B-cell acute lymphoblastic leukemia (B-ALL) is the most common malignant disease in childhood with a peak age at 2-6 years old. A two-step model has been proposed for the development of childhood B-ALL.1 The first step is an early somatic genetic rearrangement, such as the ETV6::RUNX1 fusion gene, followed by a broad range of secondary mutational events driven by environmental stimuli (including infection and abnormal cytokine release from immunologically untrained cells).1 The involvement of at least two discrete steps suggests that B-ALL may be preventable in infants with a genetically initiated step, who could be protected from harmful postnatal environmental stimuli.
In the past decades, infections have been regarded as the environmental stimuli with the most impact in the etiology of childhood B-ALL. Common pathogens may drive secondary mutations in genetically predisposed subjects.2 Experimental models of infection can be leveraged in xenograft and animal models that closely resemble the pathophysiology of childhood B-ALL. For example, two animal studies demonstrated that transgenic mice (with the ETV6::RUNX1 fusion or with Pax5+/- heterozygosity) only developed B-ALL when they were exposed to common infections, although with incomplete penetrance.3,4 These past studies indicate that infections can act as important promoters of B-ALL development in the context of genetic predispositions. However, exposure to pathogens early in life via childhood contacts (daycare, microbiome) may modulate immune reactivity and decrease risk.2 Through a serendipitous observation, we found that the impact of pinworm infection on leukemogenesis was markedly different depending on the presence or absence of a common human somatic genetic change.
Pinworms are a commonly found intestinal helminth in laboratory animals and the control of these pathogens in animal holdings is quite difficult.3 We performed a retrospective analysis of the latency and incidence of leukemia/lymphoma of two strains of mice during and after a pinworm outbreak in a specific-pathogen-free (SPF) facility (Figure 1A). Cdkn2a-/- and ETV6::RUNX1+ Cdkn2a-/- (referred to as E6R1+Cdkn2a-/- in the current work and Cre+ TA + Cdkn2a-/- in the past work4) mice were maintained on the FVB/N strain background and were age- and sex-matched for each survival experiment. In line with the details of our animal use protocol, body condition scoring, clinical signs, and a diagnosis of neoplasia were used, in consultation with veterinarians, to identify animals that had reached our predefined study endpoints. A gross necropsy was performed to identify potential sources of illness. Selected tissues were preserved through formalin-fixed paraffin embedding and stained with hematoxylin & eosin. Diagnoses were based upon gross necropsy and histopathology. If gross necropsy findings suggested a hematopoietic neoplasm, single-cell suspensions of involved tissues were cryopreserved in medium containing 10% dimethylsulfoxide. Additional diagnostic information was obtained by immunophenotyping when necessary. All experiments were performed following institutional review and approval by the University of California San Francisco Institutional Animal Care and Use Committee.
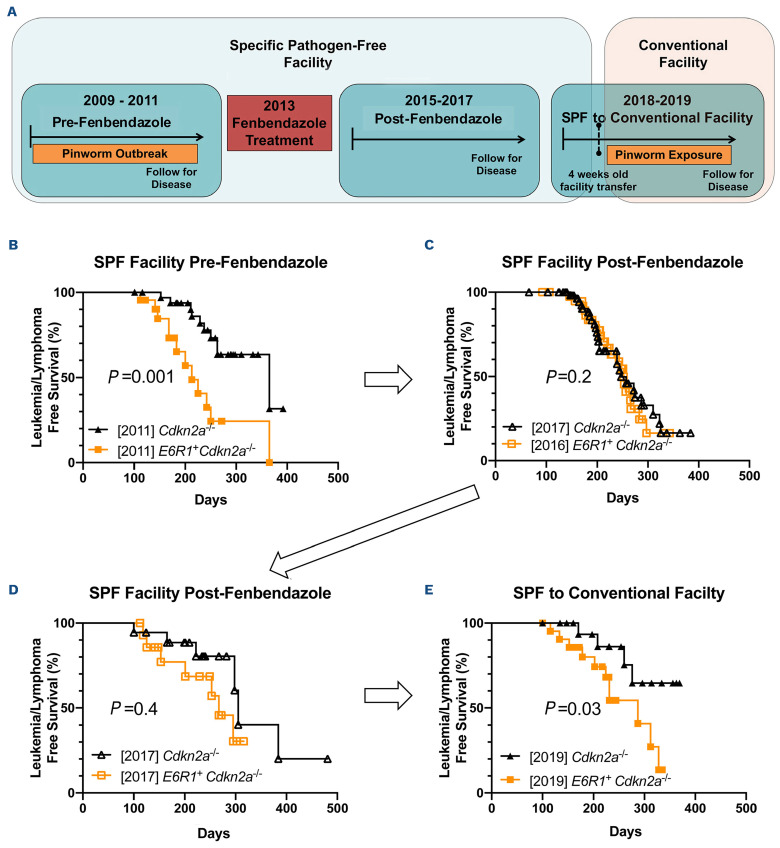
We previously reported that ETV6::RUNX1 expression cooperates with Cdkn2a deletion to promote the development of B-ALL in mice (Figure 1B). After 2013, the leukemogenic effect of E6R1 expression was no longer observed, as demonstrated by two independent experiments showing overlap (Figure 1C) or minimal separation (Figure 1D) between the Cdkn2a-/- and E6R1+ Cdkn2a-/- survival curves. Review of the infection records in the SPF facility revealed that an outbreak of the pinworm Aspicularis was detected by fecal floatation testing of sentinels during the timeframe when decreased latency for leukemia/lymphoma had been observed in E6R1+ Cdkn2a-/- mice in comparison with Cdkn2a-/- mice. Following the outbreak of Aspicularis, all mice in the room were treated with the broad spectrum antihelminthic fenbendazole. The diminished effect of E6R1 on promoting leukemia/lymphoma development was observed after the eradication of pinworm.
