Significance
Improving the long-term stability of sulfur cathodes is of great importance to the development of Na-S (sodium-sulfur) batteries. Here, we report a chemical and spatial dual-confinement engineering for reforming the stability of Na-S batteries. It secures S species through forming the robust covalent C-S bond and sealing S1-S2 small species in the confined pores as well. This promotes the formation of a coordination structure (N···Na···S) without breaking the C-S bond during the discharge process. Impressively, the Na-S batteries exhibit a good electrochemical performance featured by a high-capacity retention. This work sheds light on the design of high-energy and long-lifespan Na-S batteries and other S-based batteries.
Keywords: Na-S batteries, chemical and spatial dual-confinement, covalent sulfur, functional carbon materials, high-capacity retention
Abstract
Sodium-sulfur (Na-S) batteries are attracting intensive attention due to the merits like high energy and low cost, while the poor stability of sulfur cathode limits the further development. Here, we report a chemical and spatial dual-confinement approach to improve the stability of Na-S batteries. It refers to covalently bond sulfur to carbon at forms of C-S/N-C=S bonds with high strength for locking sulfur. Meanwhile, sulfur is examined to be S1-S2 small species produced by thermally cutting S8 large molecules followed by sealing in the confined pores of carbon materials. Hence, the sulfur cathode achieves a good stability of maintaining a high-capacity retention of 97.64% after 1000 cycles. Experimental and theoretical results show that Na+ is hosted via a coordination structure (N···Na···S) without breaking the C-S bond, thus impeding the formation and dissolution of sodium polysulfide to ensure a good cycling stability. This work provides a promising method for addressing the S-triggered stability problem of Na-S batteries and other S-based batteries.
Sodium-ion batteries (SIBs) with multiple merits of low cost, high safety, and abundant reserves have attracted worldwide attention in the large-scale energy storage. The relatively low capacity of cathode materials for SIBs, such as Na0.78Ni0.23Mn0.69O2 (1), NaVPO4F/C (2), and Na2CoFe(CN)6 (3), limits the improvement of energy density. Sodium-sulfur (Na-S) batteries with using sulfur cathode have been considered a promising battery technology due to the high theoretical specific capacity (1,672 mAh g−1) and energy density (1,274 Wh kg−1) and abundant supply of sodium and sulfur (4, 5). Despite these advantages, Na-S batteries still suffer from many challenges, including a) the inherent insulating property of sulfur leads to the poor charge transfer (~5.1*10−30 S cm−1) and low utilization (6); b) sulfur has a large volume expansion (260% from S8 to Na2S), which prevents the stable capacity output (4, 5); c) the intermediate products of the redox reaction between sulfur and sodium are mainly soluble sodium polysulfide (Na2Sx, 4 ≤ x ≤ 6), causing a serious shuttle effect (7); and d) the reaction kinetics related to the solid-state sodium sulfide is sluggish (8–10). It is necessary to develop effective strategies to address the challenges mentioned above to promote the practical use of Na-S batteries.
Carbon materials with good electrical conductivity and chemical stability and tunability demonstrate great potential in sulfur cathodes (11–13). The pore structure of carbon materials has been developed to host sulfur. A complex microporous carbon material of multichamber carbon nanospheres encapsulated by carbon shells was synthesized to achieve a high sulfur loading and provide a physical barrier for suppressing polysulfide dissolution (14). In addition, by adjusting the micropore size, the long-chain sulfur molecules (Sx, x > 4) can be selectively removed while the suitable short-chain sulfur molecules (Sx, x ≤ 4) can be retained, which avoids the irreversible dissolution of the intermediate polysulfide products to a certain extent (15). For instance, an ultramicroporous carbon (0.55 nm) was prepared by the pyrolysis of polyvinylidene fluoride (PVDF) is capable of accommodating S2-S4 small molecules (16). Nonetheless, this method of confining small sulfur in ultramicropores demands much on the design of the carbon matrix and cannot fundamentally circumvent issues regarding the formation and dissolution of polysulfides.
