Abstract
Linoleic acid hydroperoxide (LoaOOH) formed during free radical attack on long-chain unsaturated fatty acids is an important source of biomembrane damage and is implicated in the onset of atherosclerosis, hepatic diseases, and food rancidity. LoaOOH is toxic to wild-type Saccharomyces cerevisiae at a very low concentration (0.2 mM) relative to other peroxides. By using isogenic mutant strains, the possible roles of glutathione (gsh1 and gsh2), glutathione reductase (glr1), respiratory competence ([rho0] petite), and yAP-1p-mediated expression (yap1) in conferring LoaOOH resistance have been examined. Respiration-related processes were essential for maximal toxicity and adaptation, as evidenced by the fact that the [rho0] petite mutant was most resistant to LoaOOH but could not adapt. Furthermore, when respiration was blocked by using inhibitors of respiration and mutants defective in respiratory-chain components, cells became more resistant. An important role for reduced glutathione and yAP-1 in the cellular response to LoaOOH was shown, since the yap1 and glr1 mutants were more sensitive than the wild type. In addition, total glutathione peroxidase activity increased following treatment with LoaOOH, indicating a possible detoxification role for this enzyme. Yeast also showed an adaptive response when pretreated with a nonlethal dose of LoaOOH (0.05 mM) and subsequently treated with a lethal dose (0.2 mM), and de novo protein synthesis was required, since adaptation was abolished upon treatment of cells with cycloheximide (25 μg ml−1). The wild-type adaptive response to LoaOOH was independent of those for the superoxide-generating agents paraquat and menadione and also of those for the organic hydroperoxides cumene hydroperoxide and tert-butyl hydroperoxide. Pretreatment with LoaOOH induced resistance to hydrogen peroxide, while pretreatment of cells with malondialdehyde (a lipid peroxidation product) and heat shock (37°C) gave cross-adaptation to LoaOOH, indicating that yeast has effective overlapping defense systems that can detoxify fatty acid hydroperoxides directly or indirectly.
Within cells, oxidative stress can lead to potential harm by causing nucleic acid damage, protein oxidation, and lipid peroxidation (17). Reactive oxygen species (ROS) that can induce such a stress response include the superoxide anion (O2−), the hydroxyl radical (OH·), hydrogen peroxide (H2O2), and lipid hydroperoxides (17, 25). Incomplete reduction of molecular oxygen to water in the respiratory electron transport chain leads to increased production of ROS during aerobic metabolism. ROS production can also increase following the exposure of cells to certain environmental conditions (17). Oxidative stress is significant both environmentally and medically, as it has been strongly implicated in diseases such as asthma, cancer (17), and atherosclerosis (51), in the aging process (2), and in AIDS (38).
One major target of ROS attack is unsaturated lipids, leading to autocatalytic lipid peroxidation (25). This is a significant source of membrane damage, which may contribute to atherosclerosis (51). During lipid peroxidation, reactive lipid and fatty acid hydroperoxides are formed, and these contribute to ongoing autooxidation (25). Lipid hydroperoxides also play a key role in the development of eye cataracts and hepatic diseases, and they act as substrates for pathways that yield leukotrienes and lipoxins as part of the inflammatory response (2, 51). Fatty acid hydroperoxides have been shown to be more toxic than phospholipid hydroperoxides to endothelial cells (29), and it has been shown that phospholipid hydroperoxides are broken down to the fatty acid hydroperoxide moiety to exert their toxic effects (28). Under certain conditions, linoleic acid hydroperoxide (LoaOOH) can form a delocalized lipid radical (L′) which self-reacts to form dienoic dimers (L"L) or reacts with another hydroperoxide to form the peroxy radical (LOO′) (16). High levels of lipid hydroperoxides are also found in plants following environmental stress or physical injury (5), and these compounds are important in food rancidity and aging (2, 42).
Enzymes involved in the breakdown of lipid hydroperoxides may include phospholipases, glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase, which is specific to phospholipid hydroperoxides (35), thioredoxin reductase (6), and cytochrome P-450 enzymes (46). The lipoxygenase enzyme, which is used to generate lipid hydroperoxides in vitro, can also use them as a substrate (16).
The model eukaryote Saccharomyces cerevisiae is ideal for investigating oxidative-stress responses, since not only is it genetically well defined but its defense systems against ROS are well characterized, including enzymes such as superoxide dismutase and catalase, as well as nonenzymic antioxidants (34). One major nonenzymic antioxidant in yeast is glutathione (20), which is a low-molecular-weight thiol present at millimolar levels in the cell (36) and which may be important in detoxifying cellular lipid hydroperoxides. Glutathione is the substrate for enzymes such as glutathione peroxidase, which has been shown to be important for the response to lipid hydroperoxides in Hansenula mrakii (27). In addition, some toxic compounds are conjugated to glutathione by glutathione S-transferase for transport out of the cell (26).
In this study, the effects of LoaOOH will be examined by using a wild-type yeast and isogenic mutants affected in known antioxidant defense systems. Additionally, the role of respiration-related processes in both defense and adaptation to fatty acid hydroperoxides will be investigated by treating isogenic respiration-incompetent petite mutants with LoaOOH, and results will be compared to those seen with other oxidants. The possible role of glutathione in the cellular response to LoaOOH will also be determined.
MATERIALS AND METHODS
Yeast strains and growth conditions.
Studies were performed with the wild-type yeast strain CY4 (DY150 Amrad) (MATa ura3-52 leu2-3 leu2-112 trp1-1 ade2-1 his3-11 can1-100), which was the parent strain for the isogenic derivatives CY7 (glr1::TRP1), CY9 (gsh1::LEU2), CY29 (yap1::HIS3), CY102 (cox6::HIS3), CY103 (coq3::HIS3) (23), CY97 (gsh2::HIS3), and the [rho0] petite mutant CY4p (19, 21, 23, 24), and also with the wild-type yeast strain W303-1B, which was the parent strain of its isogenic coq3 and atp2 mutants (10) and its [rho0] petite mutant (generated by using ethidium bromide). All mutants were obtained by standard gene disruption techniques. Cells were grown in YEPD medium (1% [wt/vol] yeast extract, 2% [wt/vol] Bacto Peptone, 2% [wt/vol] d-glucose) at 30°C with shaking unless otherwise stated. Growth on nonfermentable carbon sources was tested on YEPG medium (YEPD medium with glucose replaced by 3% [vol/vol] glycerol).
Synthesis of LoaOOH.
