Abstract
Background and Objectives
Identifying optimal methods for evaluation and monitoring of cognitive outcomes in AE is important for clinical care and research. This scoping review aimed to evaluate neuropsychological tests (NPT) that are most frequently impaired in AE cohorts to provide recommendations for a standardized NPT battery for AE outcome.
Methods
PubMed search for studies examining NPT in patients with AE was conducted on June 9, 2023. Studies were screened for inclusion/exclusion criteria as follows: at least 1 NPT, individual NPT test scores with comparison with healthy controls or normative data and neural-IgG status, total sample size ≥5, and English manuscript available.
Results
The search yielded 5,393 studies, of which 3,359 were screened, 107 were full text reviewed, and 32 met inclusion/exclusion criteria, anti-NMDA-R (k = 18), anti-LGI1 (k = 10), anti-GABAB-R (k = 2), anti-GAD-65 (k = 4), and anti-CASPR2 (k = 3). The cognitive domains most frequently impaired were visual and verbal episodic memory, attention/working memory, processing speed, and aspects of executive functions.
Discussion
Given the dearth of literature examining NPT in AE in combination with small sample sizes and methodological differences, more research in this area is needed. However, we provide recommendations for a test battery to be used in future studies, with the aim of standardizing research in this area. Based on the available literature, we recommend the use of comprehensive NPT batteries, spanning all cognitive domains. The highest yield measures may include the tests of (1) visual and verbal learning/memory, (2) basic and sustained attention, (3) processing speed, and (4) executive functions.
Introduction
Understanding of how to measure longitudinal outcomes in patients with autoimmune encephalitis (AE) is limited. Although immunosuppressive therapy is effective in ameliorating most acute symptoms, many patients still experience long-term cognitive, psychological, and functional impairments.1 Cognitive impairment (CI) is particularly pervasive and is not captured by gross outcome measures, such as the modified Rankin Scale.2-4 Objective definition of CI is important, as it may aid in assessment of therapeutic response, development of appropriate rehabilitation strategies, and standardization of reporting in observational and randomized controlled trials. Cognitive screening tools (e.g., Mini-Mental State Examination,5 Montreal Cognitive Assessment,6 Repeatable Battery for the Assessment of Neuropsychological Status7) have been used in observational studies and allow for rapid screening. However, these measures lack the sensitivity and specificity of formal neuropsychological testing and may be insensitive to CI in AE because they were initially developed to detect mild cognitive impairment and dementia in older adults.8,9
Several neuropsychological tests (NPT) have been used to measure cognitive outcomes in AE, but there is no consensus on an optimal test battery. Although some reviews have examined NPT in AE,10-12 none have specifically focused on test-level outcomes. The aim of this scoping review was to determine the most impaired NPTs in patients with AE and provide recommendations for a standardized NPT battery for measuring AE outcomes.
Methods
Overview
A scoping review of NPT in patients with neural antibody (-IgG) seropositive AE was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews guidelines. A scoping review was chosen due to the dearth of literature on NPT in AE, the rarity of the disease, and the specific aim of describing the available evidence on NPT in adults with AE to identify current knowledge gaps and aid in developing recommendations for an optimal test battery.13
Search Strategy
Studies were identified by the literature search of PubMed, conducted on June 9, 2023. See supplement for search terms (eAppendix 1, links.lww.com/NXI/A924). We additionally examined the reference lists of included studies and 3 recent reviews/meta-analyses examining cognitive outcomes in AE.10-12
Study Selection, Inclusion/Exclusion Criteria, and Participants
Inclusion criteria were (1) standardized administration of ≥1 NPT, (2) neural-IgG status reported, (3) NPT completed after the acute period (≥1 month postsymptom onset), and (4) English language manuscript available.
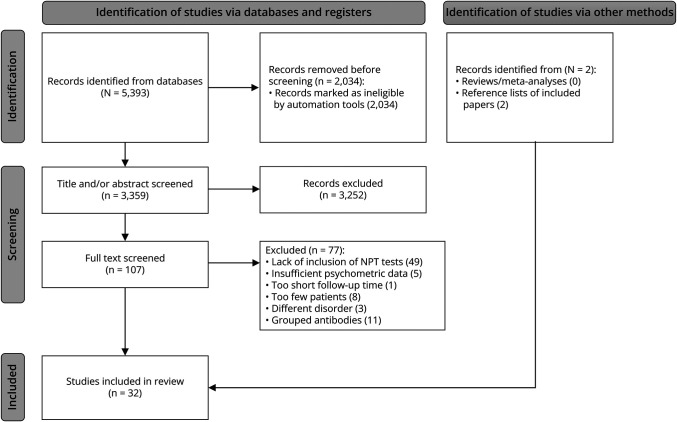
Exclusion criteria were (1) composite scores (comprised the results from multiple tests) only, (2) cognitive screening than 5 patients). Studies examining patients with VGKC-IgG were excluded if they did not report LGI1-IgGs and/or CASPR2-IgGs. See Figure 1 for details regarding study identification, screening, and inclusion.
Figure 1. PRISMA Flow Diagram of Studies Retrieved for the Review.
Extraction of Data
Studies were screened by 1 of 4 independent reviewers (R.G., T.G., J.R.A., C.S.). Studies identified for inclusion were then independently reviewed by a second reviewer (T.G. or R.G.). Data were extracted in a standardized manner, including neural-IgG, follow-up time, sample size, NPT administered, and comparative analyses to normative data and/or controls. Academic, screening, and/or experimental measures were not reported. Scores comprised multiple tests were also not reported, unless the tests included in the composite were highly similar (e.g., Trail Making Test, Delis-Kaplan Executive Function System Trail Making Test). Summary scores, which reflected multiple indices from the same test (e.g., immediate and delayed recall scores from the same list learning test), were included.
Data Synthesis and Analysis
When multiple studies reported data from overlapping patient cohorts, the study with the largest sample size was included in the analysis. When studies reported outcomes at multiple time points, the distal time point to disease onset was reported.
Study results included comparisons of performance with healthy controls and/or normative data. When comparing with controls, our threshold for significance was p < 0.05. When comparing with normative data, impairment was most often defined as at least 1.5 standard deviations below the mean (z ≤ −1.5). In studies using a different definition of impairment, we calculated the rates of impairment at z < −1.5, if possible. Otherwise, the alternative impairment definition was reported. For the 2 studies that reported equivalence and/or weighted scores (representing “significantly below average performance”), we used the authors' definition.14,15 For synthesis, significant impairment and/or difference to controls was defined as ≥25% impairment and/or significant difference relative to controls (p < 0.05).
Data Availability
Data not published within this article will be made available by request from any qualified investigator.
Results
Thirty-two studies were included in this review and examined these neural-IgG AE cohorts: anti-NMDA-R (k = 18), anti-LGI1(k = 10), anti-CASPR2 (k = 2), anti-GABAB-R (k = 2), and anti-GAD-65 (k = 4). See eTable 1 (links.lww.com/NXI/A924) for details on included studies.
