Abstract
Background:
Coronary artery calcium (CAC) scoring is often used for atherosclerotic cardiovascular disease (ASCVD) risk stratification in individuals with elevated lipoprotein(a) [Lp(a)].
Objective:
To evaluate associations between Lp(a) and baseline CAC (volume/density) and CAC progression compared to other lipid biomarkers.
Methods:
We utilized data from the Multi-Ethnic Study of Atherosclerosis (MESA), a cohort study of individuals without clinical ASCVD, excluding statin users. We evaluated the associations between Lp(a), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), triglycerides, total cholesterol, apolipoproteinB, and non-HDL-C with baseline CAC and annual CAC progression using multivariable ordinal regression with adjustment for ASCVD risk factors. Analyses were also stratified by median age.
Results:
In 5,597 participants (2,726 at median 9.5-year follow-up), Lp(a) was not associated with baseline CAC volume or density and was modestly associated with volume progression (OR 1.11, 95% CI 1.03–1.21). However, other biomarkers were positively associated with baseline volume and volume progression (LDL-C: OR 1.26, 95% CI: 1.19–1.33 and OR 1.22, 95% CI: 1.15–1.30, respectively), except HDL-C which was inversely associated. LDL-C, total cholesterol and non-HDL-C were inversely associated with baseline density. In participants <62 years of age, Lp(a) was modestly associated with baseline CAC volume (OR 1.10, 95% CI: 1.00–1.20) and volume progression (OR 1.16 95% CI: 1.04–1.30).
Conclusions:
In contrast to other lipid biomarkers, Lp(a) was not associated with baseline CAC volume or density and was only modestly associated with volume progression. Our findings suggest that Lp(a) is not as robustly associated with CAC as other lipid biomarkers.
Keywords: Lipoprotein(a), Lipids, Coronary artery calcium, Computed tomography, Primary prevention
Introduction
Lipoprotein(a) (Lp(a)) is a low-density lipoprotein (LDL)-like particle composed of apolipoprotein B-100 (apoB) linked by a disulfide bond to apolipoprotein(a) (apo(a)).1 Elevated Lp(a) levels are associated with an increased risk for several cardiovascular diseases.2 Traditional risk scoring methods do not account for Lp(a),3 however, and coronary artery calcium (CAC) scoring is often used clinically to clarify risk in individuals with elevated Lp(a).4
CAC is a robust marker of subclinical atherosclerosis and for predicting atherosclerotic cardiovascular disease (ASCVD) risk.5 Prior studies have demonstrated an association between elevated Lp(a) and CAC.6–14 However, these studies were performed in heterogenous populations, and several demonstrated only modest associations, primarily using the Agatston score. There are limited data comparing the association between Lp(a) and CAC with other lipid biomarkers, and using CAC volume and density,15 though volume and density improve risk prediction over the Agatston score.16
In this study, we investigated the association between Lp(a) and CAC measures (Agatston score, volume, density) at baseline and follow-up in comparison with other lipid biomarkers in individuals free of clinical ASCVD from the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Study population
The design of the MESA has been described previously.17 Briefly, the MESA recruited 6,814 individuals from 2000–2002 between 45–84 years of age from 6 centers across the United States and 4 ethnic groups (Caucasian, African American, Hispanic, Chinese) who were free of clinically apparent cardiovascular disease (CVD). MESA was approved by the institutional review boards at each center, and all participants provided written informed consent. For this study, those with missing data for Lp(a) or ASCVD risk factors were excluded. For the analysis of CAC progression, we additionally excluded individuals without follow-up CAC scoring. For the primary analysis, baseline statin users were excluded due to the known effects of statins on CAC, particularly density. For analyses of density, only participants with detectable CAC at baseline were included due to the need for the presence of CAC to calculate density.
CAC measurement
Participants underwent cardiac computed tomography (CT) scans for CAC scoring at baseline, at short-term follow-up (half from 2002–2004, half from 2004–2005) and long-term follow-up (2010–2011 in a subset of participants). Calcification was defined by the presence of plaque ≥ 1 mm2 with a density of ≥ 130 Hounsfield Units (HU). The Agatston score was calculated as the product of total calcified plaque area and a peak calcium density weighting factor defined by plaque attenuation in HU.18 CAC density was calculated for all participants by dividing the Agatston score by the area score (volume score / CT scanner slice thickness), as previously described.19 Progression in CAC measurements was determined by calculating the yearly change in CAC measurements from baseline to follow-up.
ASCVD risk factors and lipid biomarkers
Participants underwent standardized questionnaires, vital sign measurement and laboratory testing for characterization at baseline. For this study, relevant covariates included baseline demographics (age, sex, race/ethnicity), systolic blood pressure (SBP), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), apoB, non-HDL-C, body mass index (BMI), diabetes mellitus, smoking status, hypertension, use of anti-hypertensive medications, and use of statins. Lp(a) was measured using a latex-enhanced turbidimetric immunoassay (Denka Seiken) of mass concentration and reported in mg/dL. LDL-C was calculated by the Friede-wald equation.
Statistical analysis
Baseline characteristics were compared across 3 categories of CAC volume (0, 1–100, > 100 mm3) and CAC density (1–1.9, 2–2.9, 3–4). Continuous variables were compared using analysis of variance (ANOVA) or Kruskal-Wallis testing, and categorical variables were compared using Chi-squared tests.
For analyses of lipid biomarkers, Lp(a), total cholesterol, HDL-C, LDL-C, TG, and apoB were natural log (ln)-transformed. For the baseline analysis, the association between baseline lipid biomarkers and baseline CAC Agatston score, CAC volume, and CAC density were evaluated using multivariable ordinal regression models due to non-linearity in the associations between Lp(a) and CAC measures. For Agatston and volume, the categories were: 0, 1–100 and > 100 Agatston units (AU) and mm3, respectively. For density, the categories were 1–1.9, 2–2.9, and 3–4 (unitless). Models evaluated each biomarker individually with adjustment for ASCVD risk factors (age, sex, race/ethnicity, SBP, diabetes, smoking, hypertension treatment). For Lp(a) and LDL-C, models were also evaluated with additional adjustment for HDL-C and TG. Overlapping biomarkers (such as Lp(a) and LDL-C or total cholesterol / apoB / non-HDL-C and Lp(a) / LDL-C / HDL-C / TG) were not included in the same models. Models for density were additionally adjusted for CAC volume. Odds ratios (OR) are presented per standard deviation (SD).
For the progression analysis, the association between Lp(a), other lipid biomarkers, and progression of CAC at short-term and long-term follow-up was evaluated using multivariable ordinal regression. For Agatston and volume, categories of annual change of 0, 1–20, > 20 AU or mm3, respectively, were used. For density, categories of annual change were 0, 0–0.25 and > 0.25 (unitless). Again, categories were chosen due to the non-linear association between lipid biomarkers and annual change in CAC. Model 1 and Model 2 included the same covariates as for the baseline analysis. OR are presented per SD.
