Significance
Anti-viral and anti-inflammatory drugs have shown benefit against ARDS/COVID but more effective pharmacology is needed. Necroptosis, a proinflammatory form of cell death downstream of a signalling cascade that includes Z-nucleic acid-binding protein 1 (ZBP1), receptor interacting protein kinase 3 (RIPK3), and mixed lineage kinase domain-like protein (MLKL), is part of the immune pathology of ARDS/COVID. Here we report that GHRHR antagonist MIA-602 neutralizes ZBP1/MLKL signalling, blocks necroptosis and confers protection against lung injury in a mouse model of ARDS/COVID driven by VSV-SARS-CoV-2 spike protein. The results show that by targeting a central pathway of immune dysregulation, MIA-602 provides benefit over current anti-inflammatory agents and warrants clinical/translational development for the treatment of pneumonia/ARDS associated with severe COVID-19 infection.
Keywords: GHRH-R, SARS-CoV-2, COVID-19, ARDS, Necroptosis
Abstract
COVID-19 pneumonia causes acute lung injury and acute respiratory distress syndrome (ALI/ARDS) characterized by early pulmonary endothelial and epithelial injuries with altered pulmonary diffusing capacity and obstructive or restrictive physiology. Growth hormone–releasing hormone receptor (GHRH-R) is expressed in the lung and heart. GHRH-R antagonist, MIA-602, has been reported to modulate immune responses to bleomycin lung injury and inflammation in granulomatous sarcoidosis. We hypothesized that MIA-602 would attenuate rVSV-SARS-CoV-2-induced pulmonary dysfunction and heart injury in a BSL-2 mouse model. Male and female K18-hACE2tg mice were inoculated with SARS-CoV-2/USA-WA1/2020, BSL-2-compliant recombinant VSV-eGFP-SARS-CoV-2-Spike (rVSV-SARS-CoV-2), or PBS, and lung viral load, weight loss, histopathology, and gene expression were compared. K18-hACE2tg mice infected with rVSV-SARS-CoV-2 were treated daily with subcutaneous MIA-602 or vehicle and conscious, unrestrained plethysmography performed on days 0, 3, and 5 (n = 7 to 8). Five days after infection mice were killed, and blood and tissues collected for histopathology and protein/gene expression. Both native SARS-CoV-2 and rVSV-SARS-CoV-2 presented similar patterns of weight loss, infectivity (~60%), and histopathologic changes. Daily treatment with MIA-602 conferred weight recovery, reduced lung perivascular inflammation/pneumonia, and decreased lung/heart ICAM-1 expression compared to vehicle. MIA-602 rescued altered respiratory rate, increased expiratory parameters (Te, PEF, EEP), and normalized airflow parameters (Penh and Rpef) compared to vehicle, consistent with decreased airway inflammation. RNASeq followed by protein analysis revealed heightened levels of inflammation and end-stage necroptosis markers, including ZBP1 and pMLKL induced by rVSV-SARS-CoV-2, that were normalized by MIA-602 treatment, consistent with an anti-inflammatory and pro-survival mechanism of action in this preclinical model of COVID-19 pneumonia.
Clinical presentation of SARS-CoV-2 infection (COVID-19) varies from asymptomatic to severe critical illness (1). Although COVID-19 is a multisystemic disease, the lungs are the primary source of infection and injury, resulting in pneumonia and, in severe cases, acute respiratory distress syndrome (ARDS) that can progress to fibrosis, a condition associated with persistent pulmonary dysfunction, reduced quality of life, and increased mortality (2–8). A substantial proportion, up to 40% in some cohorts of individuals hospitalized for COVID-19, progress to severe pneumonia and/or ARDS, and such progression remains one of the highest predictors of COVID-19 mortality (7–11). Sepsis, the predominant cause of ARDS-related death, is characterized by excessive release of inflammatory cytokines, termed “cytokine storm” that precipitates multiple organ failure (12, 13). Recent work identified necroptosis, a lytic and proinflammatory form of cell death downstream of a signalling cascade that includes Z-nucleic acid-binding protein 1 (ZBP1), receptor interacting protein kinase 3 (RIPK3), and mixed lineage kinase domain-like protein (MLKL), as part of the underlying immune pathology of ARDS/COVID-19 (14–16). Standardized management of COVID-19 pneumonia and ARDS involving fluid management, lung-protective ventilation, and pharmacotherapy with steroidal and nonsteroidal anti-inflammatories, immunoglobulin, antibiotics, anti-viral, and anti-fibrosis drugs have improved outcomes in some cohorts (17–20). However, mortality of severe cases is still high, persistent pulmonary consequences common in survivors, and alternative remedies urgently needed (9).
Growth hormone–releasing hormone (GHRH) is secreted primarily from the hypothalamus and produced locally in the lungs. GHRH stimulates the release and secretion of growth hormone (GH) by the pituitary and regulates hepatic production of insulin-like growth factor-1 (IGF-1), master regulators of cell growth, proliferation, and metabolism. GHRH-receptors (GHRH-R), including a truncated SV1 variant, are expressed in the human and rodent heart and lungs (21–23). GHRH-R agonists can promote malignancies and inflammation, while administration of antagonist analogues, including MIA-602, a synthetic 30-amino acid peptide (24), suppresses tumor growth and confer anti-inflammatory, and anti-oxidative effects (25–27). Of special relevance to COVID-19-related lung injury, and the present study, MIA-602 was shown to reduce both inflammation and fibrosis in models of intraperitoneal, bleomycin-induced lung fibrosis (28), and granulomatous sarcoidosis, an inflammatory disease that targets the lung (29). By inhibiting JAK2/STAT3, NF-κB, and MAPK pathways, MIA-602 decreased levels of proinflammatory cytokines and infiltrating CD68+ monocytes/macrophages in these models. Fibrosis was reduced secondarily to ameliorated inflammation and directly by inhibition of fibroblast proliferation. GHRH-R antagonists can also protect against endothelial barrier dysfunction by similar anti-inflammatory actions (25, 30), linking GHRH-dependent signalling with the major inflammatory pathways of ARDS and sepsis (31, 32).
The goals of the current work were to validate a mouse BSL-2 COVID-19 model of ALI/ARDS by comparing infection of K18-hACE2tg mice with VSV-eGFP-SARS-CoV-2-Spike (rVSV-SARS-CoV-2) versus SARS-CoV-2/USA-WA1/2020, and demonstrate equivalent infectivity and pathology. SARS-CoV-2/USA-WA1/2020 is an established strain of virus widely used by the research community that was recovered from a patient who returned to Washington from China in January 2020 (33). Second, we used the model to quantify lung protection and mechanism of action by GHRH-R antagonist MIA-602. We hypothesised that the anti-inflammatory actions of GHRH-R antagonists, protection against sepsis, fibrosis, and endothelial barrier dysfunction in different models of lung injury translate into protection against COVID-19-induced ALI/ARDS by targeting common immune dysfunction mechanisms. The results corroborate that daily treatment with MIA-602 effectively attenuates pulmonary dysfunction and heart injury via normalized expression/phosphorylation of inflammation/necroptosis markers ZBP1, MLKL, interferon-γ, and SOCS1. The results are consistent with suppression of inflammation by MIA-602 and support development of MIA-602 or related GHRH-R antagonists as therapeutics for COVID-19-related pneumonia/ARDS.
Results
Higher Dose of rVSV-SARS-CoV-2 Produces Infection Similar to Native SARS-CoV-2 in K18-hACE2tg Mice.