Covalent chemical bond exhibits a strong anchoring ability of sulfur species for protecting sulfur cathode. The chemical method of grafting sulfur onto carbon materials by covalent bonds is an effective method to inhibit the polysulfide shuttle (17). It has been reported that benzodithiophene-4,8-dione and sulfur powder were mixed and pyrolyzed to construct covalent sulfur-based carbonaceous materials (18). The formation of Na2Sx (4 ≤ x ≤ 8) was avoided to a certain degree, whereas the cycling performance is still poor featured by a low-capacity retention of 61.6% after only 50 cycles. A covalent sulfur–carbon bridged hybrid was synthesized using phenylphosphinic acid as the carbon source/catalyst and sodium sulfate as the sulfur precursor/salt template (19). During the discharge process, the C-S and S-S bonds were broken to form Na2S and the carbon skeleton was irreversibly isomerized, leading to an inferior capacity retention of 78.3% after 200 cycles. Sulfur could also be covalently anchored on dopamine-coated graphene-derived nitrogen-doped two-dimensional carbon substrates by a high-temperature vapor-infiltration method (20). The short-chain sulfur reacts with Na+, and the C-S bond is broken to form Na2S during the discharge process, resulting in a low sulfur utilization rate of 75.6% (for the second cycle) due to the serious loss of sulfur. The main source of sulfur mentioned above is inorganic sulfur powder. Organic sulfur compounds have also attracted intensive attention because of their inherent S-containing covalent bond for avoiding the shuttle effect. 4, 4′-thiobisbenzenethiol as an organic sulfur cathode material was uncovered to form an organosulfur oligomer consisting of two (S-R-S)n during the charge process, thus ensuring a good stability and a reversible capacity of 210 mAh g−1 (21). However, the limited content of sulfur in organic sulfur molecules appears to impede the enhancement of the specific capacity. Organic polysulfides are likely to achieve a high specific capacity due to the presence of many sulfur species. Dimethyl trisulfide was reported to deliver a large specific capacity because the additional intermediate sulfur atoms could upgrade the capacity compared to dimethyl disulfide (22). A series of dipyridyl polysulfides (such as Py2S3, Py2S4, Py2S5, Py2S6, Py2S7, and Py2S8) were synthesized from dipyridyl disulfide and sulfur, which show a reversible electrochemical behavior (23). However, the low electrical conductivity of organosulfur compounds requires the overuse of conductive additives, which undoubtedly reduces the overall energy density of the battery. Generally, introducing covalently bonded sulfur represents a good approach to addressing the stability problem of the sulfur cathode, whereas it still encounters the challenges of capacity decay and/or low conductivity. In addition, the construction of covalent C-S bonds from the inorganic sulfur powder also meets the challenge at the atomic level because C-S bonds are longer and have lower bond energies than C-C/C=C bonds, which calls for regulating the electronic and chemical environments (24).
Herein, we report a chemical and spatial dual-confinement engineering to fundamentally address the sulfur-related problems for stable Na-S batteries. Small sulfur species (S1 and S2) are produced by thermally cutting the large S8 molecules and then sealed in the confined pores of carbon materials, and meanwhile, the covalent C-S/N-C=S bonds are constructed with the assistance of regulating the electronic and chemical environments by N atoms. The construction of the dual-confinement sulfur material is probed using the in situ Fourier transfer infrared spectroscopy (FTIR). The existence form of sulfur in carbon materials is examined by time-of-flight secondary ion mass spectrometry (TOF-SIMS). The electrochemical performance such as specific capacity and cycling stability of sulfur cathode are systematically evaluated. The evolution mechanism of the C-S covalent bond during the sodium storage process is investigated in depth by multiple spectroscopic analysis such as ultraviolet-visible (UV-vis) absorption spectroscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and theoretical calculations.
Results
Fig. 1 demonstrates the synthetic process of nitrogen-doped template-induced pitch-derived carbon (PC) loading with covalent sulfur (N/TPC-S) and the existing form of sulfur in the carbon matrix. The long-chain S8 is broken during the pyrolysis process, and with the polycondensation of polycyclic aromatic molecules in pitch, it is spatially confined in the carbon matrix in the form of S2 small molecules or single-atomic sulfur, which avoids the generation of soluble polysulfide. Simultaneously, the nitrogenous groups produced by the decomposition of urea promote the formation of C-S bonds, thus building a stable N-C=S bond. Benefitting from the spatial and chemical dual-confinement, the robust integration of sulfur species with the carbon material can maintain a good stability and afford a high electrical conductivity during the discharge and charge processes. In contrast, the conventional molten sulfur infusion method is also conducted by loading sulfur on PC (PC@S). Sulfur species are mainly physically adsorbed on PC in the form of large sulfur molecules such as S8 and S6, which are chemically/physically unstable at the sulfur–carbon interface.
Fig. 1.
Comparison of the synthetic processes (route 1: the spatial and chemical dual-confinement method; route 2: the conventional molten sulfur infusion method) for sulfur–carbon composite material.
We study the structure characteristics of PC, template-induced PC (TPC) loading with covalent sulfur (TPC-S), nitrogen-doped PC loading with covalent sulfur (N/PC-S), and N/TPC-S. PC, TPC, and TPC-S show a blocky morphology due to the polycondensation reaction of polycyclic aromatic molecules during the thermal treatment process (SI Appendix, Figs. S1 and S2). Different from PC and TPC-S, N/TPC-S exhibits a porous structure made up of lamellar units because of using polystyrene as a template. This structure can facilitate the rapid penetration of the electrolyte and shorten the migration path of Na+. In the XRD patterns, compared with PC, TPC-S, N/PC-S, and N/TPC-S show weak peaks (SI Appendix, Fig. S3), suggesting the inhibited graphitization. Compared with N/TPC-S, PC@S shows strong signals of sulfur in the XRD data, which implies that large sulfur molecules appear in PC@S while small sulfur species exist in N/TPC-S. As shown in SI Appendix, Fig. S4, the D band (disorder-induced region) and G band (graphitization region) appear at about 1,355 and 1,570 cm−1. The intensity ratio of D and G bands (ID/IG) is calculated to be 1.49 (N/PC-S) > 1.26 (TPC-S) > 0.95 (N/TPC-S) > 0.71 (PC), confirming that introducing S and N can effectively inhibit the thermal polycondensation of the polycyclic aromatic molecules and thus reduce the graphitization degree (25, 26). The high-resolution transmission electron microscopy (HRTEM) images of PC show the long-range ordered graphite layers, indicating a high degree of graphitization (SI Appendix, Fig. S5). The HRTEM images of N/TPC-S uncovers the coexistence of graphitic microcrystals and amorphous domains (SI Appendix, Fig. S6). This microcrystalline structure demonstrates the dual advantages of conductivity and ion diffusion, which are conducive to promote the insertion/extraction of Na+. The pore structure of PC, nitrogen-doped template-induced PC (N-TPC), and N/TPC-S are analyzed by the N2 adsorption/desorption experiment (SI Appendix, Fig. S7). The N/TPC-S shows a sharp increase of adsorption capacity at a low P/P0 compared with N-TPC. Different from previous sulfur-filled cathode materials, N/TPC-S exhibits an increased specific surface area after loading sulfur (SI Appendix, Table S1). This is mainly due to the large number of microporous distributed inside the material, which may be caused by the decomposition and volatilization of S molecules during the thermal treatment (27). The abundant pore structure and large specific surface area can increase the electron contact area between the electrolyte and electrode, shorten the transport distance of Na+ and electrons, and enhance the sodium storage performance. Thermogravimetric analysis results show the sulfur content of PC@S (SI Appendix, Fig. S8). In comparison, there is a slight mass change for N/TPC-S and PC, suggesting that sulfur is well protected by C-S covalent bonds and the confined pores, which is beneficial to address the issue of sulfur loss.
