Abstract
Arterial stiffness and hypertension are closely related in pathophysiology. Chronic high blood pressure (BP) can lead to arterial wall damage by mechanical stress, endothelial dysfunction, increased inflammation, oxidative stress, and renin–angiotensin–aldosterone system (RAAS) activation. Hypertension also increases collagen fiber production and accelerates elastin fiber degradation. Stiffened arteries struggle with BP changes, raising systolic BP and pulse pressure. The resulting increased systolic pressure further hardens arteries, creating a harmful cycle of inflammation and calcification. Arterial stiffness data can predict target organ damage and future cardiovascular events in hypertensive patients. Thus, early detection of arterial stiffness aids in initiating preventive measures and treatment plans to protect against progression of vascular damage. While various methods exist for measuring arterial stiffness, pulse wave velocity is a non-invasive, simple measurement method that maximizes effectiveness. Healthy lifestyle changes, RAAS blockers, and statins are known to reduce arterial stiffness. Further research is needed to ascertain if improving arterial stiffness will enhance prognosis in hypertensive patients.
Graphical Abstract
Keywords: Arterial damage, Arterial stiffness, Cardiovascular risk, Hypertension, Target organ damage
Introduction
Hypertension, commonly known as high blood pressure (BP), is a prevalent medical condition where the force of the blood against the artery walls is consistently too high. This chronic condition is a significant global health burden because it is associated with an increased risk of cardiovascular and cerebrovascular diseases, which are among the leading causes of death worldwide [1, 2].
With age or due to prolonged exposure to cardiovascular disease risk factors, such as high BP, hyperglycemia, dyslipidemia, smoking, and chronic inflammation, the arterial wall tends to stiffen [3, 4]. As arterial stiffness escalates, systolic pressure increases and diastolic pressure decreases because of the premature merging of the fast-reflected wave with the forward wave [5]. The increased systolic pressure triggers left ventricular hypertrophy (LVH), while the diminished diastolic pressure impairs coronary blood flow. Additionally, an increase in arterial stiffness contributes to an elevation in pulse pressure (PP), which can potentially damage the blood–brain barrier [6]. Furthermore, in the blood vessel wall with increased arterial stiffness, more elastic fibers are destroyed, and the pulsatile pressure from the heart is not properly buffered and transmitted to the target organ, causing organ damage [7]. Arterial stiffness and poor cardiovascular prognosis often share common risk factors, reinforcing the correlation between them. This increased in arterial stiffness, driven by these mechanisms, is associated with a poorer cardiovascular prognosis [8, 9]. Hence, gathering data on arterial stiffness is critical in predicting a patient’s cardiovascular prognosis.
Arterial stiffness and hypertension are intricately intertwined, each significantly influencing the other. By elucidating the relationship between arterial stiffness and hypertension, we can gain valuable insights that can be applied to the diagnosis, treatment, and prognosis of patients with hypertension. In this review, I will explore the complex interplay between arterial stiffness and hypertension, and discuss the clinical implications derived from this relationship.
Pathophysiology of arterial stiffening
Arterial stiffness refers to the reduced ability of an artery to expand and contract in response to pressure changes. With age and certain diseases, there are changes in the structure of the arterial wall. The arterial wall is made up of three layers: the intima (inner layer), the media (middle layer), and the adventitia (outer layer). The media is primarily made up of smooth muscle cells and elastin. Over time, there is a reduction in the elastin content and an increase in collagen, leading to stiffening of the arteries. In addition, there can be calcification within the arterial wall, contributing to increased stiffness [4]. The endothelium, the innermost lining of blood vessels, plays a key role in vascular tone by releasing substances like nitric oxide (NO), which causes vasodilation. With age and disease, there can be endothelial dysfunction, leading to decreased NO production and increased production of vasoconstrictive substances, contributing to arterial stiffness [10]. Chronic inflammation and oxidative stress are thought to play a significant role in the pathophysiology of arterial stiffness [11, 12]. Inflammatory and oxidative stress markers are often elevated in conditions associated with increased arterial stiffness, like hypertension and diabetes [13]. Increased arterial stiffness results in higher systolic BP and PP, leading to LVH and further cardiovascular complications [6].
How to measure arterial stiffness
There are numerous methodologies for evaluating arterial stiffness, among which pulse wave velocity (PWV) is the most widely accepted and validated. PWV quantifies the rate at which the arterial pulse travels through the arterial system—a faster pulse wave indicates increased arterial stiffness. The most commonly utilized PWVs are carotid-femoral PWV (cfPWV) and brachial-ankle PWV (baPWV). Initially developed and primarily used in the West, cfPWV is considered the gold standard for noninvasive arterial stiffness measurement, as it focuses on the elastic artery portion between the carotid and femoral arteries [14]. A wealth of clinical data supports its use. In contrast, baPWV was developed later in Japan and is mainly used by Asians. Since baPWV also encompasses the muscular arteries between the upper arm and ankle, it has been criticized for not exclusively reflecting the elastic components of arterial stiffness. Despite this, numerous studies have confirmed that baPWV correlates well with cfPWV values and aligns well with invasively measured results [15]. Importantly, the prognostic value of baPWV has been substantiated in many clinical studies [9, 16–27]. Further, it’s reported that baPWV may better represent the total resistance value than cfPWV, with regard to the left ventricle’s afterload [28]. While cfPWV may cause patient discomfort when locating the femoral or carotid artery and necessitates skilled examiners, baPWV is simple and convenient, requiring only a BP cuffs wrapped around the limbs. Thus, for mass screenings involving large numbers of individuals, baPWV has distinct advantages [6]. In general, it is suggested that a cfPWV ≥ 10 m/s is considered a high-risk finding [29–32]. For baPWV, values < 14 m/s are considered low risk, ≥ 14 and < 18 m/s as moderate risk, and ≥ 18 m/s as high risk [29, 31, 32]. The strengths, limitations, and reference values of cfPWV and baPWV are compared in Table 1.
