthweights (P < 0.05) were significantly lower in the SAP group than in the CSII/SMBG group. Regarding the insulin doses and the TBD in the second trimester (P < 0.05) and peripartum period (P < 0.05), the TBD/TDD ratios in the first trimester (P < 0.01), second trimester (P < 0.01) and peripartum period (P < 0.01) were significantly lower in the SAP group than in the CSII/SMBG group. Two newborns had morphological anomalies in the CSII/SMBG group; one had a cleft lip and another had a ventricular septal defect. No significant difference was observed in the TDDs between the two groups. Furthermore, in the SAP group, there was no significant difference between the TDDs in the first and second trimesters; however, the TDD in the peripartum period was significantly higher than both TDDs in the first and second trimesters (Figure 2a). In contrast, there was no significant difference in the TDDs throughout the pregnancy in the CSII/SMBG group (Figure 2b).
Table 1.
Clinical characteristics of pregnant women with type 1 diabetes and their newborns
| Clinical characteristics | SAP group n = 40 | CSII/SMBG group n = 29 | P‐value |
|---|---|---|---|
| Age at delivery (years) | 32.4 ± 5.8 | 33.6 ± 4.9 | 0.4 |
| Gravidity | 1.9 ± 1.1 | 2.2 ± 1.0 | 0.1 |
| Parity | 0.4 ± 0.7 | 0.6 ± 0.7 | 0.3 |
| BMI before pregnancy (kg/m2) | 22.1 ± 3.1 | 22.9 ± 3.0 | 0.2 |
| Weight gain during pregnancy (kg) | 11.6 ± 4.6 | 11.5 ± 4.3 | 0.9 |
| Maternal severe hypoglycemia | 1 (2.5%) | 1 (3.4%) | 1.0 |
| Laboratory findings and insulin doses | |||
| In the first trimester | |||
| HbA1c (%) | 7.1 ± 1.3 | 7.2 ± 1.1 | 0.4 |
| Glycoalbumin (%) | 19.4 ± 3.5 | 21.4 ± 3.8 † | <0.05 |
| TIR (%) | 65.9 ± 15.5 ‡ | N/D | – |
| TAR (%) | 28.1 ± 18.3 ‡ | N/D | – |
| TBR (%) | 6.0 ± 4.6 ‡ | N/D | – |
| TDD (units/day) | 40.7 ± 19.5 | 40.7 ± 12.1 | 0.4 |
| TBD (units/day) | 14.3 ± 7.5 | 17.9 ± 7.5 | 0.06 |
| TBD/TDD ratio (%) | 31.9 ± 12.1 | 44.0 ± 11.5 | <0.01 |
| In the second trimester | |||
| HbA1c (%) | 5.9 ± 0.6 | 6.2 ± 0.5 | <0.05 |
| Glycoalbumin (%) | 16.0 ± 2.4 | 17.7 ± 2.6 | <0.01 |
| TIR (%) | 69.3 ± 11.2 § | N/D | – |
| TAR (%) | 22.7 ± 14.1 § | N/D | – |
| TBR (%) | 8.0 ± 5.4 § | N/D | – |
| TDD (units/day) | 42.3 ± 15.8 | 40.1 ± 10.7 | 0.06 |
| TBD (units/day) | 12.5 ± 6.8 | 15.3 ± 5.2 | <0.05 |
| TBD/TDD ratio (%) | 28.1 ± 10.7 | 38.9 ± 8.1 | <0.01 |
| In the peripartum period | |||
| HbA1c (%) | 6.2 ± 0.7 | 6.5 ± 0.7 | <0.05 |
| Glycoalbumin (%) | 14.3 ± 1.6 | 15.2 ± 2.0 | <0.05 |
| TIR (%) | 74.4 ± 10.7 ¶ | N/D | – |
| TAR (%) | 19.3 ± 11.8 ¶ | N/D | – |
| TBR (%) | 6.3 ± 4.4 ¶ | N/D | – |
| TDD (units/day) | 57.6 ± 24.6 | 52.1 ± 19.3 | 0.6 |
| TBD (units/day) | 16.1 ± 8.3 | 22.1 ± 10.1 | <0.05 |
| TBD/TDD ratio (%) | 28.5 ± 11.5 | 43.8 ± 9.0 | <0.01 |
| GW at delivery | 37.7 ± 2.0 | 37.8 ± 1.2 | 0.9 |
| Preterm delivery <37 GW | 6 (15.0%) | 4 (13.8%) | 1.0 |
| Cesarean delivery | 14 (35.0%) | 11 (37.9%) | 1.0 |
| SD score of the birthweights | 0.6 ± 1.3 | 1.4 ± 1.3 | <0.05 |
| Morphological anomalies of newborns | 0 | 2 (6.9%) | 0.2 |
Data are expressed as the mean ± standard deviation or the number (percentage).
