Abstract
Background
Children in Kenya spend a substantial amount of time at school, including at dawn and dusk when mosquitoes are active. With changing vector behaviour towards early morning biting, it is important to determine whether there is an additional risk of transmission in schools. This study sought to understand whether late morning biting by Anopheles funestus, previously documented in households in western Kenya, was replicated in schools.
Methods
From the 4th to the 6th of August 2023, human landing collections were conducted hourly in four schools in Alego Usonga sub-County, Siaya County. The collections were conducted in and outside five classrooms in each school and ran for 17 h, starting at 18:00 until 11:00 h the next morning.
Results
Anopheles funestus was the predominant species collected, forming 93.2% (N = 727) of the entire collection, with peak landing between 06:00 and 07:00 h and continuing until 11:00 h. More than half of the collected An. funestus were either fed or gravid, potentially indicative of multiple bloodmeals within each gonotrophic cycle, and had a sporozoite rate of 2.05%.
Conclusion
School children spend up to 10 h of their daytime in schools, reporting between 06:00 and 07:00 h and staying in school until as late as 17:00 h, meaning that they receive potentially infectious mosquito bites during the morning hours in these settings. There is a need to consider vector control approaches targeting schools and other peridomestic spaces in the morning hours when An. funestus is active.
Keywords: Anopheles funestus, Malaria, Behaviour, Primary schools
Background
Malaria disproportionately affects sub-Saharan Africa, with > 93% of all malaria cases and deaths occurring in this region. While the disease affects people of all ages, children under five are particularly vulnerable, accounting for over 60% of all malaria deaths worldwide [1]. Malaria case burden extends to the 5–15-year-old age group [1, 2]. Furthermore, malaria infection prevalence is often highest in this age group [3, 4]. Malaria can cause severe anaemia, leading to fatigue and reduced concentration, impacting a child’s learning ability [5–7]. Malaria-related absences from school contribute to lower school attendance and poorer academic performance, further perpetuating the cycle of poverty [8]. Asymptomatic infections, in children aged 5–15 years often go untreated and likely serve as an important reservoir of infection for the entire community [3, 9–11].
There are limited interventions that target control of malaria in school-aged children despite their high burden of malaria infection. Most interventions target the vulnerable groups: under-fives and pregnant women. As children become older and more independent, parents have less control over when they go to bed, where they sleep, and whether they use LLINs [8]. This results in less LLIN coverage and less malaria protection in this age group [12]. School-aged children have greater anti-malarial immunity than younger children, which is acquired through repeated exposure to malaria parasites, gained with repeated infections. Because older children have greater anti-disease immunity, they are often more likely to have asymptomatic parasitaemia than younger children (and adults) and this age group often has the highest parasite prevalence [8, 13, 14]. Schools have been described as a potential avenue for malaria control in children through programmes, such as intermittent preventive treatment in school children (IPT-SC) [15, 16] and seasonal malaria chemoprevention (SMC) [17, 18], due to the logistic feasibility of reaching many eligible children in one place.
On the other hand, schools are potential risk sites for malaria transmission, especially in rural areas with a high disease burden [19]. With the change in mosquito behaviour, they have shifted to resting and biting outside the treated homes to evade the available interventions [20]. Children spend up to 10 h of their daytime at school, including dawn and dusk, when mosquitoes are active, and if schools are not adequately protected against malaria transmission, they can become hotspots for the disease [21].
Ongoing vector surveillance in Siaya County as part of an evaluation of attractive targeted sugar baits (ATSBs) has observed a peak in biting by Anopheles funestus at 06:00 h with continued biting into the later hours of the morning (Ochomo et al., unpublished).
Observations have shown that many children in this area consistently arrive at school between 06:00 and 07:00 h. Consequently, it is possible that they are exposed to potentially infectious mosquito bites while seated in class during their prep time and morning lessons. Mosquito abundance and biting behaviour was characterized in primary schools at night and in the morning hours when children would normally be in school.
