Abstract
Human gender-related behavior/psychology is shaped by a developmental system that involves numerous influences interacting over time. Understanding of the full range of elements in the system and how they interact is currently incomplete. The available evidence suggests, however, that early exposure to testosterone, postnatal socialization, e.g., by parents and peers, and self-socialization related to cognitive understanding of gender are important elements. This article focuses on prenatal and early neonatal influences of testosterone on gender-related psychological/behavioral outcomes, and contextualizes these hormonal influences within an understanding of socialization influences. There is consistent evidence that early testosterone exposure influences childhood gender role behavior, including sex-typical toy play, as well as gender identity and sexual orientation. Evidence for similar hormonal influences on spatial ability and on traits related to autism, or autistic spectrum disorder, is inconsistent. Evidence from girls exposed to elevated testosterone prenatally suggests that they experience alterations in processes of external socialization, as well as self-socialization, and that these, along with early testosterone exposure, shape gender-related outcomes.
Keywords: sex, gender, gender role behavior, childhood gender role behavior, sexual orientation, gender identity, spatial ability, mental rotation, autistic traits, autistic spectrum disorder, autism, testosterone, androgen
A search for books on human gender development will locate books suggesting strong inborn, sometimes called biological, contributions to gender-related behavior (Baron-Cohen, 2003; Brizendine, 2007), as well as books espousing a very different conclusion, that the evidence for inborn influences is flawed and gender-related behavior is caused solely by socialization, sometimes called social construction (Fine, 2010; Jordan-Young, 2010). Developmental scientists, however, view gender related behavior, like most other behavior, as the product of a developmental system involving numerous factors, interacting over time (Hines, 2015; Lickliter and Honeycutt, 2015). From this perspective, neither so called biological determinism (nature) or social construction (nurture) is the crucial factor. Instead, many types of influences are involved, and one or another of them can be made more or less powerful by changes elsewhere in the system.
Developmental science is far from understanding all of the elements of the system that shapes gender-related behavior/psychology, much less understanding how the elements interact. However, some of the component elements have been identified. They include early exposure to gonadal steroids, particularly testosterone, postnatal socialization, e.g., by parents and peers, and self-socialization related to cognitive understanding of gender.
In this article, I focus largely on prenatal and early neonatal influences of testosterone on gender-related psychological and behavioral development. However, I also contextualize these hormonal influences within an understanding of the influences of other factors, particularly socialization. To begin, I will describe gonadal hormone influences on neural and behavioral development in non-human mammals. This experimental research provides the basis for hypothesizing that testosterone influences human psychological/behavioral development. In addition, it supports specific predictions about possible hormonal influences, including which hormones might be hypothesized to be influential, when they would be hypothesized to act, and which behaviors they would be hypothesized to influence. After this, I will describe research that has examined the possibility that gonadal hormones influence human psychological and behavioral development. Finally, I will discuss how early hormonal exposure might interact with social factors to influence gender-related outcomes.
Influences of gonadal hormones on brain and behavior in non-human mammals
Literally thousands of studies of non-human mammals support the conclusion that testosterone, prenatally or neonatally, influences brain development and later behavior (Arnold, 2009). These influences were initially described in regard to reproductive behavior (Phoenix et al., 1959). Phoenix et al. treated pregnant guinea pigs with testosterone and found increased male-typical, and reduced female-typical, reproductive behavior in their female offspring. The authors described these early influences of testosterone on later behavior as organizational influences, because the influences were seen long after the hormone was gone, assumedly because early testosterone exposure had influenced the underlying organization of the brain. They contrasted these organizational influences, which were long-lasting and occurred during early critical periods of development, with what they called activational influences, which were transient and typically occurred later in life, e.g., after puberty, and waxed and waned as hormone concentrations rose and fell.
Subsequent research extended the range of behaviors that were found to be influenced by early testosterone exposure, as well as the range of species affected (Arnold, 2009; Hines, 2004). Several general principles emerged from these studies. First, the behaviors that were influenced by early exposure to testosterone included not only reproductive behaviors, but also non-reproductive behaviors that show sex differences, meaning that they differ on the average for males and females of the species. These behaviors include, for example, juvenile rough-and-tumble play and aggression, which are more characteristic of male than female animals, and nest building and grooming, which are more characteristic of females than males. Early testosterone exposure increased the behaviors more characteristic of males, and decreased those more characteristic of females. Second, the times when testosterone manipulations influenced later behaviors were found to correspond to times when testosterone is higher in developing male than female animals. These general principles have been found to apply across a range of rodent species, as well as in non-human primates, such as rhesus macaques.
Although early testosterone exposure has been found to increase male-typical outcomes (masculinize) and reduce female-typical outcomes (defeminize) later behavior, estrogen has generally not been found to have feminizing or demasculinizing effects during early development in non-human mammals. This may seem surprising, because we know that estrogen has feminizing physical effects at puberty, promoting female-typical breast development, for example. Prenatally, however, developing male and female humans, do not appear to differ in estrogen production (Wilson et al., 1981). In addition, studies of non-human mammals do not find that estrogen treatment feminizes or demasculinizes later behavior. Indeed, in rodents, estrogen treatment early in life promotes male-typical development in female animals (Hines, 2004; McCarthy, 2008). This occurs because estrogen treatment of developing female rodents mimics the situation in developing male rodents where testosterone is converted to estrogen in the brain, and then acts through neural estrogen receptors to masculinize and defeminize. The evidence from non-human animals, therefore, suggests that no feminizing or demasculinizing influence of prenatal or neonatal exposure to estrogens, such as estradiol, would be hypothesized. Also, among primates, unlike rodents, early estrogen exposure does not appear to have either masculinizing or feminizing influences.
In addition to influencing behavior, early exposure to testosterone influences the development and structure of the mammalian brain. Initial studies found that the ultrastructure of the rodent brain was influenced by exposure to gonadal steroids during critical periods of early development. For instance, Raisman and Field reported that female rats had more non-amygdaloid synapses on dendritic spines in the preoptic area than did male animals. They also found that treating female rats with testosterone during the early postnatal period reduced spine numbers, and that castrating male rats soon after birth increased them (Raisman and Field, 1973). Subsequent research reported even more dramatic influences of early testosterone exposure, including effects on the volume of certain subregions of the brain. Several cell groups that were observed to be larger in male or female animals were made more male-typical or less female-typical by early testosterone treatment. These included the sexually dimorphic nucleus of the preoptic area (SDN-POA), as well as subregions of the bed nucleus of the stria terminalis and the medial amygdala, and the anteroventral paraventricular nucleus of the preoptic area (Hines, 2020).
Does testosterone influence human brain and behavior during early development?