Given the associations between Lp(a), premature ASCVD, and race/ethnicity we also performed the baseline and progression analysis stratified by the median age in the cohort (62 years) and by race/ethnicity. The multiplicative interactions between Lp(a) level and age and between Lp(a) and race/ethnicity were tested in the baseline ordinal regression models used above for volume and density. As an additional sensitivity analysis, we performed the baseline and progression analyses including individuals with baseline statin use; in this analysis, models were additionally adjusted for baseline statin use. Of note, apoB was only available in non-statin users and was not included in this sensitivity analysis. We also performed an additional sensitivity analysis using quartiles of baseline CAC measures and quartiles of CAC change as the outcomes for the primary analyses.
Results
MESA included 6,814 participants. After excluding participants with pre-baseline events, missing Lp(a) data or missing covariates, there were 6,566 participants. For the baseline analysis, there were 5,597 participants without statin use, 4,733 participants for the short-term progression analysis and 2,726 for the long-term progression analysis. For analyses involving density, only participants with CAC (ability to calculate density) were included for the baseline (n=2,625), short-term progression (n=2,150), and long-term progression (n=1,780) analyses (Figure 1).
Figure 1.
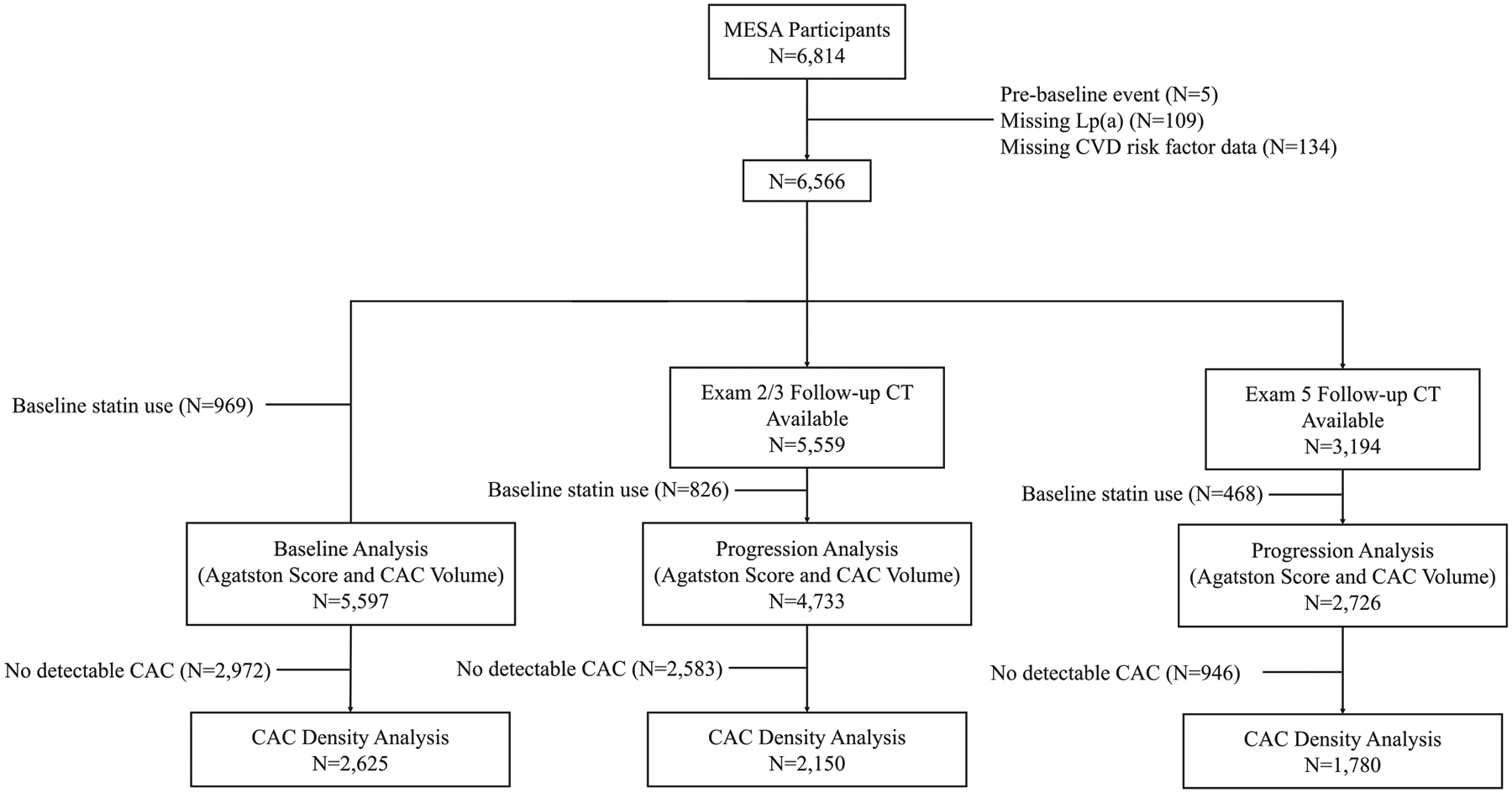
Flow diagram of study population.
Abbreviations: CAC, coronary artery calcium; CT, computed tomography; CVD, cardiovascular disease; Lp(a), lipoprotein(a); MESA, Multi-Ethnic Study of Atherosclerosis.
Baseline characteristics
Table 1 shows the baseline characteristics of the study population stratified by CAC volume and CAC density. Participants with a higher CAC volume were more likely to have increased burden of CVD risk factors including older age, male sex, hypertension, diabetes, higher SBP, and higher 10-year ASCVD risk score. With increasing level of CAC volume, greater total cholesterol, LDL-C, and TG, and lower HDL-C were observed, though trends were modest in magnitude. There was no difference in Lp(a) levels between different categories of CAC volume. Participants with higher CAC density similarly had increased burden of CVD risk factors including older age, male sex, hypertension, higher SBP and higher 10-year ASCVD risk score, but lower LDL-C. There was no difference in Lp(a) levels between different categories of CAC density.
Table 1.
Baseline Characteristics of Study Cohort by Baseline CAC Volume and Density.