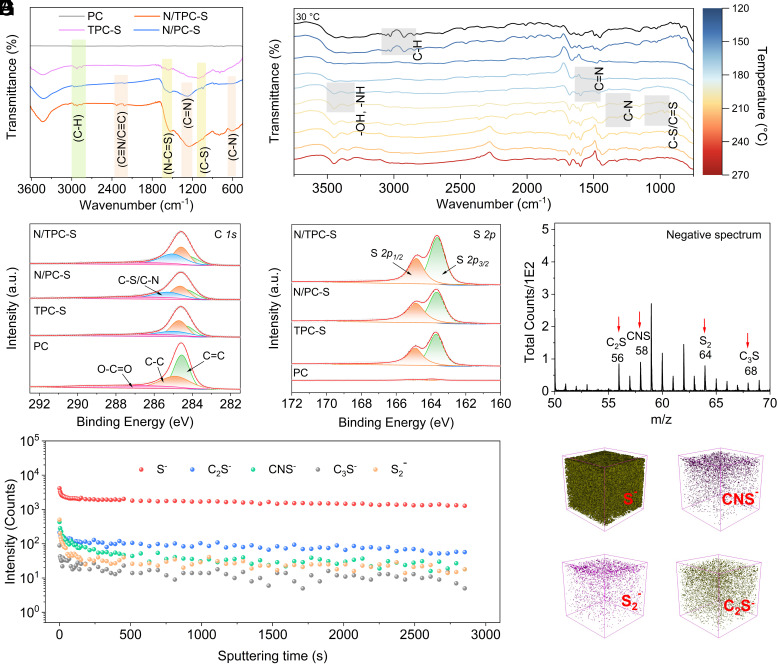
We investigate the chemical environment of sulfur in the carbon matrix using multiple spectroscopic analysis. The detailed molecular structure and chemical bond can be illustrated from the FTIR spectroscopy (Fig. 2A). The characteristic peak between 2,800 and 3,000 cm−1 corresponds to the stretching vibration of the saturated C-H bond (28). The signal peak near 1,052 cm−1 for the three materials other than PC is attributed to C-S, confirming that sulfur atoms are covalently bonded to carbon. For N/PC-S and N/TPC-S, the characteristic peaks at 612, 1,265, and 2,210 cm−1 can be attributed to C-N, C=N, and C≡N/C≡C, respectively, which indicate the presence of nitrogen in the carbon materials (29). Specifically, the significantly enhanced signals of N/PC-S and N/TPC-S at 1,537 cm−1 suggest the formation of N-C=S chemical bonds (25, 26, 30). These results imply that nitrogen can promote the binding of its neighboring carbon atom with the sulfur atom. The chemical environment of sulfur is regulated by nitrogen (N-C=S), which is beneficial to tune the electronic structure to enhance the adsorption capability for sodium ions.
Fig. 2.
Structure characterization of PC, TPC-S, N/PC-S, and N/TPC-S. (A) FTIR spectra. (B) The evolution of chemical bonds during the thermal treatment of pitch under the presence of PS microsphere, urea, and sulfur studied by in situ FTIR. (C) C 1s XPS spectra. (D) S 2p XPS spectra. (E–G) TOF-SIMS data of N/TPC-S.
The in situ FTIR is employed to real time reveal the evolution of chemical bonds during the thermal treatment of pitch under the presence of polystyrene, urea, and sulfur (Fig. 2B). When increasing the temperature from 30 to 144 °C, the peaks between 2,796 and 3,080 cm−1, which are attributed to the C-H stretching of the benzene ring, become weak or even disappear, demonstrating that polystyrene starts to interact with the polycyclic aromatic molecules in pitch (31). The peaks of -OH (3,449 cm−1), -NH (3,345 cm−1), and C=N (1,594 cm−1) appear or become stronger at a higher temperature of 168 °C (32, 33), due to the decomposition of urea to NH3 and the formation of H-N=C=O (12). The further increase of temperature to about 200 °C witnesses the appearance of two peaks at 1,328 and 1,235 cm−1 corresponding to the C-N in heterocycles, verifying that the decomposition products of urea start to combine with the polycyclic aromatic molecules (34). Meanwhile, the appearance of C=S/C-S peaks at 1,084 and 1,023 cm−1 evidences that sulfur starts to interact with carbon to form chemical bonds (25). It is confirmed by in situ FTIR that the decomposed small sulfur molecules gradually bond with the carbon material to form C-S/C=S bonds during the thermal treatment process. The formation of C-S covalent bonds can be promoted by nitrogen to form the N-C=S covalent bond. This is because the bare carbon network shows a weak affinity toward sulfur because of the low electronegativity, which can be optimized by introducing nitrogen atoms with large electronegativity to regulate the localized electronic structure.
