Abstract
Background
Prior research has demonstrated that low- and low-middle-income countries (LLMICs) bear a higher burden of critical illness and have a higher rate of mortality from critical illness than high-income countries (HICs). There is a pressing need for improved critical care delivery in LLMICs to reduce this inequity. This systematic review aimed to characterise the range of critical care interventions and services delivered within LLMIC health care systems as reported in the literature.
Methods
A search strategy using terms related to critical care in LLMICs was implemented in multiple databases. We included English language articles with human subjects describing at least one critical care intervention or service in an LLMIC setting published between 1 January 2008 and 1 January 2020.
Results
A total of 1620 studies met the inclusion criteria. Among the included studies, 45% of studies reported on pediatric patients, 43% on adults, 23% on infants, 8.9% on geriatric patients and 4.2% on maternal patients. Most of the care described (94%) was delivered in-hospital, with the remainder (6.2%) taking place in out-of-hospital care settings. Overall, 49% of critical care described was delivered outside of a designated intensive care unit. Specialist physicians delivered critical care in 60% of the included studies. Additional critical care was delivered by general physicians (40%), as well as specialist physician trainees (22%), pharmacists (16%), advanced nursing or midlevel practitioners (8.9%), ambulance providers (3.3%) and respiratory therapists (3.1%).
Conclusions
This review represents a comprehensive synthesis of critical care delivery in LLMIC settings. Approximately 50% of critical care interventions and services were delivered outside of a designated intensive care unit. Specialist physicians were the most common health care professionals involved in care delivery in the included studies, however generalist physicians were commonly reported to provide critical care interventions and services. This study additionally characterised the quality of the published evidence guiding critical care practice in LLMICs, demonstrating a paucity of interventional and cost-effectiveness studies. Future research is needed to understand better how to optimise critical care interventions, services, care delivery and costs in these settings.
Registration
PROSPERO CRD42019146802.
Critical illness represents any immediately life-threatening disease or injury that, if left untreated, can lead to death [1]. It is not specific to a single disease category, patient population or age group and may be encountered throughout the health care system, including intensive care unit (ICU) settings, emergency care units, operating rooms, post-anesthetic care units, wards and pre-hospital settings [2]. Critical care may be defined as treating severely ill patients irrespective of care setting, care provider, or specific technologies utilised [3]. The unmet need for timely and high-quality critical care interventions and services is especially pressing in low- and low-middle-income country (LLMIC) settings [4]. The burden of critical illness is often higher in LLMICs than in high-income countries (HICs), and there is also a higher risk of death and loss of disability-adjusted life years (DALYs) from critical illness in lower-income settings [3,5-9]. The provision of critical care in LLMICs is challenging due to more limited resources than in HICs. However, many critical care interventions and services, such as intravenous fluids, antibiotics, haemorrhage control, close monitoring of patients and supplemental oxygen, are feasible to deliver in resource-constrained settings[10].
Effective initial care for critically ill patients requires recognition and resuscitation, followed in some cases by access to definitive care, which necessitates temporal and spatial alignment of critical care interventions and services with patient needs and location[2,11]. Given the significant heterogeneity in the distribution of resources within and between health care systems worldwide, models for critical care intervention and service delivery tailored to local circumstances and resources are required [2,12,13]. For example, due to the lack of ICU capacity in LLMICs, critically ill patients may be cared for in non-traditional environments, such as emergency departments, hospital wards, emergency care units or pre-hospital settings. Therefore, understanding how, where and by whom critical care is currently delivered in LLMICs is essential to strengthening the delivery of critical care across the breadth of the health care system.
The primary aim of this systematic review was to characterise the range of critical care interventions and services delivered across health care systems in LLMICs, as reported in the literature. We report the health service location in which critical care is delivered, the health care professionals involved, the populations treated, and the disease or syndrome categories addressed by critical care interventions and services.
METHODS
The methods for this review have been published previously [14]. In summary, controlled vocabulary terms and text words related to critical care in LLMICs were developed. The search strategy was implemented in PubMed/MEDLINE, EMBASE, Web of Science and PROSPERO databases (Online Supplementary Document). We restricted search results to citations in English pertaining to humans published from 1 January 2008 to 1 January 2020. The Stanford University Office for Human Subjects Research and Institutional Review Board reviewed and exempted this study on 20 May 2020. Reviewers were unblinded to authors, as well as institutional details of all included citations.