We then prospectively investigated the impact of intentional pinworm exposure on leukemogenesis. To determine whether pinworm infection could restore the leukemogenic effect of E6R1 in the Cdkn2a-/- model, 4-week-old Cdkn2a-/- and E6R1+ Cdkn2a-/- mice were transferred from an SPF facility to an Aspicularis-infected conventional facility (also detected by fecal floatation testing of sentinels), where they were followed for survival (Figure 1A). In the context of pinworm infection, E6R1+ Cdkn2a-/- mice developed leukemia/lymphoma earlier and with a higher incidence than Cdkn2a-/- mice (Figure 1E). Together, these survival studies indicate a different impact of pinworm infection on the development of leukemia/lymphoma in Cdkn2a-/- and E6R1 Cdkn2a-/- mice.
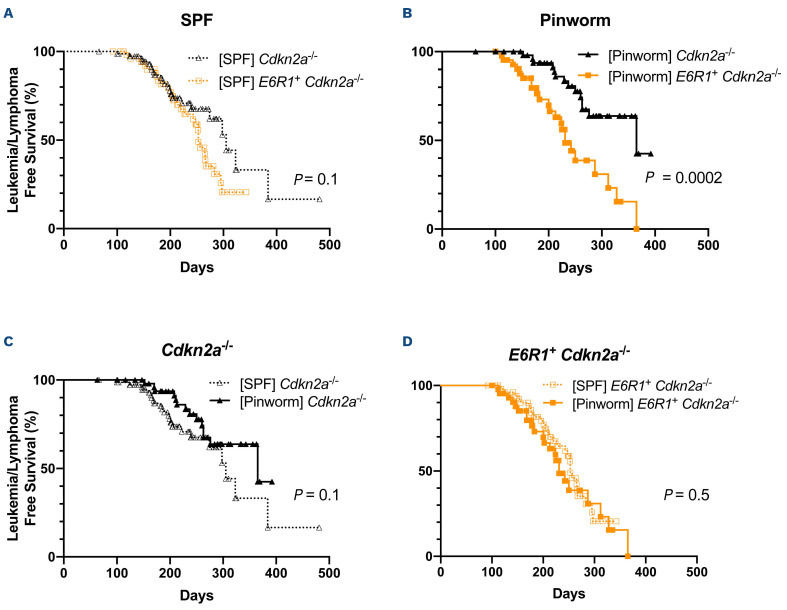
Given the well-established role of the E6R1 mutation in cooperating with radiation, chemicals, and infectious exposures to promote the development of lymphoid malignancies,3,4,6,7 we had hypothesized that pinworm infection would promote leukemogenesis and have a stronger effect in E6R1+ Cdkn2a-/- mice than in Cdkn2a-/- mice. We examined this hypothesis by aggregating the survival cohorts from the studies shown in Figure 1. Mice In a pinworm-free facility mice (cohorts shown in Figure 1C, D) were aggregated to serve as pinworm-free controls (Figure 2A). For comparison, survival cohorts of pinworm-infected mice (Figure 1B, E) were also aggregated. Consistent with individual experiments, only pinworm-infected mice demonstrated a statistically significant difference in the development of leukemia/lymphoma between E6R1+ Cdkn2a-/- mice and Cdkn2a-/- animals (Figure 2B).
Figure 1.