| CAC Volume 0 mm3 (n=2972) |
CAC Volume 1–100 mm3 (n=1448) |
CAC Volume >100 mm3 (n=1177) |
P | |
|---|---|---|---|---|
| Age, years | 57.49 (9.11) | 63.52 (9.86) | 69.11 (8.73) | <0.001 |
| Female sex, n (%) | 1879 (63.2) | 674 (46.5) | 403 (34.2) | <0.001 |
| Race / ethnicity, n (%) | <0.001 | |||
| Black, African-American | 900 (30.3) | 373 (25.8) | 255 (21.7) | |
| Chinese | 351 (11.8) | 212 (14.6) | 116 (9.9) | |
| Hispanic | 733 (24.7) | 315 (21.8) | 227 (19.3) | |
| White, Caucasian | 988 (33.2) | 548 (37.8) | 579 (49.2) | |
| Hypertension, n (%) | 978 (32.9) | 671 (46.3) | 666 (56.6) | <0.001 |
| Diabetes, n (%) | 242 (8.1) | 169 (11.7) | 189 (16.1) | <0.001 |
| Current smoking, n (%) | 401 (13.5) | 198 (13.7) | 151 (12.8) | 0.800 |
| Hypertension medication use, n (%) | 773 (26.0) | 526 (36.3) | 542 (46.0) | <0.001 |
| Systolic blood pressure, mmHg | 121.76 (20.34) | 128.46 (21.71) | 132.72 (21.41) | <0.001 |
| Total cholesterol, mg/dL | 193.00 [171.00, 215.00] | 195.00 [175.00, 218.00] | 195.00 [173.00, 219.00] | 0.007 |
| HDL cholesterol, mg/dL | 50.00 [42.00, 61.00] | 47.00 [39.00, 57.00] | 46.00 [39.00, 57.00] | <0.001 |
| LDL cholesterol, mg/dL | 116.00 [97.00, 136.00] | 122.00 [101.00, 140.00] | 120.00 [102.00, 142.00] | <0.001 |
| Triglycerides, mg/dL | 104.00 [73.00, 152.00] | 113.50 [82.00, 161.00] | 116.00 [80.00, 164.00] | <0.001 |
| apoB, mg/dL | 104.65 [88.77, 122.03] | 108.60 [93.25, 126.40] | 109.80 [94.55, 127.25] | <0.001 |
| Non-HDL-cholesterol, mg/dL | 140.00 [118.00, 163.00] | 147.00 [125.00, 168.00] | 147.00 [125.00, 169.00] | <0.001 |
| Lp(a), mg/dL | 18.10 [7.80, 40.32] | 17.00 [7.98, 37.40] | 15.30 [6.80, 38.50] | 0.062 |
| ASCVD 10-year risk score, % | 7.98 (9.18) | 14.71 (13.05) | 22.44 (14.58) | <0.001 |
| CAC Density 1–1.9 (n=344) |
CAC Density 2–2.9 (n=822) |
CAC Density 3–4 (n=1459) |
P | |
| Age, years | 61.04 (10.28) | 65.27 (9.90) | 67.63 (9.11) | <0.001 |
| Female sex, n (%) | 155 (45.1) | 356 (43.3) | 566 (38.8) | 0.029 |
| Race / ethnicity, n (%) | 0.016 | |||
| Black, African-American | 75 (21.8) | 194 (23.6) | 359 (24.6) | |
| Chinese | 44 (12.8) | 99 (12.0) | 185 (12.7) | |
| Hispanic | 77 (22.4) | 202 (24.6) | 263 (18.0) | |
| White, Caucasian | 148 (43.0) | 327 (39.8) | 652 (44.7) | |
| Hypertension, n (%) | 143 (41.6) | 429 (52.2) | 765 (52.4) | 0.001 |
| Diabetes, n (%) | 42 (12.2) | 97 (11.8) | 219 (15.0) | 0.071 |
| Current smoking, n (%) | 46 (13.4) | 112 (13.6) | 191 (13.1) | 0.936 |
| Hypertension medication use, n (%) | 110 (32.0) | 341 (41.5) | 617 (42.3) | 0.002 |
| Systolic blood pressure, mmHg | 126.82 (20.92) | 130.35 (21.71) | 131.22 (21.76) | 0.003 |
| Total cholesterol, mg/dL | 197.00 [175.75, 219.25] | 197.00 [178.00, 220.00] | 194.00 [172.00, 218.00] | 0.078 |
| HDL cholesterol, mg/dL | 46.00 [39.00, 55.25] | 46.00 [39.00, 57.00] | 47.00 [39.00, 58.00] | 0.510 |
| LDL cholesterol, mg/dL | 124.00 [104.00, 141.00] | 123.00 [103.00, 142.00] | 119.00 [100.00, 140.00] | 0.025 |
| Triglycerides, mg/dL | 117.00 [84.00, 167.25] | 115.50 [82.00, 160.00] | 113.00 [79.00, 162.00] | 0.597 |
| apoB, mg/dL | 110.80 [94.40, 130.60] | 110.60 [93.93, 127.38] | 107.70 [93.40, 125.50] | 0.122 |
| Non-HDL-cholesterol, mg/dL | 147.00 [128.00, 172.00] | 149.00 [126.00, 169.00] | 145.00 [124.00, 167.00] | 0.042 |
| Lp(a), mg/dL | 15.30 [6.73, 33.22] | 15.80 [7.30, 38.90] | 16.70 [7.70, 38.25] | 0.279 |
| ASCVD 10-year risk score, % | 12.84 (13.03) | 17.29 (14.07) | 19.93 (14.33) | <0.001 |
Data presented as n (%), mean (SD) or median [IQR].
Abbreviations: CAC, coronary artery calcium; HDL, high-density lipoprotein; LDL, low-density lipoprotein; apoB, apolipoprotein B; Lp(a), lipoprotein (a); ASCVD, atherosclerotic cardiovascular disease.
Baseline CAC
At baseline, there were 2,972 participants with CAC volume = 0 mm3, 1,448 with volume 1–100 mm3, and 1,177 with volume > 100 mm3. There were 344 participants with CAC density 1–1.9, 822 with density 2–2.9, and 1,459 with density 3–4. Lp(a) was not associated with Agatston score or CAC volume at baseline. Of the biomarkers evaluated, non-HDL-C had the strongest association with CAC volume (odds ratio (OR) per SD 1.29, 95% confidence interval (CI) 1.22–1.37). In addition, LDL-C (OR 1.26, 95% CI 1.19–1.33), TG (OR 1.13, 95% CI 1.06–1.20), total cholesterol (OR 1.22, 95% CI 1.15–1.29), and apoB (OR 1.25, 95% CI 1.17–1.34) were positively associated with CAC volume at baseline (Figure 2). HDL-C was inversely associated with volume at baseline (OR 0.84, 95% CI 0.79–0.89). Similar results were seen for the Agatston score. Lp(a) was not associated with baseline CAC density, while LDL-C (OR 0.91, 95% CI 0.83–0.99), total cholesterol (OR 0.90, 95% CI 0.83–0.99) and non-HDL-C (OR 0.90, 95% CI 0.82–0.99) were all inversely associated (Table 2). When quartiles of CAC measures were used as the outcome, results were similar, except that apoB was now significantly associated with baseline density (Table S1). When statin users were included in a sensitivity analysis, Lp(a) was associated with baseline CAC volume (OR 1.06, 95% CI 1.01–1.11, Table S2).
Figure 2.
