Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic precursor condition that precedes multiple myeloma and related disorders but has also been associated with other medical conditions. Since systematic screening is not recommended, MGUS is typically diagnosed due to underlying diseases and most cases are not diagnosed. Most previous studies on MGUS disease associations have been based on clinical cohorts, possibly resulting in selection bias. Here we estimate this selection bias by comparing clinically diagnosed and screened individuals with MGUS with regards to demographics, laboratory features, and comorbidities. A total of 75,422 participants in the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study were screened for MGUS by serum protein electrophoresis, immunofixation and free light chain assay (clinicaltrials gov. Identifier: NCT03327597). We identified 3,352 individuals with MGUS, whereof 240 had previously been clinically diagnosed (clinical MGUS), and crosslinked our data with large, nationwide registries for information on comorbidities. Those with clinical MGUS were more likely to have at least one comorbidity (odds ratio=2.24; 95% confidence interval: 1.30-4.19), and on average had more comorbidities than the screened MGUS group (3.23 vs. 2.36, mean difference 0.68; 95% confidence interval: 0.46-0.90). They were also more likely to have rheumatological disease, neurological disease, chronic kidney disease, liver disease, heart failure, or endocrine disorders. These findings indicate that individuals with clinical MGUS have more comorbidities than the general MGUS population and that previous studies have been affected by significant selection bias. Our findings highlight the importance of screening data when studying biological and epidemiological implications of MGUS.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic precursor condition that consistently precedes multiple myeloma and related disorders. It is present in approximately 4.5% of the general population over the age of 50 years.1-6 MGUS is asymptomatic and is, therefore, typically diagnosed incidentally during clinical workup for various medical conditions, while most patients remain undiagnosed.1 Although asymptomatic, MGUS has been associated with over 100 medical disorders, that are either assumed to cause or result from MGUS. These include thrombosis,7 infections,8 autoimmune diseases,9,10 fractures,11,12 neuropathies,13 as well as excess mortality.14 In some cases, there exists histopathologic evidence of a causative relationship between MGUS and disease, usually with the identification of the monoclonal immunoglobulin in the affected tissue (for example glomerulus or skin lesion).15 However, the majority of previously reported disease associations with MGUS have been found in retrospective epidemiological studies. Since systematic screening for MGUS is currently not recommended, most prior studies have been based on clinical cohorts of individuals diagnosed with MGUS as part of routine workup for other clinical signs or symptoms. Therefore, the diagnosis of MGUS is usually made due to the diagnosis of another disease, including autoimmune disorders or kidney diseases. This leads to a potential bias in the selection of individuals with more medical conditions into previously studied MGUS cohorts and, therefore, overestimation of the associations between MGUS and disease. This is supported by the only prior screening study on disease associations with MGUS, where in a screened cohort in Olmsted County, Minnesota, (n=17,398) only 14 of 75 previously reported associations were confirmed.16 However, the study included numerous distinct and specific diagnostic codes with unclear clinical correlation and thus, the observed number of cases with underlying conditions were small. This decreased statistical power and necessitated the adjustments for multiple comparisons, limiting the possibility of detecting true subtle associations.17
The Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study is a large Icelandic screening study for MGUS.18 It included both individuals with incidentally diagnosed (clinical) MGUS and people diagnosed through screening, providing a unique opportunity to explore the differences between clinical and screened MGUS with regards to selection bias in clinical cohorts. The aim of this study was to systematically estimate possible selection bias in studies on MGUS by comparing the association of demographic, clinical, and laboratory factors with clinical MGUS with their association with MGUS diagnosed by systematic screening.
Methods
iStopMM study
The iStopMM study is a population-based, nationwide screening study and randomized controlled trial of follow-up strategies that aims to evaluate the benefits and potential harms of screening for MGUS (clinicaltrials gov. Identifier: NCT03327597). The study has previously been described in detail.18 Briefly, 80,759 individuals, more than half of the Icelandic population aged ≥40 years in 2016, provided informed consent and 75,422 (>93%) of them were subsequently screened for MGUS using serum protein electrophoresis (SPEP), immunofixation and free light chain (FLC) assay. Those who had abnormal screening results, in absence of a more advanced disease, were randomized into one of three study arms, where arm 1 continued as they had never undergone screening while arm 2 and 3 followed different follow-up strategies.18 The study protocol and all information material from the iS-topMM study has been approved by the Icelandic National Bioethics Committee and the Icelandic Data Protection Authority. Access to national healthcare registries has been approved by the Icelandic Directorate of Health and the Icelandic Cancer Society.