Table 1.
The strengths, limitations, and reference values of cfPWV and baPWV
| cfPWV | baPWV | |
|---|---|---|
| Strength | Regarded as the gold standard among non-invasive methods for measuring arterial stiffness | Simpler and quicker to measure, making it suitable for mass screening |
| Supported by extensive clinical data and is the most validated measure | Offers insights into both central and peripheral arterial stiffness, providing a comprehensive assessment | |
| Provides an assessment of central aortic stiffness | The equipment for baPWV is more affordable and readily available in many clinical settings | |
| Minimally influenced by lower limb arterial disease | More reproducible with reduced variability due to its straightforward operator technique | |
| Limitation | Can be uncomfortable for subjects during measurement | Reflects a mixed assessment of both central and peripheral arterial stiffness |
| Demands specialized training and is more time-consuming to perform | Susceptible to alterations from conditions affecting peripheral arteries, particularly lower limb diseases | |
| Less suitable for mass screening | May not be universally recognized or preferred for some research or clinical contexts | |
| Does not provide insights into peripheral arterial stiffness | Lacks standardized normal values that are universally accepted | |
| Might overestimate values in individuals with short stature | Predominantly utilized in Asian countries | |
| Reference value | Abnormal ≥ 10 m/s |
Normal < 14 m/s Borderline ≥ 14 and < 18 m/s Abnormal ≥ 18 m/s |
cfPWV carotid-femoral pulse wave velocity, baPWV brachial-ankle pulse wave velocity
The augmentation index (AIx) quantifies the extent to which the reflected pulse wave enhances the primary forwarding pulse wave [33]. Influenced by both the pulse wave’s speed, which is impacted by arterial stiffness, and the timing of the wave reflection, AIx is often measured using pulse wave analysis of the radial artery waveform [34]. This waveform is subsequently employed to generate a central (aortic) pressure waveform.
PP, defined as the difference between systolic and diastolic BP, serves as a rudimentary surrogate marker of arterial stiffness [35]. Both brachial PP and central aortic PP can act as arterial stiffness indicators. However, PP can be influenced by various factors, and thus, it may not be as accurate as other measures, like PWV. There are invasive and non-invasive techniques to measure central arterial PP. Due to the costs, invasiveness, and ethical considerations, invasive methods are primarily reserved for patients undergoing invasive coronary angiography [36, 37]. Recently, a widely used non-invasive approach infers the aortic pressure by obtaining the radial artery’s waveform, which is known for its relative high accuracy [38].
Another less common method of evaluating arterial stiffness involves directly measuring changes in arterial diameter in response to BP variations. While not frequently used in clinical practice, this method may be utilized in research settings. Ultrasound techniques, computed tomography, or magnetic resonance imaging can capture these changes by measuring the maximal (systolic) and minimal (diastolic) arterial diameters [39].
Effect of hypertension on arterial stiffness
Arterial damage
Chronic hypertension can lead to arterial damage. High BP exerts additional strain on the walls of blood vessels. This augmented mechanical stress can directly damage the endothelium, the inner lining of the blood vessels, and stimulate vascular remodeling, resulting in thicker, stiffer arteries [40]. Hypertension can also impair endothelial function by potentially reducing the production of NO, a molecule vital for maintaining vascular health through its ability to relax blood vessels and inhibit inflammatory processes. This endothelial dysfunction is a key step in the development of atherosclerosis [41]. Moreover, hypertension is associated with increased inflammation and oxidative stress, both of which can further damage the blood vessels. Inflammatory cells can infiltrate the vascular wall, promoting the formation of atherosclerotic plaques, while reactive oxygen species can degrade NO, impairing its vasodilatory function [42]. Hypertension can stimulate the activation of the renin–angiotensin–aldosterone system (RAAS), a hormone system responsible for regulating BP and fluid balance. Excessive activation of this system can result in vasoconstriction, retention of salt and water, and additional vascular damage [43].