Anti-LGI1 AE
Verbal Memory
List learning tasks were the most used verbal memory measures. All studies comparing with controls found worse performance among patients,3,4,16 and normative impairment rates were relatively high for immediate (19%–30%) and delayed (30%–50%) recall. The only other verbal memory measure used was a story task, used in 1 study, which showed worse performance in patients compared with controls on delayed recall only (30% impairment).3
Visual Memory
Visual memory was assessed in most studies, all of which showed impairment in at least one aspect of visual memory testing. However, there was little overlap in the specific measures used. Two of 3 cohorts (using 3 different tasks) showed either worse performance relative to controls or ≥25% normative impairment in immediate recall, whereas 1 of the 3 showed worse delayed free recall.3,4,17 Two studies using a different figure recall task used a summary score (immediate and delayed recall combined) with impairment ranging from 63% to 75%.18,19 Finally, one study using the Cambridge Neuropsychological Test Automated Battery (CANTAB) showed worse spatial recognition compared with normative data but no differences in other visual memory measures.20
Attention/Working Memory
Simple auditory attention span (short-term memory)/working memory was most often assessed using a digit span task, with 2/3 studies showing worse performance (total, forward subtest) among patients compared with controls.3,4 Impairment rates were variable (5 total, 10% backward, 40% forward).3,17 Lower vigilance on a sustained attention task was found in patients compared with controls (43% impairment) in 1 study.3 Reaction time and divided attention were no different from controls and/or normative data in 1 study each.4,20
Processing Speed
Processing speed was most often examined using a numeric sequencing task (e.g., Trails A), with worse performance compared with controls in 2/3 studies and impairment ranging from 13-20%.3,16,17,21,22 One study used a coding measure (e.g., Symbol-Digit Modalities Test) showing impairment in 30% of patients but no difference compared with controls.3
Language
Semantic fluency was the most used language measure, with worse performance in patients relative to controls found in 2/3 studies4,16 and 11%–53% impairment relative to normative data in 3 studies.3,16,17 Phonemic fluency was worse in patients relative to controls in 1 of 2 studies and impairment ranged from 3% to 30% in 2 studies.3,17,21 Naming was examined in 3 studies but was not significantly different compared with controls or normative data. The rates of impairment relative to normative data ranged from 3% to 20%.3,17,20
Visuospatial
The Rey Complex Figure Task was the most used visuospatial measure, although with variable results. Normative impairment ranged from 11% to 75%, whereas worse performance relative to controls found in 1/2 studies.2,3,17,22 Angle estimation did not differ from controls in 1 study (10% normative impairment).3
Executive Functions
The most used executive function test was an alphanumeric switching task (e.g., Trails B) with worse performance compared with controls in 2/3 studies and 10%–31% normative impairment in 2 studies.3,16,17,21 Stroop inhibition did not differ between patients and controls in one study, while another showed only 6% normative impairment.17,21 One study found more errors compared with controls on a different inhibition task (Go/No-Go).4 No differences were found in set-shifting or reasoning, examined in 1 study each.3,20 Finally, although no differences were found compared with controls on a problem-solving task in 1 study, 44% of patients were impaired relative to normative data.3
Anti-LGI1 AE Summary
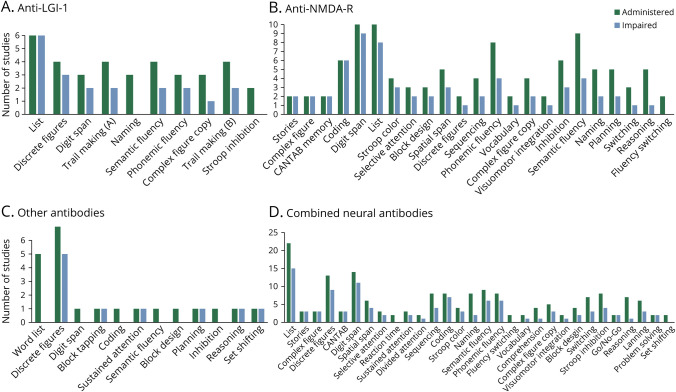
List learning, visual memory (variability in specific measures), trail making, and verbal fluency tasks were the most administered NPTs in patients with anti-LGI1 AE. Impairments were most common in verbal memory (100%), visual memory (80%), digit span (67%), and phonemic fluency (67%), followed by sequencing, semantic fluency, and reaction time measures (50% each).
Impairments were least common in complex figure copy (33%) and naming and Stroop inhibition (0% each). Other tests were only used in 1 study each, limiting conclusions about them (Figure 2A, Table 1, eTable 2, links.lww.com/NXI/A924).
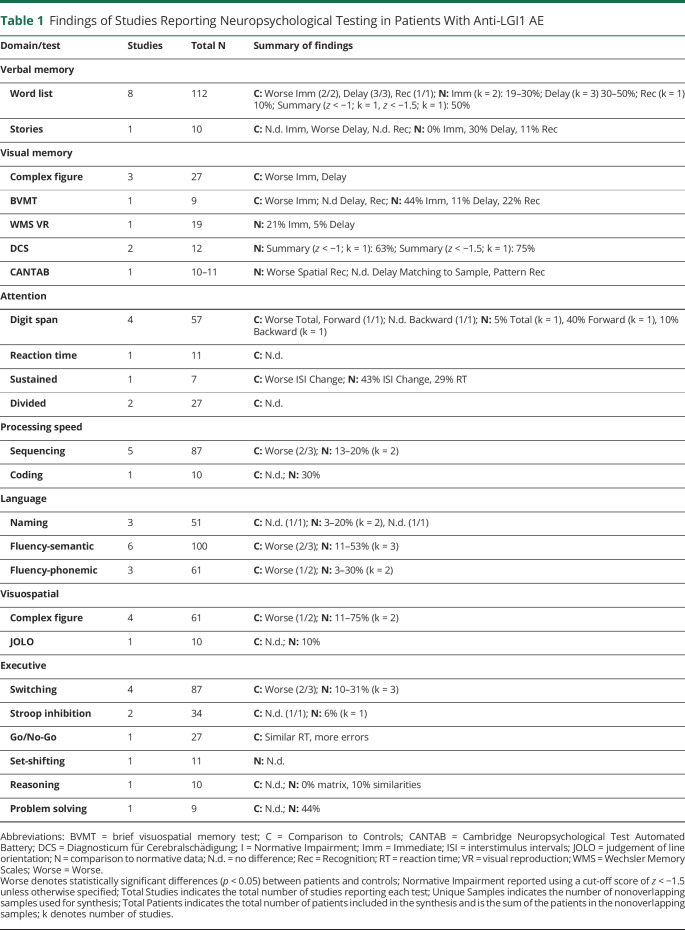
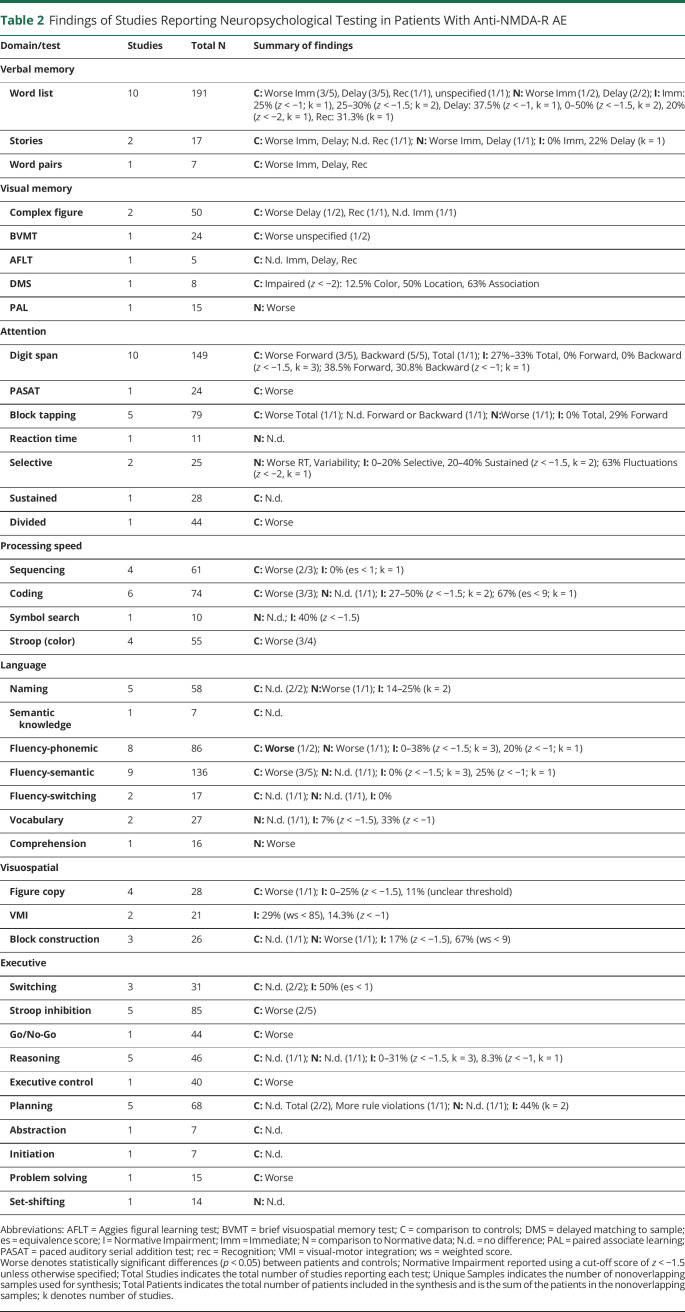
Figure 2. Total Number of Studies Which Administered (Dark Gray) and Found Impairment in (Light Gray) Each Test in Samples of Anti-LGI-1 AE (A), Anti-NMDA-R AE (B), and AE Anti-CASPR2, Anti-GABAB-R, Anti-GAD65 AE (C) Patients and Results Pooled Across Samples (D).