The eligibility criteria were set as: design (original peer-reviewed research or systematic review), setting (LLMICs as defined by the 2016 World Bank classification [15]), participants (any age group), and interventions (at least one critical care intervention or service described). The list of qualifying critical care interventions and services was selected to reflect a broad definition of critical care informed by the World Health Organization (WHO) Emergency Care Systems Framework [16]. Care delivered in operating rooms as part of a surgical procedure was excluded. Care delivered by lay providers was also excluded.
We uploaded citations generated from this search strategy to Covidence v 2.0 (Covidence, Melbourne, Australia) and completed abstract and full-text screening. We completed data extraction utilising Redcap v11.2.2 [17,18].
Reviewers were required to have medical training at a minimum level of a senior medical student and completed an orientation regarding the inclusion and exclusion criteria and the process for data extraction. Two reviewer agreement was required for the inclusion of each abstract and full-text article. Discrepancies were resolved by a third reviewer, who made the final determination regarding inclusion. A single reviewer completed data extraction for each study meeting inclusion criteria. We randomly selected five percent of included studies to be audited by lead investigators to ensure data extraction quality and consistency. Approximately 30 individuals contributed to abstract and full text review and data extraction stages. We calculated inter-rater reliability by percent agreement.
We completed data analysis using descriptive statistics utilising Microsoft Excel v16.75.2 (Microsoft Corporation, Redmond, Washington, USA). Data visualisation for Figure 2 was created utilising ArcGIS v 10.6.1(Esri, Redlands, California, USA) and data visualisation for Figure S1 in the Online Supplementary Document) was created utilising Tableau (Tableau Software, Seattle, Washington, USA). Where applicable, the review is reported according to PRISMA guidance [19].
RESULTS
Characteristics of included studies
16 510 original records were screened for inclusion; 12 940 records were excluded after abstract screening. Of the 3570 full-text reports, 159 could not be accessed. Ultimately, 1620 studies were included in the review (Figure 1). Raw inter-rater reliability for data extraction was 0.99 for audited studies. The majority of included studies had an observational study design (81%), while 93 studies (5.7%) were randomised controlled trials (Table 1). Further, 10% (163 studies) reported on the costs or economics of critical care interventions and services described.
Figure 1.
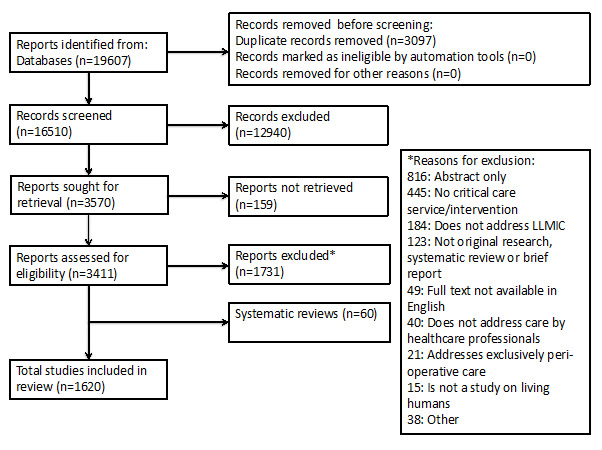
Study selection.
Table 1.
Characteristics of included studies
| Category | n (%) |
|---|---|
|
Study design (n = 1620)
|
|
| Cohort |
624 (38.0) |
| Cross-sectional |
593 (37.0) |
| Randomised controlled trial |
93 (5.7) |
| Case-control |
88 (5.4) |
| Educational |
62 (3.8) |
| Qualitative study |
24 (1.5) |
| Mixed methods |
16 (1.0) |
| Other |
121 (7.5) |
|
Population (n = 2288)*
|
|
| Paediatric |
1027 (45.0) |
| Infant |
520 (23.0) |
| Adult |
976 (43.0) |
| Geriatric |
203 (8.9) |
| Maternal |
97 (4.2) |
| Other unspecified |
188 (8.2) |
|
Healthcare workers (n = 1550)†
|
|
| Specialist doctor |
439 (60.0) |
| General doctor |
322 (44.0) |
| Specialist doctor trainee |
164 (22) |
| Medical student |
9 (1.2) |
| Advanced nursing/midlevel practitioner |
65 (8.9) |
| Nurse |
368 (50.0) |
| Nursing student |
10 (1.4) |
| Respiratory therapist |
23 (3.1) |
| Pharmacist |
28 (16.0) |
| Ambulance provider |
24 (3.3) |
| Non-clinical providers |
6 (0.8) |
| Other | 92 (13.0) |
*Overlapping categories permitted. Percentages reported with total included studies as denominator. The following definitions were utilised: pediatric (>1 y old and <18 y old), infant (<1 y old), adult (≥18 y old), geriatric (>65 y old), maternal (patient of any age during pregnancy and childbirth or within 42 d of termination of pregnancy).