Eight patients had missing data.
16 patients had missing data.
13 patients had missing data.
12 patients had missing data.
BMI, body mass index; CSII, continuous subcutaneous insulin infusion; DM, diabetes mellitus; GW, gestational weeks; HbA1c, hemoglobin A1c; N/D, not determined; SAP, sensor‐augmented insulin pump therapy; SD, standard deviation; SMBG, self‐monitoring of blood glucose; TAR, time above range; TBD, total basal insulin dose; TBR, time below range; TDD, total daily insulin dose; TIR, time in range.
Figure 2.
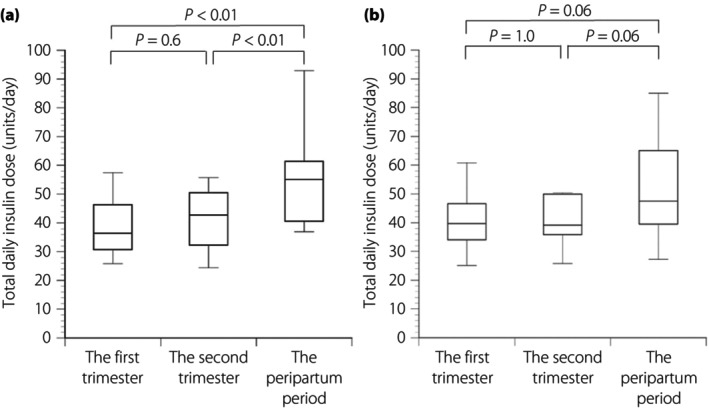
(a) The total daily insulin dose in the first and second trimesters, and the peripartum period in the sensor‐augmented insulin pump therapy group, and (b) continuous subcutaneous insulin infusion plus self‐monitoring of the blood glucose group. Statistical significance was calculated using the Mann–Whitney U‐test with a Bonferroni correction.
The obstetric and neonatal complications of the patients are presented in Table 2. The incidence rate of LGA was significantly lower in the SAP group than in the CSII/SMBG group (P < 0.05). However, no significant differences were observed in the incidence rates of HDP, SGA, RDS, neonatal hypoglycemia, polycythemia and hyperbilirubinemia between the two groups.
Table 2.
Obstetric and neonatal complications in pregnancies complicated by type 1 diabetes
| Complications | SAP group n = 40 | CSII/SMBG group n = 29 | P‐value |
|---|---|---|---|
| Hypertensive disorders of pregnancy | 9 (22.5) | 8 (27.6) | 0.8 |
| Large for gestational age | 11 (27.5) | 19 (65.5) | <0.01 |
| Small for gestational age | 2 (5.0) | 0 (0) | 0.5 |
| Respiratory distress syndrome | 2 (5.0) | 1 (3.4) | 1.0 |
| Neonatal hypoglycemia | 10 (25.0) | 8 (27.6) | 1.0 |
| Neonatal polycythemia | 4 (10.0) | 3 (10.3) | 1.0 |
| Neonatal hyperbilirubinemia | 15 (37.5) | 13 (44.8) | 0.6 |
Data are expressed as the number (percentage).
CSII, continuous subcutaneous insulin infusion; DM, diabetes mellitus; SAP, sensor‐augmented insulin pump therapy; SMBG, self‐monitoring of blood glucose.
