Abstract
In children with sickle cell anemia (SCA), early splenic complications can require splenectomy, but the benefit-to-risk ratio and the age at which splenectomy may be safely performed remain unclear. To address this question, we analyzed the rate of post-splenectomy events in children with SCA splenectomized between 2000-2018 at the Robert Debré University Hospital, Paris, France. A total of 188 children underwent splenectomy, including 101 (11.9%) from our newborn cohort and 87 referred to our center. Median (Q1-Q3) age at splenectomy was 4.1 years (range 2.5-7.3 years), with 123 (65.4%) and 65 (34.6%) children splenectomized at ≥3 years of age or <3 years of age, respectively. Median post-splenectomy follow-up was 5.9 years (range 2.7-9.2 years) yielding 1192.6 patient-years (PY) of observation. Indications for splenectomy were mainly acute splenic sequestration (101 [53.7%]) and hypersplenism (75 [39.9%]). All patients received penicillin prophylaxis; 98.3% received 23-valent polysaccharic pneumococcal (PPV-23) vaccination, and 91.9% a median number of 4 (range 3-4) pneumococcal conjugate vaccine shots prior to splenectomy. Overall incidence of invasive bacterial infection and thrombo-embolic events were 0.005 / PY (no pneumococcal infections) and 0.003 / PY, respectively, regardless of age at splenectomy. There was an increased proportion of children with cerebral vasculopathy in children splenectomized <3 years of age (0.037 / PY vs. 0.011 / PY; P<0.01). A significantly greater proportion of splenectomized than non-splenectomized children were treated with hydroxycarbamide (77.2% vs. 50.1%; P<0.01), suggesting a more severe phenotype in children who present spleen complications. If indicated, splenectomy should not be delayed in children, provided recommended pneumococcal prophylaxis is available. Spleen complications in childhood may serve as a marker of severity.
Introduction
In children with sickle cell disease, spleen function may be altered early in life. This alteration has been shown to occur as early as 3-6 months of age and to affect 87% of infants with sickle cell anemia (Hb SS or Hb S β° thalas-semia) at a mean age of 12.9 months.1,2 In the majority of children, this process is clinically silent. Over time, the spleen becomes fibrotic and loses all its main functions, namely blood filtration and immune defense against blood-borne pathogens, resulting in functional asplenia and, ultimately, to the complete loss of the organ, a process called auto-splenectomy.3,4
However, in a small proportion of children, the spleen may cause serious clinical complications such as acute splenic sequestration (ASS) or hypersplenism. ASS refers to a rapid trapping of red blood cells (RBC) in an acutely enlarged spleen, resulting in hypovolemia and severe anemia. Its prevalence is around 10-25% in children with SCA, with a median age at first occurence of 1.4 years.5 ASS is generally unpredictable, although a low fetal hemoglobin (HbF) level in the first 3-6 months of life has been correlated to its occurrence.6,7 Importantly, ASS further alters spleen function.1 Modalities of prevention of ASS are still under debate because neither chronic transfusion nor hydroxycarbamide (the major disease-modifying treatment in SCA) have demonstrated efficacy in decreasing its recurrence.8 The other frequent splenic complication is hypersplenism, a condition defined by chronic enlargement of the spleen and subsequent cytopenia.9 Its onset is generally progressive and its prevalence is ill-defined. Hypersplenism can result in failure to thrive, severe chronic anemia, and abdominal pain. Both ASS crises and hypersplenism result in, or coexist with, reduced spleen function.
The major consequence of hyposplenism is an increased susceptibility to infection from encapsulated bacteria, particularly to pneumococcal invasive infections.10,11 This risk is particularly high in very young infants, as in infancy (≤2 years of age) the spleen has still not fully matured.12 In children with SCA, this increased risk was well-established both before and after the initiation of systematic prophylactic therapy with penicillin and the introduction of pneumococcal vaccines.13-17 While these preventive measures have dramatically reduced the risk of infection, pneumococcal invasive infections remain frequent and severe, are the leading cause of death in young children with SCA in high-income countries, and presumably contribute in large measure to the high rate of mortality in children <5 years of age in low-income countries.18,19 Surgical splenectomy is the radical treatment of acute splenic events in children with SCA, but its consequences in terms of additional infectious risks are difficult to understand in the absence of a baseline measurement of the residual splenic function at the time of surgery. In other words, whether surgical splenectomy further increases the risk of pneumococcal infection in children with SCA is still an open question. In addition, the age at which splenectomy is best performed remains a subject of debate. Early publications on splenectomy in children with hemolytic disorders showed a very high risk of overwhelming sepsis in young infants.20,21 While very few studies have evaluated the risks of splenectomy with age, in many centers the lower age limit for splenectomy has been fixed at 4-5 years of age, although the evidence supporting such a recommendation is poor.16,22 Splenectomy has also been associated with an increased thrombo-embolic risk in patients with hemolytic anemias, notably in patients with non-transfusion-dependent thalassemia.23,24 SCA is a hypercoagulable state at baseline, with contributing factors including chronic inflammation, increased circulation of micro particles and thrombocytosis.25-28 At the same time, a higher incidence of venous thromboembolism has been evidenced in patients with SCA.29-31 Nevertheless, it is still unclear whether the surgical removal of the spleen further increases the thromboembolic risk.