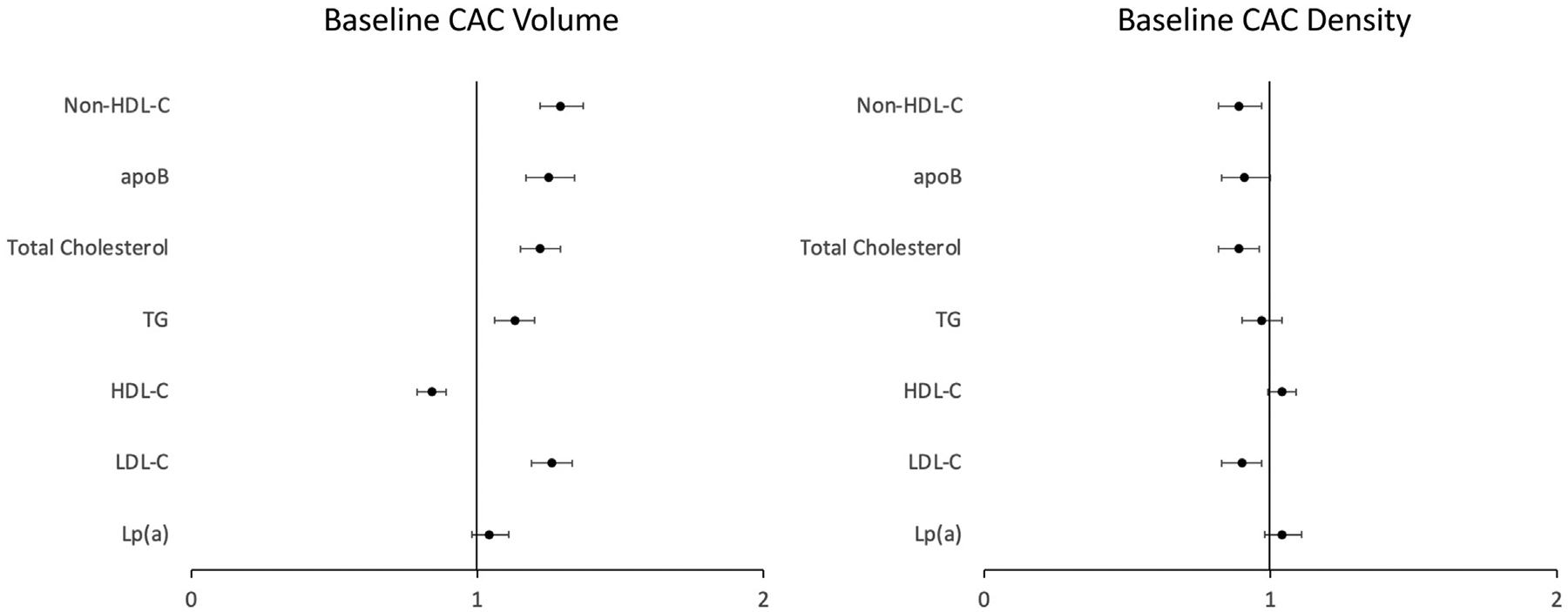
Association between lipid biomarkers and baseline coronary artery calcium (CAC) volume and density.
Abbreviations: CAC, coronary artery calcium; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; apoB, apolipoprotein B; Lp(a), lipoprotein (a); TG, triglycerides.
Table 2.
Association of lipid biomarkers with baseline CAC.
| Agatston Score | Volume Score | Density Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Lp(a) | 1.04 | 0.98, 1.10 | 0.237 | 1.04 | 0.98, 1.11 | 0.176 | 1.04 | 0.95, 1.15 | 0.365 |
| Lp(a) with adjustment for HDL-C and TG | 1.05 | 0.99, 1.11 | 0.140 | 1.05 | 0.99, 1.12 | 0.095 | 1.04 | 0.95, 1.15 | 0.374 |
| LDL-C | 1.26 | 1.19, 1.33 | <0.001 | 1.26 | 1.19, 1.33 | <0.001 | 0.91 | 0.83, 0.99 | 0.030 |
| LDL-C with adjustment for HDL-C and TG | 1.25 | 1.18, 1.32 | <0.001 | 1.25 | 1.18, 1.32 | <0.001 | 0.91 | 0.83, 0.99 | 0.031 |
| HDL-C | 0.85 | 0.80, 0.90 | <0.001 | 0.84 | 0.79, 0.89 | <0.001 | 1.03 | 0.94, 1.13 | 0.551 |
| TG | 1.11 | 1.05, 1.18 | <0.001 | 1.13 | 1.06, 1.20 | <0.001 | 0.98 | 0.89, 1.07 | 0.663 |
| Total cholesterol | 1.22 | 1.15, 1.29 | <0.001 | 1.22 | 1.15, 1.29 | <0.001 | 0.90 | 0.83, 0.99 | 0.028 |
| apoB (n=4,582) | 1.24 | 1.16, 1.33 | <0.001 | 1.25 | 1.17, 1.34 | <0.001 | 0.92 | 0.83, 1.01 | 0.082 |
| Non-HDL-C | 1.28 | 1.21, 1.36 | <0.001 | 1.29 | 1.22, 1.37 | <0.001 | 0.90 | 0.82, 0.99 | 0.025 |
All lipid biomarkers were ln-transformed. OR are presented per standard deviation. Models included individual biomarkers + CVD risk factors (age, sex, race/ethnicity, SBP, DM, smoking, hypertension treatment); For Lp(a) and LDL-C, models with additional adjustment for HDL-C and TG are shown. Models for density are adjusted for volume. Abbreviations: ApoB, apolipoprotein B; CAC, coronary artery calcium; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); OR, odds ratio; TG, triglycerides.
CAC progression
Median short-term follow-up was 2.4 [IQR 1.6, 3.1] years. At follow-up, there were 2,466 participants with yearly CAC volume progression of 0 mm3, 1325 with progression of 1–20 mm3, and 942 with progression of > 20 mm3. Lp(a) was not associated with progression of CAC volume. Non-HDL-C was most strongly associated with volume progression (OR per SD 1.26, 95% CI 1.19–1.35). LDL-C (OR 1.22, 95% CI 1.15–1.30), TG (OR 1.16, 95% CI 1.09–1.24), total cholesterol (OR 1.20, 95% CI 1.13–1.27), and apoB (OR 1.21, 95% CI 1.13–1.30) were also positively associated with volume progression. HDL-C was inversely associated with volume progression (OR 0.84, 95% CI 0.79–0.90, Figure 3). Similar results were seen for the Agatston score. None of the biomarkers evaluated were significantly associated with density progression (Table 3).
Figure 3.
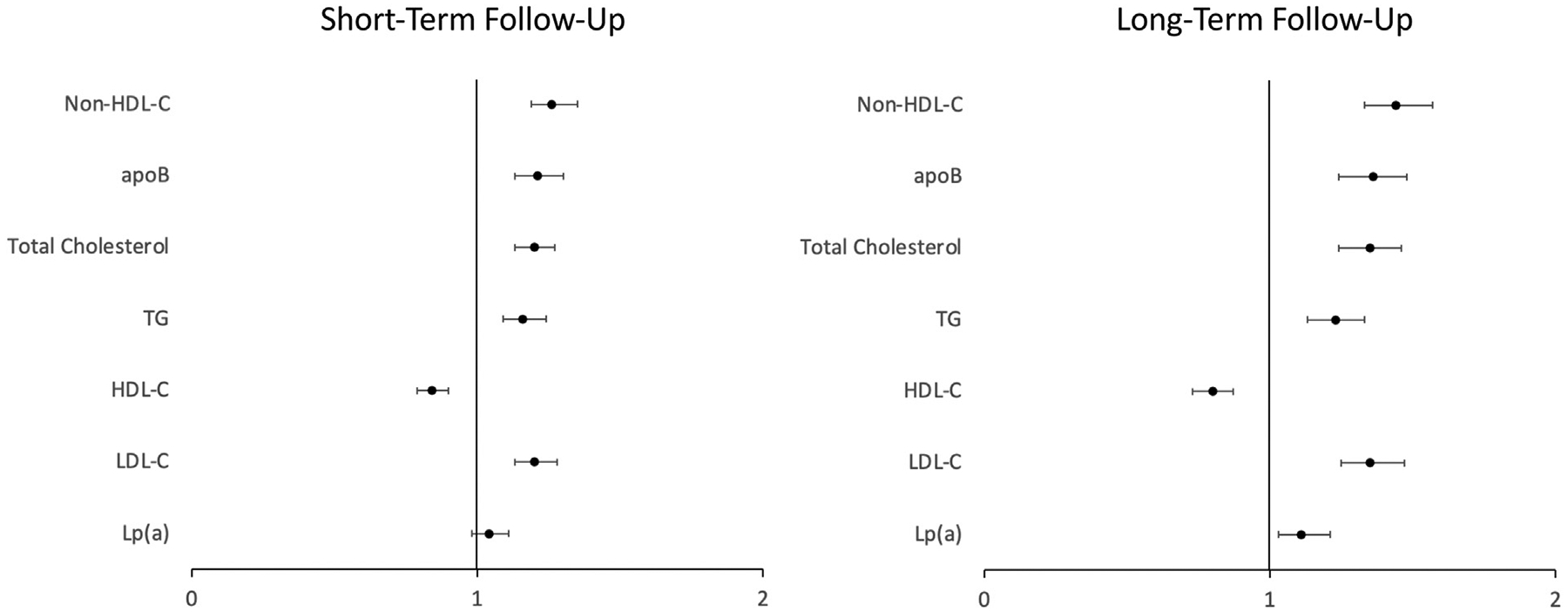
Association between lipid biomarkers and coronary artery calcium (CAC) volume progression.