XPS is conducted to study the surface chemistry of the material (SI Appendix, Figs. S9 and S10). In the high-resolution XPS spectra of C 1s, peaks centered at 284.4, 284.8, 285.2, and 286.9 eV are attributed to C=C, C-C, C-N/C-S, and O-C=O, respectively (Fig. 2C). The high-resolution S 2p XPS spectrum shows that the existence of peaks at 163.7 and 164.9 eV corresponds to the S 2p3/2 and S 2p1/2 signals of the S-S and C-S bonds, which demonstrates that sulfur is bonded to the carbon matrix via covalent bond (Fig. 2D) (35). Based on the XPS results, the sulfur contents of PC, TPC-S, N/PC-S, and N/TPC-S are calculated to be 0.81 wt%, 26.11 wt%, 27.34 wt%, and 30.88 wt%, respectively (SI Appendix, Table S2). The details regarding the existing form of sulfur are further examined by TOF-SIMS (Fig. 2E and SI Appendix, Fig. S11). The signal at m/z = 58 belongs to CNS− (36), verifying that some of the S atoms are bonded to carbon atoms adjacent to N, which agrees well with the FTIR data. The nitrogen-modulated carbon structure is rich in electron density compared with the bare carbon materials and thus can attract sulfur atoms to form chemical bonds. This can not only enhance the loading mass of sulfur in the carbon matrix but also lead to the formation of N-C=S covalent bond, which plays significant roles in improving the specific capacity and suppressing the formation of sodium polysulfides. The signals at m/z = 56 and m/z = 68 are attributed to C2S− and C3S−, further confirming the covalent bonding of S atoms with carbon atoms (20). The spectrum shows signals at m/z = 64 (S2−), while there is no signal for S3-S8, suggesting that there are only small sulfur molecules of S2 in the carbon matrix rather than large sulfur molecules of S3-S8 (36). S1-S2 small sulfur species were also detected while the large S3-S8 molecules were absent in the carbon matrix, which is beneficial to avoid the formation of sodium polysulfide. The signal intensities of S2−, C2S−, and CNS− maintain relatively stable during the sputtering time of 2,850 s, indicating that the homogeneity of sulfur species from the surface to the internal region (Fig. 2F) (37). The 3D compositional view shows the presence of S atoms in the form of chemically bonded and spatial-confined sulfur molecules (C-S, CNS−, S, and S2−) (Fig. 2G). During the thermal treatment, sulfur can be accommodated in carbon materials via the spatial and chemical dual-confinement strategies: 1) large sulfur molecules such as S8 can be decomposed into small sulfur molecules (S1-S2) and then be stored in the pore structure; 2) sulfur atoms can be integrated with the carbon matrix via the covalent bonds with the assistance of N. This is completely different from the introduction of large sulfur molecules into carbon materials by the conventional molten infusion method, inhibiting the formation of sodium polysulfide and suppressing the notorious shuttle problem of Na-S batteries.
The electrochemical performances are tested to examine the advantage of spatial and chemical dual-confinement. The cyclic voltammetry (CV) curves of N/TPC-S materials are shown in SI Appendix, Fig. S12. The small reduction peak at about 0.85 V is attributed to the decomposition of the electrolyte and the formation of solid electrolyte interphase (SEI) at the electrode/electrolyte interface (38). From the second cycle onward, the reduction peaks at 0.35 and 1 ~ 2 V are attributed to the gradual interaction of Na+ with S2 or C-S bonds, while the oxidation peak at around 1.8 V corresponds to the Na+ removal (19, 22). The charge and discharge plateaus are consistent with the results of the CV curves (SI Appendix, Fig. S13). To examine the influence of the trace amount of nitrogen and sulfur in pitch, we observe the CV curves and the galvanostatic charge–discharge (GCD) profiles of PC (SI Appendix, Figs. S14 and S15), which do not show a voltage plateau associated with the redox reaction of sulfur species. These results indicate that the trace amount of the intrinsic nitrogen and sulfur elements in pitch does not exhibit an apparent influence on the sodium storage performance. The GCD profiles at 0.05 A g−1 show that N/TPC-S delivers a much higher specific capacity (490.4 mAh g−1) than PC and TPC-S (Fig. 3A). As shown in Fig. 3 B and C, N/TPC-S exhibits a good rate capability of 460.1, 405.2, 362.3, 312.7, 260.5, 172.8, and 490.4 mAh g−1 at current densities of 0.1, 0.2, 0.5, 1, 2, 5, and 0.05 A g−1 together with typical discharge and charge plateaus. Moreover, N/TPC-S achieves a superior cycling stability as reflected by the high capacity retention of 97.64% after 1,000 cycles (Fig. 3D). In contrast, PC@S prepared by the molten infusion method shows serious specific capacity decay during the initial 10 cycles caused by the rapid loss of sulfur. TPC-S shows a good cycling stability whereas the specific capacity is as low as 125 mAh g−1 due to the small loading mass of sulfur without the assistance of N. Moreover, when increasing the current density to 5 A g−1, N/TPC-S still achieves an outstanding cycling stability featured by a large specific capacity retention of 96.45% after 1,000 cycles (Fig. 3E). The cycling