Study cohort and data
Participants who had an M-protein on SPEP or an abnormal FLC ratio and were randomized into one of our three study arms were eligible for this study. Participants who had M-protein levels ≥3.0 g/dL or an FLC ratio ≥100 or ≤0.01 were excluded as they, by definition, have smoldering myeloma or multiple myeloma.19 Data from the Icelandic Cancer Registry and laboratory records from Landspítali - the National University Hospital of Iceland (NUHI) and Læknasetrið, the only laboratories performing SPEP in Iceland, were crosslinked to the study cohort. Those who had M-protein previously identified in the clinical setting, were defined as clinical MGUS. M-protein concentration, MGUS isotype, and FLC ratio were acquired from the study screening samples. Finally, comorbidity data was acquired from two national registries: the Hospital Discharge Register and Register of Primary Health Care Contacts, where the accuracy of chronic disease diagnoses has been found to be >95%.20 International Classification of Diseases, 10th Revision (ICD-10) codes from the registries were grouped together into relevant disease categories (Online Supplementary Table S1) and their prevalence compared between clinical and screened MGUS.21
Statistical analyses
All statistical analyses compared clinical MGUS to screened MGUS. Demographic features (age, sex, and residence) were evaluated by t test and χ2 test for continuous and binary variables, respectively. When comparing laboratory features, linear regression was used to assess continuous variables (M-protein concentration, number of risk factors for progression), and logistic regression to assess binary variables (M-protein isotype, abnormal serum FLC ratio [<0.26 or >1.65]). Participants’ combined number of comorbidities was compared between the two groups by linear regression. Logistic regression was then used to compare the prevalence of each comorbidity category between the groups. All analyses were adjusted for age and sex, except for those regarding demographic differences. A P value of <0.05 was considered indicative of statistical significance. R Statistical Software (version 3.6.2; R Core Team 2021) was used for all statistical analyses.22
Results
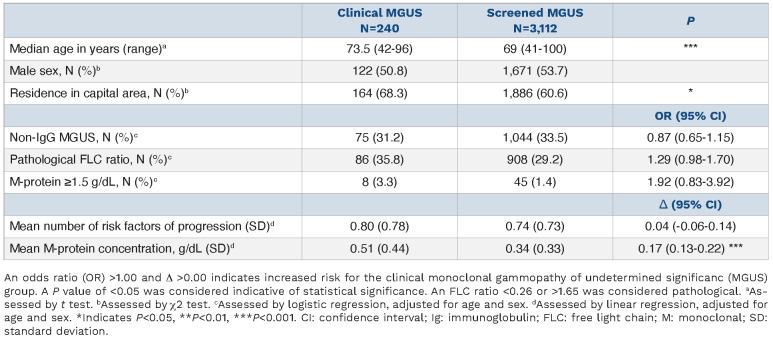
A total of 75,422 individuals were screened for MGUS in the iStopMM study, of whom 3,352 were diagnosed with MGUS based on M-proteins detected by SPEP. Of these individuals, 240 had previously been diagnosed with clinical MGUS (7.2%) (Table 1).
Demographic features
The median age of individuals with clinical MGUS was significantly higher than in the screened MGUS group (73.5 vs. 69 years, respectively; P<0.001). The clinical MGUS group was also more likely to reside in Iceland’s capital area compared with those with screened MGUS (68.3% vs. 60.6%; P=0.02). No differences in sex distribution were detected between the two groups (Table 1).