Collagen and elastin changes
Hypertension can cause significant alterations to the collagen and elastin composition within arterial walls, thereby contributing to vascular stiffness and remodeling. Collagen and elastin are two key structural proteins found in the walls of arteries. While elastin provides elasticity, allowing the arterial wall to stretch and recoil with each heartbeat, collagen provides strength and stability. Chronic high BP can stimulate the production of collagen in the arterial wall [40]. The excessive collagen can replace the more elastic fibers, making the arteries more rigid and less able to expand and recoil with blood flow. This can increase the systolic BP, and contribute to a vicious cycle of worsening hypertension and arterial stiffness. Additionally, hypertension can also accelerate the degradation of elastin fibers [44]. Loss of elastin reduces the elasticity of the arterial wall, contributing to arterial stiffness. Hypertension can also increase the activity of matrix metalloproteinases (MMPs), a family of enzymes that break down extracellular matrix proteins, including collagen and elastin [45]. While some degradation of extracellular matrix proteins is normal, excessive MMP activity can disrupt the balance between collagen and elastin, contributing to arterial remodeling and stiffness.
Effect of arterial stiffening on BP
The relationship between arterial stiffening and BP is bidirectional. As arterial stiffness increases, the capacity of the artery to expand and contract in response to fluctuations in BP decreases. Over time, this diminished elasticity can lead to an increase in systolic BP and PP. This is because arteries with greater stiffness are less capable of expanding to accommodate the surge of blood ejected from the heart during each heartbeat [46]. Moreover, in a stiffened artery, the pulse wave travels at a higher speed. As a result, the reflected wave returns to the heart more quickly, coinciding with the systolic phase (the heart’s contraction phase). This phenomenon increases systolic BP, decreases diastolic BP, and widens PP [6]. An increase in systolic BP can, in turn, further contribute to arterial stiffening. This happens through damage to the endothelial cells lining the artery walls, which triggers inflammation, fibrosis, and calcification [40]. This perpetuates a vicious cycle of arterial stiffening and high BP.
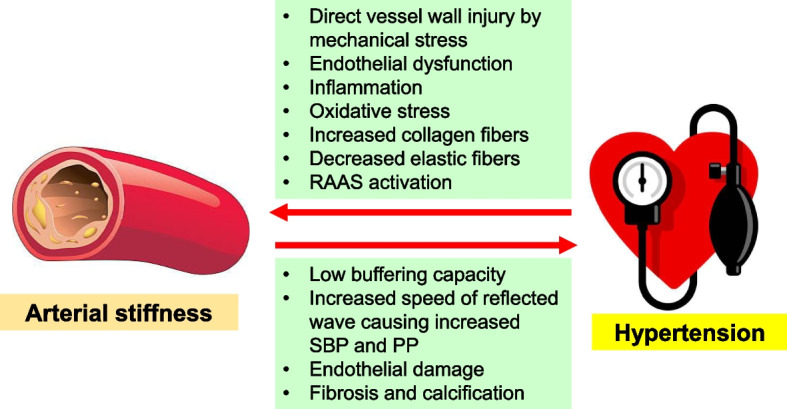
Figure 1 illustrates the pathophysiological correlation between arterial stiffness and hypertension.
Fig. 1.
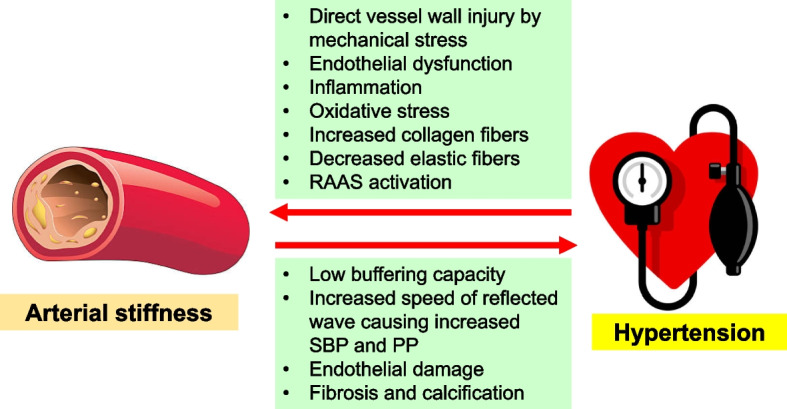
Pathophysiological correlation between arterial stiffness and hypertension. RAAS, renin–angiotensin–aldosterone system; SBP, systolic blood pressure; PP, pulse pressure
Implications of arterial stiffness in hypertensive patients
The role of arterial stiffness in predicting the future development of hypertension
Numerous studies suggest that arterial stiffness predicts future hypertension. Kaess et al. [47] discovered over a 7.8-year median follow-up that among 1,759 normotensive adults, increased arterial stiffness indicators like forward wave amplitude, AIx, and cfPWV predicted a higher hypertension risk. Another study involving 6,992 normotensive men found a 15% greater hypertension risk associated with carotid artery stiffness, regardless of baseline BP and other risk factors [48]. In a 4-year follow-up of 2,496 normotensive patients, those in the third tertile for baPWV were 3.5 times more likely to develop hypertension than those in the first tertile [49]. A study on 2,512 patients found a significant association between aortic elasticity and new hypertension cases over four years [50]. Finally, Najjar et al. [51] found that a 1 m/s PWV increase corresponded to a 10% rise in hypertension incidence among 449 normotensive individuals over 4.9 years.
These findings suggest the potential of arterial stiffness measures in early identification and prevention strategies for hypertension. However, further research is needed to determine how these measures can be best incorporated into routine clinical practice and public health efforts.