Abbreviations: CAC, coronary artery calcium; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; apoB, apolipoprotein B; Lp(a), lipoprotein (a); TG, triglycerides.
Table 3.
Association of lipid biomarkers with yearly CAC progression.
| Short-Term Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Agatston Score | Volume Score | Density Score | |||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Lp(a) | 1.04 | 0.98, 1.11 | 0.188 | 1.03 | 0.97, 1.10 | 0.309 | 0.93 | 0.85, 1.01 | 0.088 |
| Lp(a) with adjustment for HDL-C and TG | 1.05 | 0.99, 1.12 | 0.106 | 1.04 | 0.98, 1.11 | 0.179 | 0.93 | 0.85, 1.01 | 0.094 |
| LDL-C | 1.20 | 1.13, 1.28 | <0.001 | 1.22 | 1.15, 1.30 | <0.001 | 0.96 | 0.89, 1.04 | 0.354 |
| LDL-C with adjustment for HDL-C and TG | 1.19 | 1.12, 1.27 | <0.001 | 1.20 | 1.13, 1.28 | <0.001 | 0.96 | 0.88, 1.04 | 0.303 |
| HDL-C | 0.86 | 0.80, 0.92 | <0.001 | 0.84 | 0.79, 0.90 | <0.001 | 1.03 | 0.94, 1.12 | 0.547 |
| TG | 1.14 | 1.08, 1.22 | <0.001 | 1.16 | 1.09, 1.24 | <0.001 | 1.05 | 0.97, 1.15 | 0.217 |
| Total cholesterol | 1.19 | 1.12, 1.26 | <0.001 | 1.20 | 1.13, 1.27 | <0.001 | 1.01 | 0.93, 1.10 | 0.794 |
| apoB (n=3,851) | 1.19 | 1.11, 1.28 | <0.001 | 1.21 | 1.13, 1.30 | <0.001 | 1.02 | 0.94, 1.11 | 0.587 |
| Non-HDL-C | 1.25 | 1.17, 1.33 | <0.001 | 1.26 | 1.19, 1.35 | <0.001 | 0.99 | 0.91, 1.08 | 0.790 |
| Long-Term Follow-Up | |||||||||
| Agatston Score | Volume Score | Density Score | |||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Lp(a) | 1.11 | 1.02, 1.20 | 0.011 | 1.09 | 1.00, 1.18 | 0.043 | 0.95 | 0.82, 1.10 | 0.459 |
| Lp(a) with adjustment for HDL-C and TG | 1.13 | 1.05, 1.23 | 0.002 | 1.11 | 1.03, 1.21 | 0.010 | 0.95 | 0.82, 1.09 | 0.452 |
| LDL-C | 1.35 | 1.25, 1.46 | <0.001 | 1.38 | 1.27, 1.50 | <0.001 | 1.02 | 0.88, 1.18 | 0.792 |
| LDL-C with adjustment for HDL-C and TG | 1.33 | 1.22, 1.44 | <0.001 | 1.35 | 1.25, 1.47 | <0.001 | 1.02 | 0.88, 1.18 | 0.784 |
| HDL-C | 0.81 | 0.74, 0.88 | <0.001 | 0.80 | 0.73, 0.87 | <0.001 | 1.04 | 0.90, 1.20 | 0.604 |
| TG | 1.18 | 1.09, 1.27 | <0.001 | 1.23 | 1.13, 1.33 | <0.001 | 0.99 | 0.86, 1.14 | 0.902 |
| Total cholesterol | 1.31 | 1.21, 1.42 | <0.001 | 1.35 | 1.24, 1.46 | <0.001 | 1.04 | 0.90, 1.20 | 0.568 |
| apoB (n=2,135) | 1.32 | 1.21, 1.44 | <0.001 | 1.36 | 1.24, 1.48 | <0.001 | 0.99 | 0.85, 1.15 | 0.875 |
| Non-HDL-C | 1.40 | 1.29, 1.51 | <0.001 | 1.44 | 1.33, 1.57 | <0.001 | 1.02 | 0.88, 1.17 | 0.825 |
All lipid biomarkers were ln-transformed. OR and beta coefficients are presented per standard deviation. Models included individual biomarkers + CVD risk factors (age, sex, race/ethnicity, SBP, DM, smoking, hypertension treatment); For Lp(a) and LDL-C, models with additional adjustment for HDL-C and TG are shown. Models for density are adjusted for baseline volume. Abbreviations: ApoB, apolipoprotein B; CAC, coronary artery calcium; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); OR, odds ratio; TG, triglycerides.
Median long-term follow-up was 9.5 [IQR 9.2, 10.0] years. At follow-up, there were 937 participants with yearly CAC volume progression of 0 mm3, 1,210 with progression of 1–20 mm3, and 579 with progression of > 2 mm3. In general, the magnitude of associations between lipid biomarkers and CAC progression was greater at long-term follow-up than short-term follow-up. Lp(a) was significantly associated with progression of CAC volume (OR 1.11 per SD, 95% CI 1.03–1.21). Non-HDL-C was again most strongly associated with volume progression (OR per SD 1.44, 95% CI 1.33–1.57). LDL-C (OR 1.35, 95% CI 1.25–1.47), TG (OR 1.23, 95% CI 1.13–1.33), total cholesterol (OR 1.35, 95% CI 1.24–1.46), and apoB (OR 1.36, 95% CI 1.24–1.48) were also positively associated with volume progression. HDL-C was again inversely associated with volume progression (OR 0.80, 95% CI 0.73–0.87, Figure 3). Similar results were seen for the Agatston score. None of the biomarkers evaluated were significantly associated with density progression (Table 3). Results were similar when quartiles of annual CAC change were used as the outcome (Table S1). When statin users were included in a sensitivity analysis, no significant differences were noted for Lp(a) (Table S3).