†Overlapping categories permitted. Percentages reported with total included studies reporting at least one type of care provider as the denominator.
Patient populations
Included studies most commonly reported on pediatric (n = 1027, 43%) and adult (n = 976, 43%) patients. Infants (defined as less than one year old) were the next most commonly included patient group (n = 520, 23%). A smaller percentage reported on geriatric (defined as more than 65 years old, n = 203, 8.9%) and maternal patient populations (n = 97, 4.3%) (Table 1).
Healthcare workers
Among health care worker cadres described, specialist doctors (defined as physicians with critical care or other specialty training) provided care in 60% of included studies (n = 439), while general doctors provided care in 44% of included studies (n = 322). Nurses were explicitly described as providing care in 50% of included studies (n = 368). Specialist doctor trainees (n = 164, 22%), pharmacists (n = 28, 16%), advanced nursing or midlevel practitioners (n = 65, 8.9%), ambulance providers (n = 24, 3.3%) and respiratory therapists (n = 23, 3.1%) were also reported to provide care in a smaller proportion of the studies (Table 1). In 890 studies (55% of included studies), the type of care provider was not specified.
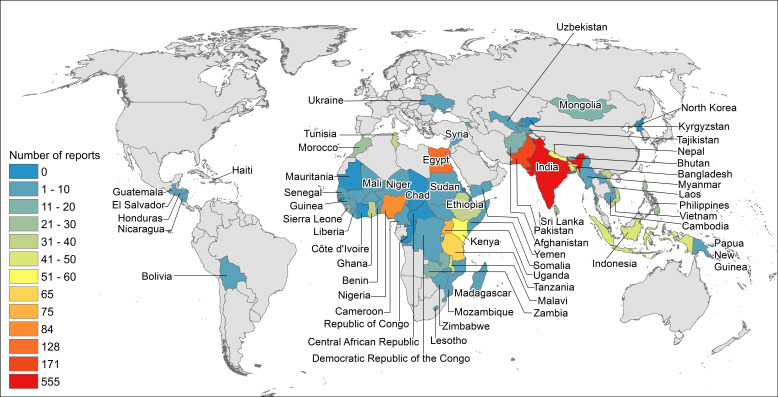
Countries
India was the LLMIC most frequently reported on (n = 555, 31%), followed by Pakistan (n = 171, 9.4%). There were no studies reporting on 17 LLMICs (20% of all LLMICs) (Figure 2).
Figure 2.
Countries reported on in included studies.
Care settings
Most of the critical care described was delivered in in-hospital settings (94%). Overall, approximately half (51%) of the care described occurred in an ICU, as self-defined, whereas the emergency department and non-ICU hospital ward each comprised approximately 13% of the care settings described (Figure S1 in the Online Supplementary Document). All combined out-of-hospital care settings, including clinic, field, ambulance and temporary health response unit settings, comprised 6.2% of reported care settings.
Critical care interventions and services
Among 6266 total critical care interventions and services reported, respiratory interventions (20%) were most frequently described. Diagnostic modalities and interventions for haemodynamic instability or organ dysfunction each comprised 15% of reported interventions and services, followed by nursing or close monitoring (13%), and multi-system processes (11%) (e.g. prognosis-based advanced care planning, care pathways and critical illness severity stratification). Critical care education and capacity-building represented 5.1% of reported interventions/services (Table 2).
Table 2.
