Histiocytoses are rare hematological disorders characterized by the proliferation and accumulation of CD68+ histiocytes in tissues.1 Previously considered as inflammatory conditions, several adult’s histiocytoses (Erdheim-Chester disease [ECD], Langerhans cell histiocytosis [LCH], RosaiDorfman disease [RDD]) are now classified as myeloid neoplasms.2 Indeed, these patients display recurrent molecular features like somatic mutations in the mitogen activating-kinase (MAP-kinase) pathway genes,1 myeloid neoplasms (chronic myelomonocytic leukemia [CMML] and essential thrombocytopenia [ET])3 or clonal hematopoiesis (CH),4 which gave rise to this paradigm shift. Despite these significant advances, the cellular origin of histiocytes is still unknown. Nevertheless, monocytes are essential to the ontogeny of histiocytic disorders.5 Circulating monocytes are divided into three subsets (“classical”, “intermediate” and “non-classical”) with a distribution influenced by the innate immune system and environmental signals.6 During histiocytoses, circulating monocytes arising from bone marrow progenitors carry most MAP-kinase gene mutations, but only “classical” monocytes can differentiate into tissue histiocytes.5 Increase in “classical” monocytes was reported in CMML7 and inflammatory states,8 while a decrease in the “non-classical” subset was described in vascular disorders.9
However, little is known about the distribution of the circulating monocyte subsets in histiocytoses10 and their differences from other myeloid neoplasms.
We, therefore, sought to evaluate the distribution of the circulating monocyte subsets in patients with histiocytoses compared to patients with myeloid neoplasms (CMML and ET) and healthy donors (HD).
Peripheral blood cells were obtained from patients diagnosed at Dijon University Hospital between 2020 and 2021, with histiocytoses (n=17), CMML (n=7) mainly subtype CMML-0 (n=6), ET (n=7), and HD (n=21), after informed consent. All blood samples were obtained at a steady state, after ruling out infection and worsening of the hemopathy. Among histiocytoses, eight patients had ECD (62.5% BRAF V600E mutated), five LCH (40% BRAF V600E mutated), and 4 RDD (50% MAP2K1 mutated). Samples were obtained at the time of diagnosis for two patients (#4, #15) and four (#12, #15, #16, #17) were treatment-naïve. Three patients had concomitant myeloid neoplasms (2 CMML and 1 ET in ECD patients), and six had concomitant CH (4 ECD, 1 LCH, 1 RDD). Patient characteristics are reported in the Online Supplementary Table S1. Blood cells were stained for CD7, CD11b, CD13, CD14, CD15, CD16, CD33 and CD45. Data were acquired on a Navios cytometer and analyzed with Kaluza software (Beckman-Coulter®). Monocytes were separated on a CD14/CD16 scatter into CD14++/CD16- (classical), CD14++/CD16+ (intermediate), and CD14+/CD16++ (non-classical) as previously reported.11 The gating procedure is described in Figure 1. Diagnosis of histiocytoses was performed according to current guidelines, expert analysis and biopsies samples were centrally reviewed by an expert pathologist (JFE) for molecular analysis. Organ involvement and disease activity were assessed by imaging including 18fluorodeoxyglucose positron emission tomography.1 Diagnosis of myeloid neoplasms and clonal hematopoiesis (CH) was assessed according to biological tests completed by bone marrow analysis.2 CH was defined by at least one myeloid gene mutation in the bone marrow with a variant allele frequency over the threshold of 2% and no morphological evidence for hematological malignancies.
The myeloid gene panel analyzed by next-generation sequencing (NGS) included: ASXL1, ASXL2, AT M, BCOR, CALR, CBL, CEBPA, DDX41, DNMT3A, EZH2, FLT3, GATA2, HRAS, IDH1, IDH2, IKZF1, JAK2, KIT, KRAS, MPL, NPM1, NRAS, PHF6, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SH2B3, SMC1A, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, WT1, ZRSR2, BRAF, ETV6, BCORL1, CCND3, EP300, EPOR, ETNK1, NFE2, PPM1D, STAG1, TERC, TERT, TET3, THPO, U2AF2, UBA1, EIF6, SRP72, SRP68, ANKRD26, RBBP6, GATA1, RPL23, PRPF8 and CSNK1A1.