Methods
Study site
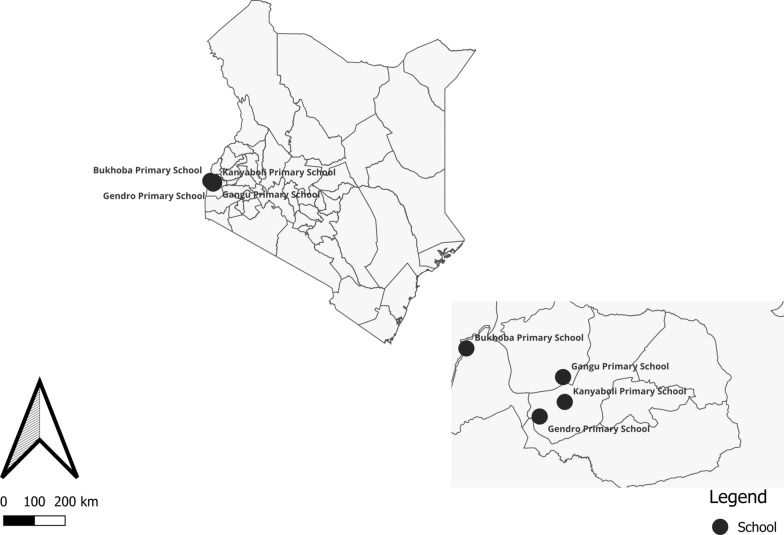
The study was conducted in four non-boarding primary schools within Alego-Usonga Sub-County, Siaya County, western Kenya: Bukhoba, Gangu, Kanyaboli, and Gendro. Siaya County is characterized by high, year-round malaria transmission with peak seasons after the long rains (March-June) and short rains (October–November). The primary malaria vectors in the region are Anopheles gambiae sensu lato (s.l.) and An. funestus s.l. [22, 23], with An. funestus being the predominant vector. Siaya County has the second highest malaria prevalence nationally [24], and receives long-lasting insecticidal nets (LLINs) through mass campaigns and routine distribution to pregnant mothers and children through antenatal and child welfare clinics.
Students report to these schools between 06:00 and 07:00 h and leave between 16:00 and 17:00 h. The schools were selected due to the high densities of Anopheles mosquitoes observed in the surrounding villages during quarterly mosquito surveillance conducted as part of the ATSB trial [25]. Bukhoba Primary School is situated on a rice plantation, while Gangu, Kanyaboli, and Gendro are located around Lake Kanyaboli, an oxbow lake with surrounding papyrus swamps that provide stable mosquito habitats, particularly for An. funestus (Fig. 1).
Fig. 1.
Map of Alego Usonga, Sub County showing the location of the primary schools in this study
Mosquito collection
Mosquitoes were collected using human landing catches (HLC) in five classrooms per school for 2 days in August 2023. Thirty adult males who were members of the community were enrolled and consented to participate as paid mosquito collectors for this study. Prior to the initial collection, the collectors were tested for malaria using the First Response® Malaria Ag. pLDH/HRP2 Combo Card Test rapid diagnostic tests (RDTs), and those who were positive for malaria were treated using artemether/lumefantrine (AL) (Coartem®). The collectors were given daily malaria chemoprophylaxis with doxycycline capsules (100 mg). Mosquito sampling was done hourly from 18:00 h overnight until 11:00 h, indoors and outdoors to match ongoing collections in neighbouring households. Collections were done for the first 45 min and the collectors took a 15-min break within every collection hour to fill out tablet questionnaires indicating the collection date, hour, and location and prepare for the next hour of collection.
During collection, the mosquito collectors wore shorts or rolled-up trousers and clothing that covered their bodies and upper limbs to protect them from unnecessary mosquito bites. Additionally, strict hourly supervision of the collectors was instituted to ensure they did not fall asleep so that they would not get bitten. It is worth noting that, no other mosquito borne infections have been detected in the region [26].
