Abstract
Objective:
Abdominal aortic aneurysm (AAA) is a chronic inflammatory disease. Studies of human aneurysm tissue demonstrate dense inflammatory cell infiltrates with CD4+ T cells predominating. Regulatory T cells (Tregs) play an important role in inhibiting pro-inflammatory T cell proliferation, therefore, limiting collateral tissue destruction. The aim of this study was to investigate whether ex vivo augmentation of human Tregs attenuates aneurysm formation in humanized murine model of AAA.
Methods:
Circulating Treg population in AAA patients and age- and gender-matched controls were determined by real-time polymerase chain reaction and flow cytometry. To create humanized murine model of AAA, irradiated Rag1-deficient (Rag1−/−) mice, without mature T lymphocytes, at 7 weeks of age were given 5 × 106 of human CD4+ T cells intraperitoneally. Then the mice underwent CaCl2 aneurysm induction. Aortic diameters were measured before and at 6 weeks after aneurysm induction. Aortic tissue was collected for histology and protein extraction. Verhoeff-Van Gieson stain was used for staining elastic fiber. CD4+ T cells in the aortic tissue were detected by immunohistochemical staining.
Results:
In human peripheral blood mononuclear cells, the proportion of Tregs are decreased in AAA patients compared with matched control patients with significant vascular disease. We first validated the role of Tregs in the CaCl2 model of AAA. To determine the role of human T cells in AAA formation, Rag1−/− mice, resistant to CaCl2-aneurysm induction, were transplanted with human CD4+ T cells. Human CD4+ T cells were able to drive aneurysm formation in Rag1−/− mice. We show that ex vivo augmentation of human Tregs by interleukin-2 resulted in decreased aneurysm progression.
Conclusions:
These data suggest that the ex vivo expansion of human Tregs may be a potential therapeutic strategy for inhibiting progression of AAA.
Keywords: Aneurysm, Aorta, Inflammation, Regulatory T cells
Abdominal aortic aneurysm (AAA) is a common and lifethreatening condition in the elderly.1 At present, it is one of the few cardiovascular diseases for which there is no pharmacological therapy. Current therapies for large aneurysms are based on mechanical treatment. Developing a medical therapy will require a more complete understanding of the pathogenesis of AAA. Previous work has highlighted the complex nature of aneurysmal disease. The studies of human aneurysm tissue have demonstrated dense inflammatory cell infiltrates, elevated levels of matrix metalloproteinases (MMPs), and destruction of the orderly elastic lamellae.2–7 Importantly, the striking aortic matrix damage seen in AAA is consistent with an overly aggressive and dysregulated immune response.
The development and progression of AAA is a complex process. Destruction of the orderly elastin lamellae of the vessel wall is considered the sine qui non of aortic aneurysm formation.8 Elastin degradation in adult tissues results in irreversible damage and loss of function because adult tissues cannot regenerate normal elastin fibers.9 Moreover, because the elastin degradation products are chemotactic for inflammatory cells, they serve to amplify the local injury.10,11 Typically, regulatory T cells (Tregs) would help to suppress autoimmune response to tissue degradation products. We have corroborated findings that circulating Tregs have been reported to be deficient in AAA patients.12,13 For these observations to be used therapeutically, it is critical to determine if these defects can be reversed. Because interleukin-2 (IL-2) is important to Tregs expansion and survival,14 we assessed the effects of IL-2 on the number of Tregs from AAA patients.
Among the inflammatory infiltrates found in the aortic wall of AAA, CD4+ T cells are the predominant cell type.2,3 Animal models of AAA support a role for CD4+ T cells in aneurysm pathogenesis by demonstrating that mice deficient in CD4+ T cells are resistant to aneurysm formation.15 CD4+ T cells have been categorized into two main subsets, the majority being effector T cells and a smaller proportion of Tregs. Tregs have been characterized by their expression of CD25 and Forkhead box P3 (Foxp3).16 The primary role of the Tregs is to balance the pro-inflammatory destructive properties of the effector T cells.17 Previous studies have shown that the expression of Foxp3 in aortic aneurysm tissue was significantly reduced compared with Foxp3 expression from normal human aorta.12,18 To further explore the role of Tregs, we developed a humanized murine model of AAA by engrafting human CD4+ T cells into Rag1-deficient mice. These mice have no mature T and B lymphocytes, so they can accept transplanted (human or murine) lymphocytes which then reconstitutes a more complete immune system. We found 1) human CD4+ cells transplanted into Rag1-deficient mice were capable of driving aneurysm formation in the CaCl2 model, 2) ex vivo augmentation of human CD4+ cells by IL-2 improved number of Tregs, and 3) expansion of human Tregs from AAA patients inhibited aneurysm progression.
METHODS
Human subjects.
Peripheral blood was collected from 78 AAA and 92 control patients. The control patients were seen for clinically significant peripheral vascular or carotid disease and had negative abdominal ultrasound or computed tomography scan for AAA (aortic diameter ≤3.0 cm). Because AAA is a late-onset disease, controls were chosen that were the same age or older than AAA patients to minimize the number of control patients that might go on to late aneurysm development. Patients consented to blood draw in accordance with Nebraska Medicine and Methodist Hospital institutional review board-approved protocols (#357–05, #1033, and #559). Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation through a Histopaque (Sigma-Aldrich, St. Louis, Mo) density gradient.19
Quantitative reverse transcriptase polymerase chain reaction (RT-PCR).
Total RNA from human PBMCs was extracted using Trizol reagent (Invitrogen, Carlsbad, Calif). Complementary DNA was produced using the ThermoScript RT-PCR system (Invitrogen) according to the manufacturer’s recommendations for oligo(dT)20 primed complementary DNA synthesis. Primers and probes for human CD4 and Foxp3 were designed using Primer Express (Applied Biosystems, Foster City, Calif) and purchased from Applied Biosystems. Quantitative Taqman PCR was performed on the Applied Biosystem StepOne Real-Time RCR system. Product amplification was confirmed using standard agarose gel techniques. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal reference.