Pinworm exposure drives differences in leukemia/lymphoma development between Cdkn2a-/- and E6R1+ Cdkn2a-/- mice. (A) Timeline of individual survival studies following Cdkn2a-/- and E6R1+ Cdkn2a-/- mice in specific pathogen-free (SPF) and conventional facilities relative to the 2013 fenbendazole treatment. (B) Survival curves of leukemia/lymphoma development in Cdkn2a-/-(N=34) and E6R1+ Cdkn2a-/- (N=22) mice housed in an SPF facility during a pinworm outbreak prior to fenbendazole treatment. Arrows indicate the chronological order of the survival studies. The year in brackets corresponds to the date of euthanasia of the last mouse to develop illness in each cohort. (C, D) Survival curves from two independent experiments of mice housed in an SPF facility after pinworm was eradicated with fenbendazole treatment. 2016-2017: Cdkn2a-/- (N=58) and E6R1+ Cdkn2a-/- (N=40); 2017: Cdkn2a-/- (N=18) and E6R1+ Cdkn2a -/- (N=15). (E) Survival curve from one experiment in which Cdkn2a-/- (N=20) and E6R1+ Cdkn2a -/-(N=22) mice were housed in an SPF facility for 4 weeks, then transferred to a conventional facility for exposure to pinworm bedding. The log-rank (Mantel-Cox) test was applied to the survival curves.
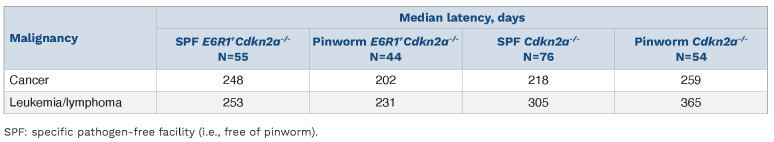
In contrast to our hypothesis, leukemia/lymphomaDfree survival curves revealed that pinworm infection elicited a protective effect in Cdkn2a-/- mice (Figure 2C) that was not observed in E6R1+ Cdkn2a-/- mice (Figure 2D). In pinworm-infected Cdkn2a-/- mice, the median latency of leukemia/lymphoma development increased from 305 days to 365 days (Table 1). In contrast, pinworm infection was associated with a decrease in the median latency of leukemia/lymphoma in E6R1+ Cdkn2a-/- mice from 253 days to 231 days (Table 1). Although there was an effect on latency, the incidence of leukemia/lymphoma was not affected by pinworm infection (Online Supplementary Table S1). Of note, pinworm exposure had a modest impact on leukemia/lymphoma-free survival curves of Cdkn2a-/- mice (Figure 2C) and E6R1+ Cdkn2a-/- mice (Figure 2D), but the cumulative bidirectional effect of pinworm resulted in a significant difference between these two mouse strains (Figure 2B). Because Cdkn2a-/- mice are susceptible to other cancers in addition to leukemia/lymphoma, we examined cancer-free survival curves: there was no difference between Cdkn2a-/- and E6R1+ Cdkn2a-/-mice in SPF conditions (Online Supplementary Figure S1A), but there was a statistically significant difference in the presence of pinworm (Online Supplementary Figure S1B). Interestingly, pinworm was associated with divergent responses: protection in Cdkn2a-/- mice (Online Supplementary Figure S1C) and a promotion in E6R1+ Cdkn2a-/- nice (Online Supplementary Figure S1D). Neither E6R1 (Online Supplementary Figure S2A, B) nor pinworm exposure (Online Supplementary Figure S2C, D) had a statistically significant impact on the development of solid tumors. Taken together, this work demonstrates a protective effect of an infection on leukemia/lymphoma development of Cdkn2a null mice in the absence of the E6R1 mutation, but that this protection is reversed in the presence of this common prenatally acquired genetic change.
Figure 2.
Impact of pinworm exposure on leukemia/lymphoma development in the absence and presence of E6R1. Cumulative survival curves showing combined data from Figure 1 of four independent experiments of mice housed in a pinworm-free (SPF) facility or pinworm-infected facility. Leukemia/lymphoma-free survival for (A) SPF-housed (open symbols) and (B) pinworm-exposed (filled symbols) Cdkn2a-/- mice (black triangles) and E6R1+ Cdkn2a-/- mice (orange squares). Leukemia/lymphoma-free survival for genotype-matched (C) Cdkn2a-/- mice and (D) E6R1+ Cdkn2a-/- mice. SPF Cdkn2a-/- mice (N=76), SPF-housed E6R1+ Cdkn2a-/- mice (N=55), pinworm-exposed Cdkn2a-/- mice (N=54), and pinworm-exposed E6R1+ Cdkn2a-/- mice (N=44). The logrank (Mantel-Cox) test was applied to the survival curves.
Table 1.