Laboratory findings
The clinical MGUS group had a 0.17 g/dL higher mean M-protein concentration than those with screened MGUS (0.51 clinical vs. 0.34 g/dL screened, mean difference 0.17 g/dL, 95% confidence interval [CI]: 0.13-0.22; P<0.001), after adjusting for age and sex (Table 1). There were no statistically significant differences in the prevalence of each risk factor for progression from MGUS to MM or related lymphoproliferative disease, as defined by the International Myeloma Working Group (M-protein isotype [non-IgG MGUS], M-protein concentration ≥1.5 g/dL, and an abnormal FLC ratio [<0.26 or >1.65]), nor in the mean number of risk factors for progression (0.80 clinical vs. 0.74 screened) (Table 1).23
Comorbidities
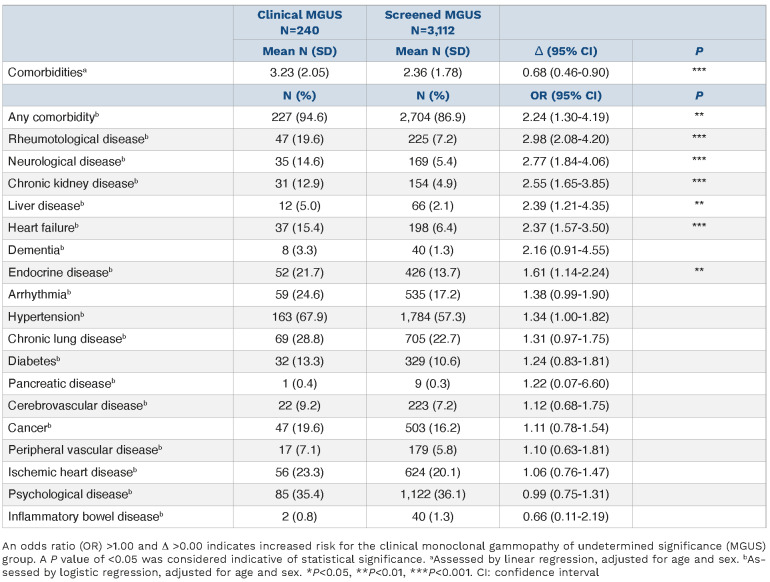
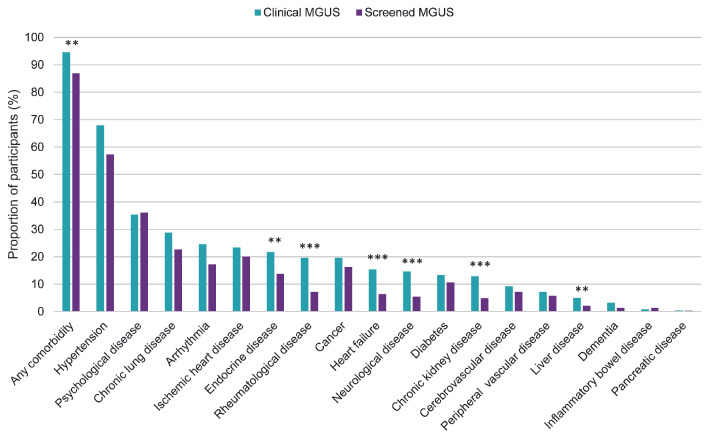
Individuals with clinical MGUS were significantly more likely to have at least one comorbidity compared with those with MGUS diagnosed by screening (n=227 [94.6%] clinical vs. n=2,704 [86.9%] screened; odds ratio [OR] =2.24; 95% CI: 1.30-4.19; P<0.01), and had a 37% higher mean number of comorbidities (3.23 clinical vs. 2.36 screened, mean difference 0.68; 95% CI: 0.46-0.90; P<0.001), in models adjusting for age and sex. Furthermore, those with clinical MGUS were more likely to have rheumatological disease (OR=2.98; 95% CI: 2.08-4.20; P<0.001), neurological disease (OR=2.77; 95% CI: 1.84-4.06; P<0.001), chronic kidney disease (OR=2.55; 95% CI 1.65-3.85; P<0.001), liver disease (OR=2.39; 95% 95% CI: 1.21-4.35; P<0.01), heart failure (OR=2.37; 95% CI:1.57-3.50; P<0.001), and endocrine disorder (OR=1.61; 95% CI: 1.14-2.24; P<0.01), after adjusting for age and sex. No significant differences in the prevalence of other disease categories were detected (Table 2; Figure 1).
Discussion
In this large population-based nationwide screening study with over 75,000 participants and detailed nationwide information on comorbidities, we analyzed the differences between individuals with clinically diagnosed and screened MGUS. We found that individuals with a clinical diagnosis of MGUS had a 37% higher number of comor-bidities compared with those found to have MGUS as part of our screening study and were two- to three-fold more likely to have been diagnosed with certain medical conditions. Our study shows that individuals diagnosed with MGUS during work-up for other medical conditions have more comorbidities than individuals diagnosed through systematic screening. This shows that studies comparing disease associations with MGUS require data from screened populations to limit selection bias.
Table 1.
Demographic and laboratory features of clinical monoclonal gammopathy of undetermined significanc (MGUS) and screened MGUS.
Table 2.
Prevalence of comorbidities in clinical monoclonal gammopathy of undetermined significanc (MGUS) and screened MGUS.
Individuals with clinical MGUS were on average 4.5 years older and had 0.17 g/dL higher M-protein concentration than those with screened MGUS. Since, by definition, those with clinical MGUS had had MGUS for a longer time, and because M-protein generally increases over time, these results were expected.24 Both groups had a relatively low mean M-protein concentration (0.51 g/dL clinical vs. 0.34 g/dL screened), a difference that, while statistically significant, is unlikely to be clinically important. Furthermore, no statistically significant differences in the prevalence of individual risk factors of MGUS progression, or in the cumulated number of risk factors, were detected in our study. According to previous studies, the risk of MGUS progression to malignancy is low at 0.5-1.5% per year.2,6,24,25 The findings of this study indicate that previous estimates of MGUS progression have not been affected by selection bias and that clinically diagnosed MGUS is similar to MGUS diagnosed by screening.