Treg isolation and analyses.
To quantify regulatory T cells in blood samples, PBMCs isolated from human blood were stained with various fluorochrome-conjugated anti-bodies and analyzed by flow cytometry. The antibodies used for staining were anti-human CD4 FITC, anti-human CD25 APC, and anti-human Foxp3 PE (eBioscience, San Diego, Calif). Cells were first stained for the cell surface markers (CD4 and CD25) for 45 minutes on ice, then fixed and made permeable by the addition of fixation/permeabilization solution (BD Biosciences, Franklin Lakes, NJ), followed by intracellular staining of Foxp3 according to the manufacturer’s instructions. Flow cytometric analysis was performed on a LSRII flow cytometer (BD Biosciences). The data were analyzed with FlowJo software (FlowJo LLC, Ashland, Ore). For accurate measurement of Foxp3 expression levels, autofluorescence and Fluorescence Minus One (FMO) controls were used establish the boundary between Foxp3-negative and positive fluorescent regions. Positive staining and gating strategy were determined by comparison to an isotype control. To analyze the prevalence of Tregs, CD25+Foxp3+ cells were evaluated after gating CD4+ (Supplementary Fig, online only).
Human Tregs and responder T cells from PBMCs were isolated using the CD4+CD25+ regulatory T-cell kit (Miltenyi Biotec, Auburn, CA). Briefly, CD4+ cells were isolated by depletion of non-CD4 cells, followed by further enrichment of CD25+ cells with CD25 microbeads according to the manufacturer’s instructions. The purity of Tregs were 80% to 90% measured by Foxp3 staining after isolation.
Generation of chimeric mice and humanized AAA model. Rag1-deficient (Rag1−/−) mice, which have no mature T and B lymphocytes, were purchased from the Jackson Laboratory (Bar Harbor, Me). They are on the C57Bl/6 genetic background and backcrossed to C57Bl/6 for 5 to 10 generations. Animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. All mouse experiments conform to the National Institutes of Health guidelines for the care and use of laboratory animals. All mice were maintained in the pathogen free animal facility.
Heterozygous female breeders of Foxp3-deficient mice were purchased from the Jackson Laboratory. They were bred with C57Bl/6 mice for five generations. Because the short lifespan of Foxp3−/− mice precluded their use in our aneurysm model, 6-week-old Rag1−/− mice were sublethally irradiated (1200 rads) and transplanted with bone marrow from Foxp3−/− (designated as Rag1−/−/Foxp3−/−) or wild-type (designated as Rag1−/−/WT) mice. Bone marrow cell suspensions were prepared from the femurs of Foxp3−/− mice or wild-type mice, and 5 × 106 cells were infused via the lateral tail veins as described previously.7 To validate the effectiveness of radiation in ablating the bone marrow, several mice per group did not receive transplanted cells; all died within one week of receiving irradiation.
CD4+ T cells from AAA patients were purified by positive selection using CD4 MicroBeads (Miltenyi Biotec Inc) following the manufacturer’s instructions. Cell purity (>95%) was verified by flow cytometry. To generate the humanized AAA murine model, irradiated Rag1−/− mice (300 rads) (7 weeks old) were given 5 × 106 of human CD4+ T cells intraperitoneally. Three days after human CD4+ T-cell transplantation, the mice underwent CaCl2 aneurysm induction. In development of the humanized model, preliminary data confirmed mouse viability and human CD4+ T cell engraftment at 4 weeks after transplantation.
Aneurysm induction.
Mice at 7 to 8 weeks of age underwent aneurysm induction as described previously.7,15 Briefly, mice were anesthetized with isoflurane in 100% oxygen with a flow of 1.5 L/mL administered by means of a facemask connected to coaxial circuit (Patterson Scientific, Waukesha, Wisc). The abdominal aorta between the renal arteries and bifurcation of the iliac arteries was isolated from surrounding retroperitoneal structures. The diameter of the aorta was measured in triplicate in the mid-infrarenal aorta using a Leica Application System (Leica Microsystems Inc, Buffalo Grove, Ill). After baseline measurements were obtained, 0.25 M CaCl2 was applied to the external surface of the aorta using a cotton applicator cut to size for 15 minutes. The aorta was then rinsed with 0.9% NaCl (sterile saline) and the incision was closed. After the operation buprenorphine (0.1 mg/kg body weight) was given subcutaneously daily for 2 days. NaCl (0.9%) was substituted for CaCl2 in sham control mice. Six weeks later, the mice underwent repeat laparotomy, dissection, and exposure of the aortas for premortem measurement. The aortic tissue was then harvested for analysis.
Induction of Tregs.
The CD4+ T cells isolated from control and AAA patients were plated onto anti-CD3-coated (1 μg/mL) plates and treated with 2 ng/mL of transforming growth factor (TGF)-β and 5 ng/mL of IL-2 for 6 days to expand the Treg population.20 The percentage CD4+CD25+Foxp3+ T cells (Tregs) was determined by flow cytometry as described previously.
Histology and immunohistochemistry.
For Verhoeff-Van Gieson (VVG) connective tissue staining, mouse abdominal aortic tissues were embedded in paraffin and stained as previously described.21 To identify tissue CD4+ T cells, sections were incubated with purified mouse anti-human CD4 (BD Biosciences) antibodies. Briefly, tissue sections on the slides were deparaffinized. The sections were incubated with a purified mouse antihuman CD4 (1:200) antibodies for 45 minutes at 37°C. The sections were then washed in citrate solution and subsequently incubated with the secondary antibody, biotin goat anti-mouse immunoglobulin G. Cell staining was examined using light microscopy. Sections incubated with secondary antibody only showed no positive staining. Three to five samples in each group were stained and evaluated.