Critical care interventions and services provided
| Category | n (%) |
|---|---|
|
Respiratory interventions
|
1240 (20.0) |
| Mechanical ventilation, invasive |
439 (7.0) |
| Support of respiratory insufficiency/failure |
351 (5.6) |
| Mechanical ventilation, non-invasive |
133 (2.1) |
| Advanced invasive airway management, non-surgical |
99 (1.6) |
| Oxygen delivery, simple (face mask, nasal prongs) |
86 (1.4) |
| Non-invasive airway management |
67 (1.1) |
| Advanced surgical airway management |
53(0.8) |
| Oxygen delivery, high flow |
12 (0.2) |
|
Diagnostic modalities
|
927 (15.0) |
| Laboratory and other rapid results reporting, including point-of-care diagnostics |
309 (4.9) |
| Microbiology and other infectious rapid results reporting |
207 (3.3) |
| Utilisation of targeted diagnostic strategy to establish timely etiology in critical illness |
152 (2.5) |
| Basic radiography |
107 (1.7) |
| Critical care ultrasound |
74 (1.2) |
| Computed tomography |
64 (1.0) |
| Magnetic resonance imaging |
14 (0.2) |
|
Interventions for haemodynamic instability/organ dysfunction
|
919 (15.0) |
| Support of haemodynamic instability and management of acute life-threatening organ dysfunction |
267 (4.3) |
| Administration of vasopressors and inotropes |
165 (2.6) |
| Intravenous fluid resuscitation |
150 (2.4) |
| Blood product transfusion |
117 (1.9) |
| Cardiopulmonary resuscitation, basic |
59 (0.9) |
| Advanced cardiac life support resuscitation |
46 (0.7) |
| Massive haemorrhage control |
26 (0.4) |
| Advanced trauma resuscitation/ trauma care checklist |
24 (0.4) |
| Advanced blood replacement therapies (e.g. plasmapheresis, plasma exchange, exchange transfusion) |
22 (0.4) |
| Anti-arrhythmic medication administration |
18 (0.3) |
| Targeted temperature management and hyperthermia/hypothermia management |
16 (0.3) |
| Spinal immobilisation |
7 (0.1) |
| Extracorporeal membrane oxygenation |
2 (0.03) |
|
Nursing and close monitoring
|
787 (13.0) |
| Frequent monitoring, surveillance and recording of clinical parameters |
358 (5.7) |
| Acuity-based triage/performance of focused assessment for critical illness state |
263 (4.2) |
| Critical care nursing services |
130 (2.1) |
| Titration of advanced parenteral therapeutics |
22 (0.4) |
| Foetal monitoring |
14 (0.2) |
|
Additional targeted therapies
|
747 (11.9) |
| Antibiotic administration in critical illness |
244 (3.9) |
| Monitoring and treatment of critical electrolyte/metabolic/acid-base derangements |
133 (2.1) |
| Renal replacement therapy |
107 (1.7) |
| Treatment of severe infections other than intravenous fluids and antibiotics |
65 (1.0) |
| Emergent poisoning detoxification/antidote |
60 (1.0) |
| Nutrition management in critically ill/injured patients |
50 (0.8) |
| Provision of prophylaxis associated with critical illness |
29 (0.5) |
| Acute reperfusion therapy, cardiac |
28 (0.5) |
| Advanced burn care |
27 (0.4) |
| Acute reperfusion therapy, Venous thromboembolism |
4 (0.1) |
|
Multi-system processes
|
661 (11.0) |
| Critical care triage/care pathways systems, clinical illness severity and/or risk stratification |
403 (6.4) |
| Coordination of specialist services for multi-system illness |
105 (1.7) |
| Prognosis-based advanced care planning |
83 (1.3) |
| Health information systems |
49 (7.8) |
| Critical care level crisis management |
21 (0.3) |
|
Neurological interventions
|
233 (3.7) |
| Acute medical stabilisation of critical neurologic illness |
114 (1.8) |
| Analgesia and sedation |
66 (1.1) |
| Acute surgical stabilisation of critical neurologic illness |
37 (0.6) |
| Acute management of agitation/delirium |
11 (0.2) |
| Acute reperfusion therapy, neurologic |
5 (0.08) |
|
Obstetrical care
|
141 (2.2) |
| Obstetric critical care |
141 (2.2) |
|
Other invasive procedures
|
133 (2.1) |
| Advanced vascular access (central venous catheters, arterial lines, pulmonary artery catheters) |
81 (1.3) |
| Peripheral venous cannulation |
34 (0.5) |
| Thoracic invasive procedures (thoracostomy, pleural drain placement, thoracentesis, pericardiocentesis, thoracotomy) |
17 (0.3) |
| Intra-osseous access |
1 (0.02) |
|
Other
|
359 (5.7) |
| Critical care education and capacity building |
318 (5.1) |
| Critical care pharmacy services |
41 (0.7) |
| Other critical care intervention/service delivery | 119 (1.9) |
*Overlapping categories permitted.