Median latency of development of malignancy in Cdkn2a-/- and E6R1+ Cdkn2a-/- mice housed in pinworm-free or pinworm-infected facilities.
The increased latency of leukemia/lymphoma in pinwormexposed Cdkn2a-/- mice is a novel in vivo demonstration of a protective role of pathogenic infection in a mouse model of B-ALL. These results build upon past findings, in which immune stimulation with CpG, a TLR9 agonist, protected against B-ALL in Eu-ret mice.5 Exposure to microbes that are capable of priming the early immune system to respond appropriately to infections has long been associated with reduced risk of childhood B-ALL.1,2 Interestingly, pinworms have immunomodulatory properties, and their decades-long decline in western societies is inversely correlated with the rising incidence of B-ALL and other childhood immune disorders.6 While the current study does not characterize the immune response to pinworm exposure in Cdkn2a-/- mice, pinworm infections are well-described to stimulate anti-inflammatory immune responses that are characterized by the production of the cytokine interleukin-10 (IL-10) and T-regulatory cells.7 As IL-10 is a protective factor for B-cell leukemia/lymphoma in humans8 and mice,9 it is possible that pinworm-induced IL-10 and/or pinworm-induced microbial diversity provide protection against B-ALL in specific genetic settings. In addition to parasites, viral and bacterial pathogens are also capable of supporting the gut microbiome and inducing production of IL-10 and T-regulatory cells.10 It will therefore be interesting to determine whether the protection garnered from parasitic infection can be acquired through other infectious pathogens that stimulate similar immune pathways.
The gut microbiome is well known for shaping immune responses through structural components or metabolites of its constituent bacteria11 and has recently been identified as a player in the development of B-ALL.12 Considering the impact of helminth infections in shaping the composition of the gut microbiome,13,14 it is likely that pinworm exerts a protective effect in Cdkn2a-/- mice by enhancing microbial diversity. Although the timing of pinworm introduction varied (from birth [Figure 1B] or from weaning age [Figure 1E]), we did not find evidence for a role of timing differences in leukemia/lymphoma development. We instead observed a strong genetic-environmental interaction, in which E6R1 expression completely inhibited the protective effect of pinworm exposure in Cdkn2a-/- mice. This result may be explained by the recently described ability of E6R1 to induce a state of microbial dysbiosis12 or the well-established role of E6R1 in converting B-cell precursors into B-ALL.2 Pinworm and probiotic interventions that are currently being investigated for the prevention of childhood autoimmune disorders may also have the capacity to prevent childhood B-ALL.2 Future studies should be aimed at understanding how genetics and infectious exposures interact to affect the gut-immune axis. This will be an important step toward leveraging the full potential of preventative strategies in children who are genetically predisposed to B-ALL.
Supplementary Material
Acknowledgments
We thank Todd Whitehead and Kamir Hiam for useful discussions.
Funding Statement
Funding: This work was supported by National Institutes of Health/National Cancer Institute grants R01 CA185058 (to SCK and JLW) and F31 CA221157 (to BF).
References
- 1.Hauer J, Fischer U, Borkhardt A. Toward prevention of childhood ALL by early-life immune training. Blood. 2021;138(16):1412-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer. 2018;18(8):471-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taffs LF. Pinworm infections in laboratory rodents: a review. Lab Anim. 1976;10(1):1-13. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Jones L, Gaillard C, et al. Initially disadvantaged, TEL-AML1 cells expand and initiate leukemia in response to irradiation and cooperating mutations. Leukemia. 2013;27(7):1570-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seif AE, Barrett DM, Milone M, Brown VI, Grupp SA, Reid GSD. Long-term protection from syngeneic acute lymphoblastic leukemia by CpG ODN-mediated stimulation of innate and adaptive immune responses. Blood. 2009;114(12):2459-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale EAM. A missing link in the hygiene hypothesis? Diabetologia. 2002;45(4):588-594. [DOI] [PubMed] [Google Scholar]
- 7.Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138(3):666-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JS, Zhou M, Buffler PA, Chokkalingam AP, Metayer C, Wiemels JL. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1736-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitch BA, Zhou M, Situ J, et al. Decreased IL-10 accelerates B-cell leukemia/lymphoma in a mouse model of pediatric lymphoid leukemia. Blood Adv. 2022;6(3):854-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771-5777. [DOI] [PubMed] [Google Scholar]
- 11.Schluter J, Peled JU, Taylor BP, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;588(7837):303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicente-Dueñas C, Janssen S, Oldenburg M, et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood. 2020;136(18):2003-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramanan D, Bowcutt R, Lee SC, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science. 2016;352(6285):608-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SC, Tang MS, Lim YAL, et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl Trop Dis. 2014;8(5):e2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.