LoaOOH was generated in vitro similarly to a method previously described (41), by incubating linoleic acid (0.3 mM) with soybean lipoxygenase (4,000 U) in 0.1 M tetra-sodium borate buffer (pH 9) at room temperature with vigorous stirring for 30 min. The enzyme catalyzes abstraction of the H-11 hydrogen, which leads to the specific formation of Loa-13-OOH (45). The reaction mixture was loaded onto an end-capped C18 reverse-phase column (Sepak cartridge), and the LoaOOH was eluted in 1.5 ml of methanol. The solution was stored at −20°C and was stable for several months. The concentration of LoaOOH was determined spectrophometrically (λ = 234 nm; ɛ = 25,000 M−1 cm−1). High-pressure liquid chromatographic analysis (C18 column RPMET4.A; 2 ml min−1 flow rate; 85% [vol/vol] acetonitrile; 15% [vol/vol] 0.2% acetic acid; 234 nm) was used to show that the resultant product was at least 95% pure and contained only trace amounts of linoleic acid (data not shown).
LoaOOH treatment and adaptation experiments.
Cells were grown to exponential phase (2 × 107 cells ml−1) in YEPD medium, and to 3-ml aliquots of the culture was added LoaOOH to a range of concentrations (0 to 0.5 mM) for 1 h. Samples from each treatment were diluted in fresh YEPD medium and plated in triplicate on YEPD in order to obtain viable counts and generate the respective dose-response curves. Adaptation was measured by pretreating cells with a nonlethal dose of LoaOOH (0.05 mM) for 1 h, followed by treatment with a lethal dose (0.2 mM) for 1 h. Untreated (receiving no pretreatment or treatment), non-pretreated (receiving the treatment dose only), and pretreated (receiving the pretreatment dose only) controls were run simultaneously. Control reactions in which either the substrate linoleic acid or the enzyme lipoxygenase was excluded from the reaction were also performed. These controls were used to treat aliquots of the same culture in order to ensure that the effect seen was a result of LoaOOH. An additional control reaction with methanol alone, equivalent to the highest concentration of LoaOOH used, was also performed on the same culture. Data are means of triplicates from a representative experiment.
Calculation of yeast cell volume.
The cells were observed with phase-contrast optics at ×400 total magnification. Volumes (V) were calculated from the major (a) and minor (b) diameters of >50 cells by assuming that they were prolate spheroids where V = πab2/6. This is reportedly accurate to ±10% (49).
Detection of petite mutants.
Yeast colonies were tested for their ability to respire by using a 10-ml overlay of 1% (wt/vol) triphenyltetrazolium chloride (TTC) in 12% (wt/vol) water agar. The solution was boiled, and after cooling it was overlaid onto YEPD plates with fresh colonies and incubated at 30°C for 1 h. Cells that formed pink colonies were able to respire and thus reduce TTC to a red dye via the electron transport chain, while petite mutants, which are unable to respire, remained white (7). The petite nature of cells in a colony was also detected by failure to grow on the nonfermentable carbon source glycerol (YEPG).
MDA treatment.
Cells were grown to exponential phase (2 × 107 cells ml−1) in YEPD medium and were harvested by centrifugation. The pellet was resuspended in 0.1 M sodium citrate buffer, pH 4.5, and cells were either pretreated with 1 mM malondialdehyde (MDA) or treated with 5 mM MDA for 1 h at 30°C (as was appropriate to the experiment) and allowed to recover in fresh YEPD medium for 1 h (47). Samples from each treatment were diluted in fresh YEPD medium and plated in triplicate on YEPD in order to obtain viable counts and generate the respective dose-response curves.
Treatment with superoxide-generating agents and other peroxides.
For cross-adaptation experiments using H2O2, tert-butyl hydroperoxide, cumene hydroperoxide, paraquat, and menadione, the pretreatment concentrations were 0.3, 1, 1, 10, and 0.2 mM, respectively. Following pretreatment with 0.05 mM LoaOOH for 1 h, treatment concentrations for these reagents were 5, 12, 4, 15, and 6 mM, respectively. All treatments were carried out for 1 h at 30°C with shaking.
Treatment with argon and oxygen.
Prior to the addition of different concentrations of LoaOOH, either pure argon or pure oxygen was bubbled through the culture aliquots for 15 min; this was continued during the 1-h treatment with LoaOOH. Samples were then diluted and plated for viability counts as previously described.
Glutathione and glutathione peroxidase assays.
Glutathione levels were determined by a microtiter plate assay described previously (48). Total, Se-dependent, and Se-independent glutathione peroxidase activity was determined by using the previously described coupled assay (31) in which oxidized glutathione, produced by the hydroperoxide-dependent oxidation of reduced glutathione, is reduced by glutathione reductase. NADPH is consumed during this reaction, and a decrease in absorbance is measured. Crude cell extract from cultures grown to exponential phase (optical density at 600 nm [OD600] = 1) was used. Specific activity is defined as nanomoles of NADPH oxidized per minute per milligram of protein.
RESULTS AND DISCUSSION
Yeast membranes do not normally contain polyunsaturated fatty acids; instead, 30 to 50% of the phospholipid bilayer consists of palmitoleic acid (9-hexadecenoic acid), which is monounsaturated (25). However, polyunsaturated fatty acids (such as linoleic acid) in the medium are incorporated into the yeast membrane (8). Lipid peroxidation can be initiated with any unsaturated fatty acid, including the monounsaturated fatty acids oleic and palmitoleic acids, resulting in products that are similar to those which occur in the presence of polyunsaturated fatty acids (25). For these reasons, it was desirable to determine the effect of a lipid peroxidation intermediate, such as LoaOOH, on S. cerevisiae, since yeast are exposed to lipid hydroperoxides in their natural environment.
LoaOOH is toxic to yeast.
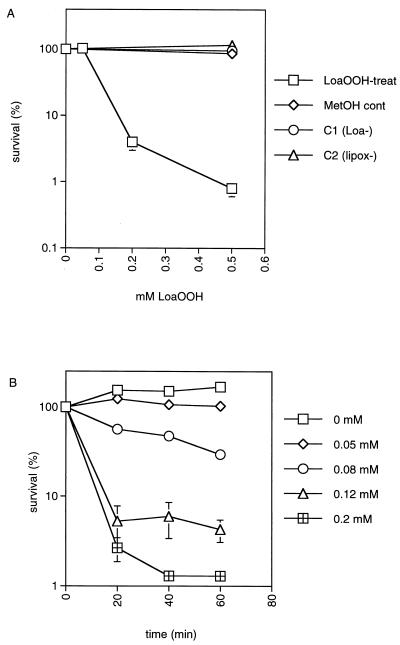
The possible toxicity of LoaOOH to the wild-type yeast strain CY4 was determined by treating cells with different doses of LoaOOH. Different concentrations of LoaOOH were added to cultures in the same growth medium (YEPD), and incubation continued with vigorous aeration at 30°C for 1 h. Concentrations greater than 0.05 mM caused a decrease in cell viability such that after 0.2 mM treatment there was less than 10% survival (Fig. 1A). Appropriate controls were run simultaneously with these experiments, and no effect was observed (Fig. 1A). These included treatment of cells with linoleic acid, which was not toxic at a concentration equivalent to the lethal dose of LoaOOH. LoaOOH is toxic at concentrations much lower than those for hydrogen peroxide or other organic peroxides. To obtain <10% survival with this strain under the same conditions, higher concentrations of H2O2 (6 mM) (21), tert-butyl hydroperoxide (15 mM), and cumene hydroperoxide (4 mM) (results of this study) are required.