Quantitative data are expressed by median with interquartile range (IQR) and were compared between groups using Mann-Whitney tests. Qualitative data are expressed by number (percentage) and were compared using Χ2 or Fisher tests, as appropriate. For multiple group comparisons (>2), we used an analysis of variance (ANOVA) test with the Kruskal-Wallis procedure followed by Dunn’s multiple comparison with histiocytoses as the reference group. Multiple linear regression analysis was performed with all variables with a P value <0.2 in simple linear regression analysis for all monocyte subsets. Statistical significance was set at P<0.05 (two-sided). Analyses were performed with GraphPad Prism software V.9 (GraphPad, San Diego, California, USA).
The study was approved by the Ethics Committee of Dijon University Hospital and is in accordance with the principles of the Declaration of Helsinki.
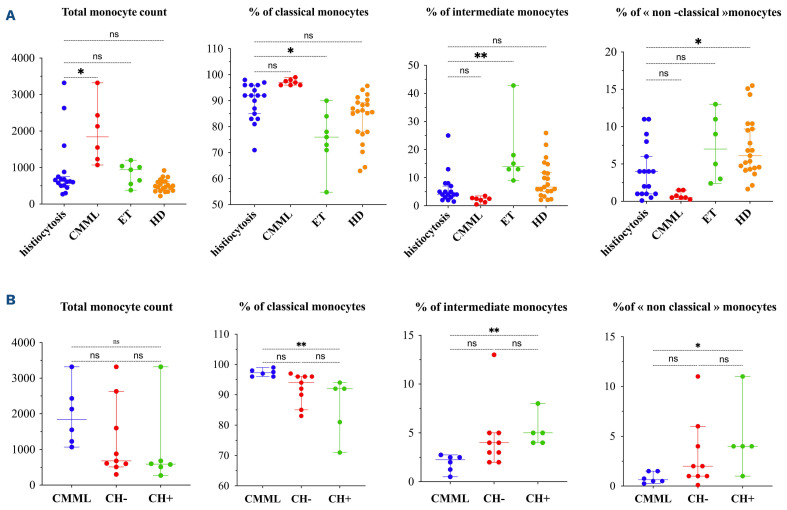
During histiocytoses, an increase in “classical” monocytes was observed, compared to ET (P=0.01) while “intermediate” (vs. ET; P=0.02) and “non-classical” monocytes (vs. HD; P=0.04) were decreased (Figure 2A; Online Supplementary Table S2). Monocyte repartition did not differ between the different types of histiocytoses. Nevertheless, ECD patients had increased “classical” monocytes compared to ET patients (93%; IQR, 83-96 vs. 76%; IQR, 71-84; P=0.032) and decreased “non-classical” monocytes compared to HD (1.5% IQR, 1-4 vs. 6.1%; IQR, 4-10; P=0.029), echoing the study published by Papo et al.10 The distribution of monocyte subsets in histiocytoses was close to that in CMML (Figure 2A), suggesting some common pathogenesis pathways between those two disorders. Two arguments may partly explain this close distribution. First, CMML is the most frequent myelodysplastic/myeloproliferative neoplasms associated with histiocytoses.3 Secondly, the involvement of the MAP-kinase pathway activation has been reported in both pathologies (mostly by BRAF or MAP2K1 gene mutations in histiocytoses and KRAS, NRAS followed by BRAF in CMML).1,12
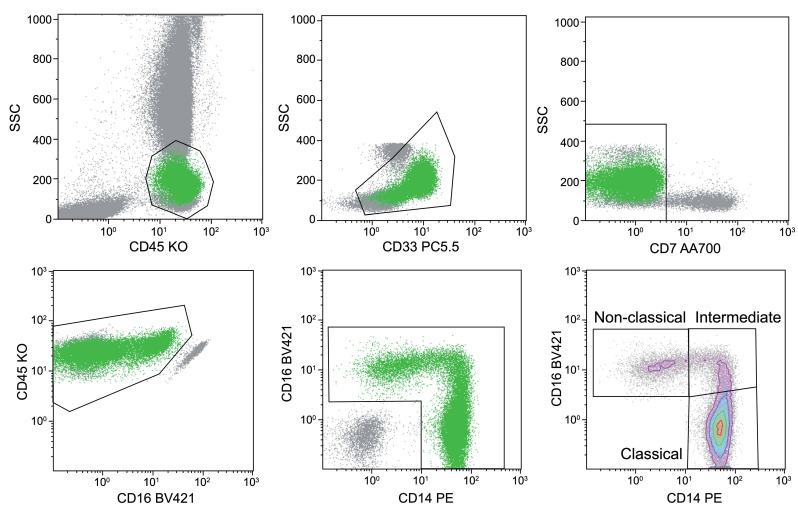
Figure 1.