Baseline CAC and progression of CAC stratified by age
There was a borderline statistically significant interaction between age and Lp(a) for baseline CAC density (p=0.05), but not CAC volume (p=0.323). Among those with age below the median of 62 years, Lp(a) was associated with baseline CAC volume (OR 1.10, 95% CI 1.00–1.20) but not CAC density. Of the other biomarkers evaluated, LDL-C, total cholesterol, apoB and non-HDL-C were all positively associated with CAC volume in younger participants, while HDL-C was inversely associated; the strongest positive association was observed with non-HDL-C (OR 1.30, 95% CI 1.18–1.42). HDL-C was positively associated with baseline CAC density (OR 1.19, 95% CI 1.02–1.39). None of the other biomarkers were associated with CAC density. In older participants (age ≥ 62 years), Lp(a) was not associated with baseline CAC volume or density. Of the other biomarkers evaluated, LDL-C, TG, total cholesterol, apoB and non-HDL-C were all positively associated with CAC volume in older participants, while HDL-C was inversely associated; the strongest positive association was observed with non-HDL-C (OR 1.29, 95% CI 1.19–1.39). LDL-C was borderline inversely associated (OR 0.89, 95% CI 0.80–1.00) and total cholesterol was significantly inversely associated (OR 0.88, 95% CI 0.79–0.99) with CAC density. None of the other biomarkers were associated with CAC density. In general, the strength of associations were similar for CAC volume in younger and older participants, while there were differential associations with CAC density (Table 4).
Table 4.
Association of lipid biomarkers with baseline CAC stratified by age.
| Age < 62 years (n=2,872) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Agatston Score | Volume Score | Density Score | |||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Lp(a) | 1.08 | 0.98, 1.18 | 0.103 | 1.09 | 0.99, 1.19 | 0.073 | 1.13 | 0.97, 1.32 | 0.121 |
| Lp(a) with adjustment for HDL-C and TG | 1.09 | 0.99, 1.20 | 0.065 | 1.10 | 1.00, 1.20 | 0.043 | 1.13 | 0.96, 1.32 | 0.138 |
| LDL-C | 1.26 | 1.15, 1.38 | <0.001 | 1.27 | 1.16, 1.39 | <0.001 | 0.91 | 0.78, 1.06 | 0.220 |
| LDL-C with adjustment for HDL-C and TG | 1.25 | 1.15, 1.37 | <0.001 | 1.27 | 1.16, 1.39 | <0.001 | 0.91 | 0.78, 1.06 | 0.219 |
| HDL-C | 0.86 | 0.78, 0.94 | 0.002 | 0.85 | 0.77, 0.93 | <0.001 | 1.19 | 1.02, 1.39 | 0.028 |
| TG | 1.07 | 0.98, 1.17 | 0.142 | 1.08 | 0.99, 1.18 | 0.100 | 0.89 | 0.76, 1.05 | 0.173 |
| Total cholesterol | 1.22 | 1.12, 1.34 | <0.001 | 1.23 | 1.13, 1.35 | <0.001 | 0.93 | 0.80, 1.08 | 0.328 |
| apoB | 1.23 | 1.11, 1.36 | <0.001 | 1.24 | 1.12, 1.37 | <0.001 | 0.86 | 0.71, 1.03 | 0.091 |
| Non-HDL-C | 1.28 | 1.17, 1.40 | <0.001 | 1.30 | 1.18, 1.42 | <0.001 | 0.87 | 0.74, 1.02 | 0.083 |
| Age ≥ 62 years (n=2,725) | |||||||||
| Agatston Score | Volume Score | Density Score | |||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Lp(a) | 1.01 | 0.93, 1.10 | 0.792 | 1.01 | 0.94, 1.10 | 0.738 | 0.99 | 0.88, 1.11 | 0.842 |
| Lp(a) with adjustment for HDL-C and TG | 1.02 | 0.94, 1.10 | 0.655 | 1.02 | 0.94, 1.11 | 0.586 | 0.99 | 0.88, 1.11 | 0.863 |
| LDL-C | 1.25 | 1.16, 1.35 | <0.001 | 1.25 | 1.16, 1.35 | <0.001 | 0.90 | 0.80, 1.00 | 0.060 |
| LDL-C with adjustment for HDL-C and TG | 1.24 | 1.15, 1.34 | <0.001 | 1.23 | 1.14, 1.33 | <0.001 | 0.89 | 0.80, 1.00 | 0.053 |
| HDL-C | 0.84 | 0.78, 0.91 | <0.001 | 0.83 | 0.76, 0.90 | <0.001 | 0.95 | 0.84, 1.06 | 0.354 |
| TG | 1.14 | 1.05, 1.23 | <0.001 | 1.16 | 1.07, 1.25 | <0.001 | 1.03 | 0.92, 1.16 | 0.579 |
| Total cholesterol | 1.21 | 1.12, 1.31 | <0.001 | 1.21 | 1.12, 1.30 | <0.001 | 0.88 | 0.79, 0.99 | 0.036 |
| apoB | 1.25 | 1.14, 1.37 | <0.001 | 1.26 | 1.16, 1.39 | <0.001 | 0.95 | 0.84, 1.06 | 0.348 |
| Non-HDL-C | 1.28 | 1.19, 1.38 | <0.001 | 1.29 | 1.19, 1.39 | <0.001 | 0.91 | 0.81, 1.02 | 0.111 |
All lipid biomarkers were ln-transformed. OR are presented per standard deviation. Models included individual biomarkers + CVD risk factors (age, sex, race/ethnicity, SBP, DM, smoking, hypertension treatment); For Lp(a) and LDL-C, models with additional adjustment for HDL-C and TG are shown. Models for density are adjusted for volume. Abbreviations: ApoB, apolipoprotein B; CAC, coronary artery calcium; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); OR, odds ratio; TG, triglycerides.
Lp(a) was associated with long-term CAC volume progression in younger participants (OR per SD 1.16, 95% CI 1.04–1.30). Of the other biomarkers evaluated, LDL-C, TG, total cholesterol, apoB and non-HDL-C were all positively associated with CAC volume progression in younger participants, while HDL-C was inversely associated; the strongest positive association was observed with non-HDL-C (OR 1.51, 95% CI 1.36–1.68). None of the biomarkers were associated with CAC density progression in younger individuals. In older participants (age ≥ 62 years), Lp(a) was not associated with CAC volume progression. LDL-C, TG, total cholesterol, apoB and non-HDL-C were all positively associated with CAC volume progression in older participants, while HDL-C was inversely associated; the strongest positive association was observed with non-HDL-C (OR 1.36, 95% CI 1.21–1.55). None of the biomarkers were associated with CAC density progression in older individuals. In general, the strength of associations was greater in younger participants than in older participants (Table S4). When short-term follow-up was evaluated, Lp(a) was not associated with CAC volume progression but was inversely associated with density progression in younger individuals (OR 0.85, 95% CI 0.73–0.98).
Baseline CAC and progression of CAC stratified by race/ethnicity
There was not a significant interaction between Lp(a) and race/ethnicity for baseline volume (p=0.944), baseline density (0.426), volume progression (p=0.322) or density progression (0.253). In general, results were consistent across racial/ethnic groups as Lp(a) was not associated with baseline CAC volume or density across groups. Similarly, Lp(a) was positively associated with volume progression across groups but was only statistically significant in Hispanic individuals (OR per SD 1.26, 95% CI 1.07–1.48). Similarly, Lp(a) was not significantly associated with density progression except in Hispanic individuals (OR per SD 0.71, 95% CI 0.52–0.95, Fig. S1).