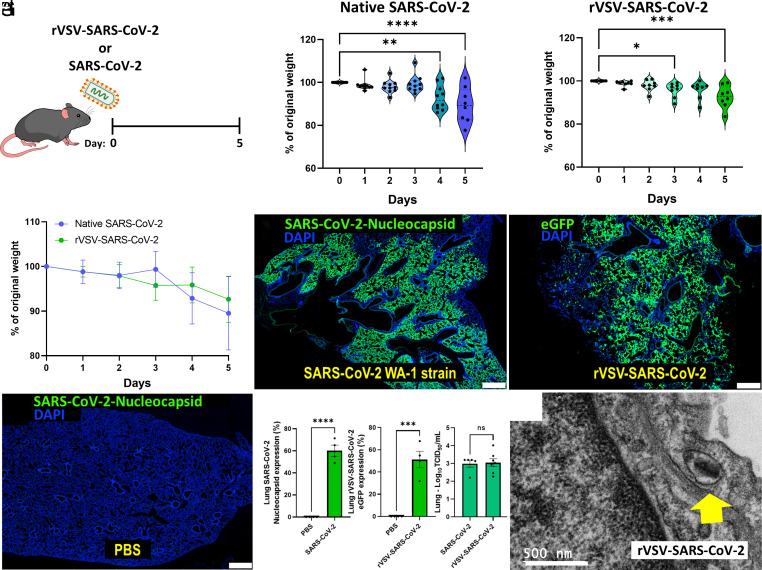
Previous works demonstrated the utility of K18 human angiotensin-converting enzyme 2 (hACE2tg) transgenic mice that express human ACE2, to mimic SARS-CoV-2 infection and recapitulate nonsevere and severe COVID-19 pathology (34, 35). In short-term studies, we established that intratracheal inoculation of K18-hACE2tg mice with 2 × 107 plaque forming units (PFU) of replication-competent chimeric virus (rVSV-SARS-CoV-2) conferred similar infection and pathophysiological patterns in lungs at 5 days post infection (dpi) as mice treated by intranasal delivery of 105 particles/10 µL/nostril of SARS-CoV-2/USA-WA1/2020 (Fig. 1A). Weight loss was 10.48% and 7.44% respectively at 5 dpi for SARS-CoV-2 and rVSV-SARS-CoV-2-S (Fig. 1 B and C). Immunostaining of SARS-CoV-2-nucleocapsid and eGFP-derived rVSV-SARS-CoV-2 revealed comparable infectivity of 60.07% vs 51.19% at 5 dpi (Fig. 1 D–G). Similarly, lung viral load RNA measurements were comparable for SARS-CoV-2 and rVSV-SARS-CoV-2 (2.986 vs. 3.043 genome copies/mL, P = 0.86), respectively (Fig. 1 G, Right). The data indicate that high-dose intratracheal rVSV-SARS-CoV-2-S mimics infection and pathology of native SARS-CoV-2 in K18-hACE2tg mice and provides a model of COVID-19 infection that can be implemented under biosafety level 2 (BSL-2) (36). Whereas previous work established that rVSV-SARS-CoV-2 reproduced SARS-CoV-2 pathology just in neonatal mice that are more susceptible to viral infection than adults (37), we document high-dose infection by rVSV-SARS-CoV-2-S in adult mice.
Fig. 1.
rVSV-SARS-CoV-2 mimics SARS-CoV-2 infectivity. (A) K18-hACE2tg mice were inoculated intranasally with ~105 PFU SARS-CoV-2 (n = 10) or intratracheally with ~2 × 107 PFU IU rVSV-SARS-CoV-2 (n = 8) for 5 d. (B and C) Weight loss was recorded daily and compared between both viruses. (D–G) Similar infection pattern with native SARS-CoV-2 Washington strain and chimeric SARS-CoV-2 in hACE2tg mice after 48 h. N = 3 to 4 mice per group. (Scale bar: 500 μm.) Quantification shown for immunostaining and viral load using the Median Tissue Culture Infectious Dose (TCID50) assay. (H) Electron microscopy shows rVSV-SARS-CoV-2 viral entry into the lung epithelial cell (yellow arrow). Data are means ± SD. The t test or ANOVA with Tukey post hoc correction was used. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Recombinant VSV-SARS-CoV-2 Causes Pulmonary Dysfunction.
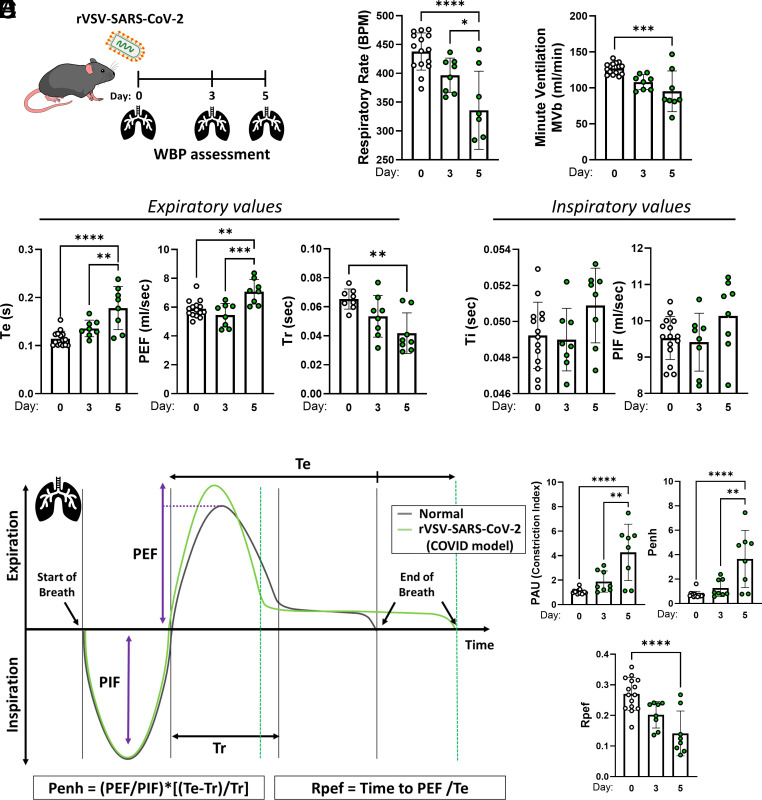
The impact of rVSV-SARS-CoV-2 infection on lung physiology was measured by unrestrained, conscious whole-body plethysmography (WBP) on days 0, 3, and 5 (n = 7 to 8) (Fig. 2A). Mice (8 to 12-wk-old) were infected with 2 × 107 PFU of rVSV-SARS-CoV-2 to induce severe acute disease. Impaired pulmonary function at 5 dpi was evidenced by decreased respiratory rate (RR) (335.8 vs. 437.8 breath/minute, P < 0.0001) and minute ventilation (MVR) (95.31 vs. 127.1 mL/min, P < 0.0001), compared to the baseline (Fig. 2B). In addition, expiratory parameters were dysregulated at 5 dpi compared to baseline: expiratory time (Te) (0.18 vs 0.11 s, P < 0.0001), end expiratory pause (EEP) (106.6 vs. 26.27 ms, P < 0.0001), estimated peak expiratory flow (PEF) (7.10 vs. 5.88 mL/s, P = 0024), and relaxation time (Tr) (0.042 vs. 0.065 s, P = 0.0026) (Fig. 2C). Inspiratory parameters did not change significantly at 5 dpi compared to the baseline: inspiratory time (Ti) and estimated peak inspiratory flow (PIF) (Fig. 2C). Unit-less indexes that reflect viral lung function and pathogenesis [SARS-CoV (2003) and influenza A virus] were analyzed to characterize the changes (38). The pause (PAU), which indicates time to expel the final 36% of the total expiratory volume compared with the time to expel the first 64% or relaxation time, increased markedly at 5 dpi (4.267 vs. 1.067, P < 0.0001), consistent with the altered changes derived from the expiratory values. Enhanced pause (Penh index), a reflection of the breathing pattern that may be related to changes in airway resistance, calculated by Penh = (PEF/PIF)*[(Te-Tr)/Tr] was also significantly increased 5 dpi (3.64 vs. 0.73, P < 0.0001). Such a change in Penh may reflect airway inflammation and secretions caused by high viral replication (38). Finally, the Rpef index that relates the ratio of PEF to expiratory time (Te) was significantly reduced at 5 dpi (0.14 vs. 0.27, P < 0.0001), a pattern also seen in SARS-CoV mouse infection (38).
Fig. 2.
rVSV-SARS-CoV-2 induces pulmonary dysfunction. (A) K18-hACE2 mice were inoculated intratracheally with ~2 × 107 PFU rVSV-SARS-CoV-2 (n = 8). On day 0, day 3, and day 5, whole-body plethysmography (WBP) was recorded. (B) Respiratory rate and minute ventilation. (C) Expiratory values (Te: expiratory time; PEF: peak expiratory flow; Tr: relaxation time) and inspiratory values (Ti: inspiratory time; PIF: peak inspiratory flow) (D and E) Schematic expiratory and inspiratory curves to determine airway obstruction measures (Penh: enhanced pause; Rpef: The location into expiration where the peak occurs as a fraction of Te). Data points represent individual animals. Green lines and points represent infected animals. Data are means ± SD. ANOVA with Tukey post hoc correction was used. **P < 0.01; ***P < 0.001; ****P < 0.0001.
GHRH-R Antagonist MIA-602 Attenuates Recombinant rVSV-SARS-CoV-2- Induced Pulmonary Dysfunction.