The role of arterial stiffness in the prediction of target organ damage of hypertensive patients
Arterial stiffness plays a significant role in the development and detection of target organ damage in hypertensive patients. Target organs in hypertension include the heart, kidneys, brain, and peripheral arteries [52]. Damage to these organs can lead to conditions such as heart disease, kidney failure, stroke, and peripheral artery disease [53]. Arterial stiffness can increase afterload, or the load against which the heart has to contract to eject blood. This can lead to LVH [6, 54], which is a thickening of the wall of the heart’s main pumping chamber. LVH is a common form of target organ damage in hypertension. Arterial stiffness can impair coronary perfusion, or the flow of blood to the heart muscle itself. This can lead to ischemic heart disease [5]. High arterial stiffness may cause high PP, which is a risk factor for cerebrovascular disease such as stroke [6]. The kidneys, as highly vascular organs, are also susceptible to damage from high BP and increased arterial stiffness, leading to kidney failure [55]. Increased arterial stiffness can also lead to peripheral artery disease, which is when the arteries supplying blood to the legs become narrowed or blocked [56]. In terms of detection, arterial stiffness can be measured using non-invasive techniques such as PWV, which is the speed at which the arterial pulse propagates through the circulatory system. An increased PWV is indicative of higher arterial stiffness. On these back grounds, some guidelines recommend PWV measurement as an optional test for early organ damage evaluation and risk prediction in hypertensive patients [30, 32, 57]. This makes it an important parameter to monitor in hypertensive patients for early detection and prevention of target organ damage.
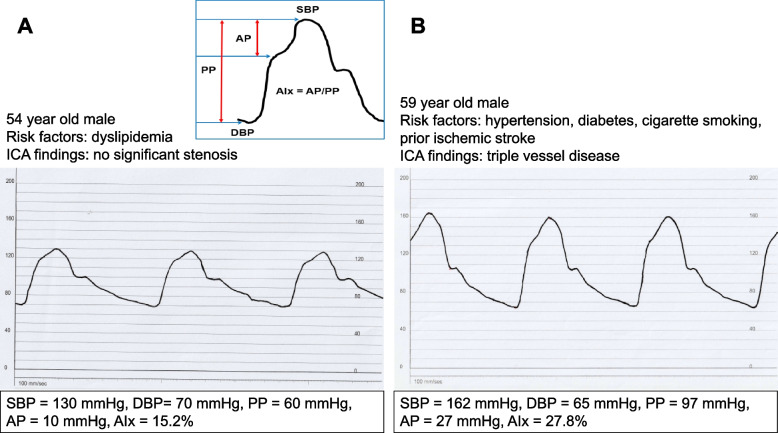
Figure 2 compares representative waveforms and hemodynamic parameters of invasively measured central aortic pressure curves between low-to-intermediate-risk and high-risk individuals. High-risk patients with multiple risk factors and three-vessel disease (Fig. 2B) exhibit higher systolic BP, PP, augmentation pressure, and AIx and lower diastolic BP due to increased aortic stiffness compared to low-to-intermediate-risk patients (Fig. 2A).
Fig. 2.
Invasively measured central aortic pressure waveforms and associated hemodynamic parameters in low-to-intermediate-risk (A) and high-risk (B) individuals. SBP, systolic blood pressure; AP, augmentation pressure; PP, pulse pressure; AIx, augmentation index; DBP, diastolic blood pressure; ICA, invasive coronary angiography
The impact of arterial stiffness on the prognosis of hypertensive patients
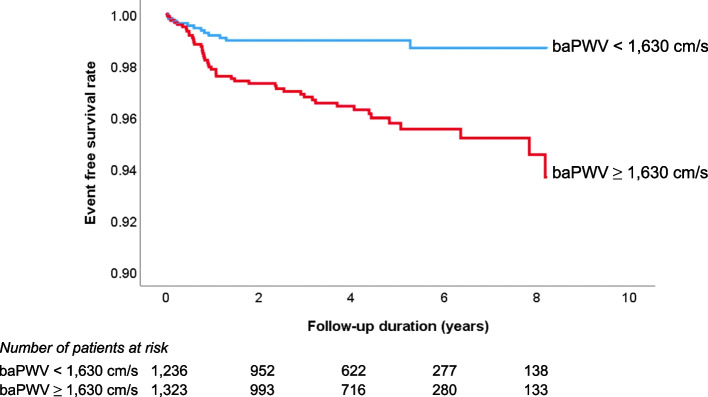
As outlined in the previous sections, arterial stiffness plays a crucial role in causing organ damage in hypertensive patients. This organ damage is intrinsically linked to the occurrence of cardiovascular events [53]. Therefore, it’s not surprising that arterial stiffness serves as a valuable predictor for cardiovascular events in patients with hypertension. Evidence from various studies affirms this relationship. Many studies have reported that an increase in arterial stiffness leads to a deterioration in the cardiovascular prognosis of hypertensive patients [16, 58, 59]. This evidence suggests that arterial stiffness is not just a by-product but an active contributor to the worsening of cardiovascular health in hypertensive patients. Recently, our research team conducted a study involving 2,561 Korean hypertensive subjects. The results of this study demonstrated that a higher baseline baPWV was independently associated with a higher risk of cardiovascular disease occurrence. This correlation was maintained even after accounting for potential confounding factors in the multivariable analysis, highlighting the robustness of this relationship. Furthermore, in this study, the authors proposed a baPWV value of 1,630 cm/s as a cut-off for predicting cardiovascular events [16] (Fig. 3). These studies highlight the importance of monitoring arterial stiffness in hypertensive patients. It is an important risk factor for cardiovascular events, contributes to the prognosis of these patients, and provides a potential target for therapeutic intervention.