Procedure for gating monocytes by flow cytometry. Monocytes were identified from peripheral blood mononucelar cells with CD45 and CD33 expression after successive exclusion of T lymphocytes, natural killer (NK) cells, residual lymphocytes and basophils. Then monocytes were analyzed regarding CD14/CD16 expression for classical, intermediate and non-classical monocytes. KO: knockout; SSC: side scatter.
Furthermore, we have evaluated the impact of bone marrow mutation, i.e., clonal hematopoiesis, in monocyte distribution in patients with histiocytoses with or without CH compared to CMML patients. The presence of CH in histiocytoses patients induced significant changes in monocyte distribution compared to CMML patients with a decrease in “classical” monocytes (92%; IQR, 76-93 vs. 97%; IQR, 96-98; P=0.002), and an increase in intermediate and “non-classical” monocytes (5%; IQR, 4.0-6.5 vs. 2.3%; IQR, 1.0-2.5; P=0.03; and 4%; IQR, 2.5-7.5 vs. 0.6%; IQR, 0.4-1.5; P=0.01) while no significant difference was observed in CH histiocytoses patients compared to CMML patients. These results suggest an impact of clonal hematopoiesis in the distribution of monocytes during histiocytoses (Figure 2B).
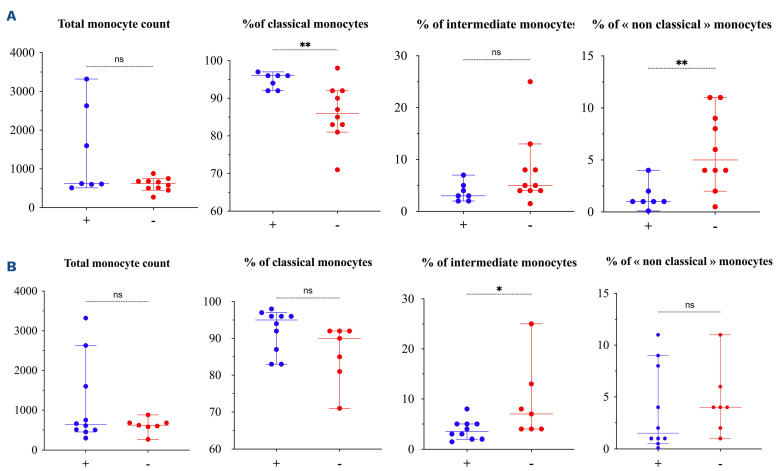
We investigated whether the distribution of the monocyte subsets was associated with specific organ damage. Seven (6 ECD including 5 BRAF V600E, 1 RDD) patients had vascular involvement. They presented an increase in classical monocytes (96.00%; IQR, 92.0-96.0 vs. 86.00%; IQR, 82.5-92.0; P=0.008) and a decrease in “non-classical” monocytes (1.00%; IQR, 1.0-2.0 vs. 5.00%; IQR, 3.50-9.50; P=0.007) (Figure 3A). The correlation between “non-classical” monocytes <4% and vascular involvement was confirmed by Pearson model (0.648; 95% confidence interval [CI]: 0.25-0.86; P=0.005) (Online Supplementary Figure S1). Our results are in line with the fact that “non-classical” monocytes are associated with vascular disorders,9 their decrease being correlated with the progression of coronary disease in atherosclerotic patients.13 Thus, our results in histiocytoses suggest that “non-classical” monocytes may be a specific marker of vascular damage, irrespective of the mechanism.