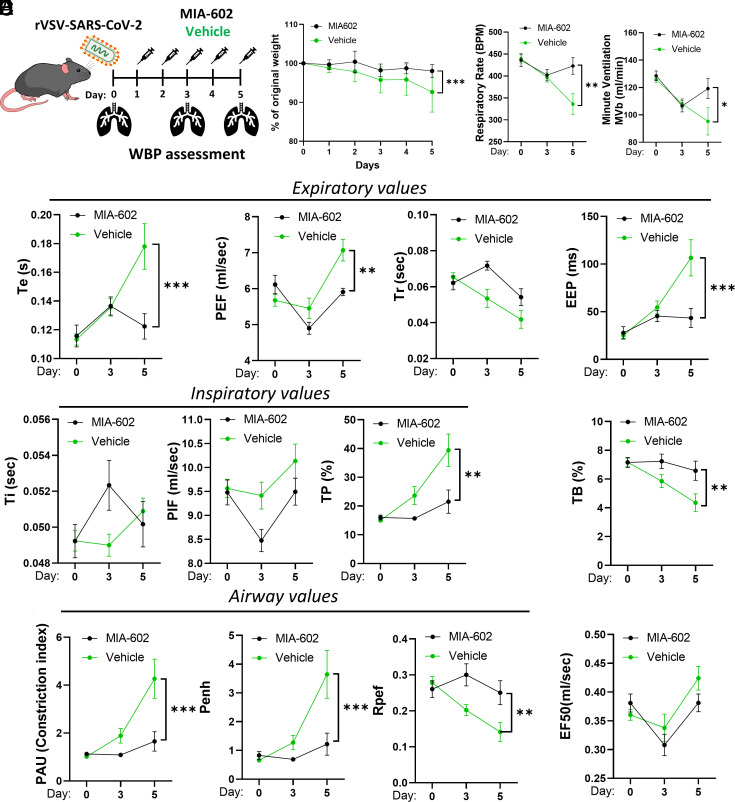
Live cell imaging of eGFP indicated that GHRH-R antagonist, MIA-602, did not affect rVSV-SARS-CoV-2-S infection (SI Appendix, Fig. S1). To evaluate the effects of MIA-602 on post infection pathology, hACE2tg mice were randomly assigned to receive daily subcutaneous MIA-602 (10 μg/mouse) or vehicle and pulmonary functions measured by WBP on dpi 0, 3, and 5 (Fig. 3A). Pulmonary functions were not affected until 5 dpi when the MIA-602 treatment group showed significant improvements over controls: respiratory rate (422.7 vs. 335.8 breath/min, P < 0.01) and minute ventilation (119.2 vs .95.3 mL/min, P < 0.05), (Fig. 3C). Expiratory parameters were similarly improved at 5 dpi: expiratory time (0.12 vs. 0.18 s, P = 0.0003), expiratory pause (EEP) (43.42 vs. 106.6 ms, P = 0.0003), estimated peak expiratory flow (PEF) (5.91 vs. 7.07 mL/s, P = 0.032). No changes were observed for relaxation time, inspiratory time, estimated peak inspiratory flow, or EF50 (expiratory flow at 50% expired volume) (Fig. 3 D and E). However, airway indices were significantly improved in the 5 dpi MIA-602 group, including reduced PAU (1.6 vs. 4.27, P = 0.0002), decreased Penh (1.22 vs. 3.64, P = 0.0005), and increased Rpef (0.25 vs. 0.14, P = 0.0096) compared to controls (Fig. 3E). The results are consistent with significant rescue of rVSV-SARS-CoV-2-induced airway dysfunction by MIA-602.
Fig. 3.
GHRH-R antagonist attenuates rVSV-SARS-CoV-2-induced pulmonary dysfunction. (A) K18-hACE2tg mice were inoculated intratracheally with ~2 × 107 PFU rVSV-SARS-CoV-2 (n = 15). Animals received daily subcutaneous GHRH-R antagonist (MIA-602; 10 μg/mouse) or vehicle. On day 0, day 3, and day 5, whole-body plethysmography (WBP) was recorded. (B) Weight loss curve. (C) Respiratory rate and minute ventilation. (D) Expiratory values (Te: expiratory time; PEF: peak expiratory flow; Tr: relaxation time; EEP: end expiratory pause) and inspiratory values (Ti: inspiratory time; PIF: peak inspiratory flow; TP: Duration of pause before inspiration) (E) Airway measures (PAU: Pause; Penh: enhanced pause; Rpef: The location into expiration where the peak occurs as a fraction of Te) and EF50: Expiratory flow at 50% expired volume; and TB: Duration of breaking. Data are means ± SD. ANOVA with Tukey post hoc correction was used. *P < 0.05; **P < 0.01; ***P < 0.001.
Treatment with MIA-602 Improves Recovery and Lung Damage in rVSV-SARS-CoV-2-Infected K18 hACE2 Mice.
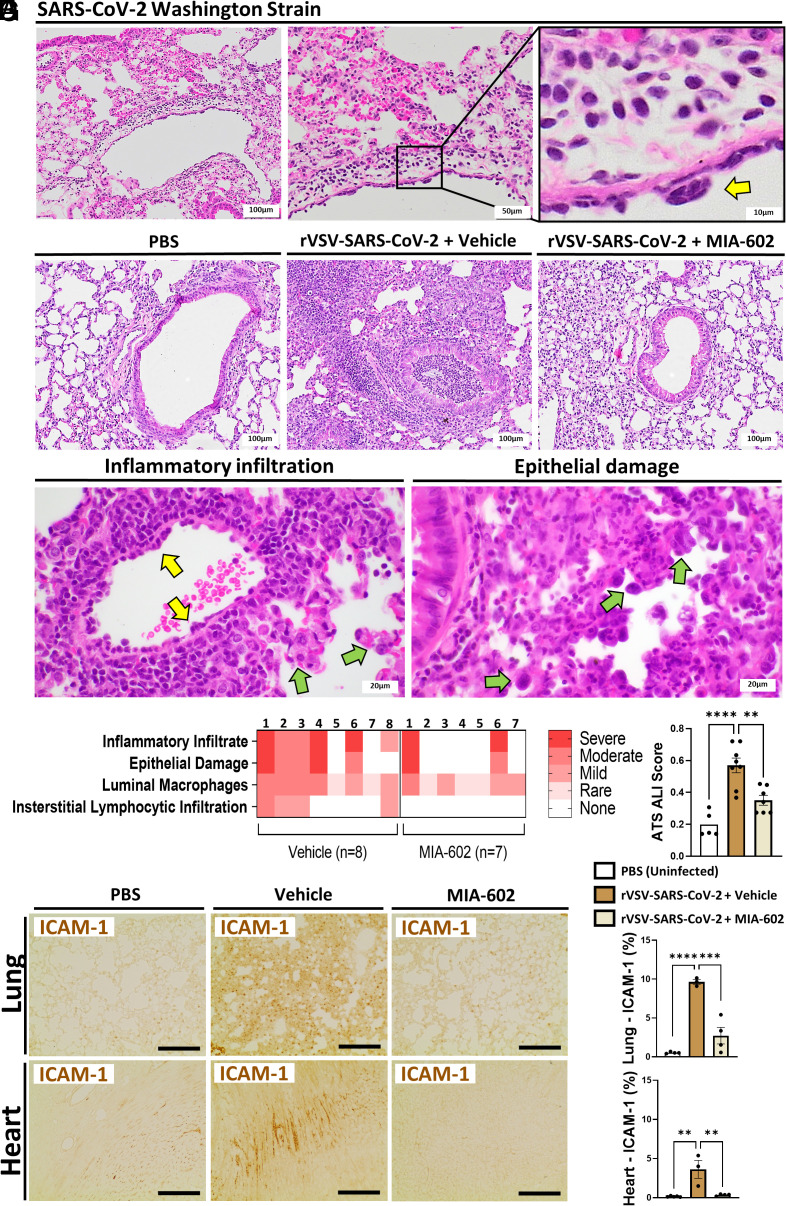
Mice in the rVSV-SARS-CoV-2 + MIA-602 treatment group maintained weight at close to normal levels and significantly greater than the rVSV-SARS-CoV-2 vehicle group at 5 dpi (Fig. 3B). Histopathological findings of inflammatory infiltrate, epithelial damage, peribronchiolar inflammation, luminal macrophages, and interstitial lymphocytic infiltration indicated acute lung injury of varying severity consistent with pneumonia and ARDS at 5 dpi in all rVSV-SARS-CoV-2 lungs, (Fig. 4 A–C). First, using standard pathology ranking, histopathological findings were ranked from 0 to 4 with zero being absent and 4 being marked/severe (39). The ranks for each of the 15 mice are displayed as representative colors in a heatmap (Fig. 4D). Second, using the American Thoracic Society (ATS) ALI scoring guidelines (40), the ALI score was clearly reduced by MIA-602 treatment, showing less lung inflammation (P < 0.01) (Fig. 4E). In addition, elevated Intracellular Adhesion Molecule-1 (ICAM-1) expression in the rVSV-SARS-CoV-2 mice was significantly reduced in the lungs (2.7% vs. 9.6%; P < 0.01) and hearts (0.4% vs. 3.6%; P < 0.05) relative to the vehicle group (Fig. 4 F and G).