Neuropsychological tests only included in 1 study were removed from figures.
Table 1.
Findings of Studies Reporting Neuropsychological Testing in Patients With Anti-LGI1 AE
| Domain/test | Studies | Total N | Summary of findings |
| Verbal memory | |||
| Word list | 8 | 112 | C: Worse Imm (2/2), Delay (3/3), Rec (1/1); N: Imm (k = 2): 19–30%; Delay (k = 3) 30–50%; Rec (k = 1) 10%; Summary (z < −1; k = 1, z < −1.5; k = 1): 50% |
| Stories | 1 | 10 | C: N.d. Imm, Worse Delay, N.d. Rec; N: 0% Imm, 30% Delay, 11% Rec |
| Visual memory | |||
| Complex figure | 3 | 27 | C: Worse Imm, Delay |
| BVMT | 1 | 9 | C: Worse Imm; N.d Delay, Rec; N: 44% Imm, 11% Delay, 22% Rec |
| WMS VR | 1 | 19 | N: 21% Imm, 5% Delay |
| DCS | 2 | 12 | N: Summary (z < −1; k = 1): 63%; Summary (z < −1.5; k = 1): 75% |
| CANTAB | 1 | 10–11 | N: Worse Spatial Rec; N.d. Delay Matching to Sample, Pattern Rec |
| Attention | |||
| Digit span | 4 | 57 | C: Worse Total, Forward (1/1); N.d. Backward (1/1); N: 5% Total (k = 1), 40% Forward (k = 1), 10% Backward (k = 1) |
| Reaction time | 1 | 11 | C: N.d. |
| Sustained | 1 | 7 | C: Worse ISI Change; N: 43% ISI Change, 29% RT |
| Divided | 2 | 27 | C: N.d. |
| Processing speed | |||
| Sequencing | 5 | 87 | C: Worse (2/3); N: 13–20% (k = 2) |
| Coding | 1 | 10 | C: N.d.; N: 30% |
| Language | |||
| Naming | 3 | 51 | C: N.d. (1/1); N: 3–20% (k = 2), N.d. (1/1) |
| Fluency-semantic | 6 | 100 | C: Worse (2/3); N: 11–53% (k = 3) |
| Fluency-phonemic | 3 | 61 | C: Worse (1/2); N: 3–30% (k = 2) |
| Visuospatial | |||
| Complex figure | 4 | 61 | C: Worse (1/2); N: 11–75% (k = 2) |
| JOLO | 1 | 10 | C: N.d.; N: 10% |
| Executive | |||
| Switching | 4 | 87 | C: Worse (2/3); N: 10–31% (k = 3) |
| Stroop inhibition | 2 | 34 | C: N.d. (1/1); N: 6% (k = 1) |
| Go/No-Go | 1 | 27 | C: Similar RT, more errors |
| Set-shifting | 1 | 11 | N: N.d. |
| Reasoning | 1 | 10 | C: N.d.; N: 0% matrix, 10% similarities |
| Problem solving | 1 | 9 | C: N.d.; N: 44% |
Abbreviations: BVMT = brief visuospatial memory test; C = Comparison to Controls; CANTAB = Cambridge Neuropsychological Test Automated Battery; DCS = Diagnosticum für Cerebralschädigung; I = Normative Impairment; Imm = Immediate; ISI = interstimulus intervals; JOLO = judgement of line orientation; N = comparison to normative data; N.d. = no difference; Rec = Recognition; RT = reaction time; VR = visual reproduction; WMS = Wechsler Memory Scales; Worse = Worse.
Worse denotes statistically significant differences (p < 0.05) between patients and controls; Normative Impairment reported using a cut-off score of z < −1.5 unless otherwise specified; Total Studies indicates the total number of studies reporting each test; Unique Samples indicates the number of nonoverlapping samples used for synthesis; Total Patients indicates the total number of patients included in the synthesis and is the sum of the patients in the nonoverlapping samples; k denotes number of studies.
Anti-NMDA-R AE
Verbal Memory
List learning tasks were the most used verbal memory measures, with worse performance in patients compared with controls and/or normative data or ≥25% normative impairment in 7/10 for immediate and delayed recall each and 2/2 for recognition.15,23-31 Story and verbal pair recall tasks were used in 2 and 1 study each, all of which showed worse performance than controls or normative data in immediate and delayed recall.31,32
Visual Memory
Visual memory was assessed in most studies, although with little overlap in the specific measures used and some variability. Complex or simple figure recall tasks were used in 5 studies, of which one found worse delayed recall23 and one found worse recognition32 compared with controls, whereas one found worse performance compared with controls but did not specify the variable of interest.27 Two studies used measures from the CANTAB with one showing 12%–63% impairment on a delayed matching to sample paradigm compared with controls2 and another found worse performance on a paired associates learning task compared with normative data.29
Attention/Working Memory
Simple auditory attention span (short-term memory)/working memory was most often assessed using a digit span task, of which 3/5 demonstrated worse forward, 5/5 showed worse backward, and 1/1 showed worse total scores in patients relative to controls,24-26,28,32-34 while impairment rates varied from 0-38.5%.14,15,30,31 Paced Auditory Serial Addition Test (PASAT) performance was worse among patients relative to controls in 1 study.27
Visual attention/working memory was most often assessed using a block tapping (or similar) test, with variable results (1/3 worse compared with controls or normative data, 0%–29% impairment).2,14,15,28,29,34 Divided attention was worse in patients compared with controls in 1 study.23 There were no differences in simple reaction time or sustained attention on a continuous performance task compared with normative or control data, respectively, in 1 study each.28,29 Selective attention was examined in 3 samples, with mixed results (0%–63% impairment across indices).14,15,29
Processing Speed
Processing speed was most often examined using a coding task, with 3/4 studies showing worse performance in patients compared with controls or normative data and impairment rates ranging from 27-67%. Numeric sequencing and speeded color naming (i.e., Stroop) tasks were showed worse patient performance relative to controls in 2/3 and 3/4 of studies, respectively.24-26,28,32,35 Symbol search scores were not different between patients and normative data in 1 study, although 40% of the sample demonstrated impairment.31
Language
Semantic fluency was the most used language measure, with patients performing worse than controls or normative data in half the studies (0%–25% impairment).14,15,25,28,30-33,35 Phonemic fluency was also commonly examined, with worse performance compared with normative data or controls was observed in 2/3 studies (0%–38% impairment).2,14,15,29-32,35 Naming was assessed in 5 studies, with worse performance compared with controls or normative data in only 1 of 3 (14%–25% impairment).14,15,29,32,34 Less commonly, language was assessed using the tests of fluency switching (0% impairment),31,32 vocabulary (7%–33% impairment),30,31 and semantic knowledge,32 none of which showed differences between patients and controls or normative data. Comprehension was worse compared with normative data in 1 study29
Visuospatial
Complex figure copy was examined in 4 studies, with worse performance relative to controls in 1/1 studies and rates of impairment ranging from 0-25%.2,14,15,32 Block construction was examined in 3 studies, with variable results.14,25,31 One study found worse performance in patients compared with normative data, whereas another found no difference between patients and controls. Another study found 67% impairment, although their cutoff for impairment differed from other studies (weighted score <9, significantly below average). Two studies used a test of visual-motor integration in pediatric samples, with 14%–29% impairment.14,30
Executive Functions
Executive functions were examined using 10 different types of tasks, most commonly using the tests of inhibition (Stroop Color-Word),2,25,26,28,32,34,35 spatial planning (e.g., Tower),2,14,28,29,32 and reasoning (e.g., Ravens Colored Matrices)2,15,30-32 (5 studies each). Worse performance among patients, compared with controls, was found in 40% of studies using Stroop, 25% of studies using a Tower (or similar) task (24%–44% impairment), and 0% of studies using a reasoning task (0%–31% impairment). Switching was examined in 3 studies, 2 of which showed no difference in patient performance relative to controls.15,32,35
All other executive function tasks were examined in only 1 study each. Worse performance relative to controls was seen for tests of executive control,36 Go/No-Go,23 and problem solving,35 whereas no differences were observed on tests of set-shifting,29 verbal abstraction,32 or initiation32 relative to controls or normative data.