Figure 2.
Distribution of monocyte subsets in histiocytoses and myeloid neoplasms and according to molecular status. (A) Distribution of monocyte subsets in patients with histiocytoses (N=17), chronic myelomonocytic leukemia (CMML) (N=7), essential thrombocythemia (ET) (N=7) and heathy donors (HD) (N=21). (B) Distribution of monocyte subsets in histiocytoses with clonal hematopoiesis (CH+) (N=6), without clonal hematopoiesis (CH-) (N=9) and CMML patients (N=7). Percentages are given among total monocytes. Horizontal bars show the median and error bars the interquartile range. P value is the result of the ANOVA test. *P<0.05; **P<0.01; ns: non-significant.
We then investigated whether the distribution of monocyte subsets correlated with disease activity. Histiocytoses patients achieving a metabolic response had a lower percentage of “intermediate” monocytes (3.5%; IQR, 2.0-5.0 vs. 7.0%; IQR, 4.0-13.0; P=0.04) and lower CRP levels (3.0 mg/L; IQR, 1.1-8.7 vs. 33.65 mg/L; IQR, 5.3-59.5 P=0.04) (Figure 3B), which is in line with the fact that “intermediate” monocytes produce higher levels of IL-12, TNF-α, IL-1β, and IL-6 than “classical” monocytes.14 Thus patients achieving a metabolic response have the lowest “inflammatory” state, as supported by low CRP levels and low “intermediate” monocyte frequency.
We then assessed whether intrinsic or extrinsic factors could influence the distribution of monocyte subsets in patients with histiocytoses. Multiple linear regression showed a relationship between clonal hematopoiesis and the percentage of “classical” monocytes (β coefficient: -10.78; 95% CI: -16.83 to -4.737; P=0.002). Monocyte regulation depends on the innate immune system and environment.6 Recently, the concept of trained immunity in myeloid cell homeostasis has emerged. This elaborated program is driven by epigenetic machinery providing memory immunity in the monocyte/macrophage system.15 As the trained immunity influences hematopoiesis and inflammation leads to clonal hematopoiesis, our data question the role of trained immunity in all histiocytoses, not only in BRAF-mutated ECD.16 Our study has some limitations. First we focused on circulating cells, whereas monocytes bearing the mutations represent a marginal proportion of mutated bone marrow-derived cells. However, in order to determine biomarkers useful to the management of patients, circulating cells remain the most convenient. Secondly, the analyses were performed at different times in the disease course, and drugs may have interfered with the distribution of monocyte subsets. Nevertheless, it is currently the best way to assess the modification of monocyte subset distribution related to disease activity. In addition, complementary molecular analysis in all the tissues available revealed a higher frequency of myeloid gene mutations in the bone marrow rather than in the MAP kinase pathway which may be explained by the combined effect of drugs17 and aging.18 Finally, we did not perform Grubb’s test and outlier exclusion because of the difficulty of obtaining samples in these rare diseases.
Figure 3.