In general, the benefit-to-risk ratio of splenectomy can be challenging to define in young children with SCA. Recent studies have indicated there is no increased risk, but these have concerned either small numbers of patients with SCD, or a later age at splenectomy in relation to a large proportion of patients with Sβ+ genotypes.17,32 As a result, the lower limit of age at which splenectomy may be safely performed remains to be determined. The main objective of this study was, therefore, to analyze the occurrence of infectious complications related to encapsulated bacteria as well as of thrombo-embolic events in a large cohort of children with SCA who underwent splenectomy during childhood. The secondary objectives were to analyze the effect of age at splenectomy on the occurrence of medical events in the post-splenectomy period, and to compare the rate of sickle cell-related events with a non-splenectomized population.
Methods
The Robert Debré University Hospital is a tertiary hospital in Paris, France, with expertise in the care of children with SCA and a national reference center for this disease population. The center manages the follow-up of one of the largest cohorts of children with this diagnosis in Europe. This study included patients from the new born cohort as well as patients referred to the surgical department of our center. The new born cohort includes all patients who have been followed-up at our institution since diagnosis after new born screening. The non-splenectomized patients of the new born cohort served as a comparison group for the secondary objectives of the study. This retrospective study included all children with SCA (homozygous sickle cell anemia or Sβ° thalassemia) splenectomized at our center between January 1st 2000 and December 31st 2018. The observation period began at the date of splenectomy and ended either the day of the last known visit to our center or the date of hematopoietic stem cell transplant or the date of death or July 1st 2021, whichever came first.
During the study period, all children received daily penicillin prophylaxis (50-100,000 UI/kg/day) until 10 years of age, as well as a 23-valent polysaccharic pneumococcal vaccination after 2 years of age (with booster shots every 3-5 years), as part of our institutional protocol. In addition, after 2000, children were immunized with a 7-valent conjugated pneumococcal vaccine, and this was replaced by a 13-valent conjugated pneumococcal vaccine after 2010. Treatment with hydroxycarbamide was initiated in those children who after 2 years presented with recurrent painful crises or acute chest syndrome, as recommended in the national guidelines at the time of study.33 All medical events treated in our hospital and/or reported in the local medical file prior to and following surgical splenectomy were recorded, including indication for splenectomy. Primary outcomes of interest (invasive infectious episode and thrombo-embolic events) were recorded post splenectomy. An invasive infectious episode was defined as a septic episode with a positive bacterial identification (culture or PCR).34-36 Episodes of pneumonia without bacterial identification were not considered invasive infections. Thrombo-embolic events included deep vein thrombosis and/or pulmonary embolism diagnosed by ultrasound (US) or computed tomography (CT) scan. The occurrence of medical events related to SCA such as vaso-occlusive events (VOE), acute chest syndrome (ACS), delayed post-transfusional reaction (DHTR), or cerebral vasculopathy (CV) were also collected post splenectomy. The definition of CV included the occurrence of an abnormal transcranial Doppler and/ or a vascular stenosis of a major cerebral artery on MRA and/or an overt stroke. Events that occurred within one month of surgery were further categorized as post-operative complications. This study was approved by the local ethics committee (CEER-RD n. 2021-552) and was registered at the Assistance Publique des Hôpitaux de Paris (AP-HP) (n. 20210408110925).