Statistical analysis.
Measurements of aortic diameter, Treg cell population, and CD4+ T-cell infiltration were expressed as the mean value ± SD. If the data were normally distributed, the Student t-test (comparison between two groups) or analysis of variance with the appropriate post hoc test were used. Statistical significance was accepted at a P < .05.
RESULTS
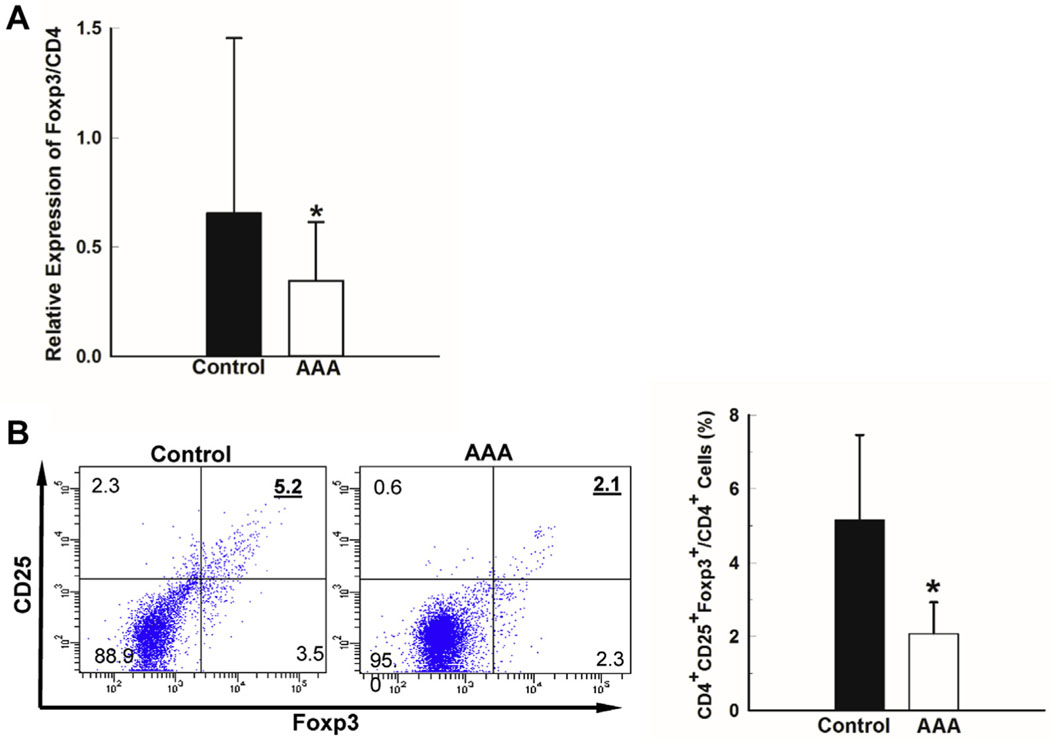
Treg population was decreased in AAA patients compared with controls. In this study, we hypothesized that a decrease in the Treg population in AAA patients could contribute to an aggressive inflammatory response that leads to matrix destruction and AAA formation. Control and AAA patients were matched for gender, age, and smoking history (Table). The aortic diameters were determined in all subjects by computed tomography scan or ultrasound. In control patients, all aortic diameters were less than 3.0 cm, whereas AAA patient aortic diameters were greater than 3.5 cm. The groups were well matched but, as has been noted in previous studies, there was a higher incidence of chronic obstructive pulmonary disease (COPD) in AAA patients compared with controls.22,23 RT-PCR was used to examine Foxp3 expression in CD4+ T cells. The Foxp3 expression relative to total CD4 expression in PBMCs from AAA patients (n = 48) was significantly lower than in control patients (n = 52; Fig 1, A). To confirm this decrease in the percentage of Tregs, PBMCs from a subset of control and AAA patients were evaluated by flow cytometry. The percentage of CD4+CD25+Foxp3+ Tregs in PBMCs was higher in control patients compared with AAA patients (Fig 1, B). The flow cytometric and PCR analyses were consistent in demonstrating a two-fold difference between control and AAA patients. Using two separate techniques and two different patient cohorts, we corroborated significant deficiency in proportion of Tregs in AAA patients.
Table.
Characteristics for abdominal aortic aneurysm (AAA) and control patients
| Control (n = 92) | AAA (n = 78) | P value | |
|---|---|---|---|
|
| |||
| Age, years | 75.2 ± 0.6 | 73.5 ± 0.7 | .0683 |
| Male | 69 (75) | 57 (73) | .8609 |
| Hypertension | 70 (76) | 58 (74) | .8591 |
| Diabetes | 18 (20) | 12 (15) | .5475 |
| Smoking history | 82 (89) | 71 (91) | .7997 |
| COPD | 9 (10) | 22 (28) | .0026 |
| β-blocker | 48 (52) | 37 (47) | .6444 |
| ACE inhibitor | 35 (38) | 23 (29) | .2597 |
| Aspirin | 69 (75) | 54 (69) | .4916 |
| Ca++ channel blocker | 22 (24) | 11 (14) | .1224 |
| AAA size, cm | 2.18 ± 0.07 | 5.06 ± 0.13 | <.0001 |
| Family AAA | 9 (10) | 11 (14) | .4755 |
| Anti-inflammatory drug | 7 (8) | 11 (14) | .2136 |
ACE, Angiotensin-converting enzyme; COPD, chronic obstructive pulmonary disease.