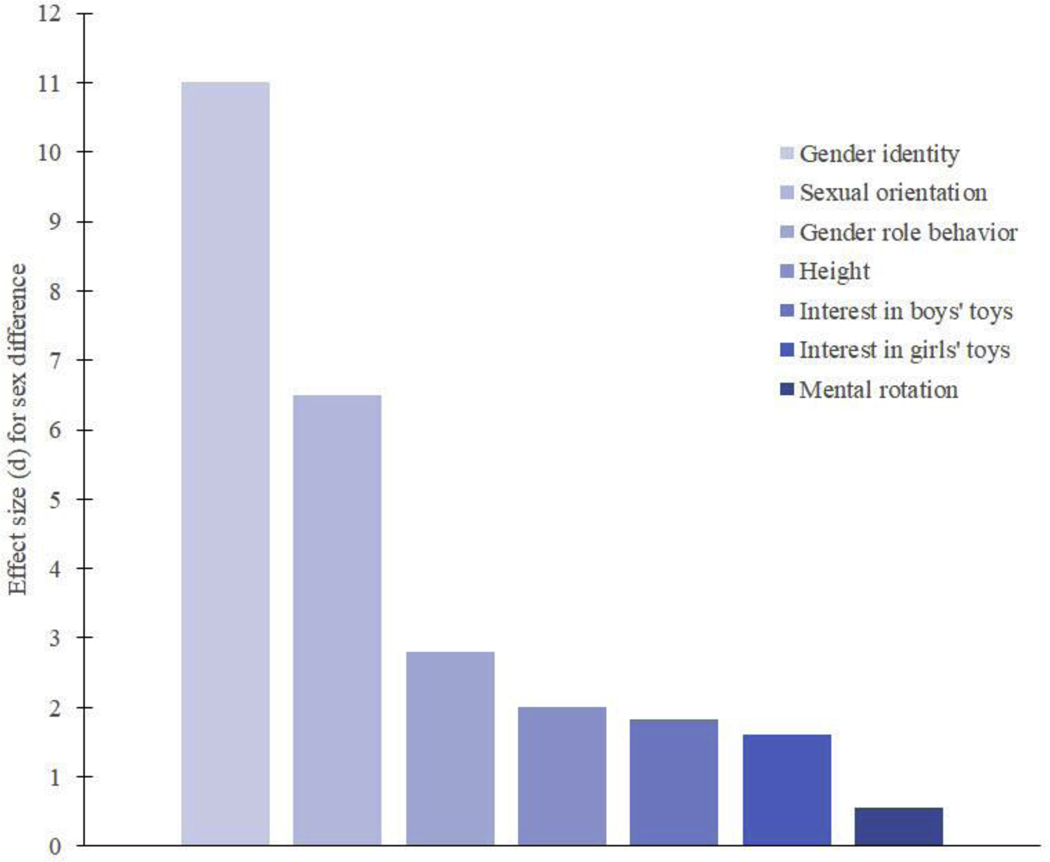
In human beings, the times when testosterone is markedly higher in developing males than females include a prenatal period from about week 8 to about week 16 to 24 of gestation, and an early postnatal period from about the first to the third month of infancy (Forest et al., 1973; Kuiri-Hanninen et al., 2011; Smail et al., 1981). These then are the likely critical periods when testosterone would be hypothesized to exert organizational influences on later human behavior/psychology. In addition, because research in other species suggests that testosterone influences characteristics that show sex differences, when formulating hypotheses and interpreting findings, it is useful to know which aspects of human behavior/psychology show sex differences and how large these differences are. Figure 1 summarizes behavioral/psychological characteristics that have been found to show relatively large and reliable sex differences in humans, and that have been studied in relation to early testosterone exposure. The figure also illustrates the size of the sex difference in adult human height. Height is included to provide a familiar comparator for contextualizing the sizes of the behavioral/psychological sex differences.
Figure 1.
Effect size (d) values for human behavioral/psychological characteristics that show relatively large and reliable sex differences, and that have been studied in relation to early testosterone exposure. The effect size for the sex difference in height is included as a familiar comparator.
Like the sex difference in height, sex differences in behavior/psychology are average differences. For example, we know that the average height of men is greater than that of women. At the same time, however, we know that some women are taller than some men. Thus, there is overlap between the distributions of height for men and women. The sizes of average group differences, such as that in the height of men and women, can be quantified using standard deviation units, or “d” values (Cohen, 1988). Conventionally, d values of 0.8 or greater for group differences in human behavior/psychology are considered large, those of about 0.5 are considered moderate, those of about 0.2 are considered small, and those below 0.2 are considered negligible. The d value for the sex difference in height is 2.0. Most human behavioral/psychological sex differences are smaller in magnitude than the sex difference in height, but a few are larger. For instance, the sex differences in gender identity and sexual orientation are larger, as are sex differences in children’s gender role behavior (Hines, 2015). Each of these three types of large behavioral/psychological sex differences is described in more detail below. In addition, the overlap between distributions of scores for groups of males and females for these characteristics is illustrated in Figure 2, again along with similar information for height
Figure 2.
Illustration of the overlap in distributions of scores for males and females for psychological/behavioral characteristics that show large and reliable sex differences. The overlap for height is included as a familiar comparator.
Gender identity is a person’s sense of self as male, female, both, or neither. Most people who have male-typical external genitalia (penis and scrotum) think of themselves as boys or men, and most people who have female-typical external genitalia (clitoris and labia) do not. This sex difference in gender identity is very large with an approximate d value greater than 10.0 (Hines et al., 2003a; Hines et al., 2004). Sexual orientation refers to the direction of a person’s erotic interests, e.g., in males, females, both or neither. Most people who have female-typical external genitalia are interested primarily in male partners, whereas most people who have male-typical external genitalia are not. This sex difference also is large, with an approximate d value of more than 5.0 (Hines et al., 2003a; Hines et al., 2004; Meyer-Bahlburg et al., 2008). Gender role behavior refers to a range of behaviors that differ on average for boys and girls, including the toys and activities they engage with, and the sex of their favorite playmates. One commonly used standardized measure of gender role behavior is the Pre-School Activities Inventory (PSAI). A population study of over 15,000 children suggests that the d value for the sex difference on the PSAI is about 2.8 (Golombok et al., 2008; Hines et al., 2002b). Also, meta-analytic findings suggest that children’s toy interests show large sex differences, d = 1.6 to 3.5 (Davis and Hines, 2020). In addition, for dolls and toy vehicles considered individually, d values for the sex differences are even larger d = 4.1 and 2.4, respectively.
Other behaviors/psychological characteristics that can show large sex differences, include some measures of physical aggression, personality traits, such as dominance and empathy, and spatial abilities. It is important to note that for these constructs large differences are seen only on specific measures. For example, a standardized paper and pencil personality questionnaire, the Thurstone 16 Personality Factor questionnaire, shows large sex differences on its subscales for dominance and empathy, but other standardized paper and pencil measures of the same personality constructs show smaller sex differences (Costa et al., 2001; Feingold, 1994; Hyde, 2005). Similarly, the Vandenbergh and Kuse measure of 3-dimensional mental rotation ability shows a large sex difference (d = 0.94) when scored using a particular scoring system (Voyer et al., 1995). However, other measures of mental rotation ability show smaller sex differences (d = 0.56 across all measures of mental rotation), as do other scoring systems (Voyer et al., 1995). For spatial ability broadly, the effect size for the sex difference is small to moderate in size (d = 0.37) (Voyer et al., 1995). Other behaviors generally show smaller sex differences than those included in Figure 1. In addition, most human behavioral/psychological characteristics, show small to negligible sex differences (Hyde, 2005).
Research studying hormonal influences on brain and behavior in non-human mammals has involved rigorous experimental procedures. Animals are assigned at random to treatment with hormones or placebos. They also can have their adult hormone environments made similar, for example, by gonadal removal and hormone replacement, to separate effects of early hormones (organizational effects) from effects of later hormones (activational effects). Ethical considerations do not allow similar rigorous experimental procedures in studies of hormonal influences on human development. Instead, researchers have investigated outcomes in individuals exposed to unusual hormone environments early in life, for example because of genetic variants. In addition, researchers have tried to measure variability in the early hormone environment in typically-developing individuals and then relate this hormonal variability to variability in later behavior/psychology.