and rate performance of N/TPC-S outperforms most of the reported cathodes for Na-S batteries (SI Appendix, Figs. S16 and S17). This good long-term cyclability is attributed to the spatial and chemical dual-confinement, which confines the small sulfur molecules in the carbon materials and covalently locks sulfur in the form of C-S bonds, avoiding the loss of sulfur species. The CV curves at different scan rates are measured and fitted (SI Appendix, Fig. S18). The capacitive contribution can be quantified according to the equation i (V) = k1 ν + k2 ν0.5, where k1 and k2 are appropriate values, and k1 ν and k2 ν0.5 correspond to the contribution of pseudocapacitive and diffusion, respectively (39, 40). As the scan rate increases from 0.2 to 1 mV s−1, the proportion of pseudocapacitive contribution gradually increases from 76 to 88% (SI Appendix, Fig. S19). This reflects the favorable charge transfer and the fast ion diffusion kinetics in the electrode. According to the galvanostatic intermittent titration technique and the electrochemical impedance spectrum results, it is found that N/TPC-S possesses a large Na+ diffusion coefficient and a small charge transfer resistance (SI Appendix, Figs. S20 and S21). This is related to the C-S/N-C=S covalent bonds and the N-containing groups in N/TPC-S, which improve the ion transfer capability and reduce the charge transfer resistance at the interface.
Fig. 3.
Electrochemical performance of PC, TPC-S, and N/TPC-S. (A) GCD profiles at 0.05 A g−1. (B) Rate performance. (C) GCD profiles at different current densities of N/TPC-S. (D) Cycling performance at 1 A g−1. (E) Cycling performance of N/TPC-S at 5 A g−1 and the corresponding coulombic efficiency.
We use a homemade cell with the visualization function to directly observe the discharge process of N/TPC-S and PC@S. It can be found that the electrolyte of the N/TPC-S system remains colorless after discharging for 6 h, while the electrolyte of the PC@S system shows a color change from apparent to yellow, demonstrating that N/TPC-S well suppresses the issue of sulfur species dissolution in the electrolyte (Fig. 4A). The UV-vis spectrum detects the existence of Na2S2, Na2S4, and Na2S6 in the electrolyte of the PC@S system (Fig. 4B). In contrast, the electrolyte of the N/TPC-S system does not exhibit an apparent signal of sodium polysulfide after discharging for 6 h, evidencing that the spatial and chemical dual-confinement can fundamentally avoid the formation of sodium polysulfide. Fig. 4C displays the GCD curves of N/TPC-S and the ex-situ XRD spectra corresponding to specific voltages. There are no characteristic diffraction peaks of sodium sulfides such as Na2S, Na2S2, Na2S4, and Na2S6, which indicates that the small sulfur molecules and the C-S chemical bonds have not been transformed into sodium polysulfide and sodium sulfide. The spatial confinement regarding host S1-S2 small sulfur species in the nanopores of carbon materials is beneficial to suppress the formation of soluble polysulfide production. Moreover, these spatially distributed S2 small molecules are simultaneously bonded to the carbon structure with C-S-S-C, and during the sodium storage process, the S-S bond is broken while the C-S bond is not broken, which avoids the generation of polysulfide. As for the chemical confinement, rather than the traditional adsorption of sulfur on carbon materials, sulfur in this work is chemically bonded with the carbon materials via the C-S covalent bonds. In addition, the intermediate and the final product of the N/TPC-S cathode during the discharge process are analyzed by ex-situ XPS (Fig. 4D). When discharging to 1.4 V, the characteristic peaks of S 2p3/2 and S 2p1/2 shift from 163.8 and 165.0 eV to 162.9 and 163.3 eV, respectively. This is because of the interaction of the sodium ion with the sulfur atom, which changes the charge state around the C-S bond (41). Meanwhile, two peaks appear at 168.3 and 169.4 eV, which can be ascribed to the S-containing organic/inorganic composite of the formed SEI (27). After discharging to 0.01 V, two peaks of S 2p3/2 and S 2p1/2 appear at 163.4 and 164.2 eV, respectively, due to the interaction of Na+ with the C-S bond for regaining charge equilibrium (42). The shift to higher binding energy indicates that Na+ with the electron-absorbing property interacts with the S atom and forms the R-S-Na bond (R refers to the carbon structure), generating the reduction of charge density. This could also be confirmed by the obvious redox peaks in the CV curves. During the entire discharge process, there is no signal of C-S bond breakage to form Na2S, which once again exhibits the unique advantages of the small sulfur molecules and the covalently bonded sulfur. According to the above-mentioned results, it is proposed that during the discharge process, Na+ combines with the C-S bond without causing the bond breakage to produce sodium polysulfides (Fig. 4E). Specifically, the N-C=S chemical bond will form a coordination structure (N···Na···S) with the sodium ion, which is then transformed into the “R-S-Na” product. The proposed mechanism can also be supported by subsequent theoretical calculations.