Continuous data are presented as mean ± standard deviation and categorical data as number (%). Boldface values indicate statistical significance.
Fig 1.
Treg proportion in the peripheral blood of abdominal aortic aneurysm (AAA) patients was significantly decreased compared with control patients. Human peripheral blood mononuclear cells were isolated from peripheral blood of AAA and control patients. Total RNA was extracted and messenger RNA levels of Foxp3 and CD4 genes were analyzed by reverse transcriptase polymerase chain reaction (RT-PCR). A, Relative messenger RNA expression levels of Foxp3 to CD4 in human Peripheral blood mononuclear cells (PBMCs) from control (n = 52) and AAA (n = 48) patients. The ratio of Foxp3 to CD4 mRNA levels is shown. B, Percentage of Tregs (CD4+CD25+FoxP3+) in the peripheral blood of control (n = 12) and AAA (n = 10) patients determined by flow cytometry; representative dot plot (left). Data represented as mean 6 standard deviation, *P < .05.
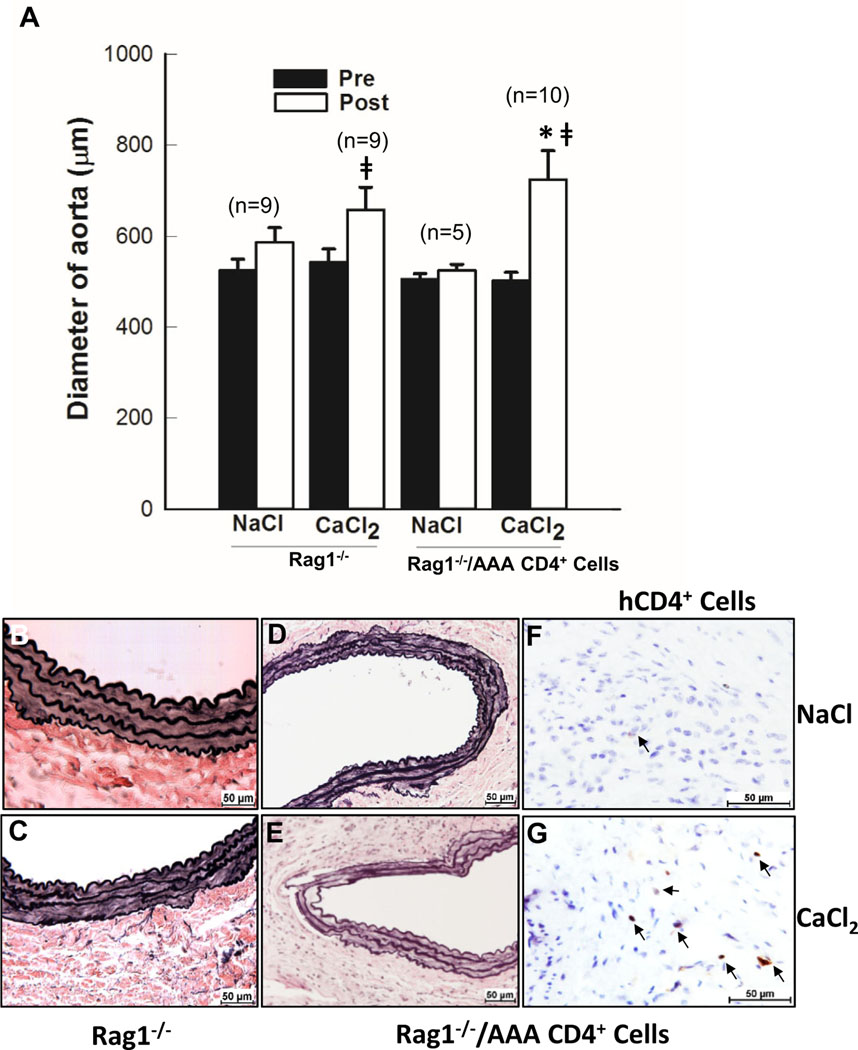
Mouse aneurysm formation could be driven by human CD4+ T cells. To further understand the role of human CD4+ cells in AAA, we next sought to develop a murine model regulated by human T cells (Fig 2). Rag1−/− mice have no mature T lymphocytes. As expected, Rag1−/− mice are resistant to CaCl2-induced aneurysm formation and elastin degradation (Fig 2, A and C). Although aortic enlargement was seen in Rag1-deficient mice after CaCl2 induction compared with NaCl-treated mice, none of the mice had >50% increase in aortic size. Human CD4+ T cells (5 × 106) from AAA patients were infused into Rag1−/− mice before aneurysm induction. Six weeks after aneurysm induction, aortic diameter was significantly increased compared with CaCl2-treated Rag1-deficient mice (Fig 2, A). Four out of ten mice had 50% increase in aortic diameter compared with its baseline. Aortic connective tissue staining showed elastic fiber damage in CaCl2-treated CD4+ T-cell infused Rag1−/− mice (Fig 2, E). Furthermore, immunohistochemical staining with human CD4-antibody confirmed that transplanted human CD4+ T cells migrated into the aorta (Fig 2, F and G). These data demonstrated that human CD4+ T cells were able to drive aneurysm formation, in part, through inflammatory modulation.
Fig 2.
Human CD4+ cell infusion promoted aneurysm formation in Rag1−/− mice. A, The bar graph shows aortic diameters at baseline (Pre) and 6 weeks after (Post) NaCl or CaCl2 incubation. Verhoeff-Van Gieson (VVG) staining of aortic tissue from (B) NaCl- or (C) CaCl2-treated Rag1−/− mice and (D) NaCl- or (E) CaCl2-treated Rag1−/− mice infused with CD4+ cells from abdominal aortic aneurysm (AAA) patients. F and G, Immunostaining of human CD4+ cells in the aortic tissue sections with corresponding VVG staining of (D and E). Three different aortas from each group were stained and representative sections are shown. Positive cells are indicated by arrows. *P < .05 compared CaCl2-treated Rag1−/− mice; ǂP < .05 compared NaCl-treated mice.