Anti-NMDA-R AE Summary
List learning/memory, visual memory (variability in specific measures), digit span, and verbal fluency tests were the most frequently administered NPTs in anti-NMDA-R AE patients. Impairments were most common in verbal memory (92%), visual memory (71%), attention/working memory (auditory—91%, visual—60%), and processing speed (80%), followed by block construction and selective attention (67% each), phonemic and/or semantic fluency (50% and 40% respectively), complex figure copy (50%), and response inhibition (57%). Impairments were less common on the tests of language, including naming, planning, switching, and reasoning (20%–40%). The other tests were only used in 1–2 studies each, making it difficult to draw conclusions (see Figure 2B, Table 2, eTable 3, links.lww.com/NXI/A924).
Table 2.
Findings of Studies Reporting Neuropsychological Testing in Patients With Anti-NMDA-R AE
| Domain/test | Studies | Total N | Summary of findings |
| Verbal memory | |||
| Word list | 10 | 191 | C: Worse Imm (3/5), Delay (3/5), Rec (1/1), unspecified (1/1); N: Worse Imm (1/2), Delay (2/2); I: Imm: 25% (z < −1; k = 1), 25–30% (z < −1.5; k = 2), Delay: 37.5% (z < −1, k = 1), 0–50% (z < −1.5, k = 2), 20% (z < −2, k = 1), Rec: 31.3% (k = 1) |
| Stories | 2 | 17 | C: Worse Imm, Delay; N.d. Rec (1/1); N: Worse Imm, Delay (1/1); I: 0% Imm, 22% Delay (k = 1) |
| Word pairs | 1 | 7 | C: Worse Imm, Delay, Rec |
| Visual memory | |||
| Complex figure | 2 | 50 | C: Worse Delay (1/2), Rec (1/1), N.d. Imm (1/1) |
| BVMT | 1 | 24 | C: Worse unspecified (1/2) |
| AFLT | 1 | 5 | C: N.d. Imm, Delay, Rec |
| DMS | 1 | 8 | C: Impaired (z < −2): 12.5% Color, 50% Location, 63% Association |
| PAL | 1 | 15 | N: Worse |
| Attention | |||
| Digit span | 10 | 149 | C: Worse Forward (3/5), Backward (5/5), Total (1/1); I: 27%–33% Total, 0% Forward, 0% Backward (z < −1.5, k = 3); 38.5% Forward, 30.8% Backward (z < −1; k = 1) |
| PASAT | 1 | 24 | C: Worse |
| Block tapping | 5 | 79 | C: Worse Total (1/1); N.d. Forward or Backward (1/1); N:Worse (1/1); I: 0% Total, 29% Forward |
| Reaction time | 1 | 11 | N: N.d. |
| Selective | 2 | 25 | N: Worse RT, Variability; I: 0–20% Selective, 20–40% Sustained (z < −1.5, k = 2); 63% Fluctuations (z < −2, k = 1) |
| Sustained | 1 | 28 | C: N.d. |
| Divided | 1 | 44 | C: Worse |
| Processing speed | |||
| Sequencing | 4 | 61 | C: Worse (2/3); I: 0% (es < 1; k = 1) |
| Coding | 6 | 74 | C: Worse (3/3); N: N.d. (1/1); I: 27–50% (z < −1.5; k = 2); 67% (es < 9; k = 1) |
| Symbol search | 1 | 10 | N: N.d.; I: 40% (z < −1.5) |
| Stroop (color) | 4 | 55 | C: Worse (3/4) |
| Language | |||
| Naming | 5 | 58 | C: N.d. (2/2); N:Worse (1/1); I: 14–25% (k = 2) |
| Semantic knowledge | 1 | 7 | C: N.d. |
| Fluency-phonemic | 8 | 86 | C: Worse (1/2); N: Worse (1/1); I: 0–38% (z < −1.5; k = 3), 20% (z < −1; k = 1) |
| Fluency-semantic | 9 | 136 | C: Worse (3/5); N: N.d. (1/1); I: 0% (z < −1.5; k = 3), 25% (z < −1; k = 1) |
| Fluency-switching | 2 | 17 | C: N.d. (1/1); N: N.d. (1/1), I: 0% |
| Vocabulary | 2 | 27 | N: N.d. (1/1), I: 7% (z < −1.5), 33% (z < −1) |
| Comprehension | 1 | 16 | N: Worse |
| Visuospatial | |||
| Figure copy | 4 | 28 | C: Worse (1/1); I: 0–25% (z < −1.5), 11% (unclear threshold) |
| VMI | 2 | 21 | I: 29% (ws < 85), 14.3% (z < −1) |
| Block construction | 3 | 26 | C: N.d. (1/1); N: Worse (1/1); I: 17% (z < −1.5), 67% (ws < 9) |
| Executive | |||
| Switching | 3 | 31 | C: N.d. (2/2); I: 50% (es < 1) |
| Stroop inhibition | 5 | 85 | C: Worse (2/5) |
| Go/No-Go | 1 | 44 | C: Worse |
| Reasoning | 5 | 46 | C: N.d. (1/1); N: N.d. (1/1); I: 0–31% (z < −1.5, k = 3), 8.3% (z < −1, k = 1) |
| Executive control | 1 | 40 | C: Worse |
| Planning | 5 | 68 | C: N.d. Total (2/2), More rule violations (1/1); N: N.d. (1/1); I: 44% (k = 2) |
| Abstraction | 1 | 7 | C: N.d. |
| Initiation | 1 | 7 | C: N.d. |
| Problem solving | 1 | 15 | C: Worse |
| Set-shifting | 1 | 14 | N: N.d. |
Abbreviations: AFLT = Aggies figural learning test; BVMT = brief visuospatial memory test; C = comparison to controls; DMS = delayed matching to sample; es = equivalence score; I = Normative Impairment; Imm = Immediate; N = comparison to Normative data; N.d. = no difference; PAL = paired associate learning; PASAT = paced auditory serial addition test; rec = Recognition; VMI = visual-motor integration; ws = weighted score.