Fig. 3.
Prognostic value of baPWV in the prediction of cardiovascular events in hypertensive patients [16]. baPWV, brachial-ankle pulse wave velocity
Therapeutic strategies targeting arterial stiffness in hypertensive patients
There are several benefits of reducing arterial stiffness in hypertensive patients. Arterial stiffness is a known independent risk factor for cardiovascular diseases [8, 9]. As such, its reduction can lead to a decreased risk of these diseases, including heart attack, stroke, and heart failure. Stiff arteries can contribute to high systolic BP, especially in older adults [7]. By lowering arterial stiffness, it may be possible to achieve better control of BP, a key component in managing hypertension and reducing related complications. Both hypertension and arterial stiffness can result in damage to important organs such as the heart, brain, and kidneys [3, 39]. By reducing arterial stiffness, the pressure load on these organs can be lessened, potentially preventing or slowing the progression of organ damage. Additionally, a reduction in arterial stiffness can improve blood flow throughout the body, leading to improved overall function and possibly reducing symptoms related to inadequate blood flow, such as fatigue or shortness of breath [60, 61].
How to reduce arterial stiffness in hypertensive patients?
Although not exclusive to hypertensive patients, there are several methods available to reduce arterial stiffness [5]. The cornerstone of these interventions is lifestyle modification. A balanced and healthy diet can contribute substantially towards reducing arterial stiffness. Consuming a diet rich in fresh fruits, vegetables, lean proteins, whole grains, and low-fat dairy products, while keeping sodium intake in check, is essential. These principles form the foundation of the Dietary Approaches to Stop Hypertension (DASH) diet, which is a scientifically validated method for controlling hypertension and enhancing cardiovascular health [62, 63]. Consuming a diet rich in fresh fruits, vegetables, lean proteins, whole grains, and low-fat dairy products provides essential anti-oxidants and anti-inflammatory compounds [64]. These reduce oxidative stress and inflammation, both of which contribute to arterial stiffness by damaging the endothelial lining and promoting the formation of plaques. Reducing sodium intake and adopting diets like the DASH diet can help lower BP [62, 63], which in turn reduces the mechanical strain on arterial walls. Omega-3 fatty acids, prevalent in fish, have anti-dyslipidemic properties [65]. Dyslipidemia can lead to atherosclerotic plaque buildup, further enhancing arterial stiffness. Physical exercise has also shown efficacy in reducing arterial stiffness. It appears that the consistency and regularity of exercise are more critical in lowering arterial stiffness than the specific type or intensity of the workout [66]. Meta-analysis demonstrated that exercise interventions based on aerobic, combined or isometric exercise significantly reduced PWV value in hypertensive subjects [67]. Regular physical activity improves endothelial function, promotes the release of nitric oxide, and stimulates antioxidant defense mechanisms, helping arteries remain flexible [68]. Weight management is another significant factor. By maintaining a healthy weight, individuals can stave off numerous health complications, including increased arterial stiffness and high BP [69]. However, it is important to keep in mind that the association between body mass index and arterial stiffness is not linear [70]. Moreover, proactive approaches to quitting smoking is vital, as these habits can worsen arterial stiffness and result in other deleterious health effects [71, 72]. Alcohol’s impact on arterial stiffness varies depending on the quantity consumed, and large amounts or binge drinking are known to exacerbate arterial stiffness [73]. Stress management, an often-overlooked aspect of overall health, also plays a significant role. Chronic stress is a known contributor to hypertension and arterial stiffness [74], and implementing stress management strategies like meditation, deep-breathing exercises, yoga, and ensuring adequate sleep, is vital for arterial health [75].
Pharmacological interventions can be also applied for reducing arterial stiffness. Drugs with established cardiovascular protective effects, such as RAAS blockers [76] and statins [77], have been demonstrated to be effective in this regard. Continuous positive airway pressure therapy has also shown efficacy in reducing arterial stiffness among patients with obstructive sleep apnea [78]. Despite these advancements, the current body of research on the reduction of arterial stiffness predominantly comprises small-scale studies. There is a definitive need for large-scale, comprehensive studies in the future to further validate these methods and possibly uncover more effective strategies for reducing arterial stiffness.