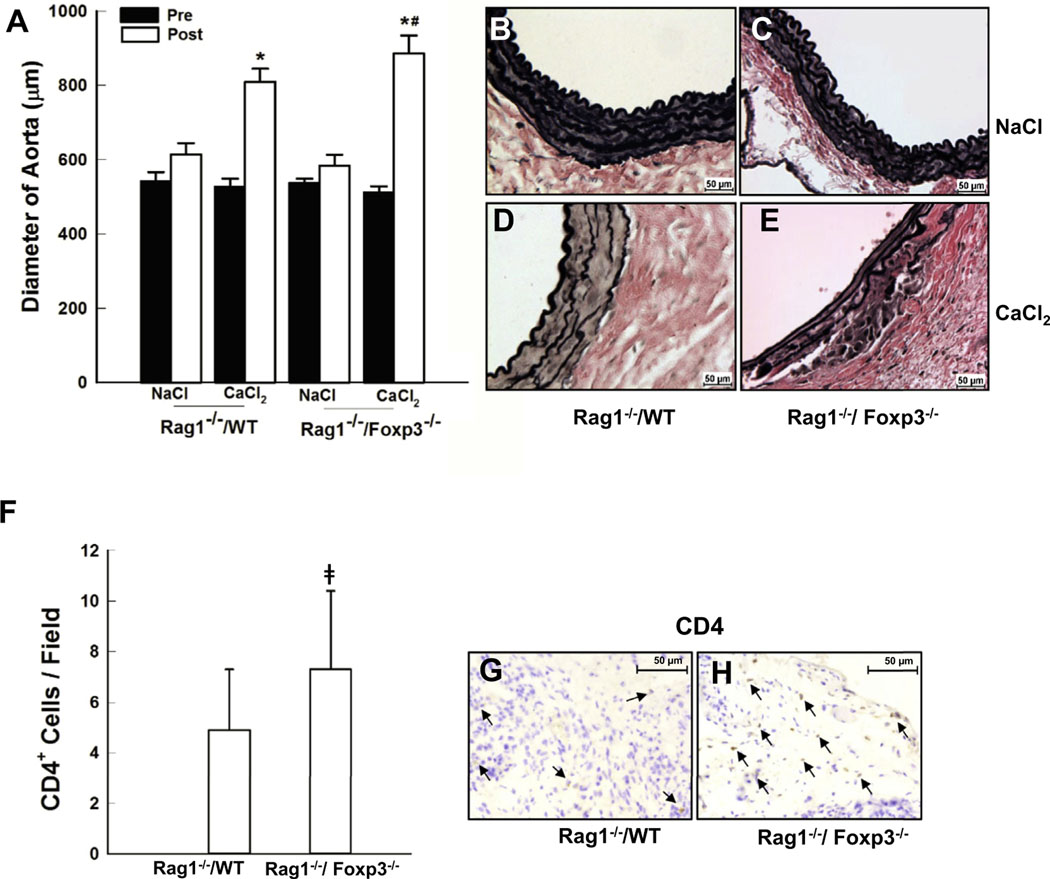
Treg augmentation from AAA patients inhibited AAA formation. The decreased population and function of Tregs in AAA patients suggests that Tregs could play an important role in AAA formation or expansion. It is well known that Foxp3 is essential for the development and immuno-suppressive activity of Tregs; it is currently the most reliable molecular marker for Tregs.24 To more precisely determine the contribution of Tregs to AAA in our CaCl2 model, we assessed the effects of Foxp3 deficiency in myeloid cells. Because of the short lifespan (16–25 days) of Foxp3-deficient mice, chimeric mice were generated by transplanting Foxp3−/− marrow into sublethally, γ-irradiated Rag1−/− mice (ie, Rag1−/−/Foxp3−/−). A group of γ-irradiated Rag1−/− mice transplanted with wild-type bone marrow (ie, Rag1−/−/WT) were used as controls. After recovery from bone marrow transplantation, mice underwent CaCl2 aneurysm induction. The relative ability of WT-derived versus Foxp3−/−-derived myeloid cells to support AAA formation was assessed in the respective chimeric mice (Fig 3). Rag1−/−/WT mice developed aneurysms similar in diameter to WT mice as previously reported (Fig. 3, A).7,15 Compared with Rag1−/−/WT mice, Rag1−/−/Foxp3−/−mice developed larger aneurysms (Fig 3, A). Furthermore, although the CaCl2-treated Rag1−/−/WT mice exhibited significant disruption of the elastic lamellae (Fig 3, D), the elastin damage was much more severe in the Rag1−/−/ Foxp3−/− mice (Fig 3, E). As expected, the architecture of the aortic walls of the NaCl-treated control mice from both groups was intact (Fig 3, B and C). Inflammatory cell infiltration, predominately T cells, plays a crucial role in aneurysm formation. The CD4+ T cells in the aortic tissues were examined. Both CaCl2-treated Rag1−/−/WT and Rag1−/−/Foxp3−/− mouse aortas showed marked CD4+ T cell infiltration (Fig 3, G and H). Furthermore, CaCl2-treated Rag1−/−/Foxp3−/− mouse aortic tissue contained significantly more CD4+ T cells than Rag1−/−/WT mice (Fig 3, F). These results demonstrate that Treg deficiency exacerbates aneurysm enlargement through its role in downregulating the immune response associated with aneurysm formation.
Fig 3.