In a subanalysis of pregnancies in which SAP was used in the present study, the obstetric and neonatal outcomes of 16 pregnancies in which 10 patients switched from MDI/SMBG to SAP or six patients switched from CSII/SMBG to SAP at 5–19 GW (the switching to SAP group) were compared with those in 24 pregnancies in which SAP was used before and throughout pregnancy (the consecutive SAP group). The clinical characteristics of pregnant women and their newborns are presented in Table 3. The maternal age at delivery (P ≤ 0.01), gravidity (P ≤ 0.01) and parity (P ≤ 0.05) were significantly lower in the switching to SAP group than in the consecutive SAP group. Both the GA (P < 0.05) and HbA1c levels (P < 0.05) during the first trimester were significantly lower in the consecutive SAP group than in the switching to SAP group. Furthermore, the incidence rate of HDP was lower in the switching to SAP group than in the consecutive SAP group, but the difference was not significant (P = 0.06; Table 4). However, no significant differences were observed in the incidence rates of neonatal complications between the two groups.
Table 3.
Clinical characteristics of pregnant women with type 1 diabetes using sensor‐augmented insulin pump therapy and their newborns
| Clinical characteristics | Switching to SAP group, n = 16 | Consecutive SAP group, n = 24 | P‐value |
|---|---|---|---|
| Age at delivery (years) | 29.5 ± 5.8 | 34.4 ± 5.0 | <0.01 |
| Gravidity | 1.1 ± 0.4 | 2.4 ± 1.2 | <0.01 |
| Parity | 0.1 ± 0.3 | 0.7 ± 0.8 | <0.05 |
| BMI before pregnancy (kg/m2) | 21.4 ± 2.3 | 22.5 ± 3.6 | 0.7 |
| Weight gain during pregnancy (kg) | 11.2 ± 4.3 | 12.0 ± 4.8 | 0.6 |
| Maternal severe hypoglycemia | 0 (0%) | 1 (4.2%) | 1.0 |
| Laboratory findings and insulin doses | |||
| In the first trimester | |||
| HbA1c (%) | 7.7 ± 1.7 | 6.7 ± 0.7 | <0.05 |
| Glycoalbumin (%) | 21.1 ± 4.0 | 18.5 ± 2.8 | <0.05 |
| TIR (%) | 73.0 ± 14.1 † | 63.5 ± 15.5 ¶ | 0.2 |
| TAR (%) | 19.4 ± 17.0 † | 31.0 ± 18.2 ¶ | 0.2 |
| TBR (%) | 7.6 ± 5.6 † | 5.4 ± 4.3 ¶ | 0.3 |
| TDD (units/day) | 35.5 ± 13.0 | 43.1 ± 22.1 | 0.4 |
| TBD (units/day) | 13.7 ± 7.6 | 14.4 ± 7.5 | 0.7 |
| TBD/TDD ratio (%) | 32.1 ± 13.4 | 31.6 ± 11.4 | 0.9 |
| In the second trimester | |||
| HbA1c (%) | 5.9 ± 0.7 | 5.9 ± 0.5 | 1.0 |
| Glycoalbumin (%) | 16.3 ± 3.2 | 15.7 ± 1.7 | 0.6 |
| TIR (%) | 70.7 ± 13.6 ‡ | 68.6 ± 10.3 †† | 0.7 |
| TAR (%) | 20.0 ± 18.7 ‡ | 23.8 ± 12.1 †† | 0.5 |
| TBR (%) | 9.3 ± 7.2 ‡ | 7.5 ± 4.6 †† | 0.5 |
| TDD (units/day) | 42.5 ± 16.4 | 42.2 ± 15.3 | 0.9 |
| TBD (units/day) | 11.0 ± 5.5 | 13.4 ± 7.5 | 0.6 |
| TBD/TDD ratio (%) | 25.7 ± 10.5 | 29.3 ± 10.7 | 0.3 |
| In the peripartum period | |||
| HbA1c (%) | 6.0 ± 0.8 | 6.2 ± 0.9 | 0.5 |
| Glycoalbumin (%) | 14.4 ± 2.1 | 14.2 ± 1.2 | 0.8 |
| TIR (%) | 81.6 ± 7.7 § | 71.1 ± 10.4 †† | <0.05 |
| TAR (%) | 11.5 ± 6.4 § | 23.0 ± 12.0 †† | <0.05 |
| TBR (%) | 6.9 ± 4.8 § | 6.0 ± 4.3 †† | 0.6 |
| TDD (units/day) | 55.2 ± 27.2 | 59.3 ± 22.6 | 0.2 |
| TBD (units/day) | 13.8 ± 6.8 | 17.6 ± 8.8 | 0.2 |
| TBD/TDD ratio (%) | 25.5 ± 11.6 | 30.4 ± 11.0 | 0.2 |
| GW at delivery | 37.7 ± 2.5 | 37.8 ± 1.8 | 0.8 |
| Preterm delivery <37 GW | 4 (25.0%) | 2 (8.3%) | 0.2 |
| Cesarean delivery | 5 (31.3%) | 9 (37.5%) | 0.7 |
| SD score of birthweights | 0.6 ± 1.3 | 0.6 ± 1.4 | 1.0 |
Data are expressed as the mean ± standard deviation or the number (percentage).