Statistical analysis
Results are expressed as numbers and percentages for categorical variables and as median (25th quartile; 75th quartile) for continuous variables. Overall and age-specific incidence of events were calculated as the number of events divided by total patient-years (PY) at risk. The univariate comparison of percentages was performed using the χ2 test and the Mann-Whitney test to compare the distribution of the continuous variables. Poisson regression analyses were performed to compare incidence of medical events according to the age at splenectomy (before or after 3 years of age). This age threshold was set because it is increasingly applied in clinical practice and because, in our center, where a substantial number of children underwent splenectomy between the ages of 2 and 3 years, it allowed for an acceptable sample size for comparative purposes.37,38 Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
General results
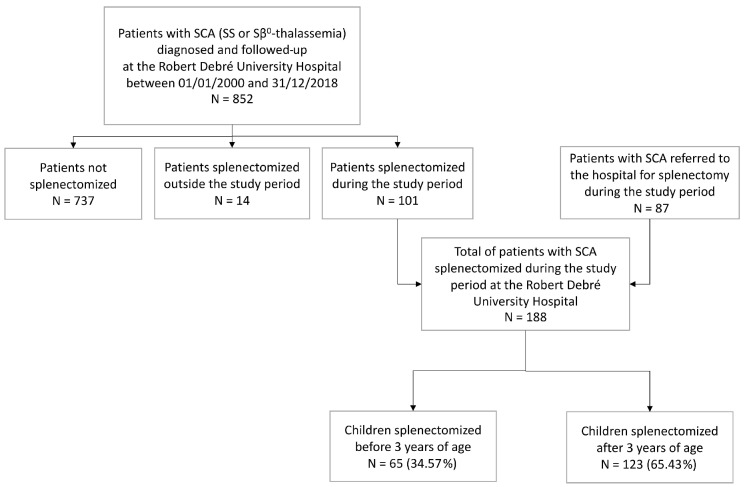
During the study period, a total of 1167 children with sickle cell disease were followed-up at the Robert Debré University Hospital subsequent to new born screening, including 852 children with SCA (either HbSS or HbS β° thalassemia) (Figure 1). A total of 101 (11.9%) children (all with SCA) underwent splenectomy. In addition, 87 children with SCA were referred to our hospital for splenectomy. Altogether, 188 splenectomized children were included in this analysis. All patients underwent total splenectomy.
General characteristics of the cohort
In this cohort, 55.3% were male. The majority of patients were homozygous for HbS (164; 87.2%), 23 (12.2%) were compound heterozygotes Hb Sβ°-thalassemia, and one patient had an undetermined genotype (SS or Sβ°-thalassemia). G6PD status was known in 158 (84.0%) patients, with a deficiency evidenced in 27 (17.1%). Median (Q1-Q3) follow-up of the cohort was 11.8 years (range 7.5-16.5 years), with a median post-splenectomy follow-up of 5.9 years (range 2.7-9.2 years), altogether yielding 1192.6 PY.
Median age at splenectomy was 4.1 years (range 2.5-7.3 years), including 3 children splenectomized before 2 years of age: at 1.8, 1.9, and 1.9 years of age, respectively (see the Online Supplementary Appendix for details). Median age of the cohort at the time of analysis was 14.4 years (range 9.9-18.3 years). Splenectomy was performed by laparoscopic procedure in 179 (95.2%) cases and laparotomy in 9 (4.8 %) cases.
During follow-up, one death (0.5%) occurred, in a boy of 9 years of age who had undergone splenectomy at the age of 29 months. He died from a posterior stroke that occurred during a severe episode of ACS requiring mechanical ventilation. His death was considered unrelated to splenectomy. A total of 9 patients benefited from a hematopoietic stem cell transplant (HSCT) with an HLA-matched sibling donor. (Indications for HSCT are detailed in Online Supplementary Table S1).
Pneumococcal prophylaxis
All the children in this cohort (100%) were receiving penicillin prophylaxis at the time of the surgery. Regarding the pneumococcal conjugated vaccine (PCV 7 or 13), of 172 (95.0%) patients with available data and eligible for PCV vaccination during the study period (PCV 7 licensure in 2000 and PCV 13 licensure in 2010), 158 (91.9%) had received at least one dose, while the median number of doses of PCV vaccine received at the time of splenectomy was 4 (range 3-4). Regarding the polysaccharidic pneumococcal vaccine (PPV-23), among 173 patients with available data and eligible for vaccination (age >2 years), 170 (98.3%) were appropriately vaccinated: 166 (95.9%) before splenectomy and 4 (2.3%) shortly (<3 months) after splenectomy. Median age at immunization was 2.1 years (range 1.8-3.3 years), and a median of 2 (range 1-3) doses were given during follow-up.
Indication for splenectomy
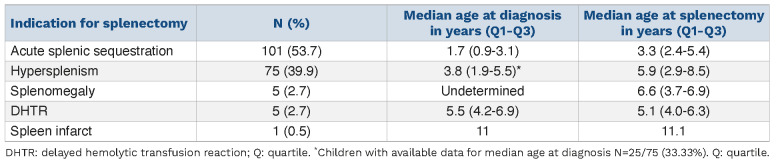
Indications for splenectomy are shown in Table 1. Prevention of ASS recurrence was the main indication in 101 (53.7%) cases. Median age at splenectomy was significantly younger in children splenectomized for ASS (P<0.01). Regarding splenomegaly and hypersplenism, in many cases, median age at diagnosis was not determined, in relation with the progressive onset of these complications. Of note, in 5 patients who had presented prior ASS, splenectomy was performed as an emergency procedure due to the occurrence of DHTR, suspected because of an acute post-transfusion drop of hemoglobin concentration, a low level of post transfusion hemoglobin A, signs of hyperhemolysis, and an acutely enlarged spleen.