FIG. 1.
Sensitivity of yeast cells to LoaOOH. (A) Wild-type cells grown to an OD600 of 1 in YEPD medium were treated with LoaOOH for 1 h at 30°C. Cells were diluted and plated in triplicate onto YEPD medium to monitor cell viability. Percent survival is expressed relative to the untreated control culture (100%). Symbols: □, LoaOOH-treated cells; ◊, methanol-treated control; ○, linoleic acid control; ▵, lipoxygenase control. (B) The kinetics of cell death was determined by sampling untreated and treated cultures at 20-min intervals for 1 h, using different concentrations of LoaOOH as indicated. Data are means of triplicates from a representative experiment.
To determine the kinetics of the loss of cell viability, the wild-type strain was treated with different concentrations of LoaOOH and samples were taken from the culture at 20-min intervals over 1 h (Fig. 1B). The results indicate that, unlike the exponential death obtained when cell populations were treated with H2O2 (11), there was an initial rapid loss of cell viability followed by a marked decrease in the rate of kill. This may have resulted from the presence of two populations with differing sensitivities to LoaOOH in the starting culture. More than 90% of the survivors after 1 h were shown to be respiration deficient by the TTC reduction test and also by their failure to grow on nonfermentable carbon sources (data not shown). This indication that petite mutants may be more resistant to LoaOOH is discussed later. In subsequent experiments, treatment of yeast with 0.2 mM LoaOOH for 1 h was chosen as a lethal dose and treatment with 0.05 mM LoaOOH for 1 h was chosen as the sublethal pretreatment dose, since it resulted in approximately 80% survival.
Yeast can adapt to LoaOOH.
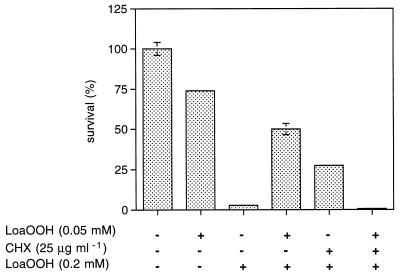
Given that lipid hydroperoxides may be formed in aerobic cells with unsaturated fatty acids in their membrane lipids, the question arises whether cells have a mechanism to respond to the presence of these compounds and detoxify them. Yeast cells are able to adapt to a range of oxidants and free radical-generating agents (11, 15) as well as to the lipid peroxidation product MDA (47). Adaptation is noted when cells display increased tolerance to a lethal dose of a particular compound following pretreatment with a sublethal dose, and this is indicated by a higher percentage of cell survival relative to survival of the non-pretreated control. The number of viable cells in the untreated control after the same time period was considered 100% cell survival. The adaptive response was observed when CY4 cells were pretreated with a sublethal dose of LoaOOH (0.05 mM; 1 h) and found to be more resistant to a subsequent treatment with a lethal dose (0.2 mM; 1 h) than those which received no pretreatment (Fig. 2). This result indicates that there may be an inducible system which allows yeast either to detoxify LoaOOH directly or to mount a cellular defense against this compound. Either mechanism would likely involve alteration in the expression of several genes and/or modification of proteins, and this can be partly determined by using inhibitors of protein synthesis.
FIG. 2.
Adaptation of wild-type cells to a lethal dose of LoaOOH. Cells can mount an inducible adaptive response to LoaOOH which is dependent on protein synthesis. Cells were pretreated with a sublethal dose of LoaOOH (0.05 mM; 1 h) prior to challenge with a lethal dose (0.2 mM; 1 h). The cytosolic protein synthesis inhibitor CHX was used at a concentration of 25 μg ml−1. Data are means of triplicates from a representative experiment.
De novo protein synthesis is required for adaptation.
To determine if ongoing cytoplasmic protein synthesis was required to elicit the adaptive response, cells of the same culture were pretreated with 25 μg of the inhibitor cycloheximide (CHX) ml−1, 0.05 mM LoaOOH, or both of these together (Fig. 2). These cells were subsequently treated with the lethal concentration of LoaOOH, as was the non-pretreated control. Control LoaOOH-pretreated cells displayed the usual adaptive response. Interestingly, CHX pretreatment alone led to protection of the cells (to almost the same degree as LoaOOH); this has been observed for other stress responses and indicates that cells may have some posttranslational change that confers resistance to LoaOOH, or that recovery of cells from LoaOOH treatment can be improved by CHX pretreatment, since it may lead to induction of repair processes once the CHX and LoaOOH are removed. It has been reported that CHX can lead to increased transcription of several genes (32), which, through action during the recovery phase, may account for the adaptive response seen. Given the above results, it is also interesting that those cells pretreated with both CHX and LoaOOH together were found to be more sensitive than the non-pretreated control (Fig. 2), which indicated that de novo protein synthesis in the cytoplasm is necessary for LoaOOH adaptation, while the cells are exposed to LoaOOH.
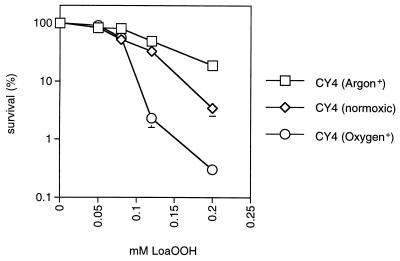
Presence of oxygen is required for maximal toxicity.
Lipid peroxidation is an autocatalytic process which requires the involvement of molecular oxygen. Hence, to examine the effect of oxygen on LoaOOH toxicity, cells were treated with different concentrations of LoaOOH in the presence of pure oxygen or argon (see Materials and Methods). With the wild-type grande strain, resistance to LoaOOH decreased markedly in the presence of oxygen and increased in the presence of argon (Fig. 3); the petite strain behaved similarly to the grande strain under the same conditions (data not shown). These results indicate that oxygen contributes significantly to the increased toxicity of LoaOOH. Lipid hydroperoxides can also break down to produce toxic carbonyl compounds, including MDA, and the question arises whether this product of lipid peroxidation can induce the defense system against LoaOOH.