Additionally, HLC is actually a beneficial method of collection for the participants as research conducted in the same region earlier showed lower malaria incidence in HLC volunteers compared to other adults of the same demographic living in the same area [27]. This is because they are usually on malaria prophylaxis during this time. The collectors sat on a chair and collected mosquitoes that landed on their exposed legs using a mouth aspirator. Mosquitoes were aspirated into paper cups labelled using a tablet-generated code indicating the collection date, hour, and location and provided with cotton soaked in a 10% sugar solution. The next morning, mosquitoes were transported to a field laboratory for processing.
Morphological identifications were performed on the Anopheles mosquitoes following taxonomic keys [28] to differentiate between An. funestus s.l. and An. gambiae s.l. and other secondary malaria vectors. They were then separated by species, sex, and abdominal status (blood-fed, non-blood-fed, half-gravid, or gravid) for females and counted by location and hour of collection. A subset of the non-blood fed, non-desiccated An. gambiae s.l. and An. funestus s.l. females were dissected for parity determination as an indicator of their age and a sample of these were identified to species using PCR [29] and sporozoites detected using enzyme linked immunosorbent assay [30]. Culicine mosquitoes were counted and sexed by location and hour of collection and then discarded.
Data collection and analysis
The data were collected using CommCare v. 2.52.1. Vector abundance was assessed using descriptive statistics (totals, proportions and means). Line plot visualizations were used to show the trend of vector biting rates over the collection period. All data analysis and visualization tasks were performed in the R statistical computing environment.
Results
Anopheles funestus was the predominant species collected overall (n = 727; 93.2%), and in each of the primary schools (range 78.8% to 95.4, Table 1). Other species collected included An. gambiae (n = 49; 6.3%), An. coustani (n = 2, 0.25%) and An. ziemanni (n = 2, 0.25%). Anopheles gambiae s.l. represented 4.7% to 21.2% of collections in the schools, while An. coustani and An. ziemanni were collected only in Bukhoba primary school. The distance between these schools ranged between 3.14 and 14.16 km. The An. funestus eligible for dissection had an overall parity rate of 89.2% (n = 157). The total number of culicines collected comprised of 2247 females and 4 males. The breakdown of the bionomics is presented in Table 1.
Table 1.
The number (%) of Anopheles collected detailing the school categorized by location, species, abdominal, and parity status
| School | Gendro | Kanyaboli | Gangu | Bukhoba |
|---|---|---|---|---|
| Collection location/number collected | 66 | 109 | 138 | 467 |
| Indoor | 52 (79%) | 93 (85%) | 96 (70%) | 353 (76%) |
| Outdoor | 14 (21%) | 16 (15%) | 42 (30%) | 114 (24%) |
| Species | ||||
| An. funestus | 52 (79%) | 104 (95%) | 130 (94%) | 441 (94%) |
| An. gambiae | 14 (21%) | 5 (4.6%) | 8 (5.8%) | 22 (4.7%) |
| An. coustani | 0 | 0 | 0 | 2 (0.4%) |
| An. ziemanni | 0 | 0 | 0 | 2 (0.4%) |
| Abdominal status | ||||
| Fed | 28 (42%) | 69 (63%) | 49 (36%) | 224 (48%) |
| Gravid | 6 (9.1%) | 11 (10%) | 13 (9.4%) | 15 (3.2%) |
| Half gravid | 1 (1.5%) | 2 (1.4%) | 6 (1.3%) | |
| Unfed | 31 (47%) | 29 (27%) | 74 (54%) | 222 (48%) |
| Parity (Anopheles funestus only)/number dissected | 17 | 10 | 33 | 97 |
| Nulliparous | 2 (11.8%) | 1 (10%) | 0 | 14 (14.4%) |
| Parous | 15 (88.2%) | 9 (90%) | 33 (100%) | 83 (85.6%) |
| Culicines collected | Females | |||
| Indoor | 357 | 29 | 108 | 537 |
| Outdoor | 480 | 50 | 75 | 606 |
| Males | ||||
| Indoor | 0 | 0 | 0 | 0 |
| Outdoor | 1 | 0 | 1 | 2 |
All of the An. funestus s.l. samples were identified as An. funestus sensu stricto (s.s.) (N = 727) by PCR and had a sporozoite rate of 2.05% while 98% (n = 49) of An. gambiae s.l. were identified as An. arabiensis and the rest as An. gambiae s.s. None of the An. gambiae s.l. tested positive for sporozoite infectivity.