Regarding children whose indication for splenectomy was ASS, the first episode occurred at 1.7 years of age (range 0.9-3.1 years) with a median 3 episodes (range 2.0-4.0). The majority of children had experienced more than one episode before splenectomy, with a number of episodes of ASS before splenectomy of one, 2-5, and > 5 episodes in 4 (4.0%), 89 (88.1%), and 6 (5.9%) cases, respectively. All 4 patients splenectomized after one single episode of ASS presented with severe threatening acute anemia (Hb ranging from 1.5 to < 4.7g/dL). (Details of these cases are available in the Online Supplementary Appendix).
Management
All except one allo-immunized child benefited from either a 'top-up' transfusion or exchange transfusion prior to surgery. In 84 (45.2%) children, this was part of a prophylactic chronic transfusion therapy initiated prior to surgery, with a median duration of 15.0 months (range 8.0-22.0 months). Median ferritin level at the time of splenectomy was available in 50 (59.5%) patients and was 395 ng/mL (range 159-783 ng/mL), with 12 patients (14.6%) receiving iron chelation therapy. Only 36 patients (19.1%) were treated with hydroxycarbamide before splenectomy.
Incidence of infectious and thrombo-embolic complications
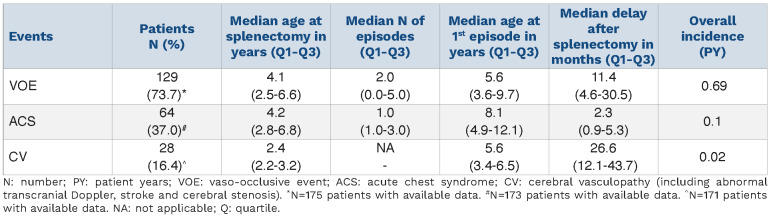
Six cases of invasive bacterial infection occurred in 5 patients during follow-up, yielding an incidence of 0.005 PY. Of note, there was no infection with an encapsulated bacterium. These infections included 5 episodes of septicemia and one episode of osteoarticular infection. The bacteria isolated during these episodes were salmonella (n=3), Klebsiella (n=1), E. Coli (n=1), and Enterobacter cloacae (n=1). These events occurred with a median delay of 10.3 months (range 8.0-49.9 months) following splenectomy.
A thrombo-embolic complication occurred in 4 cases during follow-up, including one case in the post-operative period, yielding an incidence of 0.003 PY. These events (all deep vein thrombosis) occurred at Day 4, 12 months, 15 months, and 34 months after surgical splenectomy.
Clinical course post splenectomy
During the post-operative period, 8 complications occurred in 6 patients (0.03%) despite prior exchange transfusion: VOE n=3, ACS n=2, and ACS + left subphrenic hematoma + portal thrombosis in one patient. These complications were not associated with age at splenectomy.
Figure 1.
Flow chart of the study. SCA: sickle cell anemia; N: number.
Table 1.
Indication for splenectomy in the cohort of children with sickle cell anemia.
Table 2.
Description of main sickle cell anemia-related events following splenectomy.
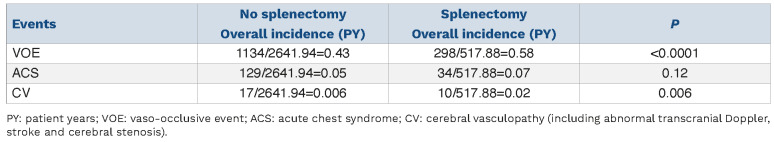
Occurrence of relevant SCA-related events (VOE, ACS, DHTR, CV) following splenectomy is detailed in Table 2. There was no difference in incidence of VOE, ACS or CV according to the indication for splenectomy. DHTR was recorded in 7 patients following splenectomy with a median (min-max) delay of 5.8 years (range 1.1-11.7 years). A total of 28 (16.4%) patients developed CV, yielding an overall incidence of 0.02 PY; delay post splenectomy ranged from 5 to 82 months. Cerebral vasculopathy consisted of abnormal transcranial Doppler (TCD) in 19, cerebral arterial stenosis on MRI in 8, and stroke in one child, 16 months after splenectomy.
A total of 138 (78.41 %) children were treated with hydroxycarbamide at last follow-up, out of 176 patients for whom data were available. Median age at initiation was 5.4 years (range 3.5-8.3 years).
Comparison of clinical profile according to splenectomy
In order to further investigate the profile of patients who underwent splenectomy and its association with clinical phenotype, we compared the frequency of events among splenectomized and non-splenectomized children who underwent follow-up at our institution. Both groups had a comparable median age: 12 years (range 9.0-17.8 years) versus 13.5 years (range 6.9-19.2 years) (P=0.59). While there was an increase in the use of hydroxycarbamide over time, there was a significantly greater proportion of patients treated in the splenectomized group both at each time period and overall: 78/101 (77.2%) versus 369/737 (50.1%) (P<0.01). Median age at hydroxycarbamide initiation was also significantly younger in splenectomized patients: 4.9 years (range 3.3-7.3 years) versus 6.7 years (range 4.0-10.3 years) (P=0.001).