Foxp3 deficiency exacerbated aneurysm expansion. A, Aortic diameter changes in Rag1−/−/WT and Rag1−/−/Foxp3−/− mice before and after aneurysm induction. B-E, Histological changes in the mouse aortas as seen with Verhoeff-Van Gieson (VVG) stain. Sections were photographed and shown with the lumen at the top. Five different aortas from each of the four groups were stained and representative sections are shown. F, Immunostaining for mouse CD4+ T cells in Rag1−/−/WT (G) and Rag1−/−/Foxp3−/− (H) aortic tissue 6 weeks after aneurysm induction (n = 4/group). CD4+ T cell number was quantified by a reviewer blinded to the mouse genotype. The bar graph (F) demonstrates the quantitative differences in CD4+ cells. Data are shown as the mean ± standard deviation. *P < .05, compared with NaCl-treated controls, #P < .05, compared with CaCl2-treated Rag1−/−/WT mice, ǂP < .05, compared with CaCl2-treated Rag1−/−/WT mice. Representative stainings are shown in the left panels. CD4+ cells are indicated by arrows.
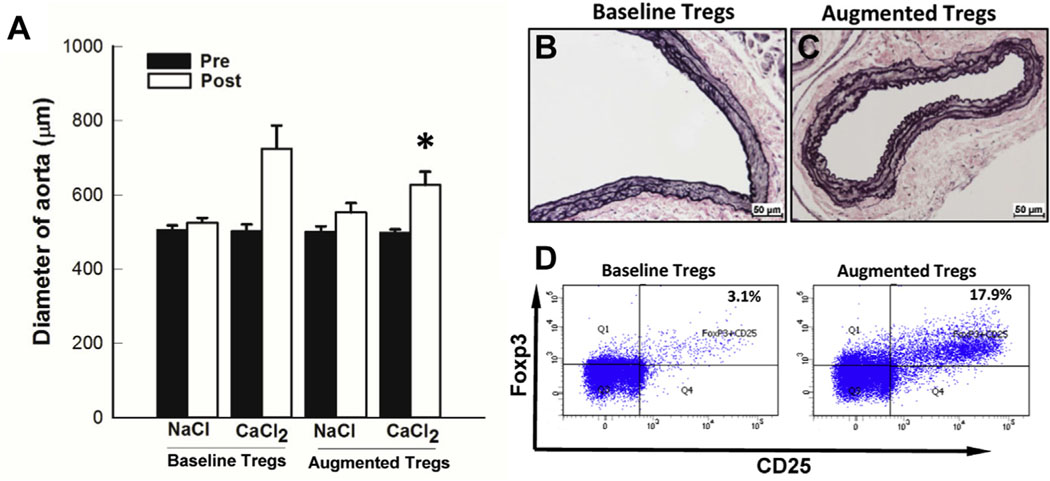
Transplantation of CD4+ T cells isolated from AAA patients was able to drive AAA formation (Fig 2). This finding was consistent with low levels of Tregs from AAA patients. We postulated that ex vivo expansion of Tregs could inhibit aneurysm formation. To test this hypothesis, human CD4+ T cells isolated from AAA patients were expanded using TGF-β and IL-2. The Treg population was increased to 16.4 ± 3.7% (n = 6; Fig 4). After transplantation of augmented cells, aneurysms were induced with CaCl2 treatment (Fig 4). Rag1−/− mice engrafted with augmented cells from AAA patients demonstrated a significant inhibition of aortic enlargement (44.6 ± 3.9% vs 25.3 ± 2.0%; Fig 4, A). Connective tissue staining showed that aortic tissue from mice receiving expanded human AAA T cells had minimal disruption of the elastic lamellae (Fig 4, C). Taken together, we have demonstrated successful development of a humanized murine model of AAA that allowed us to assess the function of T cells derived from AAA patients. We found that ex vivo treatment of IL-2 could increase the ratio of Tregs (Fig 4, D). Furthermore, these augmented cells are able to inhibit aneurysm expansion. These findings suggest a potential patient specific cell therapy to inhibit aneurysm expansion.
Fig 4.
Treg augmentation by interleukin-2 (IL-2) treatment inhibited aneurysm formation and elastin degradation. A, Aortic diameter changes in CaCl2-treated Rag1−/− mice infused without (baseline Tregs; n = 10) and with IL-2-treated (augmented Tregs; n = 6) human CD4+ T cells from abdominal aortic aneurysm (AAA) patients. Histological changes in the mouse aortas as seen with VVG stain. CaCl2-treated Rag1−/− infused with untreated (B) and IL-2-treated (C) CD4+ T cells from AAA patients. *P < .001 compared with the mice with augmented Tregs. D, Tregs (CD4+CD25+FoxP3+) population with (augmented) and without (baseline) IL-2 stimulation in PBMC of AAA patients (n = 6) determined by flow cytometry.
DISCUSSION
Analysis of human aneurysm tissue has revealed the presence of a large number of activated T lymphocytes.25,26 The abundance of CD4+ T cells in late stage AAA tissues suggests they may have a role in regulating the chronic inflammatory process. Previous studies using a murine model of AAA have confirmed the specific contributions of CD4+ T cells.15 These studies have shown that the absence of CD4+ T cells prevents aneurysm development. The CD4+ T-cell population is known to consist of a number of subsets, characterized by the expression of specific cell surface molecules. The majority of CD4+ T cells are effector T cells whose role is regulating the acute inflammatory response as part of a normal host defense mechanism. Effector T cells can also secrete and induce the secretion of proteases that allow inflammatory cell transmigration into tissues.27 This proinflammatory and proteolytic milieu is thought to play a significant role in the extracellular matrix destruction seen in chronic inflammatory diseases. Conversely, a small subset of CD4+ T cells, CD4+CD25+Foxp3+ or Tregs, assume the critical function of counterbalancing this proinflammatory process. Outnumbered 20-fold, this small proportion of Tregs can effectively limit collateral damage and allow matrix repair. The mechanisms through which they prevent dysregulation of the immune response include blocking proliferation of effector T cells, inhibiting inflammatory infiltration, and removing autoreactive T cells.28,29 This latter group of cells may generate an autoimmune response by presenting matrix degradation products not typically exposed to the innate immune system. All of these mechanisms suggest a possible important role of Tregs during aneurysm progression.