Fig. 4.

(A) Photographs of vial cells with PC@S and N/TPC-S cathodes at initial state and discharged for 0.5, 1, 2, 4, and 6 h. (B) UV-vis spectra of PC@S and N/TPC-S discharged for 6 h. (C) The initial GCD curves of N/TPC-S at 200 mA g−1 and the corresponding ex-situ XRD patterns. (D) High-resolution S 2p XPS spectra of N/TPC-S for different charge/discharge states. (E) The schematic diagram of the mechanism of sodium storage of PC@S and N/TPC-S (gray ball: C atom, dark blue ball: N atom, yellow ball: S atom, bright blue ball: sodium ion).
Theoretical calculations are performed to get an insight into the mechanism of interaction between the N/TPC-S and sodium ions. The optimized configurations of three models represent PC, TPC-S, and N/TPC-S, respectively (SI Appendix, Fig. S22). Molecular electrostatic potentials of PC, TPC-S, and N/TPC-S are calculated to unravel how electron density changes around the dopant atoms. As shown in Fig. 5A, the blue region denotes a more negative charge, which is the most favorable region for electrophilic attack (43). The red region represents a more positive charge. The specific distribution of Mulliken charge on every atom shows that the electrons mainly aggregate near the C atoms for PC, S atoms for TPC-S, and N/S atoms for N/TPC-S, manifesting that these sites prefer to take place the electrophilic reactions. Meanwhile, the interaction ability of Na+ with the C atoms, S atoms, and the N/S atoms on the structures of PC, TPC-S, and N/TPC-S is −280.1, −341.0, and −349.0 kJ mol−1, respectively. Thus, the modulation of the chemical environment of sulfur through the functionalization by nitrogen atoms can tune the distribution of the surrounding electric field to promote the sodium ion storage performance. Fig. 5B presents the total densities of states (TDOSs) for the optimized structure of PC@S and N/TPC-S. For the PC@S structure, the corresponding DOS value at the Fermi level is zero, so it has poor conductivity. Compared to the PC@S structure, the N/TPC-S structure shows a shift of the Fermi level to the conduction band, which is favorable for enhancing the electronic conductivity and chemical reactivity (24). On the other hand, the differential charge density (DCD) maps clearly demonstrate that there is no significant charge transfer between S8 and carbon in the structure of PC@S, whereas the significant charge transfer occurs in the structure of N/TPC-S due to the presence of N-C=S covalent bond. These results confirm that the binding interaction of N and S with C atoms improves the electrical conductivity of N/TPC-S. The energy gap between the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) energies of PC, TPC-S, and N/TPC-S are calculated to compare the capability of these three structures interacting with Na+ after the discharge. As presented in Fig. 5C, the HOMO-LUMO energy gap follows the order of PC (1.01 eV) > TPC-S (0.73 eV) > N/TPC-S (0.70 eV), indicating that the structure of N/TPC-S can exhibit excellent performance for interaction with Na+, which is attributed to the presence of strong electron-acceptor, namely the N-C=S group. Electron localization function (ELF) analysis for the structure of N/TPC-S exhibits that Na+ primarily localizes between N and S atoms at a stable state of coordination (N···Na···S) (Fig. 5D). The average binding energy of Na+ with N/TPC-S is −313.1 kJ mol−1. Additionally, the typical bond lengths and bond angles of N, C, and S atoms in the structures of N/TPC-S just have a slight change, indicating that the structure of N/TPC-S can be restored to its original state after the charge (SI Appendix, Table S3). Moreover, as shown in Fig. 5 D–F, the electron density around the N and S atoms shrinks slightly due to the activation of the Na+, and then due to the effect of charge balance, the sodium atom is gradually close to the S atom and the “S-Na” is formed between sodium and sulfur, which is manifested by the increase of “Na-N” bond length or the decrease of Na-S bond length in the model, followed by returning back to its original state when the sodium ions are removed. These results agree well with the sodium storage mechanism proposed according to the experimental results and further confirm that the C-S chemical bond is not broken during the repeated charge and discharge processes, and the chemical confinement plays an important role in enhancing the long-term stability of the sulfur cathode.
Fig. 5.

(A) The electrostatic potential of PC, TPC-S, and N/TPC-S. (B) The TDOS and the optimized structures and the corresponding DCD maps (isosurface level = 0.005) of PC@S and N/TPC-S. (C) Electron density and energy (eV) of the LUMO and HOMO for the structures of PC, TPC-S, and N/TPC-S. The ELF analysis and optimized configurations of N/TPC-S in the (D) early stage of discharge, (E) late stage of discharge, and (F) charge processes.
Discussion
We report a chemical and spatial dual-confinement approach to synthesizing an advanced S cathode (N/TPC-S) and investigate the Na-S battery performance and the sodium storage mechanism. A systematic investigation of multiple spectroscopic analysis reveals that S is covalently bonded to the carbon atoms (C-S/N-C=S bonds) with the assistance of N atom regulating the electronic and chemical environments of the carbon matrix. In addition, the S8 large molecules are transformed into S1-S2 small molecules and sealed in the confined pores of carbon materials. As a result, the N/TPC-S cathode exhibits a good electrochemical performance featured by a high-capacity retention of approximately 100% after 1,000 cycles, outperforming current S cathodes for Na-S batteries. UV-vis, ex situ XRD, ex situ XPS, and the optical observation demonstrate the prohibition of soluble polysulfides and Na2S, thus affording a good cycling stability. The systematic theoretical calculations reveal that the sodium ion forms a coordination structure (N···Na···S) at the sites between N and S atoms without breaking the C-S bond during the discharge process, thus eliminating the formation of Na2S during the repeated charge and discharge processes. The chemical and spatial confinement engineering provides an effective methodology for locking the active S species to address the S-related challenges for Na-S batteries and other S-based batteries.