Worse denotes statistically significant differences (p < 0.05) between patients and controls; Normative Impairment reported using a cut-off score of z < −1.5 unless otherwise specified; Total Studies indicates the total number of studies reporting each test; Unique Samples indicates the number of nonoverlapping samples used for synthesis; Total Patients indicates the total number of patients included in the synthesis and is the sum of the patients in the nonoverlapping samples; k denotes number of studies.
Anti-CASPR2, Anti-GABAB-R, and Anti-GAD-65 AE
Anti-CASPR2 AE
NPT was examined in only 2 samples of patients with anti-CASPR2 AE, involved samples of only 3 and 5 patients, and was limited to visual and verbal memory summary scores.18,19 Two of the 3 patients demonstrated impairment (z < −1) on a visual memory test involving a series of figures, whereas none were impaired on a list learning task.18 In another study, no patients demonstrated impairment on either of these measures using the traditional z < −1.5 cutoff.19
Anti-GABAB-R AE
NPT was only examined in 2 samples of patients with anti-GABAB-R AE, using samples of 5 and 10 patients.37,38 One study found impairment in at least 30% of the sample on measures of visual attention/working memory, abstract visual reasoning, sustained attention, and problem solving.37 However, they used a limited cognitive battery. The other found no differences compared with controls on any measure.38
Anti-GAD-65 AE
Four studies in 3 unique samples have examined NPT in anti-GAD-65 AE, but only included measures of visual and verbal memory. In 2 studies using a more lenient impairment cutoff (z < −1), impairment has ranged from 20% to 71% on list learning tasks and 57–75% on figure recall, although these studies either used a summary score or combined the results of all parameters.39,40 One study using the more typical cutoff (z < −1.5) showed 11% and 37% impairment on list learning and figure recall summary scores.19
Anti-CASPR2, Anti-GABAB-R, and Anti-GAD65 AE Summary
Overall, it is difficult to draw conclusions regarding CI associated with anti-CASPR2, anti-GABAB-R, and anti-GAD-65, given significantly limited data and methodological heterogeneity (Figure 2C, Table 3, eTable 4, links.lww.com/NXI/A924).
Table 3.
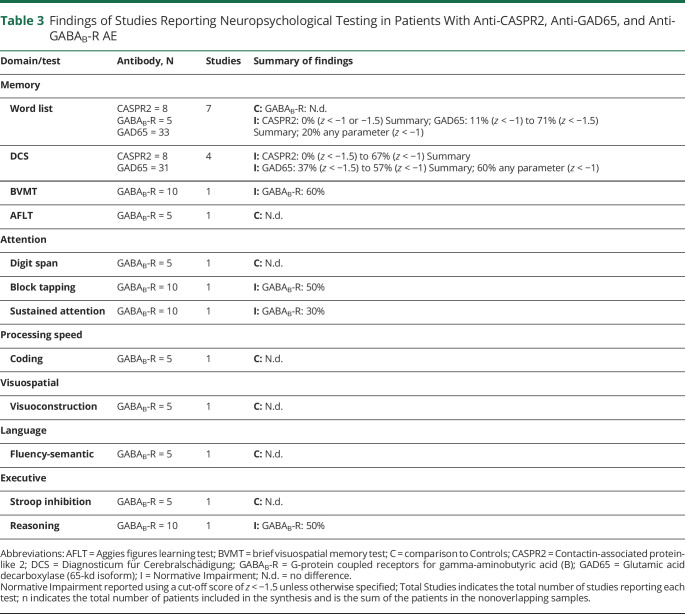
Findings of Studies Reporting Neuropsychological Testing in Patients With Anti-CASPR2, Anti-GAD65, and Anti-GABAB-R AE
| Domain/test | Antibody, N | Studies | Summary of findings |
| Memory | |||
| Word list | CASPR2 = 8 GABAB-R = 5 GAD65 = 33 |
7 |
C: GABAB-R: N.d. I: CASPR2: 0% (z < −1 or −1.5) Summary; GAD65: 11% (z < −1) to 71% (z < −1.5) Summary; 20% any parameter (z < −1) |
| DCS | CASPR2 = 8 GAD65 = 31 |
4 |
I: CASPR2: 0% (z < −1.5) to 67% (z < −1) Summary I: GAD65: 37% (z < −1.5) to 57% (z < −1) Summary; 60% any parameter (z < −1) |
| BVMT | GABAB-R = 10 | 1 | I: GABAB-R: 60% |
| AFLT | GABAB-R = 5 | 1 | C: N.d. |
| Attention | |||
| Digit span | GABAB-R = 5 | 1 | C: N.d. |
| Block tapping | GABAB-R = 10 | 1 | I: GABAB-R: 50% |
| Sustained attention | GABAB-R = 10 | 1 | I: GABAB-R: 30% |
| Processing speed | |||
| Coding | GABAB-R = 5 | 1 | C: N.d. |
| Visuospatial | |||
| Visuoconstruction | GABAB-R = 5 | 1 | C: N.d. |
| Language | |||
| Fluency-semantic | GABAB-R = 5 | 1 | C: N.d. |
| Executive | |||
| Stroop inhibition | GABAB-R = 5 | 1 | C: N.d. |
| Reasoning | GABAB-R = 10 | 1 | I: GABAB-R: 50% |
Abbreviations: AFLT = Aggies figures learning test; BVMT = brief visuospatial memory test; C = comparison to Controls; CASPR2 = Contactin-associated protein-like 2; DCS = Diagnosticum für Cerebralschädigung; GABAB-R = G-protein coupled receptors for gamma-aminobutyric acid (B); GAD65 = Glutamic acid decarboxylase (65-kd isoform); I = Normative Impairment; N.d. = no difference.
Normative Impairment reported using a cut-off score of z < −1.5 unless otherwise specified; Total Studies indicates the total number of studies reporting each test; n indicates the total number of patients included in the synthesis and is the sum of the patients in the nonoverlapping samples.
Summary of Frequency of NPT Applied to Pooled AE Cohorts
Across different neural-IgG AE cohorts, the most administered NPTs were list learning (k = 22), digit span (k = 14), semantic fluency (k = 14), and phonemic fluency (k = 11). Visual memory measures were administered in most studies, although different measures were used. Specifically, 13 studies used a task that involved a series or display of figures, 3 studies used a complex figure task, and 3 studies used a visual memory measure from CANTAB. Processing speed (sequencing k = 8, coding k = 8, Stroop color naming k = 4, symbol search k = 1); naming, reasoning, and Stroop inhibition (k = 8 each); block tapping; and shifting (k = 7) were also commonly administered.
Summary of Impairment Across NPT in Pooled AE Cohorts
Across different neural-IgG AE cohorts, impairments were most common on the measures of visual and verbal memory (complex figure recall, story memory, CANTAB memory measures, word pairs—100%, word list—70%, discrete figures—69%), processing speed (symbol search—100%, coding—88%, Stroop color naming—75%, sequencing—50%), visual and auditory attention/working memory (digit span—79%, block tapping—71%), sustained (75%) and selective (67%) attention, several measures of executive functions (problem solving, executive control, Go/No-Go—100% each, Stroop inhibition, planning—50% each), and complex figure copy (60%). The least common impairments were found on the measures of naming, reasoning, comprehension (25% each), reaction time, verbal fluency switching, semantic knowledge, visuoperception, abstraction, and initiation (0% each).