Disease and syndrome categories
Non-communicable diseases comprised 32% of disease categories addressed while infectious diseases comprised 27% (Table 3). Reproductive, maternal and newborn health represented the single most common disease category addressed (10%), followed by an integrated approach to respiratory distress and respiratory failure (8.8%). The most common individual infectious disease processes cited included sepsis (7.1%) and respiratory infections (4.5%). Malaria was addressed in 1.4% of studies, tuberculosis in 0.7% and human immunodeficiency virus (HIV) in 0.6%. The most common non-communicable disease processes addressed included injuries, envenomation and toxic exposures (7.0%), respiratory diseases (5.8%), cardiovascular diseases (5.0%) and neurologic disorders (4.4%).
Table 3.
Disease and syndrome processes addressed by included studies
| Disease process | n (%)* |
|---|---|
|
Infectious disease
|
1033 (27) |
| Integrated approach to sepsis† |
271 (7.1) |
| Infectious disease, not otherwise specified |
228 (6.0) |
| Respiratory infections |
172 (4.5) |
| Other infectious disease processes |
137 (3.6) |
| Disease prevention and surveillance |
55 (1.4) |
| Malaria |
53 (1.4) |
| Neglected tropical diseases |
37 (1.0) |
| Gastrointestinal infections |
28 (0.7) |
| Tuberculosis |
25 (0.7) |
| HIV |
24 (0.6) |
| Viral hepatitides |
3 (0.1) |
|
Non-communicable disease
|
1232 (32) |
| Injuries, envenomations, poisoning and toxic exposures |
268 (7.0) |
| Respiratory diseases |
221 (5.8) |
| Cardiovascular diseases |
191 (5.0) |
| Neurologic disorders |
169 (4.4) |
| Genitourinary and renal disorders |
96 (2.5) |
| Non-communicable disease, not otherwise specified |
69 (1.8) |
| Diseases of the gastrointestinal/digestive system |
68 (1.8) |
| Endocrine, metabolic and immune disorders |
56 (1.5) |
| Anemia, blood dyscrasis and coagulation disorders |
41 (1.1) |
| Neoplasms |
34 (0.9) |
| Mental health |
12 (0.3) |
| Diseases of the sense organs |
4 (0.1) |
| Skin and hair diseases |
3 (0.1) |
| Chronic joint and spine disorders |
0 (0.0) |
|
Other
|
1556 (41) |
| Reproductive, maternal and newborn health |
397 (10) |
| Integrated approach to respiratory distress and respiratory failure† |
336 (8.8) |
| Other severe illness or injury |
245 (6.4) |
| Integrated approach to shock† |
171 (4.4) |
| Unspecified disease process |
153 (4.0) |
| Post-surgical disease process |
126 (3.3) |
| Integrated approach to altered mental status† |
81 (2.1) |
| Nutritional deficiencies |
34 (0.9) |
| Older adult health needs | 13 (0.3) |
*Overlapping categories permitted.
†“Integrated approach” is defined as management of an undifferentiated patient where the cause of their presenting signs and symptoms has not yet been determined.
Summary of systematic reviews
The search strategy identified 60 systematic reviews. As these were not amenable to the same data extraction process as individual reports but contain important information for the field, these systematic reviews are summarised in Table S1 in the Online Supplementary Document.
Of the reviews identified, the most common focus areas were: critical care education and capacity building (n = 17); critical care triage, care pathways systems, clinical illness severity and/or risk stratification (n = 9); and non-invasive mechanical ventilation (n = 6). Other topics addressed included invasive mechanical ventilation, acute reperfusion therapy for ischaemic stroke and ischaemic heart disease, trauma care, management of advanced vascular access, critical care ultrasound, intravenous fluid resuscitation, massive haemorrhage control, obstetrical critical care management, and prognosis-based advanced care planning.
DISCUSSION
This review represents a comprehensive synthesis of critical care delivery in LLMIC settings, providing an important baseline for practitioners, researchers and policymakers dedicated to improving the care of critically ill patients. This is particularly important given that the 76th World Health Assembly passed a resolution in 2023 calling for global efforts to strengthen the planning and provision of emergency, critical and operative (ECO) care services as part of universal health coverage [20].