Distribution of monocyte subsets in histiocytoses according to vascular injury and disease activity. Comparison of the distribution of monocyte subsets according to (A) the presence of vascular injury (N=7) or not (N=10), and (B) to the achievement of a metabolic response (N=10) or not (N=7). Disease activity was established using the last metabolic evaluation with 18fluorodeoxyglucose positron emission tomography-computed tomography according to PERCIST criteria. Complete metabolic response was defined by normalization of all lesions to at or below standardized uptake value (SUV) of liver background. Partial metabolic response was defined by a ≥50% decrease in the sum of all target lesion baseline SUV. Progressive metabolic disease was defined by a ≥50% increase in the nadir sum of all target or new evaluable lesion SUV. Stable metabolic disease was defined as condition that did not meet previous criteria. Patients with "complete metabolic response" and "partial metabolic response" were considered as responders and patients with "stable metabolic disease" and "progressive metabolic disease" considered as non-responders.
However, our work highlights for the first time the difference in monocyte distribution in histiocytoses compared to myeloid malignancies and HD. It also showed a correlation between “non-classical” monocytes and vascular involvement, which can be helpful for both initial staging and follow-up. As the decrease in “intermediate” monocytes was associated with lower CRP levels and metabolic response, its accuracy in response assessment will be prospectively explore to determine which of "intermediate" monocytes or CRP variation is more effective in predicting relapse.
In summary, circulating monocytes may be the precursors of pathogenic histiocytes in tissues. Their subset distribution is singular in histiocytoses compared to other myeloid neoplasms and is influenced by clonal hematopoiesis. The decrease in the “non-classical” subset could represent a surrogate marker of vascular involvement, while the decrease of the intermediate fraction is associated with a metabolic response.
Supplementary Material
References
- 1.Emile JF, Cohen-Aubart F, Collin M, et al. Histiocytosis. Lancet. 2021;398(10295):157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papo M, Diamond EL, Cohen-Aubart F, et al. High prevalence of myeloid neoplasms in adults with non-Langerhans cell histiocytosis. Blood. 2017;130(8):1007-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen Aubart F, Roos-Weil D, Armand M, et al. High frequency of clonal hematopoiesis in Erdheim-Chester disease. Blood. 2021;137(4):485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durham BH, Roos-Weil D, Baillou C, et al. Functional evidence for derivation of systemic histiocytic neoplasms from hematopoietic stem/progenitor cells. Blood. 2017;130(2):176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selimoglu-Buet D, Wagner-Ballon O, Saada V, et al. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood. 2015;125(23):3618-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fingerle G, Pforte A, Passlick B, et al. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82(10):3170-3176. [PubMed] [Google Scholar]
- 9.Chimen M, Yates CM, McGettrick HM, et al. Monocyte subsets co regulate inflammatory responses by integrated signaling through TNF and IL-6 at the endothelial cell Interface. J Immunol. 2017;198(7):2834-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papo M, Corneau A, Cohen-Aubart F, et al. Immune phenotyping of Erdheim-Chester disease through mass cytometry highlights decreased proportion of non-classical monocytes and increased proportion of Th17 cells. Ann Rheum Dis. 2020;79(11):1522-1524. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74-80. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Singh RR, Patel KP, et al. BRAF kinase domain mutations are present in a subset of chronic myelomonocytic leukemia with wild-type RAS. Am J Hematol. 2014;89(5):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang J, Han Y, Xu D, et al. Comparison of circulating dendritic cell and monocyte subsets at different stages of atherosclerosis: insights from optical coherence tomography. BMC Cardiovasc Disord. 2017;17(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74(7):2527-2534. [PubMed] [Google Scholar]
- 15.Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molteni R, Biavasco R, Stefanoni D, et al. Oncogene-induced maladaptive activation of trained immunity in the pathogenesis and treatment of Erdheim-Chester disease. Blood. 2021;138(17):1554-1569. [DOI] [PubMed] [Google Scholar]
- 17.Coombs CC, Zehir A, Devlin SM, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlush L. Age-related clonal hematopoiesis. Blood. 2018;131(5):496-504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.