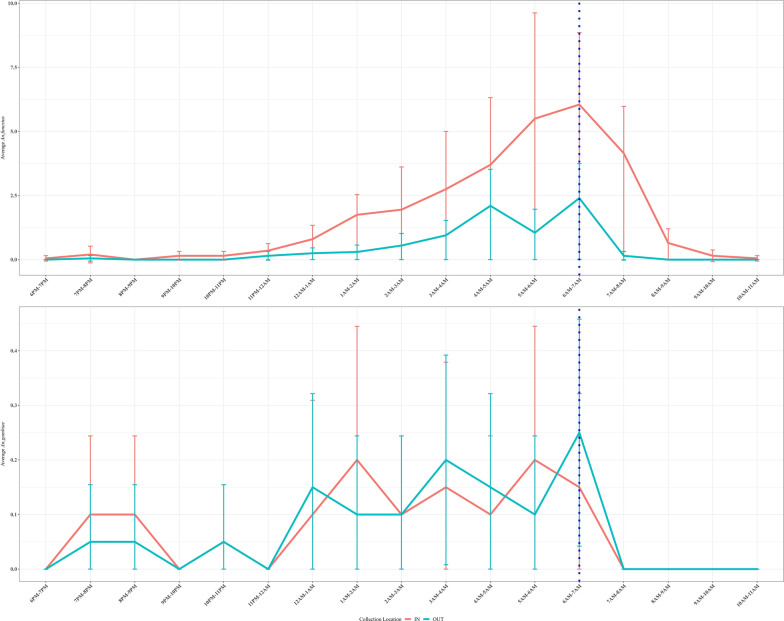
Anopheles funestus landing rates indoors increased steadily between 23:00 and midnight, peaking between 06:00 and 07:00 h and declined rapidly, with biting continuing at lower levels until the end of collections at 11:00 h. Inside the classrooms, collectors received an average of 1.8 An. funestus landings between 18:00 and 07:00 h and 1.25 bites between 07:00 and 11:00 h. Outdoors, they received an average of 0.6 landings between 18:00 and 07:00 h and only an average of 0.04 landings after 07:00 h. The collectors received an average of 0.1 An. arabiensis landings indoors between 18:00 and 07:00 h and 0.09 landings outdoors. Anopheles arabiensis did not land on the collectors after 07:00 h. In outdoor settings, An. funestus exhibited two distinct peaks, the first between 04:00 and 05:00 h and the second and a slightly larger peak coincident with the indoor peak between 06:00 and 07:00 h, but no landing was recorded after 09:00 h. Anopheles gambiae densities were much lower (averaging 0.2 mosquitoes per hour at peak) with no discernable differences in density between the indoor and outdoor collections. Anopheles gambiae activity was high between 23:00 and 08:00 h with no clear peaks indoors or outdoors (Fig. 2). Although a few An. gambiae were collected between 08:00 and 09:00 h, landing by this species largely ceased after 07:00 h.
Fig. 2.
The mean number of An. funestus and An. gambiae s.l. collected hourly between 18:00 to 11:00 h
Discussion
This study describes the collection of high densities of Anopheles mosquitoes, 93.2% of which were An. funestus, in primary schools in a malaria endemic area of western Kenya. Despite being only a 2-day sampling effort in August 2023, which is not peak mosquito season, the study yielded high densities of An. funestus averaging > 17 An. funestus females per collector, per hour. Anopheles funestus is one of the primary vectors in the area, having re-emerged about a decade ago [23], but it has a heterogeneous distribution evidenced by the collection of more than half of the mosquitoes in one school. Surprisingly, more than half of all the mosquitoes collected landing on the collectors were either already fed or gravid, suggesting that the vector has a repeat feeding behaviour, which has been documented in An. arabiensis in Zambia [31] but not in An. funestus, and certainly not at this scale. Lower densities of An. gambiae were observed, while only two each of An. coustani and An. ziemanni were collected. Importantly, An. funestus mosquitoes were collected indoors and outdoors, landing on HLC volunteers in high numbers up to 9 a.m. After 9 a.m., no mosquitoes were collected outdoors, with indoor mosquito numbers tapering off until 11 a.m., indicating that school children are at risk of malaria infection during the day.