10 patients had missing data.
Eight patients had missing data.
Seven patients had missing data.
Six patients had missing data.
Five patients had missing data.
BMI, body mass index; DM, diabetes mellitus; CSII, continuous subcutaneous insulin infusion; GW, gestational weeks; HbA1c, hemoglobin A1c; SAP, sensor‐augmented insulin pump therapy; SD, standard deviation; SMBG, self‐monitoring of blood glucose; TAR, time above range; TBD, total basal insulin dose; TBR, time below range; TDD, total daily insulin dose; TIR, time in range.
Table 4.
Obstetric and neonatal complications in pregnancies complicated by type 1 diabetes treated with sensor‐augmented insulin pump therapy
| Complications | Switching to SAP group, n = 16 | Consecutive SAP group, n = 24 | P‐value |
|---|---|---|---|
| Hypertensive disorders of pregnancy | 1 (6.3) | 8 (33.3) | 0.06 |
| Large for gestational age | 5 (31.3) | 6 (25.0) | 0.7 |
| Small for gestational age | 1 (6.3) | 1 (4.2) | 1 |
| Respiratory distress syndrome | 1 (6.3) | 1 (4.2) | 1.0 |
| Neonatal hypoglycemia | 6 (37.5) | 4 (16.6) | 0.2 |
| Neonatal polycythemia | 0 (0) | 4 (16.6) | 0.2 |
| Neonatal hyperbilirubinemia | 7 (43.8) | 8 (33.3) | 0.5 |
Data are expressed as the number (percentage).
DM, diabetes mellitus; SAP, sensor‐augmented insulin pump therapy.
DISCUSSION
In the present study, it was suggested that SAP contributes to the decrease in the incidence of LGA in pregnancies complicated by type 1 diabetes mellitus compared with CSII/SMBG. Interestingly, the incidence rate of LGA in pregnancies complicated by type 1 diabetes mellitus in which SAP was initiated after conception was not different from that in which SAP was used before conception. In previous studies, obstetric and neonatal outcomes in pregnant women with type 1 diabetes mellitus were compared for CGM users versus non‐CGM users 5 or for insulin pump users versus non‐insulin pump users 4 . However, no study compared these outcomes in pregnant women with type 1 diabetes mellitus for SAP users versus users of other methods of insulin therapy and BG monitoring. To the best of our knowledge, this is the first report showing the efficacy of SAP for preventing the incidence of LGA in pregnant women with type 1 diabetes mellitus compared with CSII/SMBG.
In previous studies, the incidence rate of LGA was reported to be 41.0–63.6% in pregnant women with type 1 diabetes mellitus using CSII 4 , 17 , 18 , 19 , 20 , 21 , and 33.6–60.0% in those using MDI 19 , 20 . Some studies have shown that the incidence rate of LGA was higher in CSII users than in MDI users 19 , 20 , whereas other studies reported that the incidence rates were not significantly different between the CSII and MDI users 4 , 17 , 18 , 21 . In contrast, there were no data regarding the incidence rate of LGA in pregnant women with type 1 diabetes mellitus using SAP. The incidence rate of LGA in the CSII/SMBG group (65.5%) in the present study was similar to those in previous studies. Notably, the incidence rate of LGA in the SAP group in this study (27.5%) was the lowest in the incident rates reported in the previous studies of pregnant women with type 1 diabetes mellitus who received CSII or MDI. Indeed, the glycemic management strategies for patients with type 1 diabetes mellitus have been developed in recent years; for example, the development of new devices, new insulin formulation, carbohydrates counting and so on. Therefore, SAP, as well as other factors, could have influenced the results of the present study. However, we believe that the results of our study can be presented as real‐world data.