However, it is difficult to interpret findings when tests were not universally administered. Only 14 measures were examined in at least 5 different studies. Of these, memory (list learning, discrete figure recall), auditory attention/working memory (digit span, spatial span), processing speed (coding, sequencing), complex figure copy, and aspects of executive functions (inhibition, planning) were the most impaired. Naming and reasoning were the least impaired (see Figure 2D).
Discussion
The most frequent impairments across neural-IgG AE cohorts were found on the measures of visual and verbal learning/memory, attention, processing speed, aspects of executive functions, and complex figure copy. Among NPTs administered in ≥5 studies, impairments were most common in list and figure learning/recall, processing speed, auditory attention/working memory, and executive functions, whereas minimal impairment was observed in most aspects of language and reasoning. Surprisingly, despite obvious temporal lobe involvement in most forms of AE, naming tests were not impaired in any study and may be of minimal utility in this population. This could be due to the fact that naming deficits more often correlate with more lateral temporal regions,41 while AE often involves more mesial temporal dysfunction. However, further study is recommended to confirm the lack of naming impairment in AE.
Although small sample sizes, methodological differences, and test variability limit definitive conclusions regarding test-level impairments and differences across neural antibody groups, some differences in NPT impairment rates between the neural-IgG AE cohorts emerged. Specifically, list learning/memory impairments were common in anti-LGI1 AE (100%) and anti-NMDA-R AE (92%) groups but were present in less than one-third of other groups. By contrast, all groups showed at least 50% impairment on visual memory tasks. Processing speed, auditory attention, response inhibition, and complex figure copy tasks seem more impaired in anti-NMDA-R AE studies (80%, 91%, 57%, and 50%, respectively) compared with anti-LGI1 AE studies (60%, 67%, 33%, and 33%, respectively). These tasks were only examined in 0–2 studies in other cohorts. The rates of verbal fluency impairment were similar between anti-NMDA-R AE and anti-LGI1 AE studies.
Table 4 presents our recommended standardized NPT for AE. At this stage, neural antibody-specific recommendations cannot be made, given the variability in test administration across neural antibodies and sparse data. Given that studies examining NPT in AE remain limited, the use of comprehensive NPT batteries spanning all cognitive domains is recommended.
Table 4.
Recommended Test Battery for Adults With AE
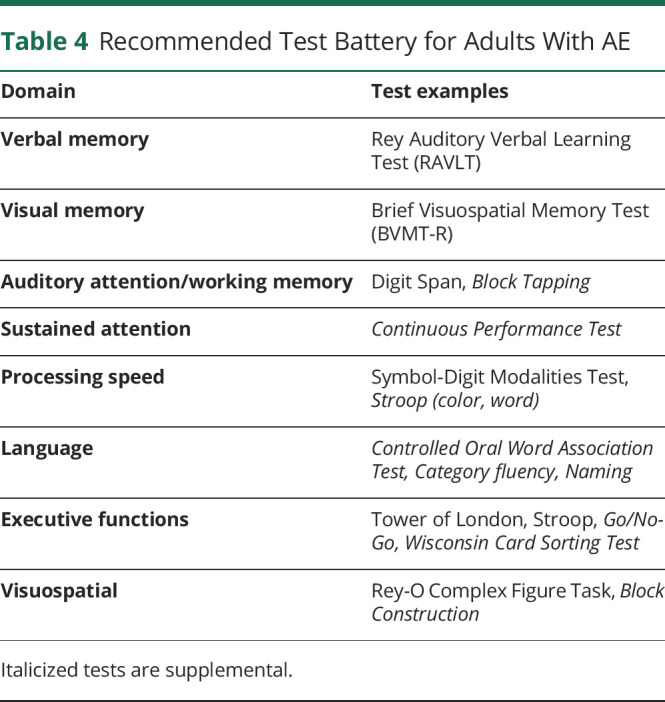
| Domain | Test examples |
| Verbal memory | Rey Auditory Verbal Learning Test (RAVLT) |
| Visual memory | Brief Visuospatial Memory Test (BVMT-R) |
| Auditory attention/working memory | Digit Span, Block Tapping |
| Sustained attention | Continuous Performance Test |
| Processing speed | Symbol-Digit Modalities Test, Stroop (color, word) |
| Language | Controlled Oral Word Association Test, Category fluency, Naming |
| Executive functions | Tower of London, Stroop, Go/No-Go, Wisconsin Card Sorting Test |
| Visuospatial | Rey-O Complex Figure Task, Block Construction |
Italicized tests are supplemental.
Specific measures which may be of highest yield include (1) tests of visual and verbal learning/memory, (2) basic attention (digit span), (3) processing speed (Symbol-Digit Modalities Test), and (4) executive functions. Regarding memory, the Rey Auditory Verbal Learning Test and Brief Visuospatial Memory Test are recommended over other memory measures given the wide availability of translations and normative data across languages. For testing executive function, we specifically recommended Stroop inhibition and spatial planning (Tower) tests, although the inclusion of multiple executive function tests, such as the Wisconsin Card Sorting Test and Go/No-Go, is encouraged to better characterize executive deficits in AE.
Additional measures which have some support and are recommended for more study include (1) visual attention/working memory (block tapping), (2) sustained attention (continuous performance), (3) verbal fluency, (4) Stroop color and word subtests, (5) block construction, and (6) complex figure copy. We have also included naming tasks in our recommendation as this represents an important domain of cognitive functioning and warrants more study.
This recommended battery may be applied to pediatric populations; however, because very few studies have examined NPT in pediatric AE cohorts and those studies have been limited to patients with anti-NMDA-R AE, more research is needed to further refine recommendations for NPT in pediatric cohorts.
We recommend that NPT be performed at standardized intervals from disease onset, ideally at 6 m, 12 m, and then yearly intervals, to minimize variability between test intervals.
One factor that can influence interpretation of these findings is the frequency of administration of each NPT as it is impossible to determine the utility of tests which have not routinely been studied. When evaluating all AE studies across the United States, Europe, Australia, and Asia as a pooled group, list learning, complex or discrete figure memory, digit span, and verbal fluency tasks were the most used NPTs. Some differences in measures used emerged between neural-IgG AE cohorts. Specifically, there were several measures commonly assessed in anti-NMDA-R AE studies, including digit span, block tapping, fluency, coding, Stroop, planning, block design, comprehension, reasoning, and selective attention, which were not often studied in other neural-IgG AE cohorts. This may reflect the larger number of studies in patients with anti-NMDA-R AE and/or preference of the researchers studying this group.
Interpretation may also be affected using normative vs control comparisons. For example, a recent study of anti-LGI-1 AE patients found that although there were no significant differences between patients and controls on a problem-solving task, 44% of patients demonstrated impairment on that task relative to normative data.3 Using normative data may improve comparability between studies and reduce issues related to small sample sizes. However, there are multiple sets of normative data for each test, and appropriate normative data may vary by country and/or region and could become outdated. In addition, one benefit for using control data is that controls may match patient samples more closely for demographic and socioeconomic factors. Cut-off scores also varied by studies, which limit comparability. It may be useful for studies to report the rates of impairment at multiple cutoffs, including 1 and 1.5 SD below the normative mean, to help determine optimal cutoffs and to improve comparability.
Timing of NPT from symptom onset of treatment may affect performance on NPTs, given expected gradual improvement over time, with greater deficits with a shorter test interval expected. Most studies did not examine the effect of the timing of assessment, except for those including multiple visits, which found improvement over time. Using blanket cut-off scores for impairment, without accounting for the impact of premorbid functioning, may limit detection of clinically significant cognitive decline.