School-based studies of malaria have been aimed at schools as avenues for conducting surveillance of malaria prevalence and incidence [14, 32–35] or schools as delivery mechanisms for chemoprevention through seasonal malaria chemoprevention [17] and intermittent preventive therapy in school children (iPTSC) [15, 36, 37]. This is informed by the often-higher parasite prevalence in children between the ages of 5–15, considered the primary school-going age, followed by children < 5 years of age, even though children < 5 years of age have the greatest burden in terms of morbidity and mortality due to an underdeveloped immune system [1]. However, this is the first investigation of Anopheles vectors as a contributor to malaria transmission within these school settings. Importantly, because children in high transmission settings have already developed immunity by this age, they remain as reservoirs of malaria infection in their communities because of greater anti-disease immunity to malaria than the younger children, but lower anti-parasite immunity than adults, thus school-aged children are at higher risk of asymptomatic parasitaemia [10, 11]. Because of this, school-aged children are less likely to seek treatment than younger children and therefore less likely to have the parasitaemia cleared with antimalaria drugs, and are also likely to have the poorest usage of nets [8, 38, 39] thus remaining important reservoirs of infection in the communities.
Several studies have reported changes in Anopheles vector biting behaviour, with changing preference towards early evening and late morning biting when people are often not under the protection of their bed nets [40–43]. Also, insecticide resistance in these mosquito populations could be leading to the survival of these mosquitoes despite exposure to pyrethroid insecticides [44]. Additionally, the early morning biting observed here points to a change in behaviour to avoid bednets. Entomological surveillance studies currently being conducted as part of a randomized control trial to evaluate the epidemiological efficacy of attractive targeted sugar baits in this study area (ClinicalTrials.gov ID NCT05219565) have reported late morning biting in An. funestus (Omondi et al., unpublished). These studies are coupled with observations of human activities and behaviour to understand the opportunities for human vector contact leading to malaria transmission. School children spend a significant portion of their time in schools, reporting between 06:00 and 07:00 h and staying in school until as late as 17:00 h.
Interestingly, the peak in biting was at 06:00 h, just as the children would normally be arriving at school. This means that children sitting in these structures are potentially being bitten during their morning classes as they sit still and pay attention during their lessons. Additionally, the high parity rates observed in all four schools suggests that a large proportion of these vectors had had prior bloodmeals and were potentially infectious. Indeed, 2.05% of the tested An. funestus were found to have Plasmodium falciparum sporozoites detected.
This study highlights the need to consider vector control approaches in schools and other peridomestic spaces where people are likely to be spending time when An. funestus mosquitoes are active during the day. The use of chemotherapeutic/chemoprophylactic approaches in combination with vector control in schools has the potential to reach a large asymptomatic reservoir of infectious individuals at a low logistical cost in line with the recent WHO’s recommendation for IPTsc in areas of moderate to high perennial or seasonal malaria transmission [43]. In particular, significant cost-benefit may be achieved with vector control approaches such as indoor residual spraying or spatial repellents protecting a large number of children attending school.
The study has several limitations, including a short and focused data collection period in August 2023, which may not capture the full seasonal variations in mosquito behaviours and malaria transmission. Using human landing catches (HLC) exposes collectors to potential health risks and may not accurately represent outdoor or animal-host-seeking mosquito behaviours. While treated and provided with chemoprophylaxis, the reliance on community members as collectors raises ethical concerns about their potential exposure to infectious mosquito bites. The study does not include a control group, making attributing changes in vector abundance challenging. Finally, the generalizability of the findings to other regions with different ecological characteristics is uncertain, given the specific conditions in Siaya County, Kenya.