FIG. 3.
The presence of oxygen is required for maximal sensitivity to LoaOOH. Pure oxygen or argon was bubbled through culture aliquots for 15 min prior to addition of different concentrations of LoaOOH for 1 h. Cell viability was determined as described for Fig. 1 and compared to that of a control which received LoaOOH treatment only. Data are means of triplicates from a representative experiment.
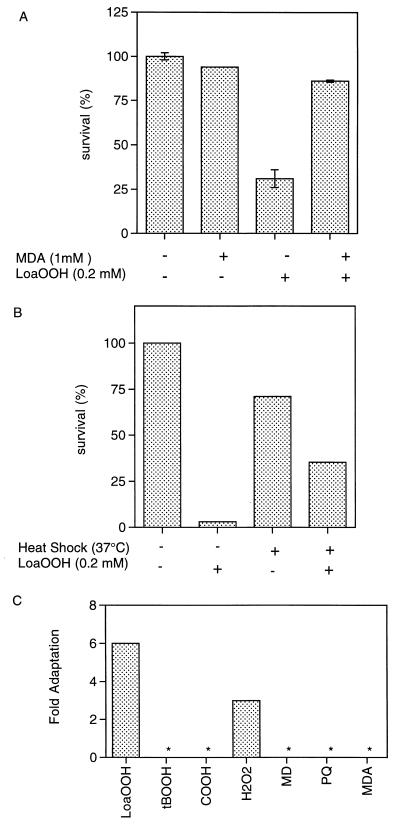
Cross-adaptation to MDA and heat shock.
Pretreatment of cells with a sublethal dose of MDA (1 mM; 1 h) conferred resistance to a subsequent treatment with 0.2 mM LoaOOH (Fig. 4A). The adaptive response with MDA pretreatment was significantly greater than that seen when cells were pretreated with LoaOOH, which indicates that an efficient system dealing with LoaOOH is triggered upon MDA exposure. However, when the cells were pretreated with 0.05 mM LoaOOH, no increased resistance to MDA was detected (data not shown). This is interesting, and a probable explanation lies with the concentrations that are required to lead to toxic effects. LoaOOH is toxic at concentrations 10-fold lower than those for MDA, and as a weak acid, MDA accumulates within cells at concentrations above those added exogenously (47). This also indicates that there may be at least two defense systems dealing with the products of lipid peroxidation and that these may overlap to some degree. Furthermore, it could be postulated that with time a breakdown product of LoaOOH may induce resistance to MDA and that this was not seen in the time course of this experiment, or that MDA induces various membrane transporters (such as PDR5 [4]), which leads to multidrug resistance and therefore to removal of LoaOOH or its breakdown products.
FIG. 4.
Cross-adaptation to other stresses indicates overlapping response systems. (A) Adaptation of wild-type cells to a lethal dose of LoaOOH (0.2 mM; 1 h) following pretreatment with a sublethal dose of MDA (1 mM; 1 h). (B) Adaptation of wild-type cells to a lethal dose of LoaOOH (0.2 mM; 1 h) following pretreatment with a mild heat shock (37°C; 1 h). (C) Fold adaptation, following pretreatment with a sublethal dose of LoaOOH (0.05 mM; 1 h), to the organic hydroperoxides cumene hydroperoxide (COOH; 4 mM) and tert-butyl hydroperoxide (tBOOH; 12 mM), the superoxide-generating agents paraquat (PQ; 15 mM) and menadione (MD; 6 mM), MDA (5 mM), and H2O2 (5 mM). An asterisk indicates that no adaptive response was detected. Data are means of triplicates from a representative experiment.
Many different stress response proteins are induced following heat shock treatment (33), including those that are able to degrade or repair damaged proteins (33). Exponentially growing cells were given a mild heat shock (37°C for 1 h) and were subsequently found to be more resistant to a 1-h treatment with 0.2 mM LoaOOH, thereby indicating that some part of the heat shock response may play a role in the defense against, or detoxification of, fatty acid hydroperoxides (Fig. 4B), or that LoaOOH may be modifying cellular protein levels.
Pretreatment with LoaOOH confers resistance to H2O2 but not to superoxide anions or other organic hydroperoxides.
Previous work has shown that there is a complex set of interactions between different forms of oxidative stress in terms of their abilities to induce cross-protection against other compounds, and therefore, distinct detoxification and adaptive responses may exist. Pretreatment with the sublethal dose of LoaOOH (0.05 mM) did not induce further resistance to organic hydroperoxides, but it did confer increased resistance to H2O2 (Fig. 4C). It is possible that the activities of enzymes involved in the detoxification of H2O2, such as catalase (34), cytochrome c peroxidase (40), and glutathione peroxidase (37) increase following treatment with LoaOOH. Of these enzymes, glutathione peroxidase activity was measured, since it has also been shown to detoxify lipid hydroperoxides under certain conditions (35). Total and Se-dependent glutathione peroxidase activity was detectable in crude cell extracts of S. cerevisiae prepared from untreated cells, and this increased following treatment with doses of LoaOOH in the range of 0.05 to 0.12 mM, where a 0.08 mM dose resulted in approximately 50% cell viability under the conditions used (Table 1). The total activity of glutathione peroxidase increases following treatment with LoaOOH, and this induction of glutathione peroxidase may therefore be the basis of the increased resistance to H2O2. Putative glutathione peroxidase genes have been identified within the S. cerevisiae genome, as have glutathione S-transferases, which are also known to have glutathione peroxidase activity (39), and it is the aim of future studies to determine the role of these genes in the LoaOOH response.
TABLE 1.
Total and Se-dependent glutathione peroxidase activity increased while total glutathione decreased following treatment with LoaOOHa
| LoaOOH concn (mM) | GPX activity (nmol min−1 mg−1)b
|
Total glutathionec | GSSG (nmol/107 cells) | GSH/GSSG ratio | |
|---|---|---|---|---|---|
| Total | Se dependent | ||||
| 0 | 123 ± 4.2 | 96 ± 10 | 4.3 | 0.56 | 6:1 |
| 0.05 | 149 | 139 ± 7.1 | 2.8 | 0.32 | 7:1 |
| 0.08 | 611 ± 22 | 419 ± 24 | 1.45 | 0.19 | 3:1 |
| 0.12 | 593 ± 18 | 562 ± 20 | 0.62 | 0.05 | 11:1 |
| 0.2 | ND | ND | 0.43 | 0.01 | 55:1 |
Abbreviations: GPX, glutathione peroxidase; GSSG, oxidized glutathione; GSH, reduced glutathione; ND, not determined.