We found that the current published literature predominantly reported critical care delivery in the in-hospital setting, however, less than half the studies reported the provision of critical care in a self-defined ICU. Specialist physicians played a major role in critical care delivery. However, general doctors, nurses, advanced nursing/midlevel practitioners and pharmacists were also frequently involved. Critical care interventions and services were found to apply to patient populations across the entire lifespan and address care needs across a broad range of disease and syndrome categories, including infectious and non-communicable diseases. Care of sepsis, respiratory infections, maternal and newborn health, injuries and cardiovascular diseases were well-represented among disease processes addressed by included studies, which align with the largest contributors to the loss of global DALYs [21]. These findings reinforce the concept that critical care occurs across a continuum within a health care system and that there is a need for integrated planning and implementation of critical care delivery across disease and population-specific programs [20].
Our results also reveal important gaps in the existing research literature. The majority of reports included in this review were observational. Only 93 randomised controlled trials that enrolled patients in LLMIC countries were identified by our search strategy, indicating a need for far more interventional research to determine which critical care interventions and services are effective in LLMIC settings [22]. Future work should analyse the key results from existing interventional studies and studies with cost-effectiveness data identified by this review to inform research, program design and policymaking [13]. Notably, over half of the included papers did not specifically state the training of care providers involved in service delivery or support teams. Given the importance of interdisciplinary care teams for delivering high-quality critical care [23], future research in this area should specifically report the care providers involved in critical care delivery and the composition of care teams in addition to the critical care intervention or service, populations served and care setting. Regarding the distribution of available literature in this field, 17 LLMICs were not reported on at all by studies meeting inclusion criteria. While data on available critical care delivery models and effective interventions from other LLMIC settings may be extrapolated to other countries, heterogeneity of economies, health care system structure, resources and disease burden likely impact the effectiveness of models for critical care delivery when implemented in different settings. For example, studies of fluid resuscitation in African adult and pediatric populations with sepsis found worse mortality rates when more aggressive fluid resuscitation protocols (akin to USA and European standards of practice) are utilised, as compared to usual care [24,25]. As such, future research should include countries and regions with a current paucity of published reports. Additionally, given that ICU care and other specialty hospital-based care is commonly clustered in urban settings, there is an imperative to ensure that future work investigates ways to describe and provide early critical care in rural, remote and austere settings [10,26-28] Finally, there were relatively few reports focusing on geriatric or maternal critical care, and future work should strive for representation of these groups to ensure their care needs are met.
This review also provides methodological advancements for research on critical care delivery. As compared to prior work defining essential emergency and critical care, the list of critical care interventions and services developed for this review takes a more inclusive approach to critical care services across a range of expected costs and technological complexity [29]. The search strategy developed for this review therefore serves to advance research in this field by creating a replicable set of search terms encompassing the breadth of potential critical care interventions and services that may be found across health care systems and across patients’ progression from pre-hospital care to inpatient care.
This review has some limitations. First, it represents the state of published, peer-reviewed literature and does not comprehensively audit all current critical care delivery models. We expect a bias toward the publication of studies taking place in ICU settings, and therefore, non-traditional critical care delivery environments, such as the pre-hospital setting, are likely under-represented in our results. It is important to note that the paucity of reports on critical care delivery in the out-of-hospital setting does not accurately represent the population-based need for such services [30,31]. This review is also limited to studies published in English, which may have affected the countries of origin of included studies. This review includes reports published prior to 2020 and therefore does not reflect changes that have occurred in response to the coronavirus disease 2019 (COVID-19) pandemic. Instead, it provides an accurate baseline representation of critical care delivery outside of the pandemic response.
CONCLUSIONS
Critical care interventions and services are delivered widely across the health care system by individuals with many different training backgrounds and are not limited to ICU or subspecialty-trained intensivists. It is well known that countries with the fewest resources shoulder a disproportionate burden of critical illness. This review has gone a step further and detailed that the largest percentage of these patients are cared for outside of formal intensive care units. Our results support the need to ensure preparedness throughout the health care system for the recognition of and response to critical illness, in alignment with the World Health Assembly resolution on integrated emergency, critical and operative care as part of universal health coverage [20]. In addition to the actions called for by the World Health Assembly resolution, there is a desperate need for more pragmatic research regarding how to deliver the best critical care with the resources available to achieve equitable global access to quality critical care.