Fig. 4.
GHRH-R antagonist reduces rVSV-SARS-CoV-2-induced lung damage. (A) K18-hACE2tg mice were inoculated intranasally with ~105 PFU SARS-CoV-2 (n = 10). Shown are representative H&E images of the lungs with perivascular lymphocytic inflammation and reactive endothelium (yellow arrow). (B) K18-hACE2tg mice were inoculated intratracheally with ~2 × 107 PFU rVSV-SARS-CoV-2 (n = 15). Animals received daily subcutaneous GHRH-R antagonist (MIA-602; 10 μg/mouse) or vehicle. Shown are representative H&E images of the lung bronchial structure compared with the normal PBS/no-virus group. rVSV-SARS-CoV-2+ vehicle: Bronchus with marked intraluminal and peribronchial inflammation. The inflammation spills into the pulmonary interstitium. rVSV-SARS-CoV-2 + MIA-602: Normal bronchial structure and parenchyma. (C) H&E images of the lungs from the rVSV-SARS-CoV-2+ vehicle group show perivascular inflammation with endothelial reactive change (yellow arrows). There is type 2 pneumocytes hyperplasia (green arrows in the left panel) and reactive type 2 pneumocytes (green arrows in the right panel). (D) Histopathological findings using standard pathology grading were ranked from 0 to 4 with zero being absent and 4 being marked/severe. The ranks for each of the 15 mice are displayed as representative colors in a heatmap. (E) Acute Lung injury score using guidelines of the American Thoracic Society (n = 5 to 8 mice per group). (F and G) Immunostaining for ICAM-1 expression in the lung and heart (n = 3 mice per group). (Scale bar: 100 μm.) Data are means ± SD. ANOVA with Tukey post hoc correction was used. *P < 0.05; **P < 0.01.
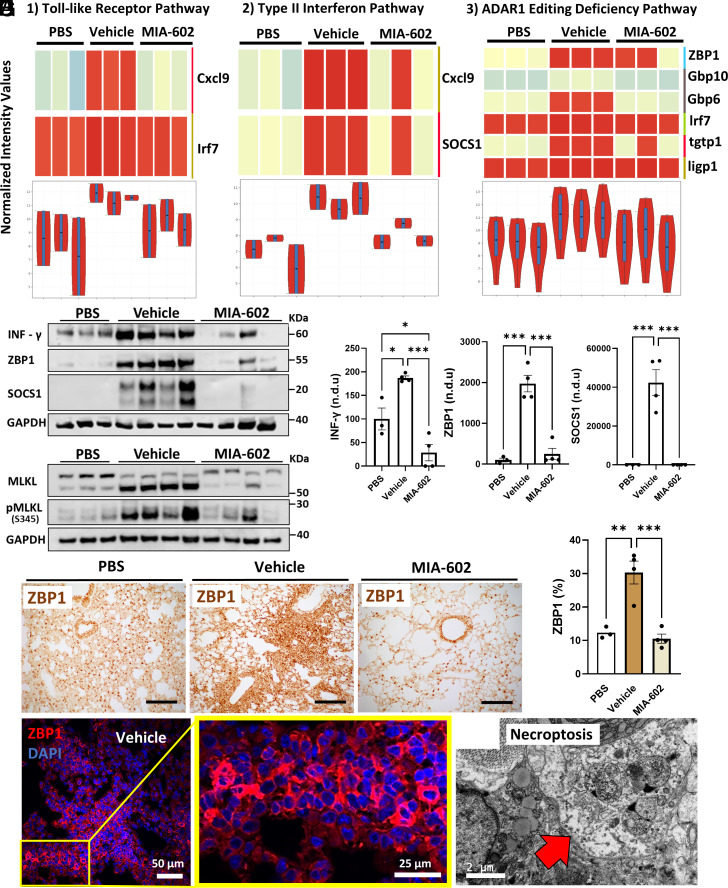
GHRH-R Antagonist MIA-602 Prevents Lung Necroptosis in rVSV-SARS-CoV-2-Infected K18 hACE2 Mice.
To investigate the mechanisms of MIA-602 protection against rVSV-SARS-CoV-2-induced pulmonary injury, we applied bulk RNA sequencing, western blot, and immunohistochemistry (IHC) to lung tissues. RNASeq of infected lung samples revealed inductions of gene expression for interferon type II, Toll-like receptors, and ADAR1 pathways that were ameliorated by MIA-602 treatment (Fig. 5A). Western blots validated these effects confirming increased protein expression of INF-γ, ZBP1, and SOCS1 by rVSV-SARS-CoV-2 that was blocked by MIA-602 treatment (Fig. 5 B and C). IHC similarly confirmed markedly enhanced ZBP1 staining in infected lungs that was similarly blocked by treatment with MIA-602 (30.3% vs. 10.6%, P < 0.001) (Fig. 5 E and F). Necroptosis mediated by ZBP1 and increased phosphorylation of MLKL were recently implicated in SARS-CoV-2 mediated inflammation and ARDS (14–16). As shown in Fig. 5D, MIA-602 treatment significantly reduced the pMLKL and MLKL relative to vehicle, in parallel with suppression of ZBP1-necroptosis. Consistent with these findings, electron microscopy (EM) identified classical patterns of necroptosis in a lung epithelial cell from the vehicle group (Fig. 5H).
Fig. 5.
GHRH-R antagonist reduces rVSV-SARS-CoV-2-induced lung necroptosis. (A) K18-hACE2tg mice were inoculated intratracheally with ~2 × 107 PFU rVSV-SARS-CoV-2. Animals received daily subcutaneous GHRH-R antagonist (MIA-602; 10 μg/mouse) or vehicle. After 5 d, lung samples were collected for transcriptomics (n = 3 mice per group). Enrichment analysis from RNASeq reveals Toll-like receptor, type II Interferon, and ADAR1Editing Deficiency pathways as the three main pathways regulated by MIA-602. Shown are normalized representations of the transcripts in each pathway by heatmap and violin plots. (B and C) Protein expression and quantification confirm INF-γ, ZBP1, and SOCS1 regulation by MIA-602 (n = 3 mice per group). (D) Protein expression of necroptosis markers pMLKL and MLKL (n = 3 mice per group). (E and F) DAB immunostaining and quantification for ZBP1 expression in lungs (n = 3 mice per group). (Scale bar: 100 μm.) (G) Immunofluorescence for ZBP1 expression in lungs. The inset shows cytoplasmic expression of ZBP1 in perivascular infiltration. (H) Electron microscopy reveals programmed cell death by necroptosis in an epithelial lung cell (red arrow). Data are means ± SD or ±SEM. ANOVA with Tukey post hoc correction was used. *P < 0.05; **P < 0.01; ***P < 0.001.
Computational Analysis Shows That Respiratory Parameters Predict Lung Histopathology.