Activity is expressed as nanomoles of NADPH oxidized per minute per milligram of protein. Data are means of duplicates from a representative experiment.
Expressed as nanomoles of GSH plus 2× GSSG per 107 viable cells for the wild-type strain.
Pretreatment with nonlethal concentrations of the superoxide-generating agents paraquat and menadione did not confer resistance to a subsequent 0.2 mM LoaOOH treatment (data not shown). This indicates that the superoxide response does not play a definitive role in the defense against fatty acid hydroperoxides and that cells treated with a low dose of superoxide-generating agent may not form enough lipid peroxide to induce subsequent resistance in the time course of this experiment. In addition, resistance to LoaOOH was not conferred following pretreatment with the lipid-soluble organic hydroperoxides tert-butyl hydroperoxide and cumene hydroperoxide. This result is interesting because it indicates that it is not just the hydroperoxide moiety which induces the adaptive response and that the yeast cells specifically adapt to LoaOOH.
Glutathione and the transcriptional activator yAP-1 play important roles in cellular defense against LoaOOH.
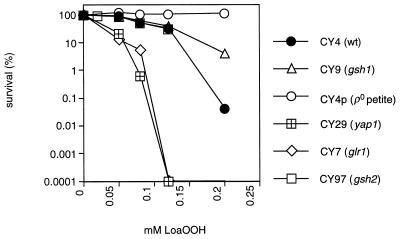
Glutathione is an abundant intracellular thiol found to be important in the response to other types of oxidative stress (20). To determine if glutathione plays a role in the cellular defense against LoaOOH, the sensitivity of a mutant unable to synthesize glutathione was tested. CY9 lacks GSH1, which encodes γ-glutamylcysteine synthetase, which is the first and rate-limiting step in glutathione synthesis (36). Surprisingly, CY9 was more resistant to LoaOOH than the wild type (Fig. 5). Since the gsh1 mutant is phenotypically petite in that it lacks mitochondrial function, it is important to compare its resistance to that of the [rho0] petite mutant generated from the wild-type parent (21) (Fig. 5). CY9 (gsh1) was more sensitive than CY4p ([rho0]), and therefore glutathione may play some role in the cellular defense against LoaOOH. The respiration-competent gsh2 mutant (CY97), which cannot form glutathione but is able to synthesize the dipeptide γ-glutamylcysteine (22), showed no difference in sensitivity from the wild type when treated with LoaOOH (Fig. 5). This finding indicates that the dipeptide can effectively substitute for glutathione in this response, and this is the first report of such a role in response to lipid hydroperoxides. From these results, it might be expected that cellular glutathione levels alter following LoaOOH treatment; hence, cells were treated as described previously and total free-glutathione levels were assayed, as well as the ratio of oxidized to reduced glutathione, which reflects the redox status of the cell (48).
FIG. 5.
Sensitivities of the wild type and oxidative-stress mutants to LoaOOH. Yeast strains CY4 (wild type), CY4p ([rho0] petite mutant), CY9 (gsh1 petite mutant), CY97 (gsh2), CY7 (glr1), and CY29 (yap1) were grown to exponential phase and treated with various concentrations of LoaOOH for 1 h. Samples were diluted and plated on YEPD to monitor cell viability. Data are means of triplicates from a representative experiment.
Free intracellular glutathione levels decreased on treatment of cells with LoaOOH (Table 1). This was unexpected, since oxidation of glutathione would not affect the total content measured in this assay. There are a number of possibilities to account for this decrease: free glutathione may be reacting directly with LoaOOH within the cell, or it may become protein bound when enzymes which use it as a substrate are induced following treatment, or, through the activity of glutathione S-transferases, glutathione may be conjugated to toxic compounds formed following treatment and may thus be exported from the cell. In all of these situations, any bound glutathione within the cell would go undetected in the assay used. Additionally, LoaOOH treatment is likely to cause membrane damage resulting in increased membrane porosity, and thus the cell could be rapidly losing low-molecular-weight molecules, such as glutathione. This would also account for the dramatic loss of volume, such as the sevenfold reduction seen with a lethal dose of LoaOOH (0.2 mM). The proportion of cells losing volume increased as the dose of LoaOOH increased (data not shown). When cells were treated with 0.5 mM LoaOOH, cell volume was greatly reduced and flocculation resulted (data not shown).
Glutathione reductase catalyzes the formation of reduced glutathione from the oxidized form (19). The isogenic mutant CY7, in which the glutathione reductase gene (GLR1) is disrupted, was treated with different concentrations of LoaOOH. A yap1 disruptant (CY29) was also tested in this way, since yAP-1 is known to regulate the genes involved in glutathione synthesis (GSH1) (50) and reduction (GLR1) (19), as well as other related genes, such as TRX2, which have a role in oxidative stress (30). These strains were found to be very sensitive to low concentrations of LoaOOH compared to the wild type (Fig. 5). The increased sensitivities of the gsh1 mutant (compared to the [rho0] petite mutant) and the glr1 and yap1 mutants (compared to the wild type) indicate that there is a role for yAP-1-inducible genes, such as those involved in the glutathione system. This yAP-1-mediated response further supports the possible induction of membrane transporters mentioned earlier, since yAP-1 is a transcriptional activator of multidrug resistance genes (18). Overall, these results indicate an important role for glutathione in the defense and protection against LoaOOH.
Respiration-deficient cells are resistant, and inhibition of respiration can increase resistance to LoaOOH.
Previously it was found that treatment of a wild-type culture with LoaOOH led to the selection of petite mutants as survivors. This is surprising, since previous studies in yeast have shown that petite strains are generally more sensitive than the wild type to different types of stress, including oxidant exposure (11, 15, 23, 47). The finding that petite mutants were more resistant to LoaOOH raises the question of what role the mitochondrion plays in the toxicity of LoaOOH, especially since mutations in the mitochondrial genome can enhance oxidative stress (40). It is unlikely that mitochondrial DNA is a target for LoaOOH, since the frequency of petite-mutant generation did not increase during the treatments used. To investigate the role of mitochondrial function, the sensitivity of an isogenic [rho0] petite mutant (CY4p) was compared to that of the wild-type grande strain. Petite mutants do not lack all mitochondrial functions, since nuclear-encoded proteins can continue to be imported into the petite-mutant mitochondrion (3) and petite mutants can grow on minimal medium, for which some mitochondrial metabolism is required; however, [rho0] petite mutants lack mitochondrial DNA, which encodes the apoprotein of cytochrome b, some subunits of cytochrome c oxidase, and the F1-ATPase (14), and thus are impaired in respiration, mitochondrial ATP generation, and related cellular processes. The [rho0] petite mutant was found to be more resistant to LoaOOH than the wild type (Fig. 5), tolerating concentrations threefold higher than the dose of LoaOOH that was lethal for the grande strain under the same conditions (data not shown). This high intrinsic resistance indicates that mitochondrial function plays an important role in eliciting the cytotoxic effect of LoaOOH.