Materials and Methods
Materials, Material Synthesis, Material Characterizations, Electrochemical Measurements, and Density Functional Theory (DFT) Calculations are provided in SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work is supported by the National Key R&D Program of China (2022YFB4101600), the National Natural Science Foundation of China (U2003216, 22209007), the Fundamental Research Funds for the Central Universities, China (buctrc202029, buctrc202129), the Beijing Nova Program (Z211100002121093), the fund from China Shenhua Coal to Liquid and Chemical Co., Ltd (MZYHG-22-01), and the Shenzhen Science and Technology Program (CJGJZD20210408092801005).
Author contributions
Q.Y. and J.S.Q. designed research; Y.Z., X.G., Y.S., Y.D., J.Q., and Z.S. performed research; Y.Z., Q.Y., M.Z., Y.H., Y.T., Y.L., and R.Z. contributed new reagents/analytic tools; Y.Z., X.G., Q.Y., Y.S., R.Z., B.W., and J.S.Q. analyzed data; and Y.Z., Q.Y., and J.S.Q. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Qi Yang, Email: qi.yang@mail.buct.edu.cn.
Riguang Zhang, Email: zhangriguang@tyut.edu.cn.
Jieshan Qiu, Email: qiujs@mail.buct.edu.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Ma C., et al. , Exploring oxygen activity in the high energy P2-type Na0.78Ni0.23Mn0.69O2 cathode material for Na-ion batteries. J. Am. Chem. Soc. 139, 4835–4845 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Jin T., et al. , Electrospun NaVPO4F/C nanofibers as self-standing cathode material for ultralong cycle Life Na-ion batteries. Adv. Energy Mater. 7, 1700087 (2017). [Google Scholar]
- 3.Wu X., et al. , Highly crystallized Na2CoFe(CN)6 with suppressed lattice defects as superior cathode material for sodium-ion batteries. ACS Appl. Mater. Inter. 8, 5393–5399 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Wang L., et al. , The promises, challenges and pathways to room-temperature sodium-sulfur batteries. Natl. Sci. Rev. 9, nwab050 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S., Yao Y., Yu Y., Frontiers for room-temperature sodium-sulfur batteries. ACS Energy Lett. 6, 529–536 (2021). [Google Scholar]
- 6.Wang Y. X., et al. , Room-temperature sodium-sulfur batteries: A comprehensive review on research progress and cell chemistry. Adv. Energy Mater. 7, 1602829 (2017). [Google Scholar]
- 7.Wang Y. X., et al. , Achieving high-performance room-temperature sodium-sulfur batteries With S@interconnected mesoporous carbon hollow nanospheres. J. Am. Chem. Soc. 138, 16576–16579 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Oshima T., Kajita M., Okuno A., Development of sodium-sulfur batteries. Int. J. Appl. Ceram. Technol. 1, 269–276 (2005). [Google Scholar]
- 9.Liu H., et al. , Electrocatalyzing S cathodes via multisulfiphilic sites for superior room-temperature sodium-sulfur batteries. ACS Nano 14, 7259–7268 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Yu X., Manthiram A., Highly reversible room-temperature sulfur/long-chain sodium polysulfide batteries. J. Phys. Chem. Lett. 5, 1943–1947 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Mou J., et al. , Hierarchical porous carbon sheets for high-performance room temperature sodium-sulfur batteries: Integration of nitrogen-self-doping and space confinement. J. Mater. Chem. A 8, 24590–24597 (2020). [Google Scholar]
- 12.Yang X., et al. , Kinetic enhancement of sulfur cathodes by N-doped porous graphitic carbon with bound VN nanocrystals. Small 16, e2004950 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Li H., et al. , Mesoporous nitrogen-doped carbon nanospheres as sulfur matrix and a novel chelate-modified separator for high-performance room-temperature Na-S batteries. Small 16, e1907464 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Wu S., et al. , Engineering multi-chambered carbon nanospheres@carbon as efficient sulfur hosts for lithium–sulfur batteries. J. Mater. Chem. A 6, 10891–10897 (2018). [Google Scholar]
- 15.Scholz J., et al. , Severe loss of confined sulfur in nanoporous carbon for Li–S batteries under wetting conditions. ACS Energy Lett. 3, 387–392 (2018). [Google Scholar]
- 16.Zhu Q., et al. , Ultra-microporous carbons encapsulate small sulfur molecules for high performance lithium-sulfur battery. Nano Energy 33, 402–409 (2017). [Google Scholar]
- 17.Wu T., et al. , Controllable chain-length for covalent sulfur-carbon materials enabling stable and high-capacity sodium storage. Adv. Energy Mater. 9, 1803478 (2019). [Google Scholar]
- 18.Fan L., Ma R., Yang Y., Chen S., Lu B., Covalent sulfur for advanced room temperature sodium-sulfur batteries. Nano Energy 28, 304–310 (2016). [Google Scholar]
- 19.Wu T., et al. , The bond evolution mechanism of covalent sulfurized carbon during electrochemical sodium storage process. Sci. China Mater. 62, 1127–1138 (2019). [Google Scholar]