The clinical MGUS group had statistically a significantly higher number of comorbidities compared to the screened MGUS group and was more likely to have at least one comorbidity, suggesting higher disease burden in general in clinically diagnosed individuals compared with the total MGUS population. This has previously been hypothesized but evidence has lacked until now.16 This important finding suggests selection bias in clinical cohorts of MGUS. In particular, chronic kidney disease, endocrine disorders, neurological disease, liver disease and rheumatological disease were more prevalent among those with clinically diagnosed MGUS. This is clinically important since various disorders of these disease categories have previously been associated with MGUS, including glomerulonephritis,26,27 immune deposition kidney diseases,28 hyperparathyroidism,29 peripheral neuropathies,13 hepatitis C,30,31 and rheumatoid arthritis.9 Due to these previously reported associations with MGUS, and often indistinct clinical signs and symptoms, affected individuals are more likely to undergo serum protein blood testing (e.g., SPEP, immunofixation, and FLC assays) during clinical workup, leading to the diagnosis of MGUS. For some of these conditions, SPEP is even advised during workup, according to clinical guidelines.32,33 This might also explain the higher prevalence of heart failure in the clinical MGUS group, as individuals suspected of amyloidosis causing their symptoms usually undergo SPEP and FLC assays.34 These differences suggest that at least some of the previously reported disease associations with MGUS have been overestimated as the evidence was based on clinical MGUS cohorts. Further studies to estimate true associations between MGUS and other diseases are needed on screened populations. Furthermore, multifaceted data, including information on laboratory markers and medications, in addition to careful, complex data analyses suiting each association, are essential to estimate causal relationships.
Figure 1.
Comorbidity prevalence of the study cohort. A P value of <0.05 was considered indicative of statistical significance. Assessed by logistic regression models. All analyses were adjusted for age and sex. *P<0.05, **P<0.01, ***P<0.001. MGUS: monoclonal gammopathy of undetermined significance.
Our study has several strengths. It is based on the largest screening study on MGUS to date, and it is the first one that is both population-based and nationwide. The high participation rate in the iStopMM study (54.3% of the Icelandic population ≥40 years of age) makes the study cohort highly representative of the general population. Extensive information was gathered on all participants, including M-protein concentration, M-protein isotype, and FLC ratios, which was used to confirm all MGUS diagnoses. By crosslinking our data to large national registries, where disease diagnoses are recorded prospectively with very high completeness and accuracy, we also gathered high-quality information on participants’ comorbidities, including cancer diagnoses. In addition, data was collected prospectively and in the same manner for all participants regardless of exposure status.
Our study also has some important limitations. Relatively few individuals had some of the comorbidities assessed, which may have affected the statistical power to ascertain potential association for those diseases. Additionally, since there were multiple statistical tests done in our study, some associations found may have been spurious. However, due to the hypothesis-generating nature of the study, we did not adjust for multiple testing.17 Finally, the Icelandic population is ethnically and genetically homogenous. Furthermore, the Icelandic health care system is universal, which makes access to full range health care services available for the entire population. However, this should not affect the results of the higher number of comorbidities among those with MGUS diagnosed in the clinical setting compared with those diagnosed through screening, although the prevalence of certain comorbidities may differ between countries and different health care systems.
In summary, we have shown that there is no meaningful difference in the severity of the precursor condition in individuals with a diagnosis of MGUS in the clinical setting compared with those diagnosed with MGUS in our screening study. However, there is a significant difference with regards to the presence of comorbidities. Those with a clinical diagnosis of MGUS have a higher mean number of comorbidities and are more likely to have been diagnosed with certain medical conditions. Our findings emphasize the fact that MGUS cohorts, based on clinically diagnosed populations, are inherently biased towards individuals with more comorbidities, compared to cohorts consisting of screened individuals. Furthermore, our findings support that selection bias has affected the results of many previous studies reporting on MGUS and various medical associations, and that at least some associations may not be biologically true or have been significantly overestimated. Going forward, it is imperative that screened MGUS cohorts are used to evaluate the epidemiological and biological implications of MGUS and that studies based on clinical MGUS cohorts should be interpreted with caution.
Supplementary Material
Acknowledgments
Screening tests were performed by the Binding Site. Cross-linking of study data to national registries was performed by the Icelandic Directorate of Health and the Icelandic Cancer Society. Special thanks go to the thousands of Icelanders who provided their informed consent for participation in the study.