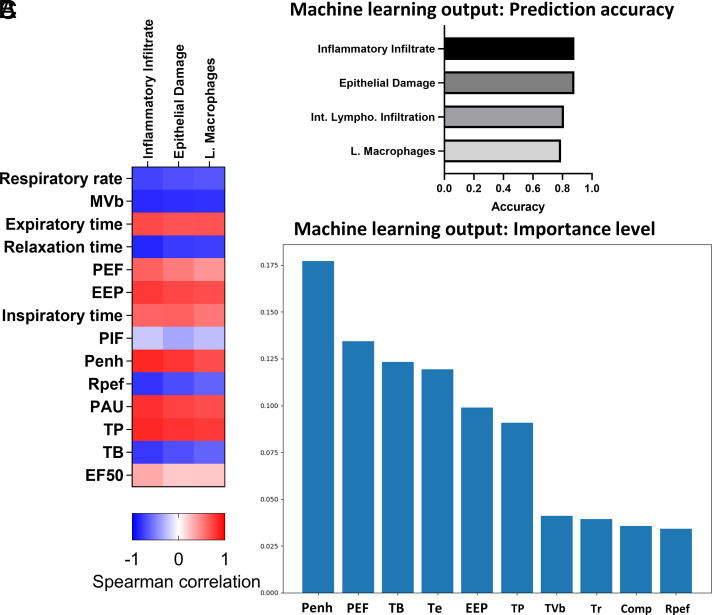
WBP has been shown to accurately assess respiratory functions in conscious mice, and detailed examination of whole-body plethysmography (WBP) parameters offers predictive insights into pulmonary inflammation and airway mucus (41). Results from WBP and histology including ICAM-1 staining independently support acute respiratory dysfunction and pulmonary inflammation of rVSV-SARS-CoV-2 mice at 5 dpi with amelioration of most indications by MIA-602. Therefore, we computed Spearman correlations between WBP and histopathological parameters, shown in Fig. 6A, and applied a machine learning approach using a random forest algorithm to determine the prognostic value of respiratory parameters. As expected, we found that multiple WBP parameters correlate significantly with histopathology. For histopathological findings of inflammatory infiltrate, respiratory rate (r = −0.74; P = 0.0019); minute ventilation (r = −0.84; P = 0.0002); Tr (r = −0.86; P < 0.0001); EEP (r = 0.78; P = 0.0008); PAU (r = 0.81; P = 0.0004); Penh (r = 0.85; P = 0.0001); Rpef (r = −0.80; P = 0.0005); TP% (r = 0.85; P = 0.0001); and TB% (r = 0.79; P = 0.0007) showed significant correlation. For histopathological findings of epithelial damage, minute ventilation (r = −0.82; P = 0.0002); Tr (r = −0.77; P = 0.0009); EEP (r = 0.72; P = 0.0026); PAU (r = 0.74; P = 0.0018); Penh (r = 0.79; P = 0.0006); and TP% (r = 0.81 P = 0.0001) showed significant correlation. For luminal macrophages, minute ventilation (r = −0.81; P = 0.0003); Tr (r = −0.76; P = 0.0012); and TP% (r = 0.78; P = 0.0007) correlated significantly (Fig. 6A). For machine learning analysis, WBP parameters and histopathological findings were used as input and output data, respectively. We built a random forest model trained on our data and observed that inflammatory infiltrate or epithelial damage obtained the highest accuracy for prediction, 0.88 and 0.88, respectively (Fig. 6B). Based on Spearman correlation data Penh, Rpef and minute ventilation were selected as guidance for posterior independent machine learning analyses. Based on the interpretability of random forest models, we show the feature importance of one trained model from our set of solutions in which Penh is the most relevant feature for prediction (Fig. 6C). The results support WBP parameters as prognostic indicators and corroborate the protective actions of MIA-602 against rVSV-SARS-CoV-2-S-induced COVID-19 injury, with the Penh index as a predictor of treatment effectiveness.
Fig. 6.
Functional and histopathological correlation and prediction analysis. (A) The respiratory parameters and histopathology scores were subjected to Spearman correlation analysis, averaged, and converted to a heat map. (B) Shown are the top four prediction accuracies of histopathologic parameter output using a random forest model with all the WBP parameters as input. (C) Shown are the importance ranking of WBP parameters that contributed to the accurate prediction of the histological scores, in the machine learning model. MVb: minute ventilation (mL/min). PEF: peak expiratory flow; EEP: end expiratory pause; PIF: estimated peak inspiratory flow; TP: duration of pause before inspiration (%); TB: duration of breaking, percentage of the breath occupied by transitioning from inspiration to expiration (%); PAU: pause; Penh: enhanced pause; Rpef: The location into expiration where the peak occurs as a fraction of Te; EF50: expiratory flow at 50% expired volume; and RH: relative humidity (%).
Discussion
Pneumonia and ARDS that can progress to sepsis and multiorgan failure are frequent sequelae of hospitalized patients with severe COVID-19. Parallel findings are reproduced in animal models using native and pseudo-typed SARS-CoV-2 viruses that recapitulate clinical and molecular features of COVID-19 disease (42–44). Here, we report that high-dose-replicating rVSV-SARS-CoV-2 infection of K18-hACE2tg mice mimics that of native SARS-CoV-2. The functional coincidence is evidenced by parallel responses of weight loss, infectivity range, and pulmonary viral load, key diagnostic criteria for SARS-CoV-2 infection (45–47). Validation of rVSV-SARS-CoV-2-infected K18-hACE2tg mice as a model of SARS-CoV-2/COVID-19 is further supported by the concurrence of histological lung injury, elevated expression of ICAM-1 in both lung and heart, the broad repertoire of dysregulated respiratory parameters incurred by the virus in mice at 5 dpi determined by whole-body plethysmography and the upregulated expressions of lung ZBP1 and Phospho-MLKL which are involved necroptosis induced by COVID-19 associated ARDS (Figs. 2–5) (14–16). ICAM-1 is a marker for IL-1, TNF-alpha, and leukocyte binding to endothelial cells, and serum ICAM-1 levels indicate systemic inflammation and constitute a prognostic marker of disease severity in COVID-19 patients (48–50).
The results are temporally and quantitatively consistent with previous reports of K18-hACE2tg mice infected with native SARS-CoV-2 Hong Kong/VM20001061/2020 (34) and SARS-CoV-2 WA-1/US (35). Whereas replacement of native SARS-CoV-2 with VSV eliminates most of the native SARS-CoV-2 genome, the chimeric VSV displays an intact Spike glycoprotein in an antigenic form that resembles native infectious SARS-CoV-2 (42, 43). The Spike glycoprotein is responsible for host cell attachment and entry as well as the host immune responses to infection, and mutations of the Spike glycoprotein are largely responsible for the altered infectivity and virulence of SARS-CoV-2 variants (51). The Spike glycoprotein also serves as a ligand for the toll-like receptor-4 (TLR4) that triggers classical NF-κB activation, induction of proinflammatory cytokines, and disruption of inflammatory homeostasis that leads to hyperinflammation, multiorgan failure, and death (51, 52). Consequently, replication-competent rVSV-SARS-CoV-2 at high dose confers Spike glycoprotein–mediated immune inflammatory responses equivalent to those of the native SARS-CoV-2 Spike glycoprotein (37, 53). In our hands, exposure of K18-hACE2tg mice to high-dose rVSV-SARS-CoV-2 conferred acute pulmonary pathology comparable with that of native SARS-CoV-2 infection in mice and humans. Application of machine learning to our results with strong positive correlations between respiration and lung damage indicated prognostic value of such pulmonary function parameters, further supporting the authenticity of the model and its clinical relevance.
Whole-body plethysmography allows noninvasive quantification of primary respiratory functions and specific airway resistance parameters in rodents and is routinely used to characterise pulmonary functions in mouse models of SARS-CoV-2 (38, 44, 54, 55). WBP of mice infected with rVSV-SARS-CoV-2 revealed a characteristic SARS-CoV pattern wherein respiratory and ventilation rates were reduced, and expiratory parameters increased. Significantly increased PAU and Penh indices indicate bronchoconstriction, and the decrease in Rpef implies airway obstruction (56). The changes are compatible with decreased pulmonary compliance reflecting faster relaxation time, reduced respiratory rate, and delayed time of breath. Together, the WBP index changes show a mixture of classic restrictive and obstructive airway disease patterns that are characteristic of patients with COVID-19, monitored by spirometry, lung volumes, and diffusion capacity (57, 58). Clinically, the observed WBP changes in this SARS-CoV-2 model are consistent with corollaries in severe COVID-19 patients that typically include diffuse alveolar epithelium destruction, capillary damage/bleeding, hyaline membrane formation, alveolar septal fibrous proliferation, and pulmonary consolidation (57). Although lower respiratory and ventilation rates are not necessarily seen in acute COVID-19 patients (59), they are consistent with a high viral dose challenge (60).