The importance and challenges of early detection of aortic stiffness
Based on what has already been described above, it is important to obtain early information on arterial stiffness in hypertensive patients. Increased arterial stiffness is a strong independent predictor of future cardiovascular events and all-cause mortality. It can help identify high-risk individuals who may require more aggressive treatment and monitoring. The detection of arterial stiffness can be useful for the early initiation of preventive and therapeutic strategies to limit the progression of vascular damage. This includes lifestyle modifications, such as dietary changes, increased physical activity, smoking cessation, and BP control, as well as the appropriate use of medication. Changes in arterial stiffness can also serve as a measure of the effectiveness of therapeutic interventions. If a particular treatment is effective in reducing arterial stiffness, it can suggest that the treatment is beneficial for vascular health and potentially reducing future cardiovascular risk. Hypertension and arterial stiffness often coexist and can exacerbate each other. Understanding the degree of arterial stiffness can provide insights into the underlying pathophysiology of an individual’s hypertension, potentially guiding more personalized treatment approaches. Studies have shown that arterial stiffness, measured by techniques like PWV, provides prognostic information beyond traditional risk factors. Patients with high arterial stiffness often have worse long-term cardiovascular outcomes, and knowing this can help shape the management plan. When patients understand that they have an increased arterial stiffness that can be improved with healthy lifestyle changes, it may provide additional motivation for them to make and maintain these changes. In summary, early detection of arterial stiffness in hypertensive patients can guide therapeutic decisions, help monitor the effectiveness of interventions, improve risk stratification, and potentially improve outcomes.
Conclusions
Hypertension and arterial stiffness are closely related to each other. When BP is high, arterial stiffness progresses, and conversely, when arterial stiffness progresses, BP also increases. Arterial stiffness information can be used in hypertensive patients to predict the cardiovascular risk. Several methods are available for measuring arterial stiffness; however, the use of non-invasive techniques makes the information highly beneficial in treating hypertensive patients. The suggestion has been made that a healthy lifestyle, RAAS blockers, and statins are beneficial in reducing arterial stiffness. Further research is required to determine if a treatment strategy that ameliorates arterial stiffness can enhance the prognosis of patients with hypertension.
Acknowledgements
None declared.
Abbreviations
- AIx
Augmentation index
- baPWV
Brachial-ankle pulse wave velocity
- BP
Blood pressure
- cfPWV
Carotid-femoral pulse wave velocity
- LVH
Left ventricular hypertrophy
- MMP
Matrix metalloproteinases
- NO
Nitric oxide
- PP
Pulse pressure
- PWV
Pulse wave velocity
- RAAS
Renin–angiotensin–aldosterone system
Author’s contributions
HLK wrote, edited and reviewed the draft.
Funding
None declared.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report From the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Huang W, Nie J, Wang Y. Hypertension and the risk of all-cause and cause-specific mortality: an outcome-wide association study of 67 causes of death in the national health interview survey. Biomed Res Int. 2021;2021:9376134. doi: 10.1155/2021/9376134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC State-of-the-art review. J Am Coll Cardiol. 2019;74(9):1237–1263. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74(11):2257–2262. doi: 10.1253/circj.CJ-10-0910. [DOI] [PubMed] [Google Scholar]
- 5.Kim HL, Weber T. Pulsatile hemodynamics and coronary artery disease. Korean Circ J. 2021;51(11):881–898. doi: 10.4070/kcj.2021.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HL, Kim SH. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. 2019;6:41. doi: 10.3389/fcvm.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurent S, Boutouyrie P. Arterial stiffness and hypertension in the elderly. Front Cardiovasc Med. 2020;7:544302. doi: 10.3389/fcvm.2020.544302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69(6):1045–1052. doi: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 10.Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, et al. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55(3):674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- 11.Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70(4):660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura T, Dohi Y, Takase H, Yamashita S, Fujii S, Ohte N. Oxidative stress is closely associated with increased arterial stiffness, especially in aged male smokers without previous cardiovascular events: a cross-sectional study. J Atheroscler Thromb. 2017;24(11):1186–1198. doi: 10.5551/jat.39289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouvreau C, Dayre A, Butkowski EG, de Jong B, Jelinek HF. Inflammation and oxidative stress markers in diabetes and hypertension. J Inflamm Res. 2018;11:61–68. doi: 10.2147/JIR.S148911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 15.Kang J, Kim HL, Lim WH, Seo JB, Zo JH, Kim MA, et al. Relationship between brachial-ankle pulse wave velocity and invasively measured aortic pulse pressure. J Clin Hypertens (Greenwich) 2018;20(3):462–468. doi: 10.1111/jch.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HL, Lim WH, Seo JB, Kim SH, Zo ZH, Kim MA. Prediction of cardiovascular events using brachial-ankle pulse wave velocity in hypertensive patients. J Clin Hypertens (Greenwich) 2020;22(9):1659–1665. doi: 10.1111/jch.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HL, Joh HS, Lim WH, Seo JB, Kim SH, Zo JH, et al. One-month changes in blood pressure-adjusted pulse wave velocity for predicting long-term cardiovascular outcomes in patients undergoing percutaneous coronary intervention. J Hypertens. 2023;41(3):437–442. doi: 10.1097/HJH.0000000000003354. [DOI] [PubMed] [Google Scholar]