The most extensively studied genetic variant involving early testosterone exposure is classical congenital adrenal hyperplasia (CAH). CAH occurs in approximately 1 in 14,000 to 1 in 18,000 births (Speiser et al., 2018), and affects both male and females. Its most common condition is caused by a mutation of the gene encoding for the 21-hydroxylase (21-OH) enzyme, and it results in reduced cortisol production, and elevation of cortisol precursors. Because the cortisol pathway is not functioning properly, these precursors are shunted into the intact adrenal androgen pathway and some of these adrenal androgens are converted to testosterone. Consequently, females with CAH are exposed to high concentrations of androgens, including testosterone, beginning prenatally. The androgen elevation virilizes the external genitalia of females with CAH to varying degrees. Based on research in other species, the elevated prenatal androgen exposure also might be hypothesized to have organizational influences on the brain and on later behavior/psychology. Specifically, females with CAH would be hypothesized to show increased male-typical characteristics and reduced female-typical characteristics. Androgen concentrations of males with CAH appear to be in the normal to high normal range prenatally (Pang et al., 1980; Pang et al., 1979). Therefore, males with CAH would not be hypothesized to show altered gender-related behaviour/psychology.
The behavioral outcome that has been studied most extensively in individuals with CAH is childhood gender role behavior. Researchers have consistently reported that girls with CAH show increased male-typical, or reduced female-typical, gender role behavior (Berenbaum and Hines, 1992; Dittmann et al., 1990b; Ehrhardt and Baker, 1974; Frisen et al., 2009; Hall et al., 2004; Hines et al., 2004; Iijima et al., 2001; Meyer-Bahlburg et al., 2006; Money and Ehrhardt, 1972; Nordenstrom and Larsson, 2003; Nordenstrom et al., 2002; Pasterski et al., 2005; Pasterski et al., 2011; Pasterski et al., 2015b; Slijper, 1984; Zucker et al., 1996). These studies have used questionnaires, as well as observation of behavior (e.g., toy choices in a playroom) and they have compared girls with CAH to their unaffected relatives of similar age, as well as to controls matched for age and other background factors. They also have come from researchers in the United States (Baltimore, Chicago, Los Angeles and New York), as well as from researchers in Canada, Germany, Japan, the Netherlands and Sweden. The results appear to be robust. In addition, the masculinization of gender role behavior in girls with CAH relates positively to the severity of the CAH disorder.
Similarly, sexual orientation and gender identity appear to be altered in females with CAH and these findings have again been replicated consistently, and by more than one research team. Although it appears that more than 50% of women with CAH are exclusively or almost exclusively heterosexual, they are more likely to be bisexual or primarily interested in female sexual partners compared to women without CAH (Dittmann et al., 1992; Frisen et al., 2009; Hines et al., 2004; Meyer-Bahlburg et al., 2008). In addition, although the great majority of women with CAH live as women, about 2% choose to live as men, despite female sex assignment and rearing (Dessens et al., 2005; Meyer-Bahlburg et al., 1996; Zucker et al., 1996). This 2% figure is markedly higher than the 1 in 100,000 (.001%) estimated for the population at large during roughly the same time period (American Psychiatric, 2000). Girls and women with CAH also have been found to report reduced strength of female gender identity compared to unaffected controls (Hines et al., 2004; Meyer-Bahlburg et al., 2006; Pasterski et al., 2015b). The results for sexual orientation and gender identity, like those for gender role behavior, also have been found to relate to CAH severity (Dittmann et al., 1990a; Frisen et al., 2009; Gastaud et al., 2007; Meyer-Bahlburg et al., 2006; Meyer-Bahlburg et al., 2008).
Outcomes for other conditions that alter androgen exposure or responsiveness have not been studied as extensively as have outcomes for individuals with CAH. However, there is some evidence for individuals with complete androgen insensitivity syndrome (CAIS). CAIS affects XY individuals and its prevalence is estimated to be one in 20,400 to one in 99,100 XY births (Hughes et al., 2012). The syndrome involves insensitivity of androgen receptors. Individuals with CAIS have normally functioning testes, but because their tissues lack functioning androgen receptors, and so are unable to respond to testosterone or other androgens, they are born with female-typical external genitalia. Based on experimental research in other mammals, XY females with CAIS would be hypothesized to show female-typical behavior/psychology, because of their inability to respond to androgens. The available research, though limited, suggests that they do show female-typical outcomes, including female-typical gender role behavior in childhood (Hines et al., 2003a; Jurgensen et al., 2007), and interest in men as sexual partners (Hines et al., 2003a; Money et al., 1984; Wisniewski et al., 2000). They also almost always have female-typical gender identity (Hines et al., 2003a; Mazur, 2005; Wisniewski et al., 2000). For instance, a review of reports on gender identity in XY individuals with CAIS published prior to 2005, reported no documented cases of gender change among the 156, XY females included in the reports. Subsequently, there has been one well-documented report of an XY individual with CAIS living as a man (T’Sjoen et al., 2011). It has been suggested that awareness of the XY karyotype, normal testes (prior to gonadal removal, if removal occurred), and other clinical consequences of CAIS, such as a rudimentary vagina, and the absence of ovaries or a uterus, might cause some to question their female identity (Meyer-Bahlburg, 2010). Alternatively, genetic mosaicism might explain rare cases of gender change in individuals with CAIS (Meyer-Bahlburg, 2010; T’Sjoen et al., 2011). Finally, XY individuals with partial androgen insensitivity (PAIS), meaning that their response to androgenic hormones is reduced, but not completely absent, or other causes of incomplete physical virilization, have also been reported to show increased male-typical and decreased female-typical childhood gender role behavior, compared to female controls, despite female sex assignment and rearing (Jurgensen et al., 2007).
Studies relating normal variability in testosterone during early development to later behavior also have looked at childhood gender role behaviors, but not at sexual orientation or gender identity. Researchers have measured testosterone in maternal blood samples taken during pregnancy, in amniotic fluid samples, and in urine or saliva samples obtained during early infancy. An initial study looked at testosterone in maternal blood samples taken for clinical reasons from several thousand women participating in a population study of pregnancy and childhood (Hines et al., 2002a). This study found that maternal testosterone during pregnancy related positively to daughters’ scores on the PSAI, a measure of gender role behavior where higher scores indicate more male-typical behavior, at age 3.5 years. A subsequent study did not find a significant association between maternal testosterone during pregnancy and later gender role behavior (van de Beek et al., 2009), but had a smaller sample and used a measure of gender role behavior that showed a smaller sex difference than the PSAI. Both of these differences would have limited the statistical power of the study to detect effects. Studies relating testosterone measured in amniotic fluid to later gender role behavior also have produced mixed results (Auyeung et al., 2009b; Knickmeyer et al., 2005; van de Beek et al., 2009), perhaps related to sample size differences or to the insensitivity of amniotic fluid measured on a single occasion at an uncontrolled time or day as a proxy for prenatal androgen exposure (Constantinescu and Hines, 2012). Two studies have explored the relationship of testosterone during early infancy to later gender role behavior. One of these suggested that testosterone measured in urine samples obtained on seven occasions across the first six months postnatal related positively to later PSAI scores in boys but not girls (Lamminmaki et al., 2012). The second suggested that penile growth from birth to three months postnatal, which correlates with testosterone concentrations at three months of age, related positively to PSAI scores in boys (Pasterski et al., 2015a). Penile measurements were not relevant for girls.