Our study documents rescue of pulmonary function and lung injury by a GHRH-R antagonist in a model of SARS-CoV-2. Previous work from our group and others documented roles for GH and IGF-1 pathways in other inflammatory conditions, including lung disease and demonstrated potent anti-inflammatory and anti-fibrosis actions of GHRH-R antagonists (27, 30, 61). In our study, GHRH-R antagonist, MIA-602, ameliorated weight loss and abrogated the pulmonary dysfunction and lung damage caused by replication-competent rVSV-SARS-CoV-2 infection of K18-hACE2tg mice. Mice treated with MIA-602 displayed WBP parameters and inflammation indicators on dpi 5 that did not differ significantly from pretreatment (dpi = 0) mice or controls (Figs. 2–4). MIA-602 abrogated lung tissue damage, reducing perivascular infiltration, epithelial damage and ICAM-1 expression, a marker of severe inflammation. Previous work documented the molecular signalling pathways whereby GH and IGF-1 activate inflammation and fibrosis in response to diverse proinflammatory stimulation and their abrogation by GHRH-R antagonism (27, 30, 61–63). Central to the molecular mechanisms of GHRH-R antagonism-mediated anti-inflammation and anti-fibrosis are the canonical JAK2/STAT3 (64), PI3K/AKT (65), and PAK1-STAT3/NF-κβ (66) signalling pathways. Whereas we did not directly demonstrate anti-fibrosis in this study, COVID-19 is primarily a Spike glycoprotein–driven inflammatory disease that in severe cases progresses to cytokine storm and sepsis, pneumonia, and associated fibrosis; therefore, anti-inflammation is the most likely mechanism of action for MIA-602 in this context as we described previously in models of intraperitoneal, bleomycin-induced lung fibrosis (28), and granuloma sarcoidosis (29).
Our biochemical, IHC and EM analyses implicate ZBP1/pMLKL signalling and necroptosis as contributing to the lung pathology of rVSV-SARS-CoV-2, that in all instances was suppressed by MIA-602 treatment. ZBP1 is a central innate immune marker of activated inflammatory signalling and necroptosis associated with viral infection, including SARS-CoV-2 (67, 68). Our results are consistent with recent preclinical reports that have assigned central roles for the ZBP1-pMLKL signaling pathway in SARS-CoV-2-induced inflammation, necroptosis, and lung damage (14–16). Therefore, our demonstration that MIA-602 targets a central pathway of SARS-CoV-2 inflammation with programmed cell death as the end stage provides a mechanism for MIA-602 with possible clinical application. Because of its unique mechanism of action, MIA-602 and other GHRH-R antagonists may prove to be superior to the armory of anti-inflammatory and anti-viral agents that, while effective in preclinical tests, have failed to provide the broad-spectrum protection required to combat severe SARS-CoV-2 infection (69–74).
Linear correlation and machine learning prediction analyses of our results revealed that minute ventilation, including the WBP indices of Penh and Rpef correlated most closely with inflammatory infiltrate and epithelial damage. Penh and Rpef are commonly used to measure pulmonary function in animal models, including those related to COVID-19 (44, 54, 55). The results are consistent with a previous report that described a dose-dependent increase of Penh and its relation to airway denudation and debris accumulation in models of SARS-CoV and influenza A virus infection (38). Hence, our machine learning analyses support the use of Penh as a prognostic indicator of COVID-19 and related viral diseases. Confirmatory analyses including additional computational experiments with cross-validation, missing information analysis, adversarial attacks, and noise in training data are warranted.
In conclusion, rVSV-SARS-CoV-2-S infection of adult hACE2tg mice provides an acute, severe COVID-19 model with viral dose dependence that can be implemented under BSL-2 conditions. The model reproduces native SARS-CoV-2 infection in permissive mice and multiple aspects of severe COVID-19 pathology in humans. By blocking the central ZBP1-pMLKL signalling pathway, treatment with MIA-602 conferred anti-inflammatory and anti-necroptosis activity that correlated with abrogated pulmonary injury and promotion of normal respiratory functions of rVSV-SARS-CoV-2-infected mice. Based on a machine learning model, the WBP Penh index represents a potential prognostic indicator of viral pathogenesis and treatment efficacy in this model.
Study Limitations.
We acknowledge a need to reproduce the effects of MIA-602 in alternative animal models using the native SARS-CoV-2 virus in a BSL-3 laboratory to include direct invasive measures to more precisely quantify lung function. Whereas human COVID-19 causes significant mortality, the absence of such mortality in our model like other murine SARS-CoV-2 models is a potential limitation.
Methods
Experimental Design and Animal Model.
K18-hACE2tg mice were purchased from Jackson. Male and female mice were housed under specific pathogen-free conditions in temperature- and humidity-controlled rooms with a 12-h light–dark cycle. Mice were provided with water and food ad libitum. Two- to three-month-old mice were inoculated either intranasally with the native SARS-CoV-2/USA-WA1/2020 at 10^5 PFU (n = 10) in BSL-3 facility or intratracheally (i.t.) with 108 IU or 2 × 107 PFU/mouse replication-competent VSV-eGFP-SARS-CoV-2-Spike recombinant virus (rVSV-SARS-CoV-2) (n = 15) in BSL-2 facility for 5 d. After 24 h post infection, mice that received the rVSV-SARS-CoV-2 received daily injections of GHRH-R antagonist MIA-602 [10 μg/mouse] (n = 8) or vehicle (DMSO/propylene-glycol) (n = 8). All the procedures were performed in mice anaesthetized with isoflurane at 3 to 5% in an induction chamber and between 0.5 and 2% for maintenance, depending on the procedure and its duration. In accordance with the protocol, animals were monitored daily for clinical signs of disease. Weight loss was monitored daily for 5 dpi. Mice losing greater than 30% of their starting body weight were immediately killed. Mice which appear to be moribund or unlikely to recover were also killed. At the end point, mice were killed with isoflurane inhalation (3 to 5%) followed by cervical dislocation, thoracotomy, exsanguination, perfusion with DEPC-PBS, and lung inflation. Lungs and hearts were collected for histology and molecular assays.
SARS-CoV-2 and rVSV-SARS-CoV-2 Virus Preparation.
SARS-CoV-2/USA-WA1/ 2020 was deposited by the Centers for Disease Control and Prevention and obtained through BEI resources, NIAID, NIH (catalog number NR-52281), and propagated as previously described (75). Quantifying infectious virus and viral genome copies was performed as previously described (76, 77). rVSV-SARS-CoV-2 expressing Spike glycoprotein of the Wuhan isolate with a 21 aa truncation at the cytoplasmic tail to enhance viral replication (42) (GenBank MN908947.3) was supplied by Sean Whelan. All viruses were propagated in 293 T-ACE2-Furin cells to generate master stocks or concentrated stocks using Lenti-X™ Concentrator - 100X concentration (Takara). The stocks were titrated by plaque-forming unit (PFU) assays (PFU) or TCID50 on VeroE6-ACE2-Furin cells (SI Appendix, Fig. S2) and working stocks were aliquoted and stored at −80 °C.
Whole Body Plethysmography for Assessment of Lung Function.
Respiratory function was monitored unrestrained in conscious mice by the Buxco small animal whole-body plethysmography system and FinePoint software (Data Science International). Prior to the measurements, each mouse was placed in separate cylindrical chambers for around 30 min/day for 3 consecutive days. Mice were recorded before infection (Day 0 or baseline), three days (Day 3), and five (Day 5) post infection. On days 0, 3, and 5, mice were acclimatized in the chamber for 10 min; then, respiratory parameters were measured continuously for the next 10 min (78). The system was calibrated prior to each recording session. Multiple parameters were recorded: respiratory frequency (breaths/min), tidal volume (TV), tidal volume (TV), minute volume (MV), enhanced pause (Penh), pause (PAU), inspiratory time (Ti), peak inspiratory flow (PIF), expiratory time (Te), peak expiratory flow (PEF), relaxation time (Tr), tidal mid expiratory flow (EF50), and total expiratory time (Rpef). Penh = (Te - Tr)/Tr*PEF/PIF
Immunostaining, Imaging Acquisition, and Analysis.