Since petite strains are defective in respiration, several different respiratory inhibitors were used with the wild type to determine what role respiration may play in the LoaOOH response. Flavone inhibits NADH dehydrogenase, which is complex I of the respiratory chain in S. cerevisiae (12). Antimycin inhibits the cytochrome reductase complex between cytochromes b and c, while both potassium cyanide (KCN) and sodium azide (NaN3) inhibit at complex IV via binding to the cytochrome a3 heme prosthetic group (44). Oligomycin removes the F0 subunit of the mitochondrial ATPase, which blocks the proton pump through this system and affects electron flow along the respiratory chain in tightly coupled mitochondria (1), and thus cells are not able to synthesize mitochondrial ATP (9). Exponentially growing cells were pretreated with a range of concentrations of the appropriate inhibitor (0.05 to 0.5 mM) at 30°C in YEPD medium, and after 1 h, a lethal concentration of LoaOOH (0.2 mM) was added to the culture, which was incubated for an additional 1 h. The results were compared to aliquots of the same culture that were untreated or treated with LoaOOH alone (no pretreatment) (Fig. 6A). The efficacy of the inhibitors was tested by growth on different carbon sources in the presence of each inhibitor. At the concentrations used, respiration was blocked (no growth on YEPG containing the inhibitor), while there was no serious loss of viability on YEPD medium (data not shown).
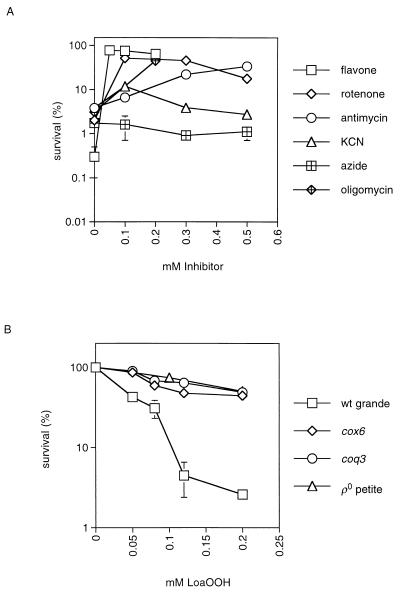
FIG. 6.
Inhibition of respiration or ATP synthesis can increase cellular resistance to LoaOOH. (A) Wild-type cells were treated with a range of concentrations of one of the respiratory inhibitors flavone (0.05 to 0.2 mM), antimycin (0.1 to 0.5 mM), KCN (0.1 to 0.5 mM), and sodium azide (0.1 to 0.5 mM) or with an inhibitor of ATP synthesis, oligomycin (0.2 mM), for 1 h prior to treatment with a lethal dose of LoaOOH (0.2 mM). (B) The wild-type strain CY4 (wt grande) and its isogenic coq3 and cox6 mutants (lacking ubiquinone and cytochrome c oxidase subunit 6, respectively) were tested for their sensitivities to LoaOOH as described for Fig. 5. Data are means of triplicates from a representative experiment.
Prior inhibition of respiration by flavone or antimycin led to a marked increase in the resistance of the wild type to LoaOOH (Fig. 6A). This resistance was similar to that obtained for the [rho0] petite mutant (CY4p) treated with the same dose of LoaOOH (data not shown). Different results were, however, obtained following pretreatment with the inhibitors KCN and NaN3. The low resistance of the wild type to LoaOOH did not change following treatment with NaN3, and KCN had little effect beyond a slight increase in resistance at 0.1 mM (Fig. 6A). Similar results were obtained when these inhibitors were used at 1 mM (data not shown). The results with flavone and antimycin indicate that a block in the respiratory chain can lead to a significant increase in resistance to LoaOOH. Those with cyanide and azide are interesting because they show that inhibition of the respiratory chain after cytochrome reductase (complex III) and before the final reduction of oxygen to water by cytochrome c oxidase (complex IV) does not increase viability, thus indicating that a component of the respiratory chain between complex III and complex IV is involved in the toxicity of LoaOOH. It is also possible that KCN and NaN3 are inhibiting other heme-containing proteins of the cell, as has been noted for catalase (44). Inhibition of ATP synthesis by oligomycin also increased resistance to LoaOOH (Fig. 6A). Another approach to analyzing the role of respiration is to use mutants specifically defective in components of the respiratory chain or in ATP synthesis.
Effect of mutations affecting the respiratory chain on cellular resistance and adaptation to LoaOOH.
Ubiquinone-deficient mutants are sensitive to linoleic acid at concentrations higher than those used as controls in this study, possibly due to the subsequent formation of lipid peroxidation products (13). A range of mitochondrial mutants were tested under the conditions for LoaOOH treatment previously described. These included the coq3 mutant (which lacks ubiquinone), the cox6 mutant (which lacks cytochrome oxidase subunit 6), and the atp2 mutant (which lacks the β subunit of the mitochondrial F1-ATPase). The effect of each mutation was compared to results for both the isogenic wild type and the isogenic [rho0] petite mutant, since all of the above nuclear pet mutants are phenotypically petite. In the CY4 strain background, mutations of cox6 and coq3 led to increased resistance approaching that of the [rho0] petite mutant (Fig. 6B). These results agree with those obtained with the respiration inhibitors. In the W303-1B background, the atp2 mutant gave results similar to those for the isogenic coq3 mutant and the [rho0] petite mutant (data not shown), and these were in agreement with the result obtained with oligomycin. Inhibition of mitochondrial ATP synthesis also led to resistance to the toxic effects of LoaOOH. The results described above indicate that mitochondrial function(s) associated with respiration is needed to elicit full toxicity of LoaOOH. One possibility is that reduced cytochrome c oxidase can donate an electron to LoaOOH and that, in the presence of oxygen, a lipid peroxy radical is formed which can initiate the chain reaction of lipid peroxidation. This is supported by the oxygen effect on sensitivity. The intrinsic resistance of the coq3 and cox6 mutants, which is similar to that of the [rho0] petite mutant, also supports this hypothesis.