Additional material
Acknowledgments
We would like to thank the following individuals for their assistance with data extraction for this review: Adelouahab Bellou, MD, Elif Cizmeci, MD, Caitlin Contag, MD, Crystal Donelan, MD, Allison Ferreira, MD, Naomi George, MD, Aditi Ghatak-Roy, Naeha Haridasa, MD, Sarah Hirner, MD, Lauren Klingman, MD, Patrick McCarville, MD, Carl Mickman, MD, Thiago Oliveira, MD, Varun Shetty, MD, Nebiyu Shukur, MD, Kate Simeon, MD, Paul Sonenthal, MD, Menbeu Sultan, MD, Jonathan Yang, MD, Finot Debebe Yayehyirad, MD. We would like to thank Swati Kittur, MSc for assistance with ArcGIS software. Other technical support was provided by the International Federation for Emergency Medicine (IFEM), and the IFEM Special Interest Group for Critical Care in Emergency Medicine.
Footnotes
Funding: The research presented in this manuscript received no external funding.
Authorship contributions: ESB completed data analysis and drafted, prepared and submitted the manuscript. AGL and TR contributed to the development of the selection criteria and search strategy. ESB and AGL developed the data extraction criteria and forms, and provided teaching and guidance of review process to the review group. NA, SM, SK,LIL, FP, ED, BAC, NB, AB, MSJ, PDS, and TR provided comments and critical revisions to the manuscript, and made substantial contributions to the conception and design of the review methodology and planned analysis. ED, SK, GN, GS, RL, MR, BS, NC, JB, SCK, RS, MD, CL, SY, MSJ, BAC, NS, PAS, ALR, NB, and NH made key contributions to the conception, design and execution of eligibility criteria and methodology, participated in the acquisition and interpretation of reference data for screening and data extraction phases, and provided critical edits to the intellectual content of the manuscript. All authors read, provided feedback and approved the final manuscript, and agree to be accountable for all aspects of the work.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and declare the following activities and relationships: NA has served as a consultant to the World Health Organization (WHO) regarding guidelines for COVID-19 and serves as a member of a WHO Technical Advisory Group for Integrated Clinical Care. NC reports receipt of the United States National Heart, Lung, and Blood Institute training grant: T32HL007287. ED serves as the director of the Emergency Care Society of South Africa. SK has received grants or contracts from the WHO for work as a consultant to the WHO Clinical Services and Systems Program, from International Medical Corps as a program lead for trauma care training in Ukraine and from CARE as a program lead to provide emergency care training in India as well as payments for travel and presentations at the annual conference of the American College of Emergency Physicians. He also holds unpaid positions as a member of the editorial advisory board of EMS World, president of the Global Emergency Medicine Academy of the Society for Academic Emergency Medicine and as the standards lead for the Critical Care in Emergency Medicine Interest Group of the International Federation for Emergency Medicine. CL reports royalties from UptoDate.com for an article on management of respiratory failure in patients with elevated ICP and payment for legal review from Dansker and Aspromonte Associates, James Newman P.C. and McEldrew Purtell Law Associates, and support from the Mount Sinai Institute of Critical Care Medicine for travel. SM reports receipt of grants from the Canadian Institutes of Health Research. MR has received the McGrevin Post-Doctoral Grant to fund an educational project in Malawi.