- 18.Kim HL, Lim WH, Seo JB, Kim SH, Zo JH, Kim MA. Prognostic value of arterial stiffness according to the cardiovascular risk profiles. J Hum Hypertens. 2021;35(11):978–984. doi: 10.1038/s41371-020-00441-z. [DOI] [PubMed] [Google Scholar]
- 19.Ishizuka K, Hoshino T, Shimizu S, Shirai Y, Mizuno S, Toi S, et al. Brachial-ankle pulse wave velocity is associated with 3-month functional prognosis after ischemic stroke. Atherosclerosis. 2016;255:1–5. doi: 10.1016/j.atherosclerosis.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Park HW, Kim HR, Kang MG, Kim K, Koh JS, Park JR, et al. Predictive value of the combination of brachial-ankle pulse wave velocity and ankle-brachial index for cardiovascular outcomes in patients with acute myocardial infarction. Coron Artery Dis. 2020;31(2):157–165. doi: 10.1097/MCA.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 21.Hwang IC, Jin KN, Kim HL, Kim YN, Im MS, Lim WH, et al. Additional prognostic value of brachial-ankle pulse wave velocity to coronary computed tomography angiography in patients with suspected coronary artery disease. Atherosclerosis. 2018;268:127–137. doi: 10.1016/j.atherosclerosis.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Song TJ, Kim EH, Lee KJ, Lee HS, Nam CM, et al. Brachial-ankle pulse wave velocity for predicting functional outcome in acute stroke. Stroke. 2014;45(8):2305–2310. doi: 10.1161/STROKEAHA.114.005576. [DOI] [PubMed] [Google Scholar]
- 23.Chen SC, Lee WH, Hsu PC, Lin MY, Lee CS, Lin TH, et al. Association of brachial-ankle pulse wave velocity with cardiovascular events in atrial fibrillation. Am J Hypertens. 2016;29(3):348–356. doi: 10.1093/ajh/hpv124. [DOI] [PubMed] [Google Scholar]
- 24.Ueki Y, Miura T, Minamisawa M, Abe N, Nishimura H, Hashizume N, et al. The usefulness of brachial-ankle pulse wave velocity in predicting long-term cardiovascular events in younger patients. Heart Vessels. 2017;32(6):660–667. doi: 10.1007/s00380-016-0919-6. [DOI] [PubMed] [Google Scholar]
- 25.Washida N, Wakino S, Hayashi K, Kuwahara T, Itoh H. Brachial-ankle pulse wave velocity predicts silent cerebrovascular diseases in patients with end-stage renal diseases. J Atheroscler Thromb. 2010;17(2):165–172. doi: 10.5551/jat.2097. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Li D, Liu R, Hu Y, Chen S, Wu S, et al. Brachial-ankle pulse wave velocity is a stronger predictor than blood pressure for atherosclerotic cardiovascular diseases and all-cause mortality: a cohort study. Hypertens Res. 2023;46:2100. doi: 10.1038/s41440-023-01313-y. [DOI] [PubMed] [Google Scholar]
- 27.Sang T, Lv N, Dang A, Cheng N, Zhang W. Brachial-ankle pulse wave velocity and prognosis in patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Hypertens Res. 2021;44(9):1175–1185. doi: 10.1038/s41440-021-00678-2. [DOI] [PubMed] [Google Scholar]
- 28.Chow B, Rabkin SW. Brachial-ankle pulse wave velocity is the only index of arterial stiffness that correlates with a mitral valve indices of diastolic dysfunction, but no index correlates with left atrial size. Cardiol Res Pract. 2013;2013:986847. doi: 10.1155/2013/986847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JB, Sharman JE, Li Y, Munakata M, Shirai K, Chen CH, et al. Expert consensus on the clinical use of pulse wave velocity in Asia. Pulse (Basel) 2022;10(1–4):1–18. doi: 10.1159/000528208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancia Chairperson G, Kreutz Co-Chair R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH) J Hypertens. 2023;41:1874. doi: 10.1097/HJH.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 31.Lee HY, Shin J, Kim GH, Park S, Ihm SH, Kim HC, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20. doi: 10.1186/s40885-019-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2019) Hypertens Res. 2019;42(9):1235–1481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 33.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45(5):980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 34.Kim HL, Koo BK, Joo SK, Kim W. Association of arterial stiffness with the histological severity of nonalcoholic fatty liver disease. Hepatol Int. 2020;14(6):1048–1056. doi: 10.1007/s12072-020-10108-z. [DOI] [PubMed] [Google Scholar]
- 35.Safar ME. Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Curr Opin Nephrol Hypertens. 2001;10(2):257–261. doi: 10.1097/00041552-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Kim HL, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Association between invasively measured central aortic pressure and left ventricular diastolic function in patients undergoing coronary angiography. Am J Hypertens. 2015;28(3):393–400. doi: 10.1093/ajh/hpu146. [DOI] [PubMed] [Google Scholar]
- 37.Kim KJ, Kim HL, Kim MJ, Kim CH, Lim WH, Seo JB, et al. Gender difference in the association between aortic pulse pressure and left ventricular filling pressure in the elderly: an invasive hemodynamic study. J Card Fail. 2017;23(3):224–230. doi: 10.1016/j.cardfail.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47(6):1203–1208. doi: 10.1161/01.HYP.0000223013.60612.72. [DOI] [PubMed] [Google Scholar]