Two recent papers have reported that Tregs in human peripheral blood samples are decreased in AAA patients compared with controls.12,13 Our initial studies used total white blood cells derived from carefully selected and screened control and AAA patients. The relative expression of Foxp3 to CD4 indicated a decrease in AAA Tregs consistent with these previous studies. Because these studies indirectly assessed the ratio of Tregs to CD4+ cells, we were concerned that expression of Foxp3 by cells other than Tregs could skew the results. For this reason, we performed flow cytometric analysis on freshly collected cells from separate AAA and control cohorts. This demonstrated a decrease in Tregs in AAA and differences between AAA and control groups that were similar to those observed by the indirect measurements used for the previous studies.
All of the control patients used in our studies had significant atherosclerosis and were matched for age, gender, and smoking history with the AAA group. By using this particular control group, we are able to demonstrate a deficiency in Tregs that is specific to AAA and is not a general marker for atherosclerosis. The only notable clinical difference between the AAA and control groups was the higher incidence of COPD in the AAA patients. Whether COPD is correlated with a decrease in Tregs is controversial; one study has shown a significant negative correlation between COPD and the percentage of Tregs, whereas a second study found no relationship.30,31 Therefore, additional studies examining lung function, AAA, and Tregs will be required to address this question.
In previous studies, we demonstrated the important role that CD4+ cells play in the CaCl2 model of AAA.15 Mice deficient in CD4+ cells do not develop aneurysms after CaCl2 exposure. Infusion of interferon-gamma restored aneurysm formation in CD4 null mice. After this, we developed a humanized murine model of AAA by engrafting human CD4+ T cells into the immunodeficient Rag1−/− mice. These mice displayed a matrix-degrading immune response that resulted in aneurysms comparable to those observed in immunocompetent wild-type mice. Conversely, Rag1−/− mice without CD4+ T cell reconstitution do not form aneurysms. This confirms the reliance of the model on the engrafted human cells for aneurysm formation. Importantly, we observed that the human CD4+ cells traffic to the aorta.
A previous study has demonstrated the role of Foxp3+ cells in the angiotensin II infusion model of AAA.12 As part of the preliminary work to create the humanized model, we sought to confirm that Tregs impact aneurysm formation in the CaCl2 model. We created chimeric mice with a deficiency of Foxp3 in myelogenous cells using Rag1−/− mice. Recombination-activating genes (Rags) encode enzymes without which the development of mature T and B cells cannot occur. By infusing the bone marrow from background matched Foxp3−/− mice into Rag1−/− mice, the immune system was completely reconstituted except for the presence of Tregs. Rag1−/−/Foxp3−/− mice developed larger aneurysms than Rag1−/− mice reconstituted with WT bone marrow. The damage to the aortic wall architecture correlated directly with aneurysm size. In the absence of Tregs, effector T-cell proliferation is uncontrolled. CD4+ T-cell infiltration was increased in aortic sections from Rag1−/−/Foxp3−/− mice compared with Rag1−/−/WT mice. These data are consistent with the ability of Tregs to suppress inflammatory cell proliferation and inhibit aneurysm progression.
To better understand how the deficiency of Tregs found in AAA patients might affect their disease, we created a humanized murine model by engrafting human CD4+ T cells into irradiated Rag1−/− mice. We demonstrated that human cells were present 4 weeks after infusion and that they trafficked to the aorta. Without engraftment of human CD4+ T cells, Rag1−/− mice are resistant to CaCl2-induced aneurysm, confirming that human CD4+ cells are able to drive aneurysm formation in the model. Our goal in developing a humanized model was to more closely recapitulate human AAA in a murine model.
Human aneurysm growth rate has been correlated with decreased Foxp3 levels in blood.12 Henkel et al32 have shown a high degree of correlation between Treg dysfunction and progression of neurological disease. The same research group has demonstrated that the anti-proliferative effects of Treg cells can be restored by ex vivo augmentation.33 Intravenous infusion of IL-2 inhibits aneurysm formation in the angiotensin II murine model of AAA partly by improvement of Treg activity.34 We demonstrate that ex vivo expansion of Tregs from AAA patients can be done by treating CD4+ T cells with IL-2 and TGF-β. Furthermore, infusion of these augmented human Tregs inhibited aneurysm formation in humanized murine model of AAA. In conclusion, our studies suggest a patient-specific approach to immunomodulation that could have an important role in medical therapy of AAA.
This study has a number of limitations. First, it is unlikely that acute animal models could precisely mimic chronic AAA formation in humans. The animal data should be interpreted cautiously. Second, not all animals achieved a 50% increase in aortic diameter in this model using the C57Bl/6 background. Although Tregs display remarkable inhibitory effect on aneurysm formation in CaCl2-induced model, future studies of Tregs’ effect on a chronic large animal model of AAA would be essential for preclinical studies.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Experimental study using a humanized murine model of abdominal aortic aneurysm (AAA)
Key Findings: Aneurysm patients are deficient in the proportion of regulatory T cells compared to nonaneurysmal control patients. The authors demonstrated that regulatory T cells from AAA patients can be augmented ex vivo. They developed a humanized murine model of AAA and showed that the augmented regulatory T cells can inhibit aneurysm formation.