REFERENCES
- 1.Baker T. Critical Care in Low Resource Settings [Dissertation]. Solna (Sweden): Karolinska Institute; 2015. [Google Scholar]
- 2.Losonczy LI, Papali A, Kivlehan S, Calvello Hynes EJ, Calderon G, Laytin A, et al. White Paper on Early Critical Care Services in Low Resource Settings. Ann Glob Health. 2021;87:105. 10.5334/aogh.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD.Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339-46. 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayambankadzanja RK, Schell CO, Mbingwani I, Mndolo SK, Castegren M, Baker T.Unmet need of essential treatments for critical illness in Malawi. PLoS One. 2021;16:e0256361. 10.1371/journal.pone.0256361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavallaro FL, Marchant TJ.Responsiveness of emergency obstetric care systems in low- and middle-income countries: a critical review of the “third delay”. Acta Obstet Gynecol Scand. 2013;92:496-507. 10.1111/aogs.12071 [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380-6. 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- 7.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259-72. 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 8.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-11. 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates MM, Ezzati M, Robles Aguilar G, Kwan GF, Vigo D, Mocumbi AO, et al. Burden of disease among the world’s poorest billion people: An expert-informed secondary analysis of Global Burden of Disease estimates. PLoS One. 2021;16:e0253073. 10.1371/journal.pone.0253073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaddeus S, Maine D.Too far to walk: maternal mortality in context. Soc Sci Med. 1994;38:1091-110. 10.1016/0277-9536(94)90226-7 [DOI] [PubMed] [Google Scholar]
- 11.Aluisio AR, Waheed S, Cameron P, Hess J, Jacob ST, Kissoon N, et al. Clinical emergency care research in low-income and middle-income countries: opportunities and challenges. BMJ Glob Health. 2019;4:e001289. Erratum in: BMJ Glob Health. 2020;4:e001289corr1. 10.1136/bmjgh-2018-001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy S, Leligdowicz A, Adhikari NK.Intensive care unit capacity in low-income countries: a systematic review. PLoS One. 2015;10:e0116949. 10.1371/journal.pone.0116949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A critical look at critical care. Lancet. 2010;376:1273. 10.1016/S0140-6736(10)61896-X [DOI] [PubMed] [Google Scholar]
- 14.Lim AG, Kivlehan S, Losonczy LI, Murthy S, Dippenaar E, Lowsby R, et al. Critical care service delivery across healthcare systems in low-income and low-middle-income countries: protocol for a systematic review. BMJ Open. 2021;11:e048423. 10.1136/bmjopen-2020-048423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The World Bank. World Bank Country and Lending Group 2022. Available:https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed: 28 September 2023.
- 16.The World Health Organization. WHO Emergency care system framework. 2018. Available: https://www.who.int/publications/i/item/who-emergency-care-system-framework. Accessed: 28 September 2023.
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Resolution WHA EB152(3): Integrated emergency, critical and operative care for universal health coverage and protection from health emergencies. Available: https://apps.who.int/gb/ebwha/pdf_files/WHA76/B152_REC1_EXT-en.pdf. Accessed: 19 November 2023.
- 21.Institute for Health Metrics and Evaluation (IHME)·GBD Compare· Seattle, WA: IHME, University of Washington. 2015. Available: http://vizhub.healthdata.org/gbd-compare. Accessed: 15 December 2022.
- 22.Razzak J, Beecroft B, Brown J, Hargarten S, Anand N.Emergency care research as a global health priority: key scientific opportunities and challenges. BMJ Glob Health. 2019;4:e001486. 10.1136/bmjgh-2019-001486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MM, Barnato AE, Angus DC, Fleisher LA, Kahn JM.The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170:369-76. 10.1001/archinternmed.2009.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483-95. 10.1056/NEJMoa1101549 [DOI] [PubMed] [Google Scholar]
- 25.Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomised Clinical Trial. JAMA. 2017;318:1233-40. 10.1001/jama.2017.10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitter CC, Rice B, Periyanayagam U, Dreifuss B, Hammerstedt H, Nelson SW, et al. What resources are used in emergency departments in rural sub-Saharan Africa? A retrospective analysis of patient care in a district-level hospital in Uganda. BMJ Open. 2018;8:e019024. 10.1136/bmjopen-2017-019024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron PA, Gabbe BJ, Smith K, Mitra B.Triaging the right patient to the right place in the shortest time. Br J Anaesth. 2014;113:226-33. 10.1093/bja/aeu231 [DOI] [PubMed] [Google Scholar]
- 28.Mehta S, Aboushi H, Campos C, Botelho R, Fernandez F, Rodriguez D, et al. Impact of a telemedicine-guided, population-based, STEMI network on reperfusion strategy, efficiency, and outcomes: Impact of telemedicine on STEMI management. AsiaIntervention. 2021;7:18-26. 10.4244/AIJ-D-18-00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schell CO, Khalid K, Wharton-Smith A, Oliwa J, Sawe HR, Roy N, et al. Essential Emergency and Critical Care: a consensus among global clinical experts. BMJ Glob Health. 2021;6:e006585. 10.1136/bmjgh-2021-006585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen K, Mock C, Joshipura M, Rubiano AM, Zakariah A, Rivara F.Assessment of the status of pre-hospital care in 13 low- and middle-income countries. Prehosp Emerg Care. 2012;16:381-9. 10.3109/10903127.2012.664245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suryanto, Plummer V, Boyle M.Boyle M. EMS Systems in Lower-Middle Income Countries: A Literature Review. Prehosp Disaster Med. 2017;32:64-70. 10.1017/S1049023X1600114X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.