Selection bias may also contribute, with patients who are adherent to therapies and follow up more often referred for NPT. In addition, patients with greater symptoms may be preferentially referred for NPT, skewing the proportion of CI in observational studies. Finally, studies examining NPT in AE have occurred in only a few centers and have primarily involved samples from the United States, Europe, and China, limiting generalizability to other regions/countries and languages.
There is a specific need for additional studies examining executive functions in AE, given significant heterogeneity in specific tests administered across studies. At this stage, inclusion of multiple executive function measures is recommended. Future studies should report test-level data, even if using domain composite scores, to further clarify the sensitivity of specific NPTs in this population. There should also be an effort to standardize analysis and reporting of normative data and cutoffs used. Adjustment for premorbid indicators and/or including measures of subjective cognitive decline could increase the detection of cognitive changes, particularly for patients at the higher end of the spectrum of premorbid ability.
Standardized follow-up intervals would improve comparability between studies. Initial assessment should occur after the acute disease phase (when many patients might not be able to perform a full cognitive assessment) and initial dynamic recovery phase (i.e., a few weeks after the initiation of first-line treatment), and follow-up may be beneficial every 6–12 months in the first 1–2 years of recovery. The number of follow-up assessments should be carefully planned to avoid unnecessary repetitions with increasing test/re-test effects.
As there are differences in pathophysiology between neural antibody-mediated AE, future research should focus on distinct and well-defined (e.g., high titer for GAD65-IgG patients) AE cohorts, rather than pooled cohorts, which may be heterogenous in their neuropsychological outcomes.
Finally, systemic and psychiatric comorbidities, which may significantly contribute to cognitive outcomes, are not typically accounted for in studies reporting cognitive outcomes in AE. Anti-LGI-1 and anti-CASPR2 cohorts may be at greatest risk for undiagnosed concomitant neurodegenerative conditions, given the typically older age of onset compared with other cohorts. Future studies should assess and adjust for these comorbidities in statistical models and use age-matched and sex-matched healthy controls. In addition, in age groups where cognitive disorders, such as mild cognitive impairment, are more prevalent, using matched controls from these cohorts may also be important.
Our search did not reveal any studies of patients with paraneoplastic neurologic disorders manifesting with encephalitis, although research is needed in these groups, to determine whether similar cognitive profiles and testing are applicable. These disorders may differ from the synaptic/extracellular neural antibody-mediated AE due to their cancer association, potential impact of cancer therapies on cognition, and that they are associated with irreversible neuronal destruction and often have a limited response to immunosuppressive therapies.
This review examined NPT's most frequently impaired in neural-IgG seropositive AE cohorts and provided recommendations for a standardized NPT battery. The most impaired NPTs were list and figure learning/recall, processing speed, auditory attention/working memory, and aspects of executive functions, while minimal impairment was observed in most aspects of language and reasoning.
We recommend the use of comprehensive NPT batteries given the dearth of data in this area. At minimum, we recommend inclusion of measures of list learning and figural memory (RAVLT, BVMT), basic attention (digit span), processing speed (SDMT), and multiple executive function measures (particularly Stroop, Tower as well as Wisconsin Card Sorting Test, Go/No-Go—secondary). Measures that may additionally be useful include block tapping, sustained attention (continuous performance), verbal fluency, block construction, and complex figure copy.
We observed several limitations affecting the cognitive research in AE to date including variability in type and frequency of NPTs used. However, these initial recommendations for NPT in AE will help standardize research in this area and facilitate collaboration and compilation of data registries. We further hope that this work encourages future work using more focused and high-yield approaches to identifying meaningful cognitive changes in AE.
Glossary
- AE
autoimmune encephalitis
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- CI
cognitive impairment
- NPT
neuropsychological tests
- PASAT
Paced Auditory Serial Addition Test
Appendix. Authors

| Name | Location | Contribution |
| Rachel Galioto, PhD | Cleveland Clinic Mellen Center for MS, OH | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Tiffany Grezmak, MA | Department of Neurology, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Carol Swetlik, MD | Department of Neurology, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content |
| Justin R. Abbatemarco, MD | Cleveland Clinic Mellen Center for MS, OH | Drafting/revision of the manuscript for content, including medical writing for content |
| Maarten J. Titulaer, MD, PhD | Neurology, Erasmus University Medical Center, Rotterdam, Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Carsten Finke, MD | Department of Neurology, Charité - Universitätsmedizin Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Amy Kunchok, MD, PhD | Mellen Center for MS, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
R. Galioto, C. Swetlik, and T. Grezmak report no disclosures relevant to the manuscript; A. Kunchok has received compensation for consulting and scientific advisory boards for Genentech, Horizon Therapeutics, and EMD Serono; J.R. Abbatemarco has served on scientific advisory boards for of EMD Serono, Genentech, Horizon; has received research support from Horizon; M.J. Titulaer has received research funds for serving on a scientific advisory board of Horizon Therapeutics and UCB; M.J. Titulaer has filed a patent for methods for typing neurologic disorders and cancer, and devices for use therein, and has received research funds for consultation at Guidepoint Global LLC and unrestricted research grants from CSL Behring and Euroimmun AG. M.J. Titulaer is supported by an E-RARE 3 grant (UltraAIE), Dioraphte (2001 0403) and the Dutch Epilepsy Foundation (NEF 19-08); CF has received grant funding Deutsche Forschungsgemeinschaft (DFG, German Research Foundation Grant Nos. FI 2309/1-1 (Heisenberg Program), FI 2309/2-1, and 327654276 (SFB 1315) and German Ministry of Education and Research (BMBF) Grant Nos. 01GM1908D, 01GM2208C, 13GW0566D, 01GM2102, and 01EP2201). Go to Neurology.org/NN for full disclosures.