Conclusion
This study was conducted in a highly endemic malaria setting in Siaya, where there is an abundance of An. funestus. These vectors have been observed to shift their biting patters, peaking between 06:00 and 07:00 h, coincident with when children generally report to non-boarding primary schools. An. funestus were observed to bite as late as 11:00 h meaning that children are receiving potentially infectious mosquito bites during the morning hours in these settings. For this reason, it is important to consider vector control approaches targeting schools and other peridomestic spaces in the morning hours when An. funestus is active.
Acknowledgements
We are sincerely grateful to the Chief Officers of the education and health departments in Siaya County for approving these collections. We are grateful to the school administrators for allowing us to conduct the study in their schools and to the entomology field and laboratory teams for their dedicated efforts in collecting and processing the samples.
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Kenya Medical Research Institute or the US Centers for Disease Control and Prevention.
Author contributions
SO, JK, GM, MM, BA and SA participated in study design, coordinated sample collection and processing. SO and EO drafted the manuscript, MM and BA performed statistical analysis and interpretation. DPM, MJD, SGS, JS, JRG, JEG participated in data interpretation, manuscript reviews and revisions, JRG, JEG, and EO conceptualized the study, supervised its implementation and analysis and offered technical support. All authors read and approved the final manuscript.
Funding
This study was funded by the US Centres for Disease Control and Prevention (CDC).
Availability of data and materials
All the study data is available and can be shared upon request to the corresponding author.
Declarations
Ethics approval and consent to participate
The study was approved by the scientific and ethics review unit (SERU) of the Kenya Medial Research Institute (protocol number KEMRI/SERU/CGHR/123/2776) and by the Institutional Review Board of the US Centers of Disease Control and Prevention (IRB 6728) through a reliance agreement with KEMRI. Written informed consent was obtained from all HLC collectors. The HLC collectors were given doxycycline malaria prophylaxis unless they tested positive for malaria in which case, they were treated with artemisinin-based combination therapy (ACT).
Consent for publication
This study was published with the consent of the KEMRI Director General.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . World malaria report 2022. Geneva: World Health Organization; 2022. [Google Scholar]
- 2.Samuels AM, Odero NA, Odongo W, Otieno K, Were V, Shi YP, et al. Impact of community-based mass testing and treatment on malaria infection prevalence in a high-transmission area of western Kenya: a cluster randomized controlled trial. Clin Infect Dis. 2021;72:1927–1935. doi: 10.1093/cid/ciaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rek J, Blanken SL, Okoth J, Ayo D, Onyige I, Musasizi E, et al. Asymptomatic school-aged children are important drivers of malaria transmission in a high endemicity setting in Uganda. J Infect Dis. 2022;226:708–713. doi: 10.1093/infdis/jiac169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z, Mitchell RM, Kariuki S, Odero C, Otieno P, Otieno K, et al. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya, 2012. Malar J. 2016;15:421. doi: 10.1186/s12936-016-1482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Rodriguez D, Katusele M, Auwun A, Marem M, Robinson LJ, Laman M, et al. Human behavior, livelihood, and malaria transmission in two sites of Papua New Guinea. J Infect Dis. 2021;223(12 Suppl 2):S171–S186. doi: 10.1093/infdis/jiaa402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . World malaria report 2021. Geneva: World Health Organization; 2021. [Google Scholar]
- 7.White NJ. Anaemia and malaria. Malar J. 2018;17:371. doi: 10.1186/s12936-018-2509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nankabirwa J, Brooker SJ, Clarke SE, Fernando D, Gitonga CW, Schellenberg D, et al. Malaria in school-age children in Africa: an increasingly important challenge. Trop Med Int Health. 2014;19:1294–1309. doi: 10.1111/tmi.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis. 2021;21:1568–1578. doi: 10.1016/S1473-3099(21)00072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coalson JE, Cohee LM, Buchwald AG, Nyambalo A, Kubale J, Seydel KB, et al. Simulation models predict that school-age children are responsible for most human-to-mosquito Plasmodium falciparum transmission in southern Malawi. Malar J. 2018;17:147. doi: 10.1186/s12936-018-2295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walldorf JA, Cohee LM, Coalson JE, Bauleni A, Nkanaunena K, Kapito-Tembo A, et al. School-age children are a reservoir of malaria infection in Malawi. PLoS ONE. 2015;10:e0134061. doi: 10.1371/journal.pone.0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kihwele F, Gavana T, Makungu C, Msuya HM, Mlacha YP, Govella NJ, et al. Exploring activities and behaviours potentially increases school-age children’s vulnerability to malaria infections in south-eastern Tanzania. Malar J. 2023;22:293. doi: 10.1186/s12936-023-04703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Malaria Control Programme. Kenya malaria indicator survey. Ministry of Health, Nairobi, Kenya. 2020.