Unlike the consistent evidence linking early androgen exposure to gender identity, sexual orientation and childhood gender role behavior, evidence of a relationship between early androgen exposure and later mental rotation performance or performance on other spatial tasks is inconsistent or lacking. Two early studies with small samples reported that girls with CAH showed enhanced spatial performance (Hampson et al., 1998; Resnick et al., 1986). However, other researchers, sometimes using larger samples, did not always find similar evidence (Baker and Ehrhardt, 1974; Collaer et al., 2016; Dittmann et al., 1993; Helleday et al., 1994; Hines et al., 2003b; Malouf et al., 2006). A 2020 meta-analysis concluded that CAH in females is not associated with improved performance on mental rotation tasks, or on other spatial tasks (Collaer and Hines, 2020). In addition, the 2020 meta-analysis found that smaller samples produced larger effect sizes than larger samples, suggesting a publication bias, where studies that produce significant results are more likely to be published than studies that do not, thus contributing to the publication of false positive findings.
Like studies of females with CAH, studies relating normal variability in testosterone to later performance on mental rotation or other spatial tasks also have produced inconsistent results. One study reported that testosterone measured in amniotic fluid showed a negative relationship to block building in girls at four years of age, a result that is in the opposite direction of that predicted (Finegan et al., 1992). In the same sample of children at age seven years there was no relationship between amniotic fluid testosterone and number correct on a measure of mental rotation (Grimshaw et al., 1995), although the researchers reported that there was a negative relationship to speed of rotation in boys, but not girls, but this relationship was only significant when some data points were removed from the data set. A second study, using a larger sample, again found no relationship between amniotic fluid testosterone and number correct on a mental rotation task (Auyeung et al., 2012). Surprisingly, however, this study found that amniotic fluid testosterone related significantly to performance on a spatial task that does not typically show a sex difference. A single study has measured testosterone during mini-puberty and found a relationship to mental rotation performance later in infancy (Constantinescu et al., 2018).
Autistic traits also have been studied in individuals with CAH, because of a suggestion that androgens prenatally might contribute to more males than females being diagnosed with autistic spectrum disorder (ASD) (Baron-Cohen, 2002). An initial study found a small increase in autistic traits, measured using a questionnaire, in girls with CAH compared to unaffected girls, although the differences was significant only with a one-tailed, not a two-tailed, statistical test (Knickmeyer et al., 2006). A second study was unable to replicate this finding, instead finding similar scores on a measure of autistic traits for females with and without CAH (Kung et al., 2016b). Autistic traits have also not been found to relate to testosterone during mini-puberty (Kung et al., 2016a), or to testosterone measured in umbilical cord blood at birth (Park et al., 2017; Whitehouse et al., 2012).
Some studies have related testosterone measured in amniotic fluid to later autistic traits. Two studies produced significant findings in the expected direction (Auyeung et al., 2009a; Auyeung et al., 2010), but a third study did not (Kung et al., 2016b). Testosterone measured in amniotic fluid has also been examined in relationship to a subsequent ASD diagnosis in boys (Baron-Cohen et al., 2014). This study found that testosterone was not a statistically significant predictor of an ASD diagnosis. However, the authors observed a pattern in their data of generally elevated steroids in boys with ASD, and they conducted a principal component analysis (PCA) of data for five hormones, progesterone, 17-alpha-progesterone, androstenedione, testosterone and cortisol. They found that the first component produced by the PCA explained 49.47% of the variance for the five hormones, and that this component related positively to an ASD diagnosis. Subsequently, the same research group reported that estrogens measured in amniotic fluid in a smaller subset from essentially the same sample related positively to a later diagnosis with ASD (Baron-Cohen et al., 2019). These are intriguing findings that could suggest some aspect of general endocrine function might be altered in male fetuses who will later be diagnosed with ASD, or in their mothers. However, neither result would be predicted based on existing animal models of influences of testosterone or estrogen on gender-related development in primates. It might be informative, however, to develop animal models of general endocrine function that could aid understanding of these surprising findings.
Influences of socialization on the development of gender-related behavior
In addition to the evidence linking early testosterone exposure to later gender-related behavior/psychology, there is extensive evidence that socialization influences gender-related outcomes. For example, children’s preferences for different types of objects (toys) have been found to relate to external socialization and to self-socialization. Research documenting these influences of socialization are described below, as is research evaluating how early testosterone exposure might interact with these same socialization processes to influence outcomes.
In regard to external socialization, parents, peers, and teachers encourage children to play with toys that society views as gender appropriate. For instance, from early infancy parents provide different toys for girls and boys (MacPhee and Prendergast, 2019; Rheingold and Cook, 1975). In addition, when observed playing with toys, parents encourage their children to play with same-gender toys more than other-gender toys and discourage them from playing with other-gender toys (Fagot, 1978; Fagot and Hagan, 1991; Langlois and Downs, 1980; Pasterski et al., 2005). A meta-analysis that included behavioral observation data, as well as other types of data (e.g., self-report), also concluded that parents encourage their children to engage in same-gender activities, including gender-typical toy play (Lytton and Romney, 1991). Similar findings of encouragement of same-gender toy play, and discouragement of other-gender toy play, have been reported for peers and for teachers (Fagot, 1977; Fagot and Patterson, 1969; Lamb et al., 1980; Lamb and Roopnarine, 1979; Langlois and Downs, 1980).
Children also self-socialize gender related behavior. Once they know that they are girls or boys, they tend to imitate or model the object choices of others of their own sex more than of the other sex (Hines et al., 2016; Masters et al., 1979; Perry and Bussey, 1979). They also have been found to prefer objects and activities that have been endorsed by children of their own sex over those endorsed by children of the other sex (Shutts et al., 2010). In addition, if told that certain objects are for their sex or for the other sex, they will subsequently say that they like the objects that they have been told are for their sex more than those that they have been told are for the other sex (Hines et al., 2016; Masters et al., 1979). They also will engage with toys that they have been told are for their own sex more than those described as for the other sex when given a choice in a playroom (Hines et al., 2016; Masters et al., 1979).
How might early testosterone exposure and socialization work together to influence gender-related outcomes?
Some research has examined the possibility that socialization processes are altered in girls with CAH. One study found that parents gave their daughters with CAH more encouragement than their daughters without CAH to play with female-typical toys, when observed in a laboratory playroom (Pasterski et al., 2005). Another study, however, found that parents of girls with CAH reported on a paper and pencil questionnaire that they encouraged their daughters with CAH to engage in more male-typical behavior than they did their daughters who did not have CAH (Wong et al., 2012). Parents of girls with CAH also self-report that they wish that their daughters were more gender-typical in their behavior (Servin et al., 2003). Taken together, these findings suggest that parents of girls with CAH wish that the girls behaved in a more gender-typical way, and that when in a playroom with a range of toys, they encourage their daughters to play with girl-typical toys. On a day to day basis, however, they encourage their daughters with CAH to engage with toys and activities that the girls enjoy, and these tend to be boy-typical toys and activities. Thus, although early androgen exposure may cause girls with CAH to show increased preferences for boy-typical toys, and reduced preferences for girl-typical toys, parents may also contribute to their toy preferences, both by encouraging more female-typical behavior when the opportunity arises, and by encouraging their daughters on a day-to-day basis to engage in the male-typical activities that their daughters enjoy.