Alternative explanations for these results include the possibility that mitochondrially generated ATP may be used to potentiate the toxic effects of LoaOOH. The results with KCN and NaN3 are not consistent with this explanation; however, these inhibitors can affect heme-containing systems in the cell other than respiration (44, 46). Another possibility is that autocatalytic lipid peroxidation is initiated by a mitochondrial protein with lipoxygenase activity using LoaOOH as the substrate, and this may not function in the absence of respiration. A protein with lipoxygenase activity has been assayed and purified from the mitochondria of S. cerevisiae, but its corresponding gene has not been identified (43).
To determine if mitochondrial function was also required for cells to adapt to LoaOOH, the isogenic petite strains CY4p and CY9 were tested as previously described. Both of these strains were unable to adapt (data not shown), which indicates that there may be a mitochondrial function required for adaptation; for example, adaptation to LoaOOH may require an active system(s) whereby energy is required at levels higher than those obtained via fermentation alone. An intriguing possibility is that petite mutants are already maximally adapted to the toxic effects of LoaOOH due to loss of a mitochondrial function. For example, the capacity of a petite strain to remove oxygen from the cytoplasm may be lower than that of the grande strain, and since oxygen is involved in toxicity, this could lead to the triggering of an adaptive response. This cannot be generally true for oxidants however, since petite mutants are more sensitive than the wild type to other ROS tested (11, 15, 47).
The results of this study have laid foundations for a more thorough understanding of how eukaryotic cells cope with lipid peroxide stress, and this has implications for both the medical and industrial fields, since lipid peroxidation plays a major role in both atherosclerosis and the rancidity of foods. Analysis has revealed several important features of the response to LoaOOH in yeast: oxygen is important for the elicitation of the compound’s cytotoxicity; the absence of mitochondrial function and specifically of respiration-related processes leads to increased resistance to LoaOOH; and glutathione and its related enzymes play key roles in the cellular defense against LoaOOH. It is the aim of future research to further elucidate the cellular response to LoaOOH.
ACKNOWLEDGMENTS
We thank W. Scott Moye-Rowley (University of Iowa) and Thomas Lisowsky (University of Dusseldorf) for the gifts of the YAP1 and GSH1 disruption plasmids, respectively, and also Catherine Clarke (University of California, Los Angeles) for kindly supplying the W303-1B wild type and isogenic mutants.
Financial support from the Cooperative Research Centre for Food Industry Innovation and the Australian Research Council is also recognized and appreciated.
REFERENCES
- 1.Babcock G T, Wikstrom M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 2.Babizhayev M. Failure to withstand oxidative stress induced by phospholipid hydroperoxides as a possible cause of the lens opacities in systemic diseases and ageing. Biochim Biophys Acta. 1996;1315:87–99. doi: 10.1016/0925-4439(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 3.Baker K, Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991;349:205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- 4.Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J Biol Chem. 1994;269:2206–2214. [PubMed] [Google Scholar]
- 5.Beeor-Tzahar T, Ben-Hayyim G, Holland D, Faltin Z, Eshdat Y. A stress-associated citrus protein is a distinct plant phospholipid hydroperoxide glutathione peroxidase. FEBS Lett. 1995;366:151–155. doi: 10.1016/0014-5793(95)00521-a. [DOI] [PubMed] [Google Scholar]
- 6.Bjornstedt M, Hamberg M, Kumar S, Xue J, Holmgren A. Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocysteine strongly stimulates the reaction via catalytically generated selenols. J Biol Chem. 1995;270:11761–11764. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- 7.Böker-Schmitt E, Francisci S, Schweyen R J. Mutations releasing mitochondrial biogenesis from glucose repression in Saccharomyces cerevisiae. J Bacteriol. 1982;151:303–310. doi: 10.1128/jb.151.1.303-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderbank J, Keenan M H J, Rose A H. Plasma-membrane phospholipid unsaturation affects expression of the general amino-acid permease in Saccharomyces cerevisiae Y185. J Gen Microbiol. 1985;131:57–65. doi: 10.1099/00221287-131-1-57. [DOI] [PubMed] [Google Scholar]
- 9.Capaldi R A, Aggeler R, Turina P, Wilkens S. Coupling between catalytic sites and the proton channel in F1F0-type ATPase. Trends Biochem Sci. 1994;19:284–289. doi: 10.1016/0968-0004(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 10.Clarke C, Williams W, Teruya J H. Ubiquinone biosynthesis in Saccharomyces cerevisiae. J Biol Chem. 1991;266:16636–16644. [PubMed] [Google Scholar]
- 11.Collinson L P, Dawes I W. Inducibility of the response of yeast cells to peroxide stress. J Gen Microbiol. 1992;138:329–335. doi: 10.1099/00221287-138-2-329. [DOI] [PubMed] [Google Scholar]
- 12.De Vries S, Grivell L A. Purification and characterization of a rotenone-insensitive NADH:Q6 oxidoreductase from mitochondria of Saccharomyces cerevisiae. Eur J Biochem. 1988;176:377–384. doi: 10.1111/j.1432-1033.1988.tb14292.x. [DOI] [PubMed] [Google Scholar]
- 13.Do T Q, Schultz J R, Clarke C F. Enhanced sensitivity of ubiquinone-deficient mutants of Saccharomyces cerevisiae to products of autoxidized polyunsaturated fatty acids. Proc Natl Acad Sci USA. 1996;93:7534–7539. doi: 10.1073/pnas.93.15.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans I H. Molecular genetic aspects of yeast mitochondria. In: Spencer J F T, Spencer D M, Smith A R W, editors. Yeast genetics. Fundamental and applied aspects. New York, N.Y: Springer-Verlag; 1983. pp. 269–370. [Google Scholar]
- 15.Flattery-O’Brien J, Collinson L P, Dawes I W. Saccharomyces cerevisiae has an inducible response to menadione which differs from that to hydrogen peroxide. J Gen Microbiol. 1993;139:501–507. doi: 10.1099/00221287-139-3-501. [DOI] [PubMed] [Google Scholar]
- 16.Garssen G J, Vliegenthart J F G, Boldingh J. An anaerobic reaction between lipoxygenase, linoleic acid and its hydroperoxides. Biochem J. 1971;122:327–332. doi: 10.1042/bj1220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gille G, Sigler K. Oxidative stress and living cells. Folia Microbiol. 1995;40:131–152. doi: 10.1007/BF02815413. [DOI] [PubMed] [Google Scholar]