Sections from lungs and hearts were incubated for 45 min at 70 degrees, dewaxed with xylene washes, and hydrated by graded ethanol washes of 100% (twice for 3 min), 95%, 80%, and 70% followed by water immersion (twice for 4 min). Next, slides were steamed for 45 min with citrate Antigen Retrieval Buffer (ab93678). Sections were rinsed with PBS, permeabilized with 0.2% triton x-100 (Sigma-Aldrich) for 30 min blocking with 10% donkey serum (45 min) and incubated overnight with primary antibody anti-ICAM (sc-390483) or anti-ZBP1 (sc-271483), in blocking solution. On the next day after the PBS washes, the slides were incubated for 1 h with HRP-conjugated or Alexa Fluor 555 secondary antibodies. Nuclei were stained with DAPI before mounting with ProLong Gold Antifade (Invitrogen P36934) and coverslips. No primary controls were used for all staining and produced close to zero signal. For imaging and quantification, slides were scanned at 20× magnification using the Olympus VS120–L100 Virtual Slide Microscope (Tokyo, Japan) or imaged with 5 z-stacks using a Confocal Zeiss Microscope. Five images from randomly selected tissue sections were used to quantify different markers using Image J where lung parenchyma and myocardium regions were selected for quantification (biomarker expression/tissue area × 100). For the ATS/ALI scoring system (40), the following parameters were analyzed: a) neutrophils in the alveolar space (none = 0, 1-5 cells = 1, > 5 cells = 2); b) neutrophils in the interstitial space/septae (none = 0, 1-5 cells = 1, > 5 cells = 2); c) hyaline membranes (none = 0, one membrane = 1; > 1 membrane = 2); d) proteinaceous debris in air spaces (none = 0, one instance = 1, > 1 instance = 2); e) alveolar septal thickening (>2× mock thickness = 0, 2-4× mock thickness = 1, >4× mock thickness = 2). Scores were calculated as follows: [(20 × a) + (14 × b) + (7 × c) + (7 × d) + (2 × e)]/100 (40). Final scores were obtained by the average of three lung parenchyma fields at 40× magnification per mouse. Image analyses were done blindedly by two independent examiners.
Machine Learning Analysis.
Computational experiments were performed using a random forest machine learning model. The dataset used to train the model was split 50%:50% for training and validation. The random forest model was set to 25 estimators with bootstrapping, obtaining overall 88% accuracy in the testing set. We programmed this model using python 3.9, and the model was taken from a scikit-learn package. The 2 python code files used were: Inference_one_model.py for one single training with a model whose most important feature is Penh, and massive_testing.py which generates the figure with 1000 of trainings to obtain the model behavior, including the SD.
RNASeq Studies.
Snap-frozen lung tissues from the three experimental groups (N = 3 per group) were subjected to Illumina RNA prep (Nugen Universal Plus) followed by RNASeq (40 Million paired-end 100 base reads per sample). Data were analyzed using GeneSpringX. Pathway analysis was applied to transcripts selected based on a P-value < 0.01 and fold change of at least 2.
Western Blots.
To validate the RNASeq results, a different set of mice were used for the western blots. Protein concentration from lung tissue lysates was measured using a Bradford assay. Samples were prepared and separated using a 4 to 12% Novex mini 15-well gradient gel (Bolt System, Life Technologies) and probed with the Zbp1 (sc-271483), SOCS1 (Cell Signaling 3950), INF-gamma (Biolegend 505801), MLKL (Cell Signaling 37705), pMLKL (Cell Signaling 37333), and GAPDH (sc-365062) antibodies. Signals were detected by means of chemiluminescence (Femto, Thomas Scientific) on photographic films. Digitized images were analyzed using Image J (NIH). Protein band densitometry was normalized to that of GAPDH, and the averaged results were plotted as normalized densitometry units (n.d.u.).
Study Approval.
All protocols and experimental procedures were reviewed and approved by the University of Miami and the John’s Hopkins University Institutional Animal Care and Use Committee and comply with all Federal and State guidelines concerning the use of animals in research and teaching as defined by The Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-234, revised 2011). IBC Protocol # 20-071. The University of Miami has received full accreditation with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International), site 001069. Dissections were performed under deep anesthesia (100 mg/kg ketamine and 20 mg/kg Xylazine via intraperitoneal injections) to collect hearts, lungs, and plasma. Experiments with SARS-CoV-2 were carried out under biosafety level-3 containment.
Statistical Analysis.
Data are reported as mean ± SEM. Statistical significance between three groups was determined by one-way or two-way ANOVA (unless otherwise indicated) followed by Tukey’s or Bonferonni’s post hoc tests, as recommended by the software. For comparisons of two groups, Student’s two-tailed t test was used. Analyses were performed using Graph Pad Prism, version 9.0.0. The null hypothesis was rejected at P < 0.05.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank the Analytical Imaging and the John P. Hussman Institute for Human Genomics core facilities at the University of Miami Miller School of Medicine for the VS120 Slide Scanner (grant 1S10OD023579-01 from the NIH) and RNASeq work, respectively. We acknowledge Vania Almeida and the University of Miami Transmission Electron Microscopy Core for EM sample preparation and assistance with the generation of EM images. We thank Drs. Diamond and Whelan for providing the rVSV-eGFP-SARS-CoV-2 virus. This work was supported by the following: NIH (1 R01 HL169608-01), the Miami Heart Research Institute and the American Heart Association (965480); Miami VAHS Research Service, and a Distinguished Medical Research Scientist Award (I01 BX005051) from the VA Office of Research and Development (A.V.S. and R.M.J.).
Author contributions
J.M.C.C., A.S., R.M.J., and L.A.S. designed research; J.M.C.C., A.K., E.R., A.G.S., T.C., A.W., J.V., W.Z., E.M., and M.J.R. performed research; R.C., W.S., and A.V.S. contributed new reagents/analytic tools; J.M.C.C., A.K., A.G.S., T.C., A.P., A.V.S., R.M.J., and L.A.S. analyzed data; and J.C., A.G.S., K.A.W., A.V.S., R.M.J., and L.A.S. wrote the paper.
Competing interests
A.V.S. and R.M.J. are listed as co-inventors on patents of GHRH analogs, which were assigned to the University of Miami and Veterans Affairs Department.
Footnotes
Reviewers: G.G., University of Antioquia; S.M., University of Alabama at Birmingham; and V.L.d.O., University of Sao Paulo School of Medicine.
Contributor Information
Andrew V. Schally, Email: andrew.schally@va.gov.
Lina A. Shehadeh, Email: lshehadeh@med.miami.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Harapan H., et al. , Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 13, 667–673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovato A., de Filippis C., Clinical presentation of COVID-19: A systematic review focusing on upper airway symptoms. Ear Nose Throat J. 99, 569–576 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Costagliola G., Spada E., Consolini R., Age-related differences in the immune response could contribute to determine the spectrum of severity of COVID-19. Immun. Inflamm. Dis. 9, 331–339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leisman D. E., et al. , Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 8, 1233–1244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ssentongo P., Ssentongo A. E., Heilbrunn E. S., Ba D. M., Chinchilli V. M., Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PloS One 15, e0238215 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A., et al. , Extrapulmonary manifestations of COVID-19. Nat. Med. 26, 1017–1032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X., et al. , Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 299, E177–E86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hama Amin B. J., et al. , Post COVID-19 pulmonary fibrosis; A meta-analysis study. Ann. Med. Surg. 77, 103590 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalski J. E., Kurche J. S., Schwartz D. A., From ARDS to pulmonary fibrosis: The next phase of the COVID-19 pandemic? Trans. Res. 241, 13–24 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spagnolo P., et al. , Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 8, 750–752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X., et al. , 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 9, 747–754 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auriemma C. L., et al. , Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 46, 1222–1231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton R. D., et al. , Causes and timing of death in patients with ARDS. Chest 128, 525–532 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Messaoud-Nacer Y., et al. , STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis. 13, 269 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S., et al. , SARS-CoV-2 Z-RNA activates the ZBP1-RIPK3 pathway to promote virus-induced inflammatory responses. Cell Res. 33, 201–214 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X. Y., et al. , ZBP1-mediated necroptosis: Mechanisms and therapeutic implications. Molecules 28, 52 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer N. J., Gattinoni L., Calfee C. S., Acute respiratory distress syndrome. Lancet (London, England) 398, 622–637 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushner P., et al. , The use of non-steroidal anti-inflammatory drugs (NSAIDs) in COVID-19. NPJ Prim. Care Respir. Med. 32, 35 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyriazopoulou E., et al. , Effect of anakinra on mortality in patients with COVID-19: A systematic review and patient-level meta-analysis. Lancet Rheumatol. 3, e690–e697 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh P., et al. , Nintedanib vs pirfenidone in the management of COVID-19 lung fibrosis: A single-centre study. J. R. College Phys. Edinburgh 52, 100–104 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Toogood A. A., Shalet S. M., Growth hormone replacement therapy in the elderly with hypothalamic-pituitary disease: A dose-finding study. J. Clin. Endocrinol. Metab. 84, 131–136 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Schally A. V., et al. , Agonists of growth hormone-releasing hormone (GHRH) inhibit human experimental cancers in vivo by down-regulating receptors for GHRH. Proc. Natl. Acad. Sci. U.S.A. 115, 12028–12033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gesmundo I., et al. , Growth hormone-releasing hormone attenuates cardiac hypertrophy and improves heart function in pressure overload-induced heart failure. Proc. Natl. Acad. Sci. U.S.A. 114, 12033–12038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarandi M., et al. , Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides 89, 60–70 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Barabutis N., Akhter M. S., Kubra K.-T., Jackson K., Growth hormone-releasing hormone in endothelial inflammation. Endocrinology 164, bqac209 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez R., et al. , Antagonists of growth hormone-releasing hormone suppress in vivo tumor growth and gene expression in triple negative breast cancers. Oncotarget 3, 988–997 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C., et al. , Growth hormone-releasing hormone in lung physiology and pulmonary disease. Cells 9, 2331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C., et al. , Activity of the growth hormone-releasing hormone antagonist MIA602 and its underlying mechanisms of action in sarcoidosis-like granuloma. Clin. Trans. Immunol. 10, e1310 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C., et al. , Growth hormone-releasing hormone receptor antagonist modulates lung inflammation and fibrosis due to bleomycin. Lung 197, 541–549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin M. A., et al. , GHRH antagonists support lung endothelial barrier function. Tissue Barriers 7, 1669989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhter M. S., Uddin M. A., Kubra K. T., Barabutis N., Elucidation of the molecular pathways involved in the protective effects of AUY-922 in LPS-induced inflammation in mouse lungs. Pharmaceuticals (Basel, Switzerland: ) 14, 522 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelnaser M., Alaaeldin R., Attya M. E., Fathy M., Hepatoprotective potential of gabapentin in cecal ligation and puncture-induced sepsis; targeting oxidative stress, apoptosis, and NF-kB/MAPK signaling pathways. Life Sci. 320, 121562 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Harcourt J., et al. , Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg. Infect. Dis. 26, 1266–1273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau G. B., et al. , Evaluation of K18-hACE2 mice as a model of SARS-CoV-2 infection. A. J. Tropical Med. Hygiene 103, 1215–1219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong W., et al. , The K18-human ACE2 transgenic mouse model recapitulates non-severe and severe COVID-19 in response to an infectious dose of the SARS-CoV-2 virus. J. Virol. 96, e0096421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.C. f. D. Control, Prevention. Interim laboratory biosafety guidelines for handling and processing specimens associated with coronavirus disease 2019 (COVID-19) (2021).