What about self-socialization? One study compared girls with CAH to other children in their response to information that certain neutral toys are for girls or for boys (Hines et al., 2016). In this study, children, some of whom had CAH and some of whom were unaffected relatives of children with CAH, were taught that balloons and xylophones of one color were for girls and that those of another color were for boys. They then were asked which balloons and xylophones they preferred, and they were observed in a playroom where balloons and xylophones of both colors were available. Results suggested that, although girls with CAH, like other children, remembered which objects were for girls and which were for boys, this information did not influence their behavior. Other children reported liking objects in the colors that they had been taught were for their own sex as opposed to the other sex, and engaged more with these objects when observed in a playroom. In contrast, girls with CAH did not. Thus, processes of self-socialization appear to be altered in girls with CAH. This too could be an additional mechanism by which testosterone leads to reduced interest in girl-typical toys and activities, and increased interest in boy-typical toys and activities, in girls with CAH.
What about pink?
Another behavior that shows a large sex difference in children involves preference for the color pink. This sex difference has received relatively little scientific attention, and there are no meta-analytic findings, or population findings, that can provide a reliable size for the difference. One study of two hundred children ages 3 to 12 years, however, found that about 52% of girls, but only 5% of boys, named pink/purple as their favorite color (Chiu et al., 2006).
The small amount of available research suggests that the sex difference in preference for pink becomes apparent at a later age than do sex differences in preferences for toys. One study found that children ages 12 to 24 months already showed statistically significant sex differences in preferences for dolls and toys vehicles, but that they did not yet show a sex difference in preference for pink versus blue (Jadva et al., 2010). Subsequent research suggests that the sex difference in preference for pink emerges between 24 and 36 months of age (LoBue and DeLoache, 2011; Wong and Hines, 2015). At about this same age, children have become aware that they are girls or boys, and over the next several years they gain a more sophisticated understanding of their sex assignment, learning that this will not change over time or if they engage in other-gender activities (Ruble et al., 2007; Slaby and Frey, 1975; Thompson, 1975). During this same period the size of the sex difference in preference for pink grows (LoBue and DeLoache, 2011; Wong and Hines, 2015). By adulthood, the sex difference in this color preference is diminished, with women showing a slightly greater liking of pink than men do, but with both sexes preferring blue to pink (Palmer, 2010; Wong and Hines, 2015).
Given the changes in preference for pink over the lifespan, it seems unlikely that this particular aspect of human color preference is evolutionarily determined, although this has been suggested by some researchers. For instance, some theorists have suggested that females evolved to like pinkish/reddish colors because these are the colors of fruits or berries for which they foraged, and that this evolved behavioral adaptation is wired into the different visual systems of males and females (Alexander, 2003; Hurlbert and Ling, 2007). If this were the case, the sex difference in preference for pink might be expected to be larger in adults than in children, since adults would do most of the foraging, but, on the contrary, the sex difference appears to be larger in children than in adults.
An alternative theory suggests that color preferences can be acquired through recent experience (Palmer, 2010). This ecological valence theory proposes that people learn to like colors that are associated with pleasant affective experiences, and dislike those that are associated with unpleasant affective experiences. For instance, most adults, both men and women, might like blue, because it is associated with clear skies and clean water. Some support for the ecological valence theory came from evidence that people’s affective valence estimates of objects of given colors correlated with other people’s liking for the colors (Palmer, 2010). A study of the color preferences of students at Stanford University and at the University of California Berkeley provided additional support. The school colors at Stanford are red and white, whereas those at Berkeley are blue and gold. Not only do students at Stanford like red and white more than students at Berkeley, and students at Berkeley like blue and gold more than students at Stanford, the discrepancy in color preference correlates with students’ identification with their University (Schloss et al., 2011). Extrapolating to girls’ preference for pink, children may learn to like the colors of the toys that are typed for their own sex, pink for girls, and anything but pink for boys, contributing to the stronger preference for pink in girls than in boys. Girls also may identify strongly with their assigned sex during early childhood, and so may show particularly strong preferences for pink compared to older ages. With the fuller understanding of gender that comes with age, and with increased engagement with objects that are not strongly color coded, girls’ preference for pink might diminish.
Summary
Gender is multi-dimensional and its causes are multi-faceted. We are far from a full understanding of the developmental systems involved in the development of gender-related characteristics. For human gender development, current evidence suggests that early testosterone exposure contributes to the development of gender role behavior, particularly gender-typed object (toy) preferences, as well as to sexual orientation and gender identity. Evidence for similar influences on mental rotation or other aspects of spatial ability is inconsistent, and positive findings may be spurious. In addition, evidence that early testosterone exposure contributes to autistic traits or a diagnosis with ASD is inconsistent both across studies and with predictions based on how gonadal steroids relate to behavior in other species. Although early exposure to testosterone contributes to gender-typed toy preferences, socialization processes also are influential. Some evidence suggests that external socialization as well as self-socialization might be altered in girls who were exposed prenatally to elevated testosterone concentrations because of CAH. Thus, early androgen exposure might act in concert with socialization processes to increase male-typical interests and reduce female-typical interests. Finally, the sex difference in preference for the color pink appears at a later age than sex differences in toy interests. The available evidence suggests that it may be caused, at least in part, by girls having positive affective experiences engaging with toys and other objects that are color coded pink to indicate that they are for girls.
Highlights.
Early testosterone (T) exposure and socialization influence gender development
Early T influences gender role behavior, gender identity and sexual orientation
Evidence that early T influences spatial skill or autistic traits is inconsistent
Early T leads to altered processes of socialization
Acknowledgements
This work was supported in part by the National Institutes of Health [Grant number R01HD-081720].
I thank Dr. Debra Spencer for help creating the figures for the manuscript.