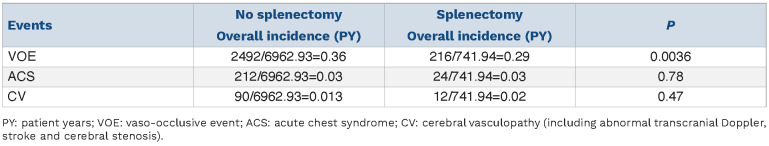
In order to take into account the effect of treatment, we compared the incidence of complications in treated and non-treated children according to spleen status (splenectomy vs. no splenectomy) (Tables 3 and 4). While the incidence of ACS did not vary, the incidence of VOE was higher in non-treated non-splenectomized children while it was greater in treated splenectomized children, suggesting a more severe profile in the latter. Regarding CV, there was a greater incidence of events in the splenectomized group, with a trend in untreated children, and a significant difference in treated children.
Comparison of patients’ characteristics and events according to age at splenectomy
General characteristics
In the global cohort of 188 children, 65 (34.6%) underwent splenectomy before the age of 3 years, and 123 (65.4%) after the age of 3 years (Figure 1). Median (Q1-Q3) post-splenectomy follow-up was 5.0 (range 3.6-8.5) and 6.4 (range 2.5-9.7) years in the groups splenectomized <3 and ≥3 years of age, respectively. Of note, there was a significant difference in rates of splenectomy before 3 years of age between the different time periods of our study (P=0.046): 36.7%, 20%, 34.1%, and 51.5% during the 2000-2005, 2005-2010, 2010-2015, and 2015-2018 time periods, respectively.
Table 3.
Incidence of complications in untreated children according to splenectomy or no splenectomy.
Table 4.
Incidence of complications in children treated with hydroxycarbamide according to splenectomy or no splenectomy.
Indication for splenectomy before the age of 3 years was ASS in the majority of cases (n=44, 67.7%), a proportion significantly greater than in children splenectomized after the age of 3 years (n=57 [46.3]; P<0.01). This group of patients benefited from a transfusion program in a larger proportion than older children (70.8% vs. 31.4%; P<0.01). Similarly, this younger group was treated in a larger proportion with hydroxycarbamide (88.5% vs. 73.0%; P=0.02), at a younger age (3.5 [range 2.8-5.0] years vs. 7.3 [range 4.9-9.5] years; P<0.001), particularly when the indication for hydroxycarbamide initiation was anemia (38.9% vs. 7.1% in the older group; P<0.01). Of note, all children except three splenectomized before the age of 3 years were started on hydroxycarbamide following splenectomy, and not before, ruling out the possibility that treatment may have been the cause of spleen complications and the need for subsequent early splenectomy.
Comparison of post-splenectomy events in children according to age at splenectomy
There was no statistical difference between the two groups in the incidence of post-splenectomy invasive infections (0.005 PY in both groups; P=0.96) or thromboembolic events (0.003 PY before the age of 3 years vs. 0.004 PY after the age of 3 years; P=0.70)
Regarding other events, while the median age at first episode of VOE was younger in those splenectomized earlier, the overall incidence of VOE did not differ according to age at splenectomy (0.68 PY vs. 0.69 PY, respectively; P=0.93). Similarly, the median age at first episode of ACS was younger in those splenectomized before the age of 3 years but there was no difference in overall incidence between groups (0.09 / PY vs. 0.10 / PY, respectively; P=0.65). In contrast, there was a statistically increased frequency of cerebral vasculopathy in children splenectomized before the age of 3 years compared to the others (0.04 / PY vs. 0.01 / PY; P<0.01), despite a higher proportion of children treated with hydroxycarbamide.