In the first trimester, the TDD and TBD were not different between the SAP and CSII/SMBG groups. In the second trimester and peripartum period, the TDD was not different between the two groups, whereas the TBD was lower in the SAP group than in the CSII/SMBG group. The TBD/TDD ratio was lower in the SAP group than in the CSII/SMBG group throughout pregnancy. In contrast, the TBD/TDD ratio during the first trimester in the SAP group (31.9 ± 12.1%) was similar to that in the previous reports of Japanese non‐pregnant type 1 diabetes mellitus patients whose basal insulin levels were properly controlled by using SAP (28 ± 8% 22 and 30.9 ± 14.4% 23 ). Whereas, the TBD/TDD ratio during the first trimester in the CSII/SMBG group (44.0 ± 11.5%) was higher than that in the previous reports of Japanese non‐pregnant type 1 diabetes mellitus patients whose basal insulin levels were properly controlled by using CSII (27.7 ± 6.9% 24 , 27.3 ± 6.0% 25 and 29 ± 10% 22 ). The patients in the SAP group might have good glycemic control from early pregnancy. In addition, in both the SAP and CSII/SMBG groups, the TDD in the peripartum period was significantly higher than those in the first and second trimesters. These results show that SAP can closely monitor the changes in the BG levels, and SAP can maintain the minimal insulin concentrations required to keep normal basal BG levels. Furthermore, SAP can achieve the necessary and sufficient insulin concentrations required to maintain normal postprandial BG levels. Tight control of BG levels in pregnant women with type 1 diabetes mellitus using SAP might help prevent the incidence of LGA. Indeed, in the present study, no significant differences were observed in the HbA1c levels in the first trimester between the SAP and CSII/SMBG groups, whereas the HbA1c levels in the second trimester and peripartum period were lower in the SAP group than in the CSII/SMBG group. The lower HbA1c levels in the second trimester and the peripartum period might indicate better glycemic control during pregnancy, which could lead to the reduction in the incidence of LGA in the SAP group. In addition, there was no significant difference in the frequency of maternal severe hypoglycemia during pregnancy between the SAP and CSII/SMBG group (2.5% vs 3.4%).
Regarding the percentage of target glycemic range, TIR of >70%, TAR of <25% and TBR of <4% are recommended for glycemic control during pregnancy with type 1 diabetes mellitus 16 . In the present study, however, some patients had missing data, the mean TIR in the peripartum period was >70%, but the means of TIR in the first and second trimester were 65.9% and 69.3%, respectively. The mean TAR in the second trimester and the peripartum period were <25%, but the mean TAR in the first trimester was 28.1%. Furthermore, the mean TBR in all three periods was >4%. These results suggested that the BG levels in pregnant women with type 1 diabetes mellitus in the SAP group were likely to be controlled strictly and to be maintained lower.
A subanalysis of pregnant women enrolled in the SAP group in the present study showed that SAP initiated before and continued after conception can lead to lower serum levels of HbA1c and GA in the first trimester than SAP initiated after conception. Notably, no differences were observed in the HbA1c and GA levels during the peripartum period and the incidence rates of LGA between the consecutive SAP group and the switching to SAP group. These results show that the initiation of SAP therapy even after conception might help prevent the incidence of LGA.