Footnotes
Declarations of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, 2003. An evolutionary perspective of sex-typed toy preferences: pink, blue, and the brain. Archives of Sexual Behavior 32, 7–14. [DOI] [PubMed] [Google Scholar]
- American Psychiatric A, 2000. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR: Fourth Edition Text Revision. American Psychiatric Publishing, Washington. [Google Scholar]
- Arnold AP, 2009. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and Behavior 55, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, 2009a. Fetal testosterone and autistic traits. British Journal of Psychology 100, 1–22. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Chapman E, Knickmeyer R, Taylor K, Hackett G, Hines M, 2009b. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychological Science 20, 144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Knickmeyer R, Ashwin E, Taylor K, Hackett G, Baron-Cohen S, 2012. Effects of fetal testosterone on visuospatial ability. Archives of Sexual Behavior 41, 571–581. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Taylor K, Hackett G, Baron-Cohen S, 2010. Foetal testosterone and autistic traits in 18 to 24-month-old children. Molecular Autism 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SW, Ehrhardt AA, 1974. Prenatal androgen, intelligence and cognitive sex differences, in: Friedman RC, Richart RN, Vande Wiele RL (Eds.), Sex differences in behavior. Wiley, New York, pp. 53–76. [Google Scholar]
- Baron-Cohen S, 2002. The extreme male brain theory of autism. Trends in Cognitive Science 6, 248–254 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, 2003. The essential difference: the truth about the male and female brain. Basic Books, New York. [Google Scholar]
- Baron-Cohen S, Auyeung B, Norgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Cohen AS, Chakrabarti B, Ruta L, Lombardo MV, 2014. Elevated fetal steroidogenic activity in autism. Molecular Psychiatry doi: 10.1038/mp.2014.48, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Tsompanidis A, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah M, Cohen A, Pohl A, 2019. Foetal oestrogens and autism. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M, 1992. Early androgens are related to childhood sex-typed toy preferences. Psychological Science 3, 203–206. [Google Scholar]
- Brizendine L, 2007. The female brain. Bantam, London. [Google Scholar]
- Cohen J, 1988. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hillsdale, N.J. [Google Scholar]
- Collaer ML, Hindmarsh P, Pasterski V, Fane BA, Hines M, 2016. Reduced short term memory in congenital adrenal hyperplasia (CAH) and its relationship to spatial and quantitative performance. Psychoneuroendocrinology 64, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaer ML, Hines M, 2020. No Evidence for Enhancement of Spatial Ability with Elevated Prenatal Androgen Exposure in Congenital Adrenal Hyperplasia: A Meta-Analysis. Archives of Sexual Behavior 49, 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu M, Hines M, 2012. Relating prenatal testosterone exposure to postnatal behavior in typically developing children: methods and findings. Child Development Perspectives 6, 407–413. [Google Scholar]
- Constantinescu M, Moore DS, Johnson SP, Hines M, 2018. Early contributions to infants’ mental rotation abilities. Developmental science 21, e12613. [DOI] [PubMed] [Google Scholar]
- Costa PT, Terracciano A, McCrae RR, 2001. Gender differences in personality traits across cultures: Robust and surprising findings. Personality and Social Psychology 81, 322–331. [DOI] [PubMed] [Google Scholar]
- Davis JTM, Hines M, 2020. How large are gender differences in toy preferences: a systematic review and meta-analysis of toy preference research. Archives of Sexual Behavior 49, 373–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens AB, Slijper FME, Drop SLS, 2005. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Archives of Sexual Behavior 34, 389–397. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes ME, Kappes MH, 1992. Sexual behavior in adolescent and adult females with congenital adrenal hyperplasia. Psychoneuroendocrinology 17, 153–170. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, 1993. Cognitive functioning in female patients with 21-hydroxylase deficiency. European Child and Adolescent Psychiatry 2. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Borger D, Meyer-Bahlburg HFL, Stegner H, Willig RH, Wallis H, 1990a. Congenital adrenal hyperplasia II: Gender-related behavior and attitudes in female salt-wasting and simple virilizing patients. Psychoneuroendocrinology 15, 421–434. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Borger D, Stegner H, Willig RH, Wallis H, 1990b. Congenital Adrenal Hyperplasia I: Gender-related behavior and attitudes in female patients and sisters. Psychoneuroendocrinology 15, 401–420. [DOI] [PubMed] [Google Scholar]
- Ehrhardt AA, Baker SW, 1974. Fetal androgens, human central nervous system differentiation, and behavior sex differences, in: Friedman RC, Richart RM, van de Wiele RL (Eds.), Sex differences in behavior. Wiley, New York, pp. 33–52. [Google Scholar]
- Fagot BI, 1977. Consequences of moderate cross-gender behavior in preschool children. Child Development 48, 902–907. [Google Scholar]
- Fagot BI, 1978. The influence of sex of child on parental reactions to toddler children. Child Development 49, 459–465. [Google Scholar]
- Fagot BI, Hagan R, 1991. Observations of parent reactions to sex-stereotyped behaviors: Age and sex effects. Child Development 62, 617–628. [DOI] [PubMed] [Google Scholar]
- Fagot BI, Patterson GR, 1969. An in vivo analysis of reinforcing contingencies for sex-role behaviors in the preschool child. Developmental Psychology 5, 563–568. [Google Scholar]
- Feingold A, 1994. Gender differences in personality: A meta-analysis. Psychological Bulletin 116, 429–456 [DOI] [PubMed] [Google Scholar]
- Fine C, 2010. Delusions of gender. Norton, New York. [Google Scholar]
- Finegan JK, Niccols GA, Sitarenios G, 1992. Relations between prenatal testosterone levels and cognitive abilities at 4 years. Developmental Psychology 28, 1075–1089. [Google Scholar]
- Forest MG, Cathiard AM, Bertrand JA, 1973. Evidence of testicular activity in early infancy. Journal of Clinical Endocrinology and Metabolism 41, 751–760. [DOI] [PubMed] [Google Scholar]
- Frisen J, Nordenstrom A, Falhammar H, Filipsson H, Holmdahl G, Janson PO, Thoren M, Hagenfeldt K, Moller A, Nordenskjold A, 2009. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. Journal of Clinical Endocrinology and Metabolism 94, 3432–3439. [DOI] [PubMed] [Google Scholar]
- Gastaud F, Bouvattier C, Duranteau L, Brauner R, Thibaud E, Kutten F, et al. , 2007. Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 92, 1391–1396. [DOI] [PubMed] [Google Scholar]
- Golombok S, Rust J, Zervoulis K, Croudace T, Golding J, Hines M, 2008. Developmental trajectories of sex-typed behavior in boys and girls: A longitudinal general population study of children aged 2.5 – 8 years. Child Development 79, 1583–1593. [DOI] [PubMed] [Google Scholar]
- Grimshaw GM, Sitarenios G, Finegan JK, 1995. Mental rotation at 7 years: Relations with prenatal testosterone levels and spatial play experiences. Brain and Cognition 29, 85–100. [DOI] [PubMed] [Google Scholar]
- Hall CM, Jones JA, Meyer-Bahlburg HFL, Dolezal C, Coleman M, Foster P, Price DA, Clayton PE, 2004. Behavioral and physical masculinization are related to genotype in girls with congenital adrenal hyperplasia. The journal of Clinical Endocrinology and Metabolism 89, 419–424. [DOI] [PubMed] [Google Scholar]
- Hampson E, Rovet J, Altmann D, 1998. Spatial reasoning in children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Developmental Neuropsychology 14, 299–320. [Google Scholar]