Discussion
Individuals with elevated Lp(a) are at significantly increased risk for several cardiovascular diseases. CAC scoring is used clinically to evaluate and individualize overall ASCVD risk. In our study, however, Lp(a) was not associated with baseline CAC volume or density and was weakly associated with CAC volume progression. In younger individuals, Lp(a) was only modestly associated with baseline CAC volume and CAC volume progression, and was the only biomarker associated (inversely) with density progression (over short-term follow-up). This is in contrast to other lipid biomarkers which were more strongly associated with CAC. Our findings suggest that Lp(a) is not as robustly associated with CAC as other lipid biomarkers.
Given that Lp(a) is strongly associated with ASCVD risk, it may be surprising that it is, at best, modestly associated with CAC, which represents subclinical atherosclerosis and is also strongly associated with ASCVD risk. A recent study utilizing coronary computed tomography angiography (CCTA) may help explain these results. In this study of 191 individuals, those with high Lp(a) ≥70 mg/dL had accelerated progression of low attenuation plaque volume (density <30 HU), which is a marker for necrotic core and a powerful predictor of future events.20 In another study evaluating young patients with acute coronary syndrome (ACS) who had higher levels of Lp(a) than age-matched healthy controls, individuals with higher levels of Lp(a) had decreased fibrous cap thickness on optical coherence tomography (OCT) imaging compared to those with lower Lp(a) levels.21 Thus, it is possible that Lp(a) is more associated with non-calcified or low attenuation plaque, which is associated with increased risk but not captured by CAC scoring. This is reflected by the inverse association between Lp(a) and CAC density progression in younger individuals in our study.
Indeed, these results align with another recent study involving data from MESA and the Dallas Heart Study (DHS). In asymptomatic individuals, elevated Lp(a) and CAC were independently associated with ASCVD risk without a significant interaction between the two. Additionally, when elevated Lp(a) and CAC were present, they appeared to be additive for risk.22 Together, these findings suggest that CAC may not be sufficient to risk stratify individuals with elevated Lp(a), as Lp(a) was independently associated with risk and is associated with low-attenuation plaque, as noted above. Furthermore, Lp(a) has additional properties associated with atherosclerotic risk, which may not be captured by CAC, including the pro-thrombotic and pro-inflammatory properties associated with apo(a) and oxidized phospholipids (OxPL).23 In a recent study, high Lp(a) was found to be associated with various high-risk characteristics on CCTA, including not only higher total plaque volume but also inflammatory attributes reflected by a higher fat attenuation index. Causal mediation analyses also showed that approximately 40% of the prognostic effect of Lp(a) was mediated by high-risk attributes such as high-risk plaque, high-risk inflammatory attributes, and high-risk physiological attributes.24
Lp(a) is a risk factor for premature ASCVD. We observed a borderline statistically significant interaction between Lp(a) and age for CAC density but not volume. This may be due to density representing plaque age / stability (i.e. older participants with elevated Lp(a) may be more likely to have denser plaque than younger participants). Thus, the age-stratified results are particularly relevant given the potential selection bias as individuals who were older with elevated Lp(a) at the time of MESA recruitment may have been selected out due to already having an ASCVD event. We observed that Lp(a) was associated with baseline CAC volume and volume progression in younger individuals but not older individuals. Again, this association was modest, particularly when compared to other lipid biomarkers. Interestingly, Lp(a) was also inversely associated with density progression in younger individuals over short-term follow-up. This aligns with the previously discussed propensity for Lp(a) to develop less dense plaque and with higher density being inversely associated with ASCVD risk.
Prior studies have observed an association between Lp(a) and CAC, and many have methodologic differences from the present study. Most of these studies used the Agatston score8–10, 12, 13, 25–28 and the associations were generally modest. These studies were also performed in varied populations including individuals with chest pain,9 randomly selected individuals,25 specific racial/ethnic groups,10, 26 individuals with familial hypercholesterolemia,8 or those with family history of ASCVD.27 One study utilized single-nucleotide polymorphisms of the LPA gene rather than Lp(a) levels.28 Additionally, some of these studies did not include extensive multivariable adjustment.9, 10, 26 A recent study from the atherosclerosis risk in communities study (ARIC) demonstrated a stronger association between Lp(a) > 50 mg/dL and CAC14; there are several potential reasons for the differences from our study – in MESA, Lp(a) testing was performed on baseline blood samples during the same time period as baseline CAC scoring and repeat CAC scoring was performed at subsequent exams. In the ARIC study, Lp(a) was measured at visit 4 and CAC scoring was performed at visit 7, participants were significantly older in ARIC when undergoing CAC scoring with a much higher prevalence of CAC, and a large number of participants did not undergo CAC scoring. Conversely, other studies have failed to show an association between Lp(a) and prevalent CAC.28, 29 A study from MESA observed that elevated Lp(a) was not associated with prevalent CAC but was associated with more rapid CAC progression, again using the Agatston score.11 In this study, progression was only assessed among those with a change in CAC score, while our study included those with no change in CAC. Another study in MESA demonstrated that elevated Lp(a) was associated with modest progression in CAC volume but not density.15 This study, however, did not report the association with baseline measures of CAC, and used different statistical methods. Our study focused on individuals free of known ASCVD, for whom CAC scoring is most appropriate, and utilized CAC volume and density which are stronger predictors of risk than the Agatston score. Additionally, our study provides context for our findings by contrasting the negative or modest associations between Lp(a) with CAC to the robust associations seen with other lipid biomarkers. Our study also stratified by age as Lp(a) is a risk factor for premature ASCVD.
Our study has limitations. As previously mentioned, our results may be subject to selection bias. Given that individuals with elevated Lp(a) are at risk for premature ASCVD and may have events at a younger age, such individuals may not have been eligible for recruitment in MESA. We attempted to address this potential bias by performing analyses stratified by age. Our study utilized a peak density factor based on the available data in MESA. However, use of a continuous assessment of mean density may be more predictive.30 Additionally, though CAC volume and density provide better risk assessment than the Agatston score, the Agatston score is the current clinical standard. However, results for CAC volume were generally consistent with results for CAC Agatston and may be similarly applied. Additionally, Lp(a) has a more skewed distribution than other lipid biomarkers, which may have impacted our ability to detect significant associations. Furthermore, as an observational study, there is the potential for residual confounding.
Conclusions
In this study of individuals free of clinical ASCVD, Lp(a) was not associated with baseline CAC volume or density and was modestly associated with CAC volume progression. In younger individuals, Lp(a) was associated with baseline CAC volume and volume progression, but this association was much more modest compared with other lipid biomarkers, and Lp(a) was the only biomarker inversely associated with CAC density progression (over the short-term). Our findings suggest that Lp(a) is not strongly associated with CAC, particularly when compared to other lipid biomarkers. Further studies are needed to identify the most effective ways to risk stratify this population and to guide the use of preventive therapies.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
HB was supported by National Institutes of Health, Grants 1KL2TR001444 and 5T32HL079891. ST is supported by NIH R01 HL159156. The MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declarations of Competing Interest: HB received consulting fees from Kaneka Medical America. ST is a co-inventor and receives royalties from patents owned by UCSD and is a co-founder and has an equity interest in Oxitope and Kleanthi Diagnostics, and has a dual appointment at UCSD and Ionis Pharmaceuticals. Although these relationships have been identified for conflict-of-interest management based on the overall scope of the project, the research findings included in this particular publication may not necessarily relate to the interests of the above companies. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. The other co-authors have nothing to disclose.