Take Home Message: Regulatory T-cell deficiency identified in AAA patients is an important driver of aneurysm formation.
Acknowledgments
We thank Lijun Sun and the UNMC Tissue Science Facility for assisting with VVG staining and the UNMC Flow Cytometry Core Facility for assisting with flow cytometric analysis.
This work was supported by the National Institutes of Health NHLBI grants HL062400 (to B.T.B.) and HL130623 (to W.X.).
Footnotes
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Author conflict of interest: none.
REFERENCES
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet 2005;365:1577–89. [DOI] [PubMed] [Google Scholar]
- 2.Galle C, Schandene L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, et al. Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm. Clin Exp Immunol 2005;142:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duftner C, Seiler R, Klein-Weigel P, Gobel H, Goldberger C, Ihling C, et al. High prevalence of circulating CD4+CD28- T-cells in patients with small abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2005;25:1347–52. [DOI] [PubMed] [Google Scholar]
- 4.Chan WL, Pejnovic N, Hamilton H, Liew TV, Popadic D, Poggi A, et al. Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ Res 2005;96:675–83. [DOI] [PubMed] [Google Scholar]
- 5.Ciavarella C, Alviano F, Gallitto E, Ricci F, Buzzi M, Velati C, et al. Human vascular wall mesenchymal stromal cells contribute to abdominal aortic aneurysm pathogenesis through an impaired immunomodulatory activity and increased levels of matrix metalloproteinase-9. Circ J 2015;79:1460–9. [DOI] [PubMed] [Google Scholar]
- 6.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest 1998;102:1900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 2002;110:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation 2007;115:1729–37. [DOI] [PubMed] [Google Scholar]
- 9.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol 2004;62:153–88. [DOI] [PubMed] [Google Scholar]
- 10.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 2006;116:753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, et al. Elastin-Derived peptides promote abdominal aortic aneurysm formation by modulating M1/M2 macrophage polarization. J Immunol 2016;196:4536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Wu W, Lindholt JS, Sukhova GK, Libby P, Yu X, et al. Regulatory T cells in human and angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc Res 2015;107: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin M, Zhang J, Wang Y, Wang S, Bockler D, Duan Z, et al. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2010;30:1825–31. [DOI] [PubMed] [Google Scholar]
- 14.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4–1BB signaling. J Immunol 2007;179:7295–304. [DOI] [PubMed] [Google Scholar]
- 15.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol 2004;172:2607–12. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int Rev Immunol 2005;24:211–26. [DOI] [PubMed] [Google Scholar]
- 18.Meng X, Yang J, Zhang K, An G, Kong J, Jiang F, et al. Regulatory T cells prevent angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E knockout mice. Hypertension 2014;64:875–82. [DOI] [PubMed] [Google Scholar]
- 19.Batra R, Suh MK, Carson JS, Dale MA, Meisinger TM, Fitzgerald M, et al. IL-1beta (Interleukin-1beta) and TNFalpha (tumor necrosis factor-alpha) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler Thromb Vasc Biol 2018;38:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis GI, Reneer MC, Velez-Ortega AC, McCool A, Marti F. Generation of induced regulatory T cells from primary human naive and memory T cells. J Vis Exp 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT. MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ Res 2012;110:e92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijer CA, Kokje VB, van Tongeren RB, Hamming JF, van Bockel JH, Moller GM, et al. An association between chronic obstructive pulmonary disease and abdominal aortic aneurysm beyond smoking: results from a case-control study. Eur J Vasc Endovasc Surg 2012;44:153–7. [DOI] [PubMed] [Google Scholar]
- 23.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 2000;160:1425–30. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005;6:345–52. [DOI] [PubMed] [Google Scholar]
- 25.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, et al. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol 1990;137:1199–213. [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce WH, Koch AE. Cellular components and features of immune response in abdominal aortic aneurysms. Ann N Y Acad Sci 1996;800:175–85. [DOI] [PubMed] [Google Scholar]
- 27.Abraham M, Shapiro S, Karni A, Weiner HL, Miller A. Gelatinases (MMP-2 and MMP-9) are preferentially expressed by Th1 vs. Th2 cells. J Neuroimmunol 2005;163:157–64. [DOI] [PubMed] [Google Scholar]
- 28.Huu DL, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, et al. FTY720 ameliorates murine sclerodermatous chronic graft-versus-host disease by promoting expansion of splenic regulatory cells and inhibiting immune cell infiltration into skin. Arthritis Rheum 2013;65: 1624–35. [DOI] [PubMed] [Google Scholar]
- 29.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol 2007;179:4323–34. [DOI] [PubMed] [Google Scholar]
- 30.Jin Y, Wan Y, Chen G, Chen L, Zhang MQ, Deng L, et al. Treg/IL-17 ratio and Treg differentiation in patients with COPD. PloS one 2014;9:e111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou J, Sun Y, Hao Y, Zhuo J, Liu X, Bai P, et al. Imbalance between subpopulations of regulatory T cells in COPD. Thorax 2013;68:1131–9. [DOI] [PubMed] [Google Scholar]
- 32.Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med 2013;5:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alsuliman A, Appel SH, Beers DR, Basar R, Shaim H, Kaur I, et al. A robust, good manufacturing practice-compliant, clinical-scale procedure to generate regulatory T cells from patients with amyotrophic lateral sclerosis for adoptive cell therapy. Cytotherapy 2016;18:1312–24. [DOI] [PubMed] [Google Scholar]
- 34.Yodoi K, Yamashita T, Sasaki N, Kasahara K, Emoto T, Matsumoto T, et al. Foxp3+ regulatory T cells play a protective role in angiotensin II-induced aortic aneurysm formation in mice. Hypertension 2015;65:889–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.