- 18.Gounalaki N, Thireos G. Yap1p, a yeast transcriptional activator that mediates multidrug resistance, regulates the metabolic stress response. EMBO J. 1994;13:4036–4041. doi: 10.1002/j.1460-2075.1994.tb06720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant C M, Collinson L P, Roe J, Dawes I W. Yeast glutathione reductase is required for protection against oxidative stress and is a target for yAP-1 transcriptional regulation. Mol Microbiol. 1996;21:171–179. doi: 10.1046/j.1365-2958.1996.6351340.x. [DOI] [PubMed] [Google Scholar]
- 20.Grant C M, Dawes I W. Synthesis and role of glutathione in protection against oxidative stress in yeast. Redox Rep. 1996;2:223–229. doi: 10.1080/13510002.1996.11747054. [DOI] [PubMed] [Google Scholar]
- 21.Grant C M, MacIver F H, Dawes I W. Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr Genet. 1996;29:511–515. doi: 10.1007/BF02426954. [DOI] [PubMed] [Google Scholar]
- 22.Grant C M, MacIver F H, Dawes I W. Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide γ-glutamylcysteine. Mol Biol Cell. 1997;8:1699–1707. doi: 10.1091/mbc.8.9.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant C M, MacIver F H, Dawes I W. Mitochondrial function is required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410:219–222. doi: 10.1016/s0014-5793(97)00592-9. [DOI] [PubMed] [Google Scholar]
- 24.Grant C M, MacIver F H, Dawes I W. Stationary-phase induction of GLR1 expression is mediated by the yAP-1 transcriptional regulatory protein in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1996;22:739–746. doi: 10.1046/j.1365-2958.1996.d01-1727.x. [DOI] [PubMed] [Google Scholar]
- 25.Gunstone F D. Fatty acid and lipid chemistry. London, United Kingdom: Blackie Academic and Professional; 1996. [Google Scholar]
- 26.Hayes J D, Pulford D J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 27.Inoue Y, Ichiryu T, Yoshikawa K, Tran L, Murata K, Kimura A. Induction of glutathione peroxidase by linoleic acid hydroperoxide in Hansenula mrakii. Agric Biol Chem. 1990;54:3289–3293. [Google Scholar]
- 28.Kaneko T, Baba N, Matsuo M. Cytotoxicity of phosphatidylcholine hydroperoxides is exerted through decomposition of fatty acid hydroperoxide moiety. Free Radical Biol Med. 1996;21:173–179. doi: 10.1016/0891-5849(96)00025-1. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko T, Baba N, Matsuo M. Phospholipid hydroperoxides are significantly less toxic to cultured endothelial cells than fatty acid hydroperoxides. Life Sci. 1994;55:1443–1449. doi: 10.1016/0024-3205(94)00684-9. [DOI] [PubMed] [Google Scholar]
- 30.Kuge S, Jones N. yAP1-dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994;13:655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence R A, Burk R F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y J, Erdos G, Hou Z Z, Cho J M. Cycloheximide decreases nascent polypeptides level and affects up-regulation of HSP70 gene expression. J Therm Biol. 1994;19:327–333. [Google Scholar]
- 33.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 34.Mager W H, Moradas Ferreira P. Stress response of yeast. Biochem J. 1993;290:1–13. doi: 10.1042/bj2900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinho H S, Antunes F, Pinto R E. Role of glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase in the reduction of lysophospholipid hydroperoxides. Free Radical Biol Med. 1997;22:871–883. doi: 10.1016/s0891-5849(96)00468-6. [DOI] [PubMed] [Google Scholar]
- 36.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 37.Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase and Cu/Zn-SOD for cell survival against oxidative stress. Free Radical Biol Med. 1994;17:235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 38.Pace G W, Leaf C D. The role of oxidative stress in HIV disease. Free Radical Biol Med. 1995;19:523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 39.Prohaska J R. The glutathione peroxidase activity of glutathione S-transferase. Biochim Biophys Acta. 1980;611:87–98. doi: 10.1016/0005-2744(80)90045-5. [DOI] [PubMed] [Google Scholar]
- 40.Richter C, Schweier M. Oxidative stress in mitochondria. In: Scandalios J G, editor. Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 169–220. [Google Scholar]
- 41.Roveri A, Maiorino M, Ursini F. Enzymatic and immunological measurements of soluble and membrane-bound phospholipid hydroperoxide glutathione peroxidase. Methods Enzymol. 1994;233:202–212. doi: 10.1016/s0076-6879(94)33023-9. [DOI] [PubMed] [Google Scholar]
- 42.Sanders T. Nutritional aspects of rancidity. In: Allen J, Hamilton R, editors. Rancidity in foods. New York, N.Y: Elsevier; 1989. pp. 125–139. [Google Scholar]
- 43.Shechter G, Grossman S. Lipoxygenase from baker’s yeast: purification and properties. Int J Biochem. 1983;15:1295–1304. doi: 10.1016/0020-711x(83)90019-8. [DOI] [PubMed] [Google Scholar]
- 44.Slater E C. Application of inhibitors and uncouplers for a study of oxidative phosphorylation. Methods Enzymol. 1967;10:48–57. [Google Scholar]
- 45.Streckert G, Stan H. Conversion of linoleic acid hydroperoxide by soybean lipoxygenase in the presence of guaiacol: identification of the reaction products. Lipids. 1975;10:847–854. doi: 10.1007/BF02532331. [DOI] [PubMed] [Google Scholar]
- 46.Thompson J A, Schullek K M, Turnipseed S B, Ross D. Role of cytochrome P450 in the metabolism and toxicity of hydroperoxides in isolated rat hepatocytes. Arch Biochem Biophys. 1995;323:463–470. doi: 10.1006/abbi.1995.0068. [DOI] [PubMed] [Google Scholar]
- 47.Turton H E, Dawes I W, Grant C M. Saccharomyces cerevisiae exhibits a yAP-1-mediated adaptive response to malondialdehyde. J Bacteriol. 1997;179:1096–1101. doi: 10.1128/jb.179.4.1096-1101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandeputte C, Guizon I, Genestie-Denis I, Vannier B, Lorenzon G. A microtitre plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturised protocol. Cell Biol Toxicol. 1994;10:415–421. doi: 10.1007/BF00755791. [DOI] [PubMed] [Google Scholar]
- 49.Wheals A E. Size control models of Saccharomyces cerevisiae cell proliferation. Mol Cell Biol. 1982;2:361–368. doi: 10.1128/mcb.2.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu A-L, Moye-Rowley W S. GSH1, which encodes γ-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol Cell Biol. 1994;14:5832–5839. doi: 10.1128/mcb.14.9.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yagi K. Lipid peroxides in hepatic, gastrointestinal, and pancreatic diseases. In: Armstrong D, editor. Free radicals in diagnostic medicine. New York, N.Y: Plenum Press; 1994. pp. 165–169. [DOI] [PubMed] [Google Scholar]