- 20.Hu P., et al. , Covalent encapsulation of sulfur in a graphene/N-doped carbon host for enhanced sodium-sulfur batteries. Chem. Eng. J. 443, 136257 (2022). [Google Scholar]
- 21.Tang S., et al. , Size effect of organosulfur and in situ formed oligomers enables high-utilization Na-organosulfur batteries. Adv. Mater. 33, e2100824 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Wu M., et al. , Organotrisulfide: A high capacity cathode material for rechargeable lithium batteries. Angew. Chem. Int. Ed. 55, 10027–10031 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Wang D. Y., Si Y., Guo W., Fu Y., Long cycle life organic polysulfide catholyte for rechargeable lithium batteries. Adv. Sci. 7, 1902646 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan J., et al. , Enhanced Na+ pseudocapacitance in a P, S co-doped carbon anode arising from the surface modification by sulfur and phosphorus with C-S-P coupling. J. Mater. Chem. A 8, 422–432 (2020). [Google Scholar]
- 25.Wu J., Ding S., Ye S., Lai C., Grafting polymeric sulfur onto carbon nanotubes as highly-active cathode for lithium–sulfur batteries. J. Energy Chem. 42, 27–33 (2020). [Google Scholar]
- 26.Rao C. N. R., Vexkataraghavan R., Kasturi T. R., Contribution to the infrared spectra of organosulphur compounds. Can. J. Chem. 42, 36–42 (1964). [Google Scholar]
- 27.Yang G.-Z., et al. , Surface-dominated potassium storage enabled by single-atomic sulfur for high-performance K-ion battery anode. Energy Environ. Sci. 16, 1540–1547 (2023). [Google Scholar]
- 28.Li M. Y., et al. , Solvothermal conversion of coal into nitrogen-doped carbon dots with singlet oxygen generation and high quantum yield. Chem. Eng. J. 320, 570–575 (2017). [Google Scholar]
- 29.Kim H., et al. , Enhanced reversible capacity of sulfurized polyacrylonitrile cathode for room-temperature Na/S batteries by electrochemical activation. Chem. Eng. J. 426, 130787 (2021). [Google Scholar]
- 30.Qiao Y., et al. , First-principles and experimental study of nitrogen/sulfur co-doped carbon nanosheets as anodes for rechargeable sodium ion batteries. J. Mater. Chem. A 4, 15565–15574 (2016). [Google Scholar]
- 31.Zhu W., et al. , Facile synthesis of mono-dispersed polystyrene (PS)/Ag composite microspheres via modified chemical reduction. Materials 6, 5625–5638 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Z., et al. , Investigation of the role of surface chemistry and accessibility of cadmium adsorption sites on open-surface carbonaceous materials. Langmuir 24, 11701–11710 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Liu S. H., et al. , Nitrogen-rich carbon coupled multifunctional metal oxide/graphene nanohybrids for long-life lithium storage and efficient oxygen reduction. Nano Energy 12, 578–587 (2015). [Google Scholar]
- 34.Yang Y., et al. , MnO2 doped carbon nanosheets prepared from coal tar pitch for advanced asymmetric supercapacitor. Electrochim. Acta 354, 136667 (2020). [Google Scholar]
- 35.Guo Q. B., et al. , A novel one-step reaction sodium-sulfur battery with high areal sulfur loading on hierarchical porous carbon fiber. Carbon Energy 3, 440–448 (2020). [Google Scholar]
- 36.Xiao F., et al. , Covalent encapsulation of sulfur in a MOF-derived S, N-doped porous carbon host realized via the vapor-infiltration method results in enhanced sodium-sulfur battery performance. Adv. Energy Mater. 10, 2000931 (2020). [Google Scholar]
- 37.Xiong P., et al. , Room-temperature potassium-sulfur batteries enabled by microporous carbon stabilized small-molecule sulfur cathodes. ACS Nano 13, 2536–2543 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Yan Z., et al. , A high-kinetics sulfur cathode with a highly efficient mechanism for superior room-temperature Na–S batteries. Adv. Mater. 32, e1906700 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Liu T.-C., Pell W. G., Conway B. E., Roberson S. L., Behavior of molybdenum nitrides as materials for electrochemical capacitors. J. Electrochem. Soc. 145, 1882–1888 (1998). [Google Scholar]
- 40.Wang J., Polleux J., Lim J., Dunn B., Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 111, 14925–14931 (2007). [Google Scholar]
- 41.Wei S., Ma L., Hendrickson K. E., Tu Z., Archer L. A., Metal-sulfur battery cathodes based on PAN-sulfur composites. J. Am. Chem. Soc. 137, 12143–12152 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Jin Q. Z., et al. , Experimental design and theoretical calculation for sulfur-doped carbon nanofibers as a high performance sodium-ion battery anode. J. Mater. Chem. A 7, 10239–10245 (2019). [Google Scholar]
- 43.Thakur S., Borah S. M., Adhikary N. C., A DFT study of structural, electronic and optical properties of heteroatom doped monolayer graphene. Optik 168, 228–236 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.