References
- 1.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finke C, Kopp UA, Prüss H, Dalmau J, Wandinger KP, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2012;83(2):195-198. doi: 10.1136/jnnp-2011-300411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galioto R, Aboseif A, Krishnan K, Lace J, Kunchok A. Cognitive outcomes in anti-LGI-1 encephalitis. J Int Neuropsychol Soc. 2023;29(6):541-550. doi: 10.1017/S1355617722000509 [DOI] [PubMed] [Google Scholar]
- 4.Heine J, Prüss H, Kopp UA, et al. Beyond the limbic system: disruption and functional compensation of large-scale brain networks in patients with anti-LGI1 encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(11):1191-1199. doi: 10.1136/jnnp-2017-317780 [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 6.Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 7.Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 8.Bettcher BM, Gelfand JM, Irani SR, et al. More than memory impairment in voltage-gated potassium channel complex encephalopathy. Eur J Neurol. 2014;21(10):1301-1310. doi: 10.1111/ene.12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binks SNM, Veldsman M, Easton A, et al. Residual fatigue and cognitive deficits in patients after leucine-rich glioma-inactivated 1 antibody encephalitis. JAMA Neurol 2021;78(5):617-619. doi: 10.1001/jamaneurol.2021.0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeon GL, Robinson GA, Ryan AE, et al. Cognitive outcomes following anti-N-methyl-D-aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol 2018;40(3):234-252. doi: 10.1080/13803395.2017.1329408 [DOI] [PubMed] [Google Scholar]
- 11.Griffith SP, Malpas CB, Alpitsis R, O'Brien TJ, Monif M. The neuropsychological spectrum of anti-LGI1 antibody mediated autoimmune encephalitis. J Neuroimmunol. 2020;345:577271. doi: 10.1016/j.jneuroim.2020.577271 [DOI] [PubMed] [Google Scholar]
- 12.Mueller C, Elben S, Day GS, et al. Review and meta-analysis of neuropsychological findings in autoimmune limbic encephalitis with autoantibodies against LGI1, CASPR2, and GAD65 and their response to immunotherapy. Clin Neurol Neurosurg. 2023;224:107559. doi: 10.1016/j.clineuro.2022.107559 [DOI] [PubMed] [Google Scholar]
- 13.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi: 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matricardi S, Patrini M, Freri E, et al. Cognitive and neuropsychological evolution in children with anti-NMDAR encephalitis. J Neurol. 2016;263(4):765-771. doi: 10.1007/s00415-016-8056-9 [DOI] [PubMed] [Google Scholar]
- 15.Cainelli E, Nosadini M, Sartori S, Suppiej A. Neuropsychological and psychopathological profile of anti-Nmdar encephalitis: a possible pathophysiological model for pediatric neuropsychiatric disorders. Arch Clin Neuropsychol. 2019;34(8):1309-1319. doi: 10.1093/arclin/acy088 [DOI] [PubMed] [Google Scholar]
- 16.Sola-Valls N, Ariño H, Escudero D, et al. Telemedicine assessment of long-term cognitive and functional status in anti-leucine-rich, glioma-inactivated 1 encephalitis. Neurol Neuroimmunol Neuroinflammation. 2020;7(2):e652. doi: 10.1212/NXI.0000000000000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez A, Klein CJ, Sechi E, et al. LGI1 antibody encephalitis: acute treatment comparisons and outcome. J Neurol Neurosurg Psychiatry. 2022;93(3):309-315. doi: 10.1136/jnnp-2021-327302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malter MP, Frisch C, Schoene-Bake JC, et al. Outcome of limbic encephalitis with VGKC-complex antibodies: relation to antigenic specificity. J Neurol. 2014;261(9):1695-1705. doi: 10.1007/s00415-014-7408-6 [DOI] [PubMed] [Google Scholar]
- 19.Bauer T, David B, Ernst L, et al. Structural network topology in limbic encephalitis is associated with amygdala enlargement, memory performance and serostatus. Epilepsia. 2020;61(10):e140–e145. doi: 10.1111/epi.16691 [DOI] [PubMed] [Google Scholar]
- 20.van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. 2016;87(14):1449-1456. doi: 10.1212/WNL.0000000000003173 [DOI] [PubMed] [Google Scholar]
- 21.Finke C, Prüss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol. 2017;74(1):50. doi: 10.1001/jamaneurol.2016.4226 [DOI] [PubMed] [Google Scholar]
- 22.Hanert A, Rave J, Granert O, et al. Hippocampal dentate gyrus atrophy predicts pattern separation impairment in patients with LGI1 encephalitis. Neuroscience. 2019;400:120-131. doi: 10.1016/j.neuroscience.2018.12.046 [DOI] [PubMed] [Google Scholar]
- 23.Phillips OR, Joshi SH, Narr KL, et al. Superficial white matter damage in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(5):518-525. doi: 10.1136/jnnp-2017-316822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Lv X, Zhang J, et al. Gray matter atrophy and corresponding impairments in connectivity in patients with anti-N-methyl-D-aspartate receptor encephalitis. Brain Imaging Behav. 2022;16(5):2001-2010. doi: 10.1007/s11682-022-00670-5 [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Wu D, Wang K, Luo B. Cognitive function recovery pattern in adult patients with severe anti-N-Methyl-D-Aspartate receptor encephalitis: a longitudinal study. Front Neurol. 2018;9:675. doi: 10.3389/fneur.2018.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Zhou J, Wu D, Ji C, Luo B, Wang K. Altered executive control network connectivity in anti-NMDA receptor encephalitis. Ann Clin Transl Neurol. 2022;9(1):30-40. doi: 10.1002/acn3.51487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Duan Y, Zhang T, et al. Aberrant multimodal brain networks in patients with anti-NMDA receptor encephalitis. CNS Neurosci Ther. 2021;27(6):652-663. doi: 10.1111/cns.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guasp M, Rosa-Justicia M, Muñoz-Lopetegi A, et al. Clinical characterisation of patients in the post-acute stage of anti-NMDA receptor encephalitis: a prospective cohort study and comparison with patients with schizophrenia spectrum disorders. Lancet Neurol. 2022;21(10):899-910. doi: 10.1016/S1474-4422(22)00299-X [DOI] [PubMed] [Google Scholar]
- 29.de Bruijn MAAM, Aarsen FK, van Oosterhout MP, et al. Long-term neuropsychological outcome following pediatric anti-NMDAR encephalitis. Neurology. 2018;90(22):e1997-e2005. doi: 10.1212/WNL.0000000000005605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson-Smith A, Blackwell LS, Howarth RA. Neuropsychological outcomes in children and adolescents following anti-NMDA receptor encephalitis. Child Neuropsychol. 2022;28(2):212-223. doi: 10.1080/09297049.2021.1965110 [DOI] [PubMed] [Google Scholar]
- 31.Hageboutros K, Hattiangadi Thomas N, Hutchinson M, Banwell B, Baum KT. Neuropsychological functioning in children and adolescents with anti-NMDA receptor encephalitis (anti-NMDARE). J Neurol. 2023;270(1):402-412. doi: 10.1007/s00415-022-11372-9 [DOI] [PubMed] [Google Scholar]
- 32.McKeon GL, Scott JG, Spooner DM, et al. Cognitive and social functioning deficits after anti-N-methyl-D-aspartate receptor encephalitis: an exploratory case series. J Int Neuropsychol Soc. 2016;22(8):828-838. doi: 10.1017/S1355617716000679 [DOI] [PubMed] [Google Scholar]
- 33.Heine J, Kopp UA, Klag J, Ploner CJ, Prüss H, Finke C. Long-term cognitive outcome in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2021;90(6):949-961. doi: 10.1002/ana.26241 [DOI] [PubMed] [Google Scholar]
- 34.Finke C, Kopp UA, Scheel M, et al. Functional and structural brain changes in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2013;74(2):284-296. doi: 10.1002/ana.23932 [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Lv X, Wei Q, et al. Impaired neurovascular coupling and cognitive deficits in anti-N-methyl-D-aspartate receptor encephalitis. Brain Imaging Behav. 2022;16(3):1065-1076. doi: 10.1007/s11682-021-00588-4 [DOI] [PubMed] [Google Scholar]
- 36.Long Q, Lv Z, Zhao J, et al. Cerebral gray matter volume changes in patients with anti-N-methyl-D-aspartate receptor encephalitis: a voxel-based morphometry study. Front Neurol. 2022;13:892242. doi: 10.3389/fneur.2022.892242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin J, Li C, Li A, et al. Long-term cognitive and neuropsychiatric outcomes of anti-GABABR encephalitis patients: a prospective study. J Neuroimmunol. 2021;351:577471. doi: 10.1016/j.jneuroim.2020.577471 [DOI] [PubMed] [Google Scholar]
- 38.Ji C, Wu D, Chen Z, Wang K. The long‐term outcome of neuropsychological function is favorable in patients with non‐malignancy related anti-GABABR encephalitis: a case series. BMC Neurol. 2021;21(1):87. doi: 10.1186/s12883-021-02111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67(4):470-478. doi: 10.1002/ana.21917 [DOI] [PubMed] [Google Scholar]
- 40.Wagner J, Witt JA, Helmstaedter C, Malter MP, Weber B, Elger CE. Automated volumetry of the mesiotemporal structures in antibody-associated limbic encephalitis. J Neurol Neurosurg Psychiatry. 2015;86(7):735-742. doi: 10.1136/jnnp-2014-307875 [DOI] [PubMed] [Google Scholar]
- 41.Binder JR, Tong JQ, Pillay SB, et al. Temporal lobe regions essential for preserved picture naming after left temporal epilepsy surgery. Epilepsia. 2020;61(9):1939-1948. doi: 10.1111/epi.16643 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not published within this article will be made available by request from any qualified investigator.