- Helleday J, Bartfai A, Ritzen EM, Forsman M, 1994. General intelligence and cognitive profile in women with congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology 19, 343–356. [DOI] [PubMed] [Google Scholar]
- Hines M, 2004. Brain gender. Oxford University Press, New York. [Google Scholar]
- Hines M, 2015. Gendered development, in: Lerner RM, Lamb ME (Eds.), Handbook of Child Development and Developmental Science. Wiley, Hoboken, NJ, pp. 842–887. [Google Scholar]
- Hines M, 2020. Neuroscience and sex/gender: looking back and forward. Journal of Neuroscience 40, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Ahmed SF, Hughes I, 2003a. Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Archives of Sexual Behavior 32, 93–101. [DOI] [PubMed] [Google Scholar]
- Hines M, Brook C, Conway GS, 2004. Androgen and psychosexual development: Core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH). Journal of Sex Research 41, 75–81. [DOI] [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C, 2003b. Spatial abilities following prenatal androgen abnormality: Targeting and mental rotations performance in individuals with congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology 28, 1010–1026. [DOI] [PubMed] [Google Scholar]
- Hines M, Golombok S, Rust J, Johnston K, Golding J, The AST, 2002a. Testosterone during pregnancy and childhood gender role behavior: A longitudinal population study. Child Development 73, 1678–1687. [DOI] [PubMed] [Google Scholar]
- Hines M, Johnston K, Golombok S, Rust J, Stevens M, Golding J, The AST, 2002b. Prenatal stress and gender role behavior in girls and boys: A longitudinal, population study. Hormones and Behavior 42, 126–134. [DOI] [PubMed] [Google Scholar]
- Hines M, Pasterski V, Spencer D, Neufeld S, Patalay P, Hindmarsh PC, Hughes IA, Acerini CL, 2016. Prenatal androgen exposure alters girls’ responses to information indicating gender-appropriate behaviour. Philosophical Transactions of the Royal Society B 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J, 2012. Androgen insensitivity syndrome. The Lancet 380. [DOI] [PubMed] [Google Scholar]
- Hurlbert AC, Ling Y, 2007. Biological components of sex differences in color preference. Current Biology 17, R623–R625. [DOI] [PubMed] [Google Scholar]
- Hyde JS, 2005. The gender similarities hypothesis. American Psychologist 60, 581–592. [DOI] [PubMed] [Google Scholar]
- Iijima M, Ariska O, Minamoto F, Arai Y, 2001. Sex differences in children’s free drawings: A study on girls with congenital adrenal hyperplasia. Hormones and Behavior 40, 99–104. [DOI] [PubMed] [Google Scholar]
- Jadva V, Golombok S, Hines M, 2010. Infants’ preferences for toys, colors and shapes. Archives of Sexual Behavior 39, 1261–1273. [DOI] [PubMed] [Google Scholar]
- Jordan-Young RM, 2010. Brainstorm: The flaws in the science of sex differences. Harvard University Press, Cambridge, MA. [Google Scholar]
- Jurgensen M, Hiort O, Holterhus PM, Thyen U, 2007. Gender role behavior in children with XY karyotype and disorders of sex development. Hormones and Behavior 51, 443–453. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Baron-Cohen S, Fane BA, Wheelwright S, Mathews GA, Conway GS, Brook CG, Hines M, 2006. Androgens and autistic traits: a study of individuals with congenital adrenal hyperplasia. Hormones and Behavior 50, 148–153. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Wheelwright S, Hackett G, Taylor K, Raggatt P, Baron-Cohen S, 2005. Gender-typed play and amniotic testosterone. Developmental Psychobiology 41, 517–528. [DOI] [PubMed] [Google Scholar]
- Kuiri-Hanninen T, Seuri R, Tyrvainen E, Turpeinen U, Hamalainen E, Stenman UH, Dunkel L, Sankilampi U, 2011. Increased activity of the hypothalamic-pituitary-testicular axis in infancy results in increased androgen action in premature boys. Journal of Clinical Endocrinology and Metabolism 96, 98–105. [DOI] [PubMed] [Google Scholar]
- Kung KTF, Constantinescu M, Browne WV, Noorderhaven RM, Hines M, 2016a. No relationship between early postnatal testosterone concentrations and autistic traits in 18- to 30-month-old children. Molecular Autism 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung KTF, Spencer D, Pasterski V, Neufeld S, Glover V, O’Connor TG, Hindmarsh PC, Hughes IA, Acerini CL, Hines M, 2016b. No relationship between prenatal androgen exposure and autistic traits: convergent evidence from studies of children with congenital adrenal hyperplasia and of amniotic testosterone concentrations in typically-developing children. Journal of Child Psychology & Psychiatry 57, 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb ME, Easterbrooks MA, Holden GW, 1980. Reinforcement and punishment among preschoolers: characteristics, effects, and correlates. Child Development 51, 1230–1236. [Google Scholar]
- Lamb ME, Roopnarine JL, 1979. Peer influences on sex-role development in preschoolers. Child Development 50, 1219–1222. [Google Scholar]
- Lamminmaki A, Hines M, Kuiri-Hanninen T, Kilpelainen L, Dunkel L, Sankilampi U, 2012. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Hormones and Behavior 61, 611–616. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Downs AC, 1980. Mothers, fathers and peers as socialization agents of sex-typed play behaviors in young children. Child Development 51, 1217–1247. [Google Scholar]
- Lickliter R, Honeycutt H, 2015. Biology, development and human systems, in: Lerner RM, Overton WF, Molenaar PCM (Eds.), Child psychology and developmental science. 7th ed. Wiley, Hoboken, NJ, pp. 162–207. [Google Scholar]
- LoBue V, DeLoache JS, 2011. Pretty in pink: the early development of gender-stereotyped colour preferences. British Journal of Developmental Psychology 29, 656–667. [DOI] [PubMed] [Google Scholar]
- Lytton H, Romney DM, 1991. Parents’ differential socialization of boys and girls: A meta-analysis. Psychological Bulletin 109, 267–296. [Google Scholar]
- MacPhee D, Prendergast S, 2019. Room for improvement: girls’ and boys’ home environments are still gendered. Sex Roles 80, 332–346. [Google Scholar]
- Malouf MA, Migeon CJ, Carson KA, Pertrucci L, Wisniewski AB, 2006. Cognitive Outcome in Adult Women Affected by Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. Hormone Research 65, 142–150. [DOI] [PubMed] [Google Scholar]
- Masters JC, Ford ME, Arend R, Grotevant HD, Clark LV, 1979. Modeling and labelling as integrated determinants of children’s sex-typed imitative behavior. Child Development 50, 364–371. [Google Scholar]
- Mazur T, 2005. Gender dysphoria and gender change in androgen insensitivity or micropenis. Archives of Sexual Behavior 34, 411–421. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, 2008. Estradiol and the developing brain. Physiological Reviews 88, 91–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, 2010. Gender outcome in 46, XY complete androgen insensitivity syndrome: comment on T’Sjoen et al. (2010). Archives of Sexual Behavior 39, 1221–1224. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Dolezal C, Baker SW, Ehrhardt AA, New MI, 2006. Gender development in women with Congenital Adrenal Hyperplasia as a function of disorder severity. Archives of Sexual Behavior 35, 667–684. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Dolezal C, Baker SW, New MI, 2008. Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Archives of Sexual Behavior 37, 85–99. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Gruen RS, New MI, Bell JJ, Morishima A, Shimshi M, Bueno Y, Vargas I, Baker SW, 1996. Gender change from female to male in classical congenital adrenal hyperplasia. Hormones and Behavior 30, 319–332. [DOI] [PubMed] [Google Scholar]
- Money J, Ehrhardt A, 1972. Man and Woman: Boy and Girl. Johns Hopkins University Press, Baltimore. [Google Scholar]