Discussion
This study is the largest report so far on splenectomy in children with SCA and related complications. Regarding the risk of overwhelming sepsis, the period of follow-up (1192.6 PY) enables us to confidently confirm its very low incidence, in agreement with recent reports in other settings.16,22,39 Arguably, this risk has been described as lifelong, leaving a possibility of delayed events not captured by this study; however, most events are known to occur in the two first years following splenectomy. Regarding this low infectious risk, it is important to remember that the level of vaccine coverage against pneumococcal risk was high in this cohort (>90%) for both PCV and polysaccharidic vaccines, a scenario that is not always the case, including in high-income countries.40 In line with this, all children were also receiving antibiotic prophylaxis at the time of splenectomy and presumably until at least 10 years of age, as recommended at our center.41 With such anti-pneumococcal measures, splenectomy in childhood with SCA appears to be safe and does not result in an increased risk of severe invasive infection. Consequently, the risk of overwhelming infection should no longer be a major argument against surgical splenectomy whenever indicated.17,32
One important finding of this study is the absence of an increased infectious risk when splenectomy is performed before 3 years of age, with a comparable very low incidence of invasive infections in those splenectomized before or after 3 years of age. However, a large majority of children were splenectomized between 2 and 3 years of age in the younger group, with only 3 children splenectomized under the age of 2. The absence of an increased infectious risk in these younger children may be associated to the early loss of spleen function, with a subsequent risk related to surgical splenectomy that is not modified by the procedure. It has, indeed, been demonstrated that ASS negatively impacts splenic function.1 Accordingly, the majority of children who were under 3 years old at splenectomy had experienced ASS, with a median number of 3 episodes. Whatever the explanation, this finding seems to suggest that it is better to proceed to splenectomy without delay in children aged between 2 and 3 years old who have an indication of splenectomy for ASS, although such a management strategy is not currently widely accepted. In many centers, prophylactic chronic transfusion therapy is usually initiated in order to decrease the risk of severe acute anemia in case of recurrence of ASS, and this is prolonged until splenectomy can be “safely” performed, generally around 4 or 5 years of age.37,4 2 However, chronic transfusion does not prevent the recurrence of ASS, while it may cause serious complications, including iron overload, as illustrated by an elevated mean ferritin level (395 ng/mL [range 159-783 ng/mL]) at a median age of 4.1 years in our cohort.8 Not only can a strategy based on early splenectomy avoid transfusion therapy, iron overload and alloimmunization, but also, in low-income countries, blood-transmitted viral infections.43 Nevertheless, early splenectomy is only to be considered if appropriate pneumococcal prophylaxis is both prescribed and available, and when parents have been educated about the risk of infection.
This study also suggests that surgical splenectomy does not increase the risk of thrombo-embolic events and that age at splenectomy does not influence this risk.44 Admittedly, median age at last follow-up was 14.4 years (range 9.9-18.3 years) and it cannot be excluded that the risk increases as these patients grow older. Likewise, splenectomy-related complications such as pulmonary hypertension were not observed in this cohort, as these events are exceptional in young patients but may increase over time.45 Whether surgical splenectomy specifically can be considered an additional risk to the basal risk of auto splenectomy in older patients remains to be determined. Our findings raise an important new question as to the relationship between spleen complications and disease severity, particularly when these occur early in life. Overall, a greater proportion of children who underwent splenectomy thereafter required hydroxycarbamide initiation compared to those who did not (77.2% vs. 50.1%). This finding cannot be attributable to differences in management or hydroxycarbamide prescription, as this comparison was performed within the same cohort during the same study period. Importantly, a greater incidence of cerebral vasculopathy was observed in splenectomized children, particularly in those splenectomized before the age of 3 years, a majority of whom presented episodes of ASS. At least two hypotheses may be advanced. The spleen may serve as a filter or a barrier protecting the cerebral vasculature from injury caused by dense cells or fragile red cells prone to hemolysis.28 The premature destruction of the splenic filter, particularly by ASS at an age of cerebral vascular vulnerability, may therefore predispose to cerebral vasculopathy. Whether the surgical procedure further aggravates the risk is, at this point, an unanswered question. Another explanation may be related to the hemolytic phenotype of patients who experience both cerebral vasculopathy and splenic complications, particularly ASS. The latter is usually wrongly viewed as being a vaso-occlusive complication, yet in the splenic filtering beds RBC flow freely out of vessels. ASS is associated with low HbF levels and a high rate of hemolytic markers.7 Consequently, early splenic complications may represent potential markers of disease severity. However, whether ASS in particular could serve as a surrogate marker of cerebrovascular risk remains to be confirmed.
For this retrospective cohort, we used data already collected within a database, hence minimizing biases, including recall bias.46 Nevertheless, this study has the limitations of observational designs (i.e., missing data, bias, no demonstration of causality) which cannot be avoided in any study on this subject since a randomized controlled trial would not be ethical in this context. Nonetheless, the study was conducted in a single center over a period of time in which those clinical practices that had the potential to impact the occurrence of post-splenectomy events did not undergo any major change. Moreover, a comparison of patients’ characteristics was made in order to avoid potential confounding variables. Finally, all children with sufficient data were included in our study, and our sample is representative of our center's population. In the largest pediatric cohort study of splenectomized patients with SCA to date, we show that surgical splenectomy does not result in a significantly increased risk of invasive bacterial infections or thrombo-embolic complication. Splenectomy performed before 3 years of age does not further increase those risks. Splenectomy should, therefore, not be delayed in patients once there is an indication for surgical removal, provided that the children have received the recommended vaccines and continue to receive prophylactic penicillin therapy. This study also points to a more severe phenotype in patients who present with spleen complications requiring splenectomy, as well as to the overlapping phenotype of patients who experience spleen complications (particularly ASS) and develop cerebral vasculopathy. The relationship between ASS and cerebral vascular vulnerability would be particularly interesting to investigate in a prospective study.