- 37.Manangeeswaran M., et al. , BSL-2-compliant lethal mouse model of SARS-CoV-2 and variants of concern to evaluate therapeutics targeting the Spike protein. Front. Immunol. 13, 919815 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menachery V. D., Gralinski L. E., Baric R. S., Ferris M. T., New metrics for evaluating viral respiratory pathogenesis. PloS One 10, e0131451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An X., et al. , Protective effect of oxytocin on LPS-induced acute lung injury in mice. Sci. Rep. 9, 2836 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matute-Bello G., et al. , An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44, 725–738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaickus L. J., Bouchard J., Kim J., Natarajan S., Remick D. G., Oral tolerance inhibits pulmonary eosinophilia in a cockroach allergen induced model of asthma: A randomized laboratory study. Respir. Res. 11, 160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Case J. B., et al. , Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe. 28, 475–485.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Case J. B., et al. , Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis in mice. Cell Host Microbe 28, 465–474.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leist S. R., et al. , A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183, 1070–1085.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halfmann P. J., et al. , SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 603, 687–692 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosa R. B., et al. , In vitro and in vivo models for studying SARS-CoV-2, the etiological agent responsible for COVID-19 pandemic. Viruses [Internet] 13, 379 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan C., et al. , Animal models for COVID-19: Advances, gaps and perspectives. Signal Transduct. Targeted Ther. 7, 220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamza A. M., et al. , Relation between macrophage inflammatory protein-1 and intercellular adhesion molecule-1 and computed tomography findings in critically-ill saudi covid-19 patients. J. Infect. Public Health 15, 1497–1502 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yap R. X. L., et al. , Immune and coagulation profiles in 3 adults with multisystem inflammatory syndrome. Ann. Acad. Med. Singapore 52, 80–87 (2023). [PubMed] [Google Scholar]
- 50.Smith-Norowitz T. A., Loeffler J., Norowitz Y. M., Kohlhoff S., Intracellular Adhesion Molecule-1 (ICAM-1) levels in convalescent COVID-19 Serum: A case report. Ann. Clin. Lab Sci. 51, 730–734 (2021). [PubMed] [Google Scholar]
- 51.Das N. C., Chakraborty P., Bayry J., Mukherjee S., Comparative binding ability of human monoclonal antibodies against omicron variants of SARS-CoV-2: An in silico investigation. Antibodies (Basel) 12, 17 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choudhury A., Mukherjee S., In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 92, 2105–2113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah V. K., Firmal P., Alam A., Ganguly D., Chattopadhyay S., Overview of immune response during SARS-CoV-2 infection: Lessons from the past. Front. Immunol. 11, 1949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pruijssers A. J., et al. , Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep. 32, 107940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dinnon K. H., et al. , SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci. Transl. Med. 14, eabo5070 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uraki R., et al. , Characterization of SARS-CoV-2 Omicron BA.2.75 clinical isolates. Nat. Commun. 14, 1620 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mo X., et al. , Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir J. 55, 2001217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres-Castro R., et al. , Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 27, 328–337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mälberg J., Hadziosmanovic N., Smekal D., Physiological respiratory parameters in pre-hospital patients with suspected COVID-19: A prospective cohort study. PloS One 16, e0257018 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Julander J. G., Kesler K., Van Wettere A. J., Morrey J. D., Smee D. F., The use of plethysmography in determining the severity of lung pathology in a mouse model of minimally lethal influenza virus infection. Antiviral Res. 108, 10–13 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Kiaris H., Chatzistamou I., Papavassiliou A. G., Schally A. V., Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol. Metabolism 22, 311–317 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Mayo K. E., Godfrey P. A., Suhr S. T., Kulik D. J., Rahal J. O., “Growth hormone-releasing hormone: Synthesis and signaling”, in Proceedings of the 1993 Laurentian Hormone Conference. 50. Boston, Bardin C. W. (Academic Press, 1995), pp. 35–73. [DOI] [PubMed] [Google Scholar]
- 63.Qin Y. J., et al. , Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation. Proc. Natl. Acad. Sci. U.S.A. 111, 18303–18308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang W. C., et al. , Signaling mechanisms of growth hormone-releasing hormone receptor in LPS-induced acute ocular inflammation. Proc. Natl. Acad. Sci. U.S.A. 117, 6067–6074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kral J. B., et al. , Sustained PI3K activation exacerbates BLM-induced lung fibrosis via activation of pro-inflammatory and pro-fibrotic pathways. Sci. Rep. 6, 23034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gan J., et al. , Growth hormone-releasing hormone receptor antagonists inhibit human gastric cancer through downregulation of PAK1–STAT3/NF-κB signaling. Proc. Natl. Acad. Sci. U.S.A. 113, 14745–14750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kesavardhana S., et al. , The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J. Biol. Chem. 295, 8325–8330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuriakose T., Kanneganti T. D., ZBP1: Innate Sensor regulating cell death and inflammation. Trends Immunol. 39, 123–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engeroff P., Belbézier A., Monsel A., Klatzmann D., Anakinra reduces lung inflammation in experimental acute lung injury. Immun. Inflamm. Dis. 10, 123–129 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiong S., et al. , Interleukin-1RA mitigates SARS-CoV-2-induced inflammatory lung vascular leakage and mortality in humanized K18-hACE-2 mice. Arterioscler Thromb Vasc. Biol. 41, 2773–2785 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeong J. H., et al. , Combination therapy with nirmatrelvir and molnupiravir improves the survival of SARS-CoV-2 infected mice. Antiviral Res. 208, 105430 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu M. L., et al. , Combinational benefit of antihistamines and remdesivir for reducing SARS-CoV-2 replication and alleviating inflammation-induced lung injury in mice. Zool. Res. 43, 457–468 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puhl A. C., et al. , Vandetanib blocks the cytokine storm in SARS-CoV-2-infected mice. ACS Omega 7, 31935–31944 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puhl A. C., et al. , Vandetanib reduces inflammatory cytokines and ameliorates COVID-19 in infected mice. ACS Omega 2022, 7, 31935–31944 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Resnick J. D., Beer M. A., Pekosz A., Early transcriptional responses of human nasal epithelial cells to infection with Influenza A and SARS-CoV-2 virus differ and are influenced by physiological temperature. Pathogens 12, 480 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dhakