ABSTRACT
Introduction
The increasing popularity of cannabinoids for treating numerous neurological disorders has been reported in various countries. Although it reduces tetrahydrocannabinol psychoactivity, it helps patients tolerate higher doses and complements the anti-spasmodic effects of tetrahydrocannabinol. One of the most important potential of cannabinoids are related to its potential to help children with cerebral palsy, a contributor of lifelong disability. Therefore, this systematic review aimed to assess the efficacy and safety of medical cannabinoids in children with cerebral palsy.
Methods
This review adhered to The Preferred Reporting Items for Systematic Reviews and Meta-analysis 2020 guidelines. Seven databases, namely, Scopus, PubMed, EBSCO Host, ProQuest, Google Scholar, Semantic Scholar, and JSTOR, were used to identify relevant studies. Studies examining pediatric patients with cerebral palsy and reporting the efficacy and safety of medical cannabinoids through clinical trials, observational cross-sectional studies, or cohort designs were included. The outcomes of the studies included the efficacy of medical cannabinoids administered for spasticity, motor components, pain control, sleep difficulties, adverse effects, and seizure control.
Results
Of 803 identified articles, only three met the inclusion criteria for data synthesis. One study exhibited a moderate risk-of-bias. A total of 133 respondents, mainly from Europe, were investigated. Overall effectiveness and safety were considered good. However, the results are inconsistent, especially regarding spasticity treatment variables.
Conclusion
The anti-spasticity, anti-inflammatory, and anti-seizure properties of cannabinoids might be beneficial for patients with cerebral palsy, although their effectiveness has not been widely studied. Further studies with larger sample sizes and various ethnicities are warranted. Prospero database registration: (www.crd.york.ac.uk/prospero) under ID CRD42022358383.
Keywords: Cannabinoids, Child, Cerebral palsy, Efficacy, Safety, Patient safety
INTRODUCTION
Complex movement disorders are a heterogeneous group of neurological disorders characterized by different types of abnormal movements and postures, including spasticity and dystonia. These abnormal movements and postures are generally associated with severe orthopedic problems, chronic pain, eating difficulties, constipation, sleep disturbances, epilepsy, and a poor quality of life. ( 1 ) Cerebral palsy (CP) is the most common complex movement disorder, with multiple complications that begin during childhood. The estimated prevalence is 2–3 per 1,000 live births. ( 2 ) Medical cannabinoids are becoming increasingly popular in several countries. Cannabinoid-based therapies have been investigated for the treatment of various neurological disorders, particularly drug-resistant epilepsy and movement disorders. ( 3 ) The methodologies used in these studies and the derived results are controversial. Cannabinoid-based drugs, phytocannabinoids, and synthetic cannabinoids have multiple mechanisms of action including interactions with endocannabinoid receptors. ( 4 , 5 ) In addition, cannabidiol may potentiate some of the beneficial effects of tetrahydrocannabinol (THC) as it reduces the psychoactivity of THC and allows patients to tolerate higher THC doses. Cannabidiol may also complement the anti-spasmodic effects of THC ( e.g ., via local enhancement of glycine signaling; inhibition of endocannabinoid degradation; or delayed demyelination through anti-inflammatory, antioxidant, and anti-excitotoxic mechanisms). ( 5 ) Cannabinoids have therapeutic potential in movement disorders. Synthetic cannabinoids, such as nabilone, dronabinol, and Sativex, are cannabinoid receptor agonists with effects similar to those of THC. They have been approved for clinical indications including spasticity, pain, and refractory epilepsy. ( 6 , 7 ) However, its efficacy and safety in children with CP are uncertain, especially in the treatment of spasticity, pain, and seizures. This systematic review aimed to assess the efficacy and safety of medical cannabinoids in children with CP.
METHODS
Study registration and strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 guidelines were followed to develop this review. The Scopus, PubMed, EBSCO Host, ProQuest, Google Scholar, Semantic Scholar, and JSTOR databases were used to identify studies relevant to the search terms. The search terms used in each database are listed in Appendix 1 (42KB, pdf) . No time restrictions were imposed on the literature search. Manual search was also conducted by examining the citations of selected articles to identify relevant publications that were not indexed in the aforementioned databases. ( 8 )
Eligibility criteria
Studies examining pediatric patients with CP and reporting the efficacy and safety of medical cannabinoids through clinical trials, observational cross-sectional studies, or cohort designs were included. Systematic reviews, meta-analyses, narrative reviews, case reports, case series, opinion pieces, conference abstracts, and grey literature were excluded.
Study selection
The titles and abstracts of unique studies were independently screened using Rayyan QCRI, an online software used for abstract and title screening. ( 9 , 10 ) This process was performed by two reviewers, and supervised and adjudicated, if necessary, by a single reviewer. Full-text articles were obtained and two reviewers independently conducted eligibility assessments. No reviewers were blinded to the bibliographic information of the studies.
Outcomes
The outcomes of the studies included the efficacy of medical cannabinoids administered for spasticity, motor components, pain control, sleep difficulties, adverse effects, and seizure control. Cerebral palsy should be assessed using validated tests and internationally standardized diagnostic criteria.
Risk-of-bias
Several tools were used to assess the risk-of-bias based on the study type. We used the Risk-of-Bias in Non-Randomized Intervention Studies tool (ROBINS-I) to examine the potential risk-of-bias in the selected experimental nonrandomized studies. The tool can be assigned for the following areas: (i) entanglement bias, (ii) study participant selection bias, (iii) exposure measurement bias, (iv) exposure misclassification bias during follow-up, (v) bias in outcome measurement, (vi) bias in missing data, and (vii) bias in the selection of the results. The risk-of-bias was assessed on 0–4 scale points following the severity of the bias risk. The randomized studies used the RoB-2 tool. The five following domains were assessed: i) randomization, ii) deviation from the intended intervention, iii) outcome measures, iv) missing outcome data, and v) selection from reported outcomes. Furthermore, we assessed the potential bias in cross-sectional studies using the Risk-of-Bias Instrument for Cross-sectional Surveys of Attitudes and Practices contributed by the CLARITY group at McMaster University. The following five domains were assessed: i) representative population, ii) adequate response rate, iii) missing data, iv) clinically sound, and v) reliability and validity of the survey instrument.
Two reviewers independently assessed the risk-of-bias and the strength of evidence in all relevant studies. Any disagreements between the two researchers were resolved through consultation with a third researcher.
Ethics approval
The authors of this article did not conduct human or animal studies. Therefore, ethical approval was not required.
Patient and publication consent statement
The authors of this article did not conduct human or animal studies. Therefore, patient and publication consent was not required.
RESULTS
Study screening
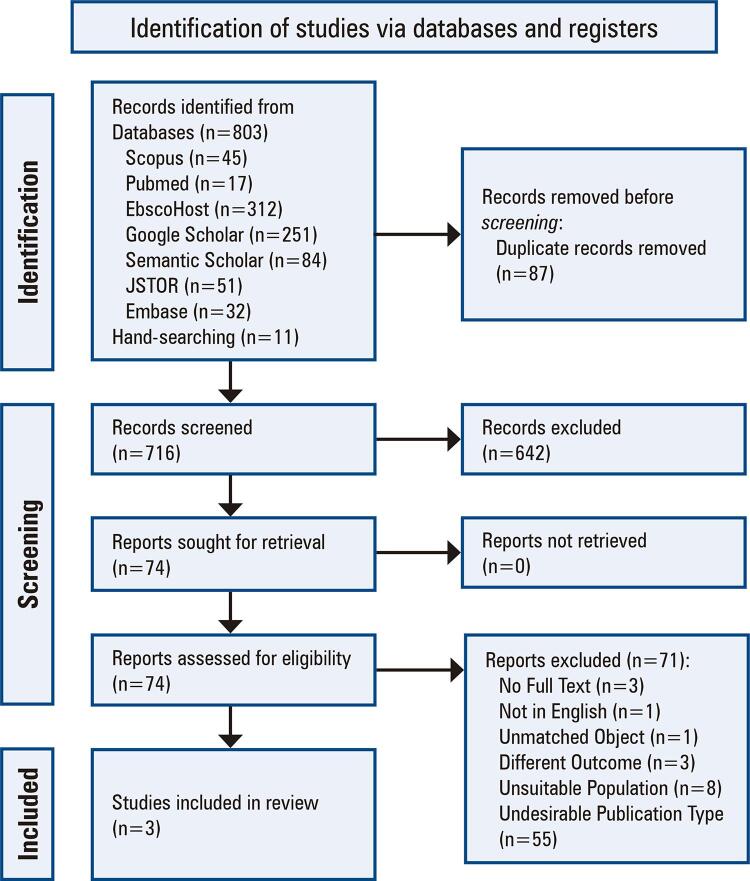
The search strategy yielded 803 articles, of which the titles and abstracts of 716 unique articles were evaluated for eligibility after deduplication. A total of 642 articles were considered ineligible and were excluded in the first screening phase. The full texts of the remaining 74 articles were reviewed to assess their eligibility for inclusion and 71 articles were excluded. Three studies met the inclusion criteria for data synthesis ( Figure 1 ).
Figure 1. Study flow diagram.
Study characteristics
Table 1 presents the characteristics of the included studies. This systematic review included three research projects, one of which was an observational study and two were experimental studies (RCT and non-RCT). There were 133 respondents (range: 19–70 participants). Each study included a European population. 11 All investigations examined the benefits and adverse effects of medical cannabis administration. Only two studies provided information on the average age of participants and duration of treatment, with a mean ages of 12.6 ( 11 ) and 6.51 ( 12 ) years and treatment durations of 12 weeks ( 11 ) and five months, ( 12 ) respectively.
Table 1. Summary of included studies.
| Author | Country | Study type | Sample size | Comparison | Mean Age (years) | Duration of treatment | Substance | Instrument | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Fairhurst et.al. ( 11 ) (2020) | United Kingdom, Israel, Czech Republic | RCT | 72 (CP: 47) | Placebo control | 12.6 | 12 weeks | Oromucosal nabiximols | NRS for spasticity | No significant difference in the spasticity between nabiximols versus placebo groups after 12 weeks (p=0.729) The substance is generally well tolerated by pediatric patients, with three cases of hallucinations |
| Libzon et.al. ( 12 ) (2018) | Israel | Non-RCT | 25 (CP: 19) | No Control Group | 6.51 | 5 months | Cannabidiol-enriched 5% oil formulation (cannabidiol-to-THC ratio 6:1 and 20:1) | NRS for spasticity, VAS for pain, dystonia scale, CPCHILDQoL questionnaire | NRS for spasticity improved from baseline in the entire study population regardless of treatment assignment. VAS score improved in addition to pain duration, frequency, and dystonia. The CPCHILDQoL improved in the study cohort. Adverse effects were rarely reported (including seizure deterioration). There were no changes in ECG or blood tests |
| Morosoli et.al. ( 13 ) (2021) | Europe, North America, Australia | Cross-sectional | 70 | N/A | N/A | N/A | Medical cannabinoids (including cannabis oil, dronabinol solution, cannabis tincture, and cannabis spray) | Questionnaire | The impact of medical cannabinoids on CP symptoms alleviation is mainly considered strong or moderate (68%) Common acute side effects of cannabinoid administration are sleepiness, restlessness, diarrhea, and nausea. No long-term side effects are witnessed |
CP: cerebral palsy; CPCHILDQoL: Cerebral Palsy Child Questionnaire for Quality of Life; ECG: electrocardiogram; NRS: numeric rating scale; RCT: Randomized Controlled Trial; VAS:visual analog scale.
Types of medical cannabinoids and administration methods
All studies used various cannabinoid substances and administration methods. Fairhurst et al. ( 11 ) used a nabiximols solution containing 2.7mg THC, 2.5mg cannabidiol (CBD), and other cannabinoid and non-cannabinoid components. The medications were administered orally or sublingually for 12 weeks. Meanwhile, Libzon et al. ( 12 ) used two products of CBD nourished with 5% oil preparation (one with CBD: THC=20:1 and the other with CBD: THC=6:1), Which were administered twice or thrice daily for five months via an oral or feeding tube route. Furthermore, an observational study by Morosoli et al. ( 13 ) revealed that only 52% of patients specified a particular cannabinoid prescription, which included dronabinol solution, cannabis oil, cannabis, Cannabis sativa spray, CBD, and Epidiolex.
Role of medical cannabis in cerebral palsy symptoms alleviation
The effects of medical cannabis on patient complaints varied. Fairhurst et al. ( 11 ) found no difference in the primary outcome of caregiver-reported spasticity between the cannabis-administered and control groups (mean difference: -0.166, p=0.729). In contrast, Libzon et al. ( 12 ) showed that irrespective of treatment allocation, the entire study population showed improved spasticity, quality of life (QOL), and gross motor coordination. Along with pain duration and frequency, pain intensity decreased, as evidenced by an improvement in the visual analog scale (VAS) score. Meanwhile, Morosoli et al. ( 13 ) reported that, while not specifically addressing the domain of improvement, more than half of the participants experienced a moderate-to-strong influence from cannabis administration, with about one-fifth reporting no improvement.
Safety profile of medical cannabis in cerebral palsy patients
Medical cannabis is particularly safe for pediatric patients with CP. The adverse events were mild-to-moderate, with no long-term consequences. ( 13 ) Tiredness, dizziness, exhaustion, xerostomia, diarrhea, nausea, vomiting, and confusion are several examples of reported symptoms. Serious adverse events included changes in seizure characteristics, ( 11 , 12 ) disorientation, euphoria, hypotonia, distress, hallucinations, psychotic symptoms, food aversion, elevated liver enzymes, and viral upper respiratory tract infections. ( 11 , 13 ) In contrast, Libzon et al. did not report any deterioration in the electrocardiogram (ECG) or blood tests, and the detrimental reactions could be controlled by reducing the dose of cannabinoids. ( 12 )
Study quality (risk-of-bias)
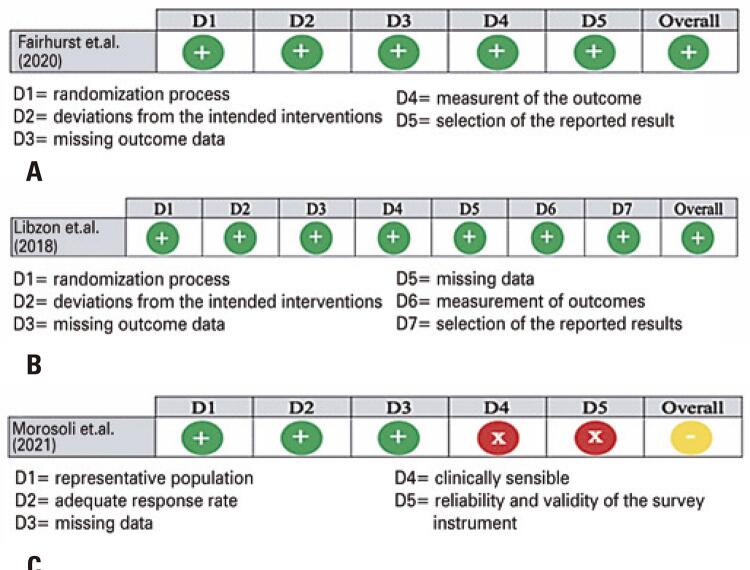
The overall risk-of-bias was moderate-to-low. The moderate results in the study by Morosoli et al. ( 13 ) were related to the lack of adequate clinical assessment and the reliability and validity of the survey instrument domains. This was not observed in the other included studies. The risk-of-bias assessment is shown in figure 2 .
Figure 2. Risk of Bias Assessment. (A) Cochrane risk-of-bias tool for randomized trials risk-of-bias 2; (B) Risk of bias in non-randomized studies - of interventions (ROBINS-I); (C) Risk of Bias for Cross-Sectional Surveys of Attitudes and Practices.
DISCUSSION
Reports on the benefits of medical cannabis are increasing. Despite the controversy over its safety and efficacy, studies have suggested that cannabis may have the therapeutic potential to improve several disorders, including neurological diseases. 14 Reduction in seizure frequency, ( 17 , 18 ) spasticity, neuropathic pain, ( 15 ) and other motor function disorders ( 16 , 18 ) are the most-reported positive impacts.
However, concerns regarding the adverse effects of cannabis have emerged. Non-serious adverse effects may occur even before cannabis initiation. 11 However, they can be generally resolved by reducing the dose of cannabis or changing the pattern of administration. ( 12 , 16 , 17 ) THC is the most important contributor to the psychoactive side-effects. Using a ‘start low and go slow’ dosing strategy and combining CBD with THC may mitigate most adverse events. ( 19 ) Therefore, the safety level is generally acceptable if the medication dose and administration are suitable.
Cannabis plants contain more than 100 known cannabinoid compounds. The psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) and the non-psychoactive CBD are the principal neuroactive components of cannabis. The term “non-psychoactive” refers to the absence of psychotropic effects compared to Δ9-THC. ( 20 ) Most cannabis retail products fall into one of the following three categories: Δ9-THC-dominant, CBD-dominant, or a balanced “hybrid” product with high concentrations of both Δ9-THC and CBD. ( 21 ) These products can be consumed through several methods, including smoke inhalation, vaporization, oral ingestion, and other routes (topical and suppository). ( 19 ) All three included studies reported on oral-ingestion of various preparations of cannabis, such as oils, tinctures, and sprays, which are associated with better convenience and less odor. ( 22 )
Cerebral palsy refers to a set of persistent movements and postural disorders that restrict activity and is caused by non-progressive disruptions in the developing brain. ( 23 ) This disorder causes motor disorders, a broad range of comorbidities, and secondary conditions such as sensation, perception, cognition, communication, and behavioral disturbances. ( 24 ) Pain is the most common secondary consequence of severe health concerns in children with CP. It is primarily related to movement disorders (the basis of CP), musculoskeletal problems, and repeated exposure to painful procedures, including surgery. ( 25 )
The effect of cannabinoid administration in patients with CP is uncertain despite its high potential. ( 26 ) There are two types of cannabinoid receptors in human cells: types 1 (CB1) and 2 (CB2). CB1 is found in the central nervous system and peripheral tissues, whereas CB2 is mainly found in the immune cells. ( 27 ) CB1 receptors are primarily located at the terminals of the central and peripheral neurons, where they regulate neurotransmitter release and psychoactive traits. They are also abundant in brain areas associated with nociceptive perception, such as the thalamus and amygdala. ( 28 ) CB1 receptor activation modifies nociceptive thresholds and exerts various biological effects by balancing excitatory and inhibitory neurotransmitters, mainly related to the Gamma-Aminobutyric Acid system, which can be used to treat seizures and spasms. ( 29 , 30 ) CB2 receptor activation hinders the release of inflammatory mediators by cells close to the nociceptive nerve terminals and prevents pain signals from entering the central nervous system. ( 28 )
Cannabinoids presynaptically hinder glutamate release. ( 31 ) The amplification of CB1 receptors reduces glutamatergic transmission in THC-exposed animals, and chronic cannabis use decreases glutamate metabolites in the human brain. Several animal studies have identified CB1 as a receptor that modulates the anti-spasticity effects of cannabinoids. ( 28 ) Owing to its effectiveness in alleviating spasticity in both animal models and humans, Δ9-THC is the most important cannabinoid that mediates the anti-spasticity effect of cannabinoid preparations. ( 32 ) Therefore, Δ9-THC is responsible for treating CP-related dystonia.
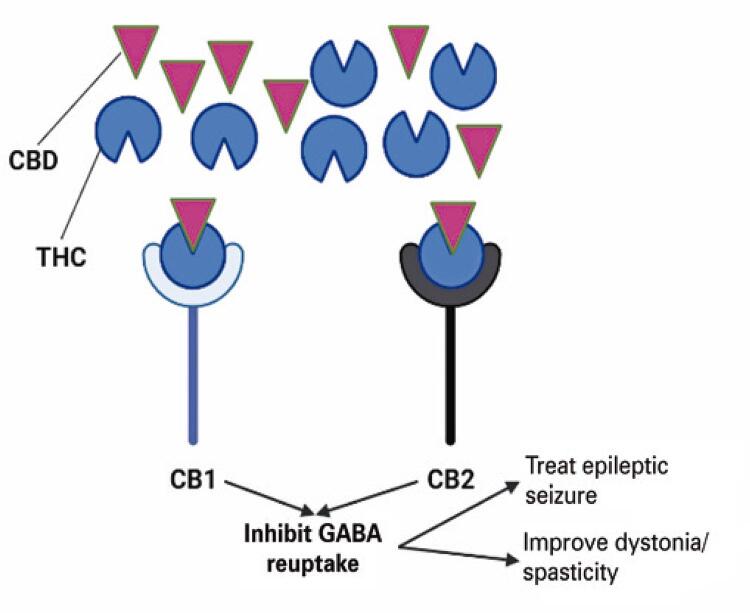
In addition to lowering spasticity, Δ9-THC and CBD have been shown to prevent seizures in animal models. These substances exhibit low toxicity and high tolerability. ( 33 ) Δ9-THC is a partial agonist of both CB1 and CB2 receptors and reduces the severity of seizures by activating CB1. Furthermore, Δ9-THC has powerful anti-inflammatory effects on microglia, which are the primary immune cells of the central nervous system. Considering the synergistic relationship between seizures and inflammation, the cannabinoid system offers a novel strategy for targeting both sectors of this feedback mechanism. ( 34 ) Cannabidiol structurally resembles Δ9-THC, but has low affinity for CB1 and CB2 receptors. Cannabidiol may exert anti-seizure effects by reducing glutamate release. CBD also minimizes epileptiform events in the hippocampus in an in vitro model in a CB1-independent, concentration-dependent, and region-specific manner. ( 35 ) The illustration of the effects of cannabinoids on spasticity and epileptic seizures is shown in figure 3 .
Figure 3. Mechanism of cannabidiol and tetrahydrocannabinol in Improving cerebral palsy-symptoms.
CBD: cannabidiol; CB1: cannabinoid receptor type 1; CB2: cannabinoid receptor type 2; GABA: gamma-aminobutyric acid; THC, tetrahydrocannabinol.
LIMITATIONS
This systematic review has some limitations. The number of studies included in this systematic review was limited to those with different study designs. Of the three studies, only one was an RCT. The small sample sizes of the included studies may not represent real-world efficacy and safety. In addition, the study focused mainly on the European population, making generalizability challenging to achieve.
CONCLUSION
Cannabinoids may be beneficial in patients with cerebral palsy; however, their effectiveness has yet to be thoroughly studied. The proposed modes of action of cannabinoids in cerebral palsy include anti-spasticity, anti-inflammatory, and anti-seizure features. Although mild-to-moderate adverse events have been reported, there have been no reports of long-term adverse events, indicating a favorable safety profile. Further research with a larger sample size, extended study period, and individuals of various ethnicities is required to determine the role of cannabinoids in cerebral palsy.
REFERENCES
- 1.Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician . 2020;101(4):213–220. Review. [PubMed] [Google Scholar]
- 2.Koy A, Lin JP, Sanger TD, Marks WA, Mink JW, Timmermann L. Advances in management of movement disorders in children. Lancet Neurol . 2016;15(7):719–735. doi: 10.1016/S1474-4422(16)00132-0. Review. [DOI] [PubMed] [Google Scholar]
- 3.Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia . 2014;55(6):791–802. doi: 10.1111/epi.12631. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadland SE, Harris SK. Youth marijuana use: state of the science for the practicing clinician. Curr Opin Pediatr . 2014;26(4):420–427. doi: 10.1097/MOP.0000000000000114. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koppel BS. Cannabis in the Treatment of Dystonia, Dyskinesias, and Tics. Neurotherapeutics . 2015;12(4):788–792. doi: 10.1007/s13311-015-0376-4. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadland SE, Knight JR, Harris SK. Medical marijuana: review of the science and implications for developmental-behavioral pediatric practice. J Dev Behav Pediatr . 2015;36(2):115–123. doi: 10.1097/DBP.0000000000000129. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhlen M, Hoell JI, Gagnon G, Balzer S, Oommen PT, Borkhardt A, et al. Effective treatment of spasticity using dronabinol in pediatric palliative care. Eur J Paediatr Neurol . 2016;20(6):898–903. doi: 10.1016/j.ejpn.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Umar TP, Samudra MG, Nashor KM, Agustini D, Syakurah RA. Health professional student’s volunteering activities during the COVID-19 pandemic: a systematic literature review. 797153 Front Med (Lausanne) . 2022;9 doi: 10.3389/fmed.2022.797153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. 210 Syst Rev . 2016;5(1) doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liana P, Liberty IA, Murti K, Hafy Z, Salim EM, Zulkarnain M, et al. A systematic review on neutrophil extracellular traps and its prognostication role in COVID-19 patients. Immunol Res . 2022;70(4):449–460. doi: 10.1007/s12026-022-09293-w. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairhurst C, Kumar R, Checketts D, Tayo B, Turner S. Efficacy and safety of nabiximols cannabinoid medicine for paediatric spasticity in cerebral palsy or traumatic brain injury: a randomized controlled trial. Dev Med Child Neurol . 2020;62(9):1031–1039. doi: 10.1111/dmcn.14548. [DOI] [PubMed] [Google Scholar]
- 12.Libzon S, Schleider LB, Saban N, Levit L, Tamari Y, Linder I, et al. Medical cannabis for pediatric moderate to severe complex motor disorders. J Child Neurol . 2018;33(9):565–571. doi: 10.1177/0883073818773028. [DOI] [PubMed] [Google Scholar]
- 13.Morosoli F, Hunziker S, Zuercher K, Tscherter A, Grunt S. Prescription practices of medical cannabinoids in children with cerebral palsy-a survey of the swiss cerebral palsy registry. medRxiv . 2021:1–26. doi: 10.1101/2021.11.18.21266388. Posted November 21, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chayasirisobhon S. The Role of Cannabidiol in Neurological Disorders. 25 Perm J . 2021;25(2) doi: 10.7812/TPP/20.156. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Amerongen G, Kanhai K, Baakman AC, Heuberger J, Klaassen E, Beumer TL, et al. Effects on spasticity and neuropathic pain of an oral formulation of δ9-tetrahydrocannabinol in patients withprogressive multiple sclerosis. Clin Ther . 2018;40(9):1467–1482. doi: 10.1016/j.clinthera.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Hosking R, Zajicek J. Cannabis in neurology-a potted review. Nature Reviews Neurology . 2014;10(8):429–430. doi: 10.1038/nrneurol.2014.122. [DOI] [PubMed] [Google Scholar]
- 17.Silvestro S, Mammana S, Cavalli E, Bramanti P, Mazzon E. Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules . 2019;24(8):1459. doi: 10.3390/molecules24081459. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain SA, Zhou R, Jacobson C, Weng J, Cheng E, Lay J, et al. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: a potential role for infantile spasms and lennox-gastaut syndrome. Epilepsy Behav . 2015;47:138–141. doi: 10.1016/j.yebeh.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 19.MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med . 2018;49:12–19. doi: 10.1016/j.ejim.2018.01.004. Review. [DOI] [PubMed] [Google Scholar]
- 20.Reddy DS, Golub VM. The Pharmacological basis of cannabis therapy for epilepsy. J Pharmacol Exp Ther . 2016;357(1):45–55. doi: 10.1124/jpet.115.230151. Review. [DOI] [PubMed] [Google Scholar]
- 21.Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol . 2019;30:98–102. doi: 10.1016/j.copsyc.2019.04.002. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules . 2018;23(10):2478. doi: 10.3390/molecules23102478. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel DR, Neelakantan M, Pandher K, Merrick J. Cerebral palsy in children: a clinical overview. Transl Pediatr . 2020;9(S1) Suppl 1:S125–S135. doi: 10.21037/tp.2020.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr Dis Treat . 2020;16:1505–1518. doi: 10.2147/NDT.S235165. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen RD, Genik L, Alriksson-Schmidt AI, Anderzen-Carlsson A, Burkitt C, Bruflot SK, et al. Pain burden in children with cerebral palsy (CPPain) survey: study protocol. Paediatr Neonatal Pain . 2021;4(1):12–22. doi: 10.1002/pne2.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zürcher K, Dupont C, Weber P, Grunt S, Wilhelm I, Eigenmann DE, et al. Use and caregiver-reported efficacy of medical cannabis in children and adolescents in Switzerland. Eur J Pediatr . 2022;181(1):335–347. doi: 10.1007/s00431-021-04202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bie B, Wu J, Foss JF, Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol . 2018;31(4):407–414. doi: 10.1097/ACO.0000000000000616. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez AJ, García-Merino A. Neuroprotective agents: cannabinoids. Clin Immunol . 2012;142(1):57–67. doi: 10.1016/j.clim.2011.02.010. Review. [DOI] [PubMed] [Google Scholar]
- 29.Maayah ZH, Takahara S, Ferdaoussi M, Dyck JR. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. 165771 Biochim Biophys Acta Mol Basis Dis . 2020;1866(7) doi: 10.1016/j.bbadis.2020.165771. Review. [DOI] [PubMed] [Google Scholar]
- 30.Pretzsch CM, Freyberg J, Voinescu B, Lythgoe D, Horder J, Mendez MA, et al. Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology . 2019;44(8):1398–1405. doi: 10.1038/s41386-019-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köfalvi A, Moreno E, Cordomí A, Cai NS, Fernández-Dueñas V, Ferreira SG, et al. Control of glutamate release by complexes of adenosine and cannabinoid receptors. 9 BMC Biol . 2020;18(1) doi: 10.1186/s12915-020-0739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manzanares J, Julian M, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol . 2006;4(3):239–257. doi: 10.2174/157015906778019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia . 2014;55(6):791–802. doi: 10.1111/epi.12631. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Löhr JM, et al. Pancreatic cancer microenvironment. Int J Cancer . 2007;121(4):699–705. doi: 10.1002/ijc.22871. Review. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg EC, Tsien RW, Whalley BJ, Devinsky O. Cannabinoids and Epilepsy. Neurotherapeutics . 2015;12(4):747–768. doi: 10.1007/s13311-015-0375-5. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]