Use of AI and AI-assisted Technologies Statement
AI or AI-assisted technologies were not used in the writing process of this manuscript
Ethical Statement
Participants in the MESA provided written informed consent at the time of recruitment, and the study was approved by the institutional review boards of each field center.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.jacl.2023.06.002.
References
- 1.Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein(a). J Lipid Res. 2016;57(8):1339–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia HS, Wilkinson MJ. Lipoprotein(a): evidence for role as a causal risk factor in cardiovascular disease and emerging therapies. J Clin Med. 2022;11(20):6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. Jun 25 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia HS, Wilkinson MJ. Lipoprotein(a): evidence for role as a causal risk factor in cardiovascular disease and emerging therapies. J Clin Med. Oct 13 2022;11(20). doi: 10.3390/jcm11206040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J.. 2018;39(25):2401–2408 07 01. doi: 10.1093/eurheartj/ehy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung YH, Lee BK, Kwon HM, et al. Coronary calcification is associated with elevated serum lipoprotein (a) levels in asymptomatic men over the age of 45 years: A cross-sectional study of the Korean national health checkup data. Med (Baltimore).. Mar 5 2021;100(9):e24962. doi: 10.1097/MD.0000000000024962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verweij SL, de Ronde MWJ, Verbeek R, et al. Elevated lipoprotein(a) levels are associated with coronary artery calcium scores in asymptomatic individuals with a family history of premature atherosclerotic cardiovascular disease. J Clin Lipidol. 2018;12(3):597–603 2018 May-June 1. doi: 10.1016/j.jacl.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Alonso R, Mata P, Muniz O, et al. PCSK9 and lipoprotein (a) levels are two predictors of coronary artery calcification in asymptomatic patients with familial hypercholesterolemia. Atherosclerosis. Nov 2016;254:249–253. doi: 10.1016/j.atherosclerosis.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Greif M, Arnoldt T, von Ziegler F, et al. Lipoprotein (a) is independently correlated with coronary artery calcification. Eur J Intern Med. Jan 2013;24(1):75–79. doi: 10.1016/j.ejim.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Kasim M, Joshi PH, et al. Abnormal lipoprotein(a) levels predict coronary artery calcification in Southeast Asians but not in Caucasians: use of noninvasive imaging for evaluation of an emerging risk factor. J Cardiovasc Transl Res. Aug 2011;4(4):470–476. doi: 10.1007/s12265-011-9273-3. [DOI] [PubMed] [Google Scholar]
- 11.Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. Mar–Apr 2021;15(2):154–160. doi: 10.1016/j.jcct.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho JH, Lee DY, Lee ES, et al. Increased risk of coronary artery calcification progression in subjects with high baseline Lp(a) levels: The Kangbuk Samsung Health Study. Int J Cardiol. Nov 1 2016;222:233–237. doi: 10.1016/j.ijcard.2016.07.219. [DOI] [PubMed] [Google Scholar]
- 13.Ida J, Kotani K, Miyoshi T, et al. High baseline lipoprotein(a) level as a risk factor for coronary artery calcification progression: sub-analysis of a prospective multicenter trial. Acta Med Okayama. Jun 2018;72(3):223–230. doi: 10.18926/amo/56067. [DOI] [PubMed] [Google Scholar]
- 14.Obisesan OH, Kou M, Wang FM, et al. Lipoprotein(a) and subclinical vascular and valvular calcification on cardiac computed tomography: the atherosclerosis risk in communities study. J Am Heart Assoc. 2022;11(11):e024870. doi: 10.1161/JAHA.121.024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong KL, McClelland RL, Allison MA, et al. Lipoprotein (a) and coronary artery calcification: prospective study assessing interactions with other risk factors. Metabolism.. Mar 2021;116:154706. doi: 10.1016/j.metabol.2021.154706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Criqui MH, Knox JB, Denenberg JO, et al. Coronary artery calcium volume and density: potential interactions and overall predictive value: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. Aug 2017;10(8):845–854. doi: 10.1016/j.jcmg.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. Nov 01 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. Mar 15 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. Jan 15 2014;311(3):271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser Y, Daghem M, Tzolos E, et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol. 2022;79(3):223–233. doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra S, Nagar S, Shukla A, et al. Correlation of lipoprotein (a) levels and plaque morphology in very young acute coronary syndrome patients using optical coherence tomography. India Heart J. 2022;74(5):357–362 Sep–Oct 2022. doi: 10.1016/j.ihj.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta A, Vasquez N, Ayers CR, et al. Independent Association of Lipoprotein(a) and coronary artery calcification with atherosclerotic cardiovascular risk. J Am Coll Cardiol. 2022;79(8):757–768 03 01. doi: 10.1016/j.jacc.2021.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsimikas S A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. Feb 14 2017;69(6):692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Dai N, Chen Z, Zhou F, et al. Association of lipoprotein (a) with coronary-computed tomography angiography-assessed high-risk coronary disease attributes and cardiovascular outcomes. Circ Cardiovasc Imaging.. Dec 2022;15(12):e014611. doi: 10.1161/CIRCIMAGING.122.014611. [DOI] [PubMed] [Google Scholar]
- 25.Erbel R, Lehmann N, Churzidse S, et al. Gender-specific association of coronary artery calcium and lipoprotein parameters: the Heinz Nixdorf Recall Study. Atherosclerosis. Aug 2013;229(2):531–540. doi: 10.1016/j.atherosclerosis.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Guo K, Chen M, Bao J, Shen C, Li Y. Serum lipoprotein(a) positively correlates with coronary artery calcification in low-risk chinese han patients: a study from a single center. PLoS One. 2013;8(8):e71673. doi: 10.1371/journal.pone.0071673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verweij SL, de Ronde MWJ, Verbeek R, et al. Elevated lipoprotein(a) levels are associated with coronary artery calcium scores in asymptomatic individuals with a family history of premature atherosclerotic cardiovascular disease. J Clin Lipidol. 2018;12(3):597–603 May – June 1. doi: 10.1016/j.jacl.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Pechlivanis S, Mahabadi AA, Hoffmann P, et al. Association between lipoprotein(a) (Lp(a)) levels and Lp(a) genetic variants with coronary artery calcification. BMC Med Genet. Mar 27 2020;21(1):62. doi: 10.1186/s12881-020-01003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huffman MD, Kandula NR, Baldridge AS, Tsai MY, Prabhakaran D, Kanaya AM. Evaluating the potential association between lipoprotein(a) and atherosclerosis (from the mediators of atherosclerosis among South Asians living in America cohort). Am J Cardiol. Mar 15 2019;123(6):919–921. doi: 10.1016/j.amjcard.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzaye O, Razavi AC, Dardari ZA, et al. Mean versus peak coronary calcium density on non-contrast CT: calcium scoring and ASCVD risk prediction. JACC Cardiovasc Imaging. Nov 6 2021;15(3):489–500. doi: 10.1016/j.jcmg.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