Funding Statement
Funding: The iStopMM study is funded by the Black Swan Research Initiative by the International Myeloma Foundation and the Icelandic Center for Research (grant no. 173857), the European Research Council (ERC) under the European Union‘s Horizon 2020 research and innovation programme (grant no. 716677). Additional funding was provided by the University of Iceland, Landspítali University Hospital, and the Icelandic Cancer Society.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362-1369. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375(9727):1721-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray D, Kumar SK, Kyle RA, et al. Detection and prevalence of monoclonal gammopathy of undetermined significance: a study utilizing mass spectrometry-based monoclonal immunoglobulin rapid accurate mass measurement. Blood Cancer J. 2019;9(12):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss BM, Hebreo J, Cordaro DV, et al. Increased serum free light chains precede the presentation of immunoglobulin light chain amyloidosis. J Clin Oncol. 2014;32(25):2699-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102(10):3759-3764. [DOI] [PubMed] [Google Scholar]
- 7.Kristinsson SY, Pfeiffer RM, Björkholm M, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010;115(24):4991-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012;97(6):854-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindqvist EK, Goldin LR, Landgren O, et al. Personal and family history of immune-related conditions increase the risk of plasma cell disorders: a population-based study. Blood. 2011;118(24):6284-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigurbergsdóttir A, Love TJ, Kristinsson SY. Autoimmunity, infections, and the risk of monoclonal gammopathy of undetermined significance. Front Immunol. 2022;13:876271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood. 2010;116(15):2651-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorsteinsdottir S, Lund SH, Lindqvist EK, et al. Bone disease in monoclonal gammopathy of undetermined significance: results from a screened population-based study. Blood Adv. 2017;1(27):2790-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rögnvaldsson S, Steingrímsson V, Turesson I, Björkholm M, Landgren O, Kristinsson SY. Peripheral neuropathy and monoclonal gammopathy of undetermined significance: a population-based study including 15,351 cases and 58,619 matched controls. Haematologica. 2020;105(11):2679-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristinsson SY, Björkholm M, Andersson TM, et al. Patterns of survival and causes of death following a diagnosis of monoclonal gammopathy of undetermined significance: a population-based study. Haematologica. 2009;94(12):1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fermand JP, Bridoux F, Dispenzieri A, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132(14):1478-1485. [DOI] [PubMed] [Google Scholar]
- 16.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84(8):685-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. [PubMed] [Google Scholar]
- 18.Rögnvaldsson S, Love TJ, Thorsteinsdottir S, et al. Iceland screens, treats, or prevents multiple myeloma (iStopMM): a population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies. Blood Cancer J. 2021;11(5):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. [DOI] [PubMed] [Google Scholar]
- 20.Rögnvaldsson S, Long TE, Thorsteinsdottir S, Love TJ, Kristinsson SY. Validity of chronic disease diagnoses in Icelandic healthcare registries. Scand J Public Health. 2023;51(2):173-178. [DOI] [PubMed] [Google Scholar]
- 21.WHO. International statistical classification of diseases and related health problems, 10th revision (ICD-10). World Health Organization; 2016. [Google Scholar]
- 22.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.r-project.org. Accessed April 20, 2023. [Google Scholar]
- 23.Kyle RA, Durie BGM, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569. [DOI] [PubMed] [Google Scholar]
- 25.Turesson I, Kovalchik SA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014;123(3):338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasr SH, Satoskar A, Markowitz GS, et al. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20(9):2055-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung N, Bridoux F, Hutchison CA, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120(22):4292-4295. [DOI] [PubMed] [Google Scholar]
- 28.Nasr SH, Valeri AM, Cornell LD, et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7(2):231-239. [DOI] [PubMed] [Google Scholar]
- 29.Arnulf B, Bengoufa D, Sarfati E, et al. Prevalence of monoclonal gammopathy in patients with primary hyperparathyroidism: a prospective study. Arch Intern Med. 2002;162(4):464-467. [DOI] [PubMed] [Google Scholar]
- 30.Andreone P, Zignego AL, Cursaro C, et al. Prevalence of monoclonal gammopathies in patients with hepatitis C virus infection. Ann Intern Med. 1998;129(4):294-298. [DOI] [PubMed] [Google Scholar]
- 31.Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 2008;111(7):3388-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.KDIGO 2021. Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4s):S1-S276. [DOI] [PubMed] [Google Scholar]
- 33.England JD, Gronseth GS, Franklin G, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2009;1(1):5-13. [DOI] [PubMed] [Google Scholar]
- 34.Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97(6):818-829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.