- Money J, Schwartz M, Lewis V, 1984. Adult erotosexual status and fetal hormonal masculinization and demasculinization: 46 XX congenital virilizing adrenal hyperplasia and 46 XY androgen-insensitivity syndrome compared. Psychoneuroendocrinology 9, 405–414. [DOI] [PubMed] [Google Scholar]
- Nordenstrom A, Larsson A, 2003. Prenatal androgens and gender-typed behavior: A study of girls with mild and severe forms of congenital adrenal hyperplasia. Developmental Psychology 39, 440–450. [DOI] [PubMed] [Google Scholar]
- Nordenstrom A, Servin A, Bohlin G, Larsson A, Wedell A, 2002. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 87, 5119–5124. [DOI] [PubMed] [Google Scholar]
- Palmer SE, 2010. An ecological valence theory of human color preference. Proceedings National Academy of Sciences 107, 8877–8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Levine LS, Cederqvist LL, Fuentes M, Riccardi VM, Holcombe JH, Nitowsky HM, Sachs G, Anderson CE, Duchon MA, Owens R, Merkatz I, New MI, 1980. Amniotic fluid concentrations of delta 5 and delta 4 steroids in fetuses with congenital adrenal hyperplasia due to 21-hydroxylase deficiency and in anencephalic fetuses. Journal of Clinical Endocrinology and Metabolism 51, 223–229. [DOI] [PubMed] [Google Scholar]
- Pang S, Levine LS, Chow DM, Faiman C, New MI, 1979. Serum androgen concentrations in neonates and young infants with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clinical Endocrinology 11, 575–584. [DOI] [PubMed] [Google Scholar]
- Park BY, Lee BK, Burstyn I, Tabb LP, Keelan JA, Whitehouse AJO, Croen LA, Fallin MD, Hertz-Picciotto I, Montgomery O, Newschaffer CJ, 2017. Umbilical cord blood androgen levels and ASD-related phenotypes at 12 and 36 months in an enriched risk cohort study. Molecular Autism 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski V, Acerini CL, Dunger DB, Ong KK, Hughes IA, Thankamony A, 2015a. Postnatal penile growth concurrent with mini-puberty predicts later gender-typed behavior: evidence for neurobehavioral effects of the postnatal androgen surge in typically developing boys. Hormones and Behavior 69, 98–105. [DOI] [PubMed] [Google Scholar]
- Pasterski VL, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M, 2005. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Development 76, 264–278. [DOI] [PubMed] [Google Scholar]
- Pasterski VL, Geffner ME, Brain C, Hindmarsh PC, Brook C, Hines M, 2011. Prenatal hormones and childhood sex segregation: Playmate and play style preferences in girls with congenital adrenal hyperplasia. Hormones and Behavior 59, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski VL, Zucker KJ, Hindmarsh PC, Hughes IA, Acerini CL, Spencer D, Neufeld S, Hines M, 2015b. Increased cross-gender identification independent of gender role behavior in girls with Congenital Adrenal Hyperplasia: results from a standardized assessment of 4–11-year-old children. Archives of Sexual Behavior 44, 1363–1375. [DOI] [PubMed] [Google Scholar]
- Perry DG, Bussey K, 1979. The social learning theory of sex difference: Imitation is alive and well. Journal of Personality & Social Psychology 37, 1699–1712. [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC, 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology (Baltimore) 65, 163–196. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM, 1973. Sexual dimorphism in the neurophil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Research 54, 1–29. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Berenbaum SA, Gottesman II, Bouchard T, 1986. Early hormonal influences on cognitive functioning in congenital adrenal hyperplasia. Developmental Psychology 22, 191–198. [Google Scholar]
- Rheingold HL, Cook KV, 1975. The content of boys’ and girls’ room as an index of parents’ behavior. Child Development 46, 459–463. [Google Scholar]
- Ruble DN, Taylor LJ, Cyphers L, Greulich FK, Lurye LE, Shrout PE, 2007. The role of gender constancy in early gender development. Child Development 78, 1121–1136. [DOI] [PubMed] [Google Scholar]
- Schloss KB, Poggesi RM, Palmer SE, 2011. Effects of university affiliation and “school spirit” on color preferences: Berkeley versus Stanford. Psychonomic Bulletin and Review 18, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin A, Nordenstrom A, Larsson A, Bohlin G, 2003. Prenatal androgens and gender-typed behavior: A study of girls with mild and severe forms of congenital adrenal hyperplasia. Developmental Psychology 39, 440–450. [DOI] [PubMed] [Google Scholar]
- Shutts K, Banaji MR, Spelke ES, 2010. Social categories guide young children’s preferences for novel objects. Developmental Science 13, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby RG, Frey KS, 1975. Development of Gender Constancy and Selective. Child Development 46, 849–856. [PubMed] [Google Scholar]
- Slijper FME, 1984. Androgens and gender role behaviour in girls with congenital adrenal hyperplasia (CAH), in: De Vries GJ, De Bruin JPC, Uylings HBM, Corner MA (Eds.), Progress in Brain Research. Elsevier, Amsterdam, pp. 417–422. [DOI] [PubMed] [Google Scholar]
- Smail PJ, Reyes FI, Winter JSD, Faiman C, 1981. The fetal hormone environment and its effect on the morphogenesis of the genital system, in: Kogan SJ, Hafez ESE (Eds.), Pediatric Andrology. Martinus Nijhoff, The Hague, pp. 9–20. [Google Scholar]
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC, 2018. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 103, 4043–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T’Sjoen G, De Cuypere G, Monstrey S, Hoebeke P, Freedman FK, Appari M, Holterhus PM, Van Borsel J, Cools M, 2011. Male gender identity in complete androgen insensitivity syndrome. Archives of Sexual Behavior 40, 635–638. [DOI] [PubMed] [Google Scholar]
- Thompson SK, 1975. Gender labels and early sex role development. Child Development 46, 339–347. [PubMed] [Google Scholar]
- van de Beek C, Van Goozen SHM, Buitelaar JK, Cohen-Kettenis PT, 2009. Prenatal sex hormones (maternal and amniotic fluid) and gender-related play behavior in 13-month-old infants. Archives of Sexual Behavior 38, 6–15. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP, 1995. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychological Bulletin 117, 250–270. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Mattes EMMT, Dissanayake C, Sawyer M, Jones RM, Pennell CE, Keelan JA, Hickey M, 2012. Perinatal teststerone exposure and autistic-like traits in the general population: A longitudinal pregnancy-cohort study. Journal of Neurodevelopmental Disorders 4, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD, George FW, Griffin JE, 1981. The hormonal control of sexual development. Science 211, 1278–1284. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Migeon CJ, Meyer-Bahlburg HFL, Gearhart JP, Berkovitz B, Brown TJ, Money J, 2000. Complete androgen insensitivity syndrome. Long-term medical, surgical, and psychosexual outcome. Journal of Clinical Endocrinology and Metabolism 85, 2664–2669. [DOI] [PubMed] [Google Scholar]
- Wong WI, Hines M, 2015. Preferences for pink and blue: the development of color preferences as a distinct gender-typed behavior in toddlers. Archives of Sexual Behavior 44, 1243–1254. [DOI] [PubMed] [Google Scholar]
- Wong WI, Pasterski VL, Hindmarsh PC, Geffner ME, Hines M, 2012. Are there parental socialization effects on the sex-typed behavior of individuals with congenital adrenal hyperplasia? Archives of Sexual Behavior DOI 10.1007/s10508-012-9997-4. [DOI] [PubMed] [Google Scholar]
- Zucker KJ, Bradley SJ, Oliver G, Blake J, Fleming S, Hood J, 1996. Psychosexual development of women with congenital adrenal hyperplasia. Hormones and Behavior 30, 300–318. [DOI] [PubMed] [Google Scholar]