- 39.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57(14):1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Renna NF, de Las HN, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. 2013;2013:808353. doi: 10.1155/2013/808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizzoni D, Agabiti-Rosei E. Structural abnormalities of small resistance arteries in essential hypertension. Intern Emerg Med. 2012;7(3):205–212. doi: 10.1007/s11739-011-0548-0. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34(4):431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 43.Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009;93(3):569–582. doi: 10.1016/j.mcna.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, et al. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107(5):615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 46.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50(1):1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 47.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension The ARIC study. Hypertension. 1999;34(2):201–206. doi: 10.1161/01.HYP.34.2.201. [DOI] [PubMed] [Google Scholar]
- 49.Takase H, Dohi Y, Toriyama T, Okado T, Tanaka S, Sonoda H, et al. Brachial-ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011;24(6):667–673. doi: 10.1038/ajh.2011.19. [DOI] [PubMed] [Google Scholar]
- 50.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45(3):426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 51.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51(14):1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 53.Devereux RB, Alderman MH. Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation. 1993;88(4 Pt 1):1444–1455. doi: 10.1161/01.CIR.88.4.1444. [DOI] [PubMed] [Google Scholar]
- 54.Weber T. The role of arterial stiffness and central hemodynamics in heart failure. Int J Heart Fail. 2020;2(4):209–230. doi: 10.36628/ijhf.2020.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, et al. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol. 2015;10(12):2190–2197. doi: 10.2215/CJN.03000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safar ME. Arterial stiffness and peripheral arterial disease. Adv Cardiol. 2007;44:199–211. doi: 10.1159/000096731. [DOI] [PubMed] [Google Scholar]
- 57.Kim HL, Lee EM, Ahn SY, Kim KI, Kim HC, Kim JH, et al. The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension. Clin Hypertens. 2023;29(1):11. doi: 10.1186/s40885-023-00234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohkuma T, Tomiyama H, Ninomiya T, Kario K, Hoshide S, Kita Y, et al. Proposed cutoff value of brachial-ankle pulse wave velocity for the management of hypertension. Circ J. 2017;81(10):1540–1542. doi: 10.1253/circj.CJ-17-0636. [DOI] [PubMed] [Google Scholar]
- 59.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39(1):10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 60.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 61.Gonzales JU, Wiberg M, Defferari E, Proctor DN. Arterial stiffness is higher in older adults with increased perceived fatigue and fatigability during walking. Exp Gerontol. 2015;61:92–97. doi: 10.1016/j.exger.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60(5):1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 64.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(7):999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193(1):1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 66.Park W, Jung WS, Hong K, Kim YY, Kim SW, Park HY. Effects of moderate combined resistance- and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int J Environ Res Public Health. 2020;17(19):7233. doi: 10.3390/ijerph17197233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopes S, Afreixo V, Teixeira M, Garcia C, Leitão C, Gouveia M, et al. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J Hypertens. 2021;39(2):214–222. doi: 10.1097/HJH.0000000000002619. [DOI] [PubMed] [Google Scholar]
- 68.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rider OJ, Tayal U, Francis JM, Ali MK, Robinson MR, Byrne JP, et al. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 2010;18(12):2311–2316. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- 70.Kim HL, Lim WH, Seo JB, Kim SH, Jo ZH, Kim MA. Association between body mass index and arterial stiffness. Cardiometab Syndr J. 2022;2(1):49–57.
- 71.Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res. 2010;33(5):398–410. doi: 10.1038/hr.2010.25. [DOI] [PubMed] [Google Scholar]
- 72.Kubozono T, Miyata M, Ueyama K, Hamasaki S, Kusano K, Kubozono O, et al. Acute and chronic effects of smoking on arterial stiffness. Circ J. 2011;75(3):698–702. doi: 10.1253/circj.CJ-10-0552. [DOI] [PubMed] [Google Scholar]
- 73.Del Giorno R, Maddalena A, Bassetti S, Gabutti L. Association between alcohol intake and arterial stiffness in healthy adults: a systematic review. Nutrients. 2022;14(6):1207. doi: 10.3390/nu14061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 75.Patil SG, Biradar MS, Khode V, Vadiraja HS, Patil NG, Raghavendra RM. Effectiveness of yoga on arterial stiffness: a systematic review. Complement Ther Med. 2020;52:102484. doi: 10.1016/j.ctim.2020.102484. [DOI] [PubMed] [Google Scholar]
- 76.Peng F, Pan H, Wang B, Lin J, Niu W. The impact of angiotensin receptor blockers on arterial stiffness: a meta-analysis. Hypertens Res. 2015;38(9):613–620. doi: 10.1038/hr.2015.51. [DOI] [PubMed] [Google Scholar]
- 77.Alidadi M, Montecucco F, Jamialahmadi T, Al-Rasadi K, Johnston TP, Sahebkar A. Beneficial effect of statin therapy on arterial stiffness. Biomed Res Int. 2021;2021:5548310. doi: 10.1155/2021/5548310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seetho IW, Asher R, Parker RJ, Craig S, Duffy N, Hardy KJ, et al. Effect of CPAP on arterial stiffness in severely obese patients with obstructive sleep apnoea. Sleep Breath. 2015;19(4):1155–1165. doi: 10.1007/s11325-015-1131-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.