In previous studies of pregnant women with type 1 diabetes mellitus using CSII, the incidence rates of obstetric and neonatal complications were as follows: HDP, 9.2–30.6% 4 , 17 , 21 ; SGA, 0.4–8.7% 4 , 17 , 18 ; RDS, 8.8–10.0% 4 , 17 , 21 ; neonatal hypoglycemia, 22.8–31.8% 4 , 17 , 21 ; and neonatal hyperbilirubinemia, 17.4–28.2% 4 , 21 . However, there are currently no data on the incidence rates of obstetric and neonatal complications in pregnant women with type 1 diabetes mellitus treated with SAP. In the present study, the incidence rates of HDP (22.5%), SGA (5.0%) and neonatal hypoglycemia (25.0%) in the SAP group were similar to those among the CSII users in previous studies. Furthermore, the incidence rate of RDS in the SAP group (5.0%) was lower than that among the CSII users in the previous studies; this might be because the preterm delivery rate in the present study (15.0%) was lower than that in previous studies (20.1–43.2%) 4 , 18 , 20 , 21 . The incidence rate of neonatal hyperbilirubinemia in the SAP group (37.5%) was higher than that among the CSII users in previous studies; this might be due to the differences in susceptibility to neonatal hyperbilirubinemia by race 26 .
The present study had some limitations. First, it was not a randomized controlled trial, but a retrospective cohort study. Furthermore, because SAP was not available until February 2015 in Japan, all patients were treated with MDI/SMBG or CSII/SMBG until this time. After SAP became available, diabetologists suggested that patients with type 1 diabetes mellitus using MDI/SMBG or CSII/SMBG before pregnancy can use SAP. If the patients accepted their suggestions and selected SAP, they switched from MDI/SMBG or CSII/SMBG to SAP. Therefore, this study could not provide strong evidence of efficacy of SAP. Second, in our present study, from April 2008 to January 2015, pregnant women with type 1 diabetes mellitus were treated with SMBG plus CSII or MDI. From February 2015, they could also be treated with SAP. Furthermore, since January 2017, pregnant women with type 1 diabetes mellitus could be treated with isCGM plus MDI or CSII. However, the number of patients who used isCGM was too small (2 patients treated with MDI/isCGM and 2 with CSII/isCGM) to analyze. Therefore, we could not compare the obstetric and neonatal outcomes between patients treated with SAP and those with CSII/CGM in these real‐world data settings. Further studies are warranted to confirm the results of this study.
In conclusion, SAP therapy might be effective in preventing the incidence of LGA in pregnant women with type 1 diabetes mellitus. The present study will provide helpful information for both obstetricians and diabetologists.
DISCLOSURE
Wataru Ogawa is an Editorial Board member of Journal of Diabetes Investigation and a co‐author of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication.
Approval of the research protocol: approved by the research ethics committee of Kobe University Graduate School of Medicine (reference number: B220081).
Informed consent: N/A. Appropriate disclosure of information about the study is made and research participants are given the opportunity to refuse enrollment in the study.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
We acknowledge and thank Kana Ozaki, Department of Obstetrics and Gynecology, Kobe University Graduate School of Medicine. We are grateful for the participation of the participants, and care provided by the staff at Kobe University Hospital. We thank the clinical and laboratory personnel who supported this study at Kobe University Hospital.
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
REFERENCES
- 1. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population‐based study. Diabetes Care 2009; 32: 2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holmes VA, Young IS, Patterson CC, et al. Optimal glycemic control, pre‐eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre‐eclampsia intervention trial. Diabetes Care 2011; 34: 1683–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maresh MJ, Holmes VA, Patterson CC, et al. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care 2015; 38: 34–42. [DOI] [PubMed] [Google Scholar]
- 4. Feig DS, Corcoy R, Donovan LE, et al. Pumps or multiple daily injections in pregnancy involving type 1 diabetes: a prespecified analysis of the CONCEPTT randomized trial. Diabetes Care 2018; 41: 2471–2479. [DOI] [PubMed] [Google Scholar]