- 14.Mathanga DP, Halliday KE, Jawati M, Verney A, Bauleni A, Sande J, et al. The high burden of malaria in primary school children in southern Malawi. Am J Trop Med Hyg. 2015;93:779–789. doi: 10.4269/ajtmh.14-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matangila JR, Mitashi P, Inocencio da Luz RA, Lutumba PT, Van Geertruyden JP. Efficacy and safety of intermittent preventive treatment for malaria in schoolchildren: a systematic review. Malar J. 2015;14:450. doi: 10.1186/s12936-015-0988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohee LM, Opondo C, Clarke SE, Halliday KE, Cano J, Shipper AG, et al. Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Glob Health. 2020;8:e1499–e1511. doi: 10.1016/S2214-109X(20)30325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thera MA, Kone AK, Tangara B, Diarra E, Niare S, Dembele A, et al. School-aged children based seasonal malaria chemoprevention using artesunate-amodiaquine in Mali. Parasite Epidemiol Control. 2018;3:96–105. doi: 10.1016/j.parepi.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konate D, Diawara SI, Toure M, Diakite SAS, Guindo A, Traore K, et al. Effect of routine seasonal malaria chemoprevention on malaria trends in children under 5 years in Dangassa, Mali. Malar J. 2020;19:137. doi: 10.1186/s12936-020-03202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mensah BA, Myers-Hansen JL, Obeng Amoako E, Opoku M, Abuaku BK, Ghansah A. Prevalence and risk factors associated with asymptomatic malaria among school children: repeated cross-sectional surveys of school children in two ecological zones in Ghana. BMC Public Health. 2021;21:1697. doi: 10.1186/s12889-021-11714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrasco D, Lefevre T, Moiroux N, Pennetier C, Chandre F, Cohuet A. Behavioural adaptations of mosquito vectors to insecticide control. Curr Opin Insect Sci. 2019;34:48–54. doi: 10.1016/j.cois.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Chilanga E, Collin-Vezina D, MacIntosh H, Mitchell C, Cherney K. Prevalence and determinants of malaria infection among children of local farmers in Central Malawi. Malar J. 2020;19:308. doi: 10.1186/s12936-020-03382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg. 2014;90:597–604. doi: 10.4269/ajtmh.13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Division of National Malaria Programme (DNMP) [Kenya] and ICF. Kenya malaria indicator survey 2020: key indicators. Nairobi, Kenya and Rockville, USA. 2021.
- 25.Attractive Targeted Sugar Bait Phase IIITG Attractive targeted sugar bait phase III trials in Kenya, Mali, and Zambia. Trials. 2022;23:640. doi: 10.1186/s13063-022-06555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karungu S, Atoni E, Ogalo J, Mwaliko C, Agwanda B, Yuan Z, et al. Mosquitoes of etiological concern in Kenya and possible control strategies. Insects. 2019;10:173. doi: 10.3390/insects10060173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coetzee M. Key to the females of afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 30.Wirtz R, Avery M, Benedict M, Sutcliffe A. Specific Anopheles techniques 3.3 Plasmodium sporozoite ELISA. Malaria Research and Reference Reagent Resource Center, 2007 (MR4); 2014.