Supplementary Material
References
- 1.El Hoss S, Cochet S, Marin M, et al. Insights into determinants of spleen injury in sickle cell anemia. Blood Adv. 2019;3(15):2328-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers ZR, Wang WC, Luo Z, et al. Biomarkers of splenic function in infants with sickle cell anemia: baseline data from the BABY HUG Trial. Blood. 2011;117(9):2614-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nardo-Marino A, Glenthøj A, Brewin JN, et al. The significance of spleen size in children with sickle cell anemia. Am J Hematol. 2022;97(12):1520-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diggs LW. Siderofibrosis of the spleen in sickle cell anemia. JAMA. 1935;104(7):538-541. [Google Scholar]
- 5.Brousse V, Elie C, Bankerrou M, et al. Acute splenic sequestration crisis in sickle cell disease: cohort of 190 paediatric patients. Br J Haematol. 2012;156(5):643-648. [DOI] [PubMed] [Google Scholar]
- 6.Emond AM, Collis R, Darvill D, Higgs DR, Maude GH, Serjeant GR. Acute splenic sequestration in homozygous sickle cell disease: natural history and management. J Pediatr. 1985;107(2):201-206. [DOI] [PubMed] [Google Scholar]
- 7.Brousse V, El Hoss S, Bouazza N, et al. Prognostic factors of disease severity in infants with sickle cell anemia: a comprehensive longitudinal cohort study. Am J Hematol. 2018;93(11):1411-1419. [DOI] [PubMed] [Google Scholar]
- 8.Owusu-Ofori S, Remmington T. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev. 2017;11(11):CD003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Totowa NJ. The complete spleen: structure, function, and clinical disorders. Can J Surg. 2022;45(3):226. [Google Scholar]
- 10.Lee GM. Preventing infections in children and adults with asplenia. Hematol Am Soc Hematol Educ Prog. 2020;2020(1):328-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yee ME, Lai KW, Bakshi N, et al. Bloodstream Infections in children with sickle cell disease: 2010-2019. Pediatrics. 2022;149(1):e2021051892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143(10):3200-3206. [PubMed] [Google Scholar]
- 13.Rankine-Mullings AE, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Syst Rev. 2021;2021(3):CD003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428-1433. [DOI] [PubMed] [Google Scholar]
- 15.Adamkiewicz T, Thomas S, Tunali A, et al. Population-based surveillance of pneumococcal infections in children with sickle cell disease before and after Prevnar 7® and Prevnar 13® licensure: implications for expanded vaccination. Blood. 2021;138(Suppl 1):763. [Google Scholar]
- 16.Lesher AP, Kalpatthi R, Glenn JB, Jackson SM, Hebra A. Outcome of splenectomy in children younger than 4 years with sickle cell disease. J Pediatr Surg. 2009;44(6):1134-1138. [DOI] [PubMed] [Google Scholar]
- 17.Pinto VM, Gianesin B, Piel FB, et al. Morbidity and mortality of sickle cell disease patients is unaffected by splenectomy: evidence from 3 decades follow-up in a high-income setting. Haematologica. 2023;108(4):1158-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desselas E, Thuret I, Kaguelidou F, et al. Mortality in children with sickle cell disease in mainland France from 2000 to 2015. Haematologica. 2020;105(9):e440-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6 Suppl 4):S398-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King H, Shumacker HB. Splenic Studies: I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952;136(2):239-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eraklis AJ, Kevy SV, Diamond LK, Gross RE. Hazard of overwhelming infection after splenectomy in childhood. N Engl J Med. 1967;276(22):1225-1229. [DOI] [PubMed] [Google Scholar]
- 22.Kalpatthi R, Kane ID, Shatat IF, Rackoff B, Disco D, Jackson SM. Clinical events after surgical splenectomy in children with sickle cell anemia. Pediatr Surg Int. 2010;26(5):495-500. [DOI] [PubMed] [Google Scholar]
- 23.Borgna Pignatti C, Carnelli V, Caruso V, et al. Thromboembolic events in beta thalassemia major: an Italian multicenter study. Acta Haematol. 1998;99(2):76-79. [DOI] [PubMed] [Google Scholar]
- 24.Cappellini MD, Poggiali E, Taher AT, Musallam KM. Hypercoagulability in β-thalassemia: a status quo. Expert Rev Hematol. 2012;5(5):505-511; quiz 512. [DOI] [PubMed] [Google Scholar]