- 5. Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet (London, England) 2017; 390: 2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor‐augmented insulin‐pump therapy in type 1 diabetes. N Engl J Med 2010; 363: 311–320. [DOI] [PubMed] [Google Scholar]
- 7. Hermanides J, Nørgaard K, Bruttomesso D, et al. Sensor‐augmented pump therapy lowers HbA(1c) in suboptimally controlled type 1 diabetes; a randomized controlled trial. Diabet Med 2011; 28: 1158–1167. [DOI] [PubMed] [Google Scholar]
- 8. Minakami H, Hiramatsu Y, Koresawa M, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2011 edition. J Obstet Gynaecol Res 2011; 37: 1174–1197. [DOI] [PubMed] [Google Scholar]
- 9. Minakami H, Maeda T, Fujii T, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res 2014; 40: 1469–1499. [DOI] [PubMed] [Google Scholar]
- 10. Itakura A, Shoji S, Shigeru A, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J Obstet Gynaecol Res 2023; 49: 5–53. [DOI] [PubMed] [Google Scholar]
- 11. Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe K, Matsubara K, Nakamoto O, et al. Outline of the new definition and classification of “hypertensive disorders of pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens Res Pregnancy 2018; 6: 33–37. [Google Scholar]
- 13. Itabashi K, Fujimura M, Kusuda S, et al. New normative birthweight among Japanese infants according to gestational week at delivery. Acta Paediatr Jpn 2010; 114: 1271–1293 (in Japanese). [Google Scholar]
- 14. Nakamura H, Yonetani M, Uetani Y, et al. Determination of serum unbound bilirubin for prediction of kernicterus in low birthweight infants. Acta Paediatr Jpn 1992; 34: 642–647. [DOI] [PubMed] [Google Scholar]
- 15. Morioka I, Nakamura H. Treatment criteria for infants with hyperbilirubinemia in Japan. Semin Perinatol 2021; 45: 151352. [DOI] [PubMed] [Google Scholar]
- 16. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chico A, Herranz L, Corcoy R, et al. Glycemic control and maternal and fetal outcomes in pregnant women with type 1 diabetes according to the type of basal insulin. Eur J Obstet Gynecol Reprod Biol 2016; 206: 84–91. [DOI] [PubMed] [Google Scholar]
- 18. Żurawska‐Kliś M, Kosiński M, Kuchnicka A, et al. Continuous subcutaneous insulin infusion does not correspond with pregnancy outcomes despite better glycemic control as compared to multiple daily injections in type 1 diabetes – significance of pregnancy planning and prepregnancy HbA1c. Diabetes Res Clin Pract 2021; 172: 108628. [DOI] [PubMed] [Google Scholar]
- 19. Kallas‐Koeman MM, Kong JM, Klinke JA, et al. Insulin pump use in pregnancy is associated with lower HbA1c without increasing the rate of severe hypoglycaemia or diabetic ketoacidosis in women with type 1 diabetes. Diabetologia 2014; 57: 681–689. [DOI] [PubMed] [Google Scholar]
- 20. Hauffe F, Schaefer‐Graf UM, Fauzan R, et al. Higher rates of large‐for‐gestational‐age newborns mediated by excess maternal weight gain in pregnancies with type 1 diabetes and use of continuous subcutaneous insulin infusion vs multiple dose insulin injection. Diabet Med 2019; 36: 158–166. [DOI] [PubMed] [Google Scholar]
- 21. Bruttomesso D, Bonomo M, Costa S, et al. Type 1 diabetes control and pregnancy outcomes in women treated with continuous subcutaneous insulin infusion (CSII) or with insulin glargine and multiple daily injections of rapid‐acting insulin analogues (glargine‐MDI). Diabetes Metab 2011; 37: 426–431. [DOI] [PubMed] [Google Scholar]
- 22. Matsuoka A, Hirota Y, Urai S, et al. Effect of switching from conventional continuous subcutaneous insulin infusion to sensor augmented pump therapy on glycemic profile in Japanese patients with type 1 diabetes. Diabetol Int 2018; 9: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katayama A, Tone A, Watanabe M, et al. The hypoglycemia‐prevention effect of sensor‐augmented pump therapy with predictive low glucose management in Japanese patients with type 1 diabetes mellitus: a short‐term study. Diabetol Int 2020; 11: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuroda A, Kaneto H, Yasuda T, et al. Basal insulin requirement is ~30% of the total daily insulin dose in type 1 diabetic patients who use the insulin pump. Diabetes Care 2011; 34: 1089–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura T, Hirota Y, Hashimoto N, et al. Diurnal variation of carbohydrate insulin ratio in adult type 1 diabetic patients treated with continuous subcutaneous insulin infusion. J Diabetes Investig 2014; 5: 48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beutler E. G6PD deficiency. Blood 1994; 84: 3613–3636. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.