- 31.Norris LC, Fornadel CM, Hung WC, Pineda FJ, Norris DE. Frequency of multiple blood meals taken in a single gonotrophic cycle by Anopheles arabiensis mosquitoes in Macha, Zambia. Am J Trop Med Hyg. 2010;83:33–37. doi: 10.4269/ajtmh.2010.09-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swana EK, Yav TI, Ngwej LM, Mupemba BN, Suprianto, Mukeng CK, et al. School-based malaria prevalence: informative systematic surveillance measure to assess epidemiological impact of malaria control interventions in the Democratic Republic of the Congo. Malar J. 2018;17:141. doi: 10.1186/s12936-018-2297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooker S, Kolaczinski JH, Gitonga CW, Noor AM, Snow RW. The use of schools for malaria surveillance and programme evaluation in Africa. Malar J. 2009;8:231. doi: 10.1186/1475-2875-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gitonga CW, Karanja PN, Kihara J, Mwanje M, Juma E, Snow RW, et al. Implementing school malaria surveys in Kenya: towards a national surveillance system. Malar J. 2010;9:306. doi: 10.1186/1475-2875-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson JC, Stresman GH, Gitonga CW, Gillig J, Owaga C, Marube E, et al. Reliability of school surveys in estimating geographic variation in malaria transmission in the western Kenyan highlands. PLoS ONE. 2013;8:e77641. doi: 10.1371/journal.pone.0077641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staedke SG, Maiteki-Sebuguzi C, Rehman AM, Kigozi SP, Gonahasa S, Okiring J, et al. Assessment of community-level effects of intermittent preventive treatment for malaria in schoolchildren in Jinja, Uganda (START-IPT trial): a cluster-randomised trial. Lancet Glob Health. 2018;6:e668–e679. doi: 10.1016/S2214-109X(18)30126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maiga H, Barger B, Sagara I, Guindo A, Traore OB, Tekete M, et al. Impact of three-year intermittent preventive treatment using artemisinin-based combination therapies on malaria morbidity in Malian schoolchildren. Trop Med Infect Dis. 2020;5:148. doi: 10.3390/tropicalmed5030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Njatosoa AF, Mattern C, Pourette D, Kesteman T, Rakotomanana E, Rahaivondrafahitra B, et al. Family, social and cultural determinants of long-lasting insecticidal net (LLIN) use in Madagascar: secondary analysis of three qualitative studies focused on children aged 5–15 years. Malar J. 2021;20:168. doi: 10.1186/s12936-021-03705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchwald AG, Walldorf JA, Cohee LM, Coalson JE, Chimbiya N, Bauleni A, et al. Bed net use among school-aged children after a universal bed net campaign in Malawi. Malar J. 2016;15:127. doi: 10.1186/s12936-016-1178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sougoufara S, Diedhiou SM, Doucoure S, Diagne N, Sembene PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moiroux N, Damien GB, Egrot M, Djenontin A, Chandre F, Corbel V, et al. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS ONE. 2014;9:e104967. doi: 10.1371/journal.pone.0104967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangbakembi-Ngounou C, Costantini C, Longo-Pendy NM, Ngoagouni C, Akone-Ella O, Rahola N, et al. Diurnal biting of malaria mosquitoes in the Central African Republic indicates residual transmission may be out of control. Proc Natl Acad Sci USA. 2022;119:e2104282119. doi: 10.1073/pnas.2104282119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO . Updated WHO recommendations for malaria chemoprevention and elimination. Geneva: World Health Organization; 2022. [Google Scholar]
- 44.The PMI VectorLink Project. Kenya annual entomological monitoring report. October 2019–June 2021. Rockville: The PMI VectorLink Project, Abt Associates Inc.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the study data is available and can be shared upon request to the corresponding author.