- 25.Brousse V, Allaf B, Benkerrou M. [Dépistage néonatal de la drépanocytose en France.] Med Sci (Paris). 2021;37(5):482-490. [DOI] [PubMed] [Google Scholar]
- 26.Leuenberger M, Sartori C. [La rate : entre mystères et découvertes.] Rev Med Suisse. 2010;6(269):2080-2085. [PubMed] [Google Scholar]
- 27.Panigrahi I, Agarwal S. Thromboembolic complications in β-thalassemia: beyond the horizon. Thromb Res. 2007;120(6):783-789. [DOI] [PubMed] [Google Scholar]
- 28.Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Ann Rev Pathol. 2019;14(1):263-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein PD, Beemath A, Meyers FA, Skaf E, Olson RE. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. Am J Med. 2006;119(10):897.e7-11. [DOI] [PubMed] [Google Scholar]
- 30.van Hamel Parsons V, Gardner K, Patel R, Thein SL. Venous thromboembolism in adults with sickle cell disease: experience of a single centre in the UK. Ann Hematol. 2016;95(2):227-232. [DOI] [PubMed] [Google Scholar]
- 31.Alkindi S, Al-Ghadani AR, Al-Zeheimi SR, et al. Predicting risk factors for thromboembolic complications in patients with sickle cell anaemia - lessons learned for prophylaxis. J Int Med Res. 2021;49(12):3000605211055385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yacobovich J, Barzilai-Birenboim S, Steinberg-Shemer O, Stark P, Pazgal I, Tamary H. Splenectomy in childhood for nonmalignant haematologic disorders - long-term follow-up shows minimal adverse effects. Br J Haematol. 2020;190(6):909-915. [DOI] [PubMed] [Google Scholar]
- 33.[Priseen charge de la drépanocytose chez l’enfant et l’adolescent.] Haute Autorité de Santé. https://www.has-sante.fr/jcms/c_272479/fr/prise-en-charge-de-la-drepanocytose -chez-l-enfant-et-l-adolescent Accessed October 25, 2019. [Google Scholar]
- 34.EPIBAC. https://www.santepubliquefrance.fr/maladies-ettraumatismes/maladies-et-infections-respiratoires/infections-a -pneumocoque/articles/epibac Accessed August 25, 2021. [Google Scholar]
- 35.Takamatsu A, Matsuzaka S, Kodama F. [Clinical characteristics of invasive pneumococcal disease of the mucoid phenotype: a case series]. Kansenshogaku Zasshi. 2017;91(2):127-131. [PubMed] [Google Scholar]
- 36.CDC. Manual for the surveillance of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/survmanual/index.html 2021 Accessed September 2, 2021. [Google Scholar]
- 37.Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol. 2014;166(2):165-176. [DOI] [PubMed] [Google Scholar]
- 38.Lesher AP, Kalpatthi R, Glenn JB, Jackson SM, Hebra A. Outcome of splenectomy in children younger than 4 years with sickle cell disease. J Pediatr Surg. 2009;44(6):1134-1138; discussion 1138. [DOI] [PubMed] [Google Scholar]
- 39.Wright JG, Hambleton IR, Thomas PW, Duncan ND, Venugopal S, Serjeant GR. Postsplenectomy course in homozygous sickle cell disease. J Pediatr. 1999;134(3):304-309. [DOI] [PubMed] [Google Scholar]
- 40.Reeves SL, Jary HK, Gondhi JP, Kleyn M, Wagner AL, Dombkowski KJ. Pneumococcal vaccination coverage among children with sickle cell anemia, sickle cell trait, and normal hemoglobin. Pediatr Blood Cancer. 2018;65(10):e27282. [DOI] [PubMed] [Google Scholar]
- 41.[ALD n° 10 - Syndromes drépanocytaires majeurs de l’enfant et de l’adolescent.] Haute Autorité de Santé. https://www.hassante.fr/jcms/c_938890/fr/ald-n-10-syndromes-drepanocytaires -majeurs-de-l-enfant-et-de-l-adolescent. Accessed November 4, 2022. [Google Scholar]
- 42.Iolascon A, Andolfo I, Barcellini W, et al. Recommendations regarding splenectomy in hereditary hemolytic anemias. Haematologica. 2017;102(8):1304-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dei-Adomakoh Y, Asamoah-Akuoko L, Appiah B, Yawson A, Olayemi E. Safe blood supply in sub-Saharan Africa: challenges and opportunities. Lancet Haematol. 2021;8(10):e770-e776. [DOI] [PubMed] [Google Scholar]
- 44.Taher A, Isma’eel H, Mehio G, et al. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost. 2006;96(4):488-491. [PubMed] [Google Scholar]
- 45.Chan KH, Rizvi SH, De Jesus-Rojas W, et al. Pulmonary hypertension screening in children with sickle cell disease. Pediatr Blood Cancer. 2023;70(1):e29980. [DOI] [PubMed] [Google Scholar]
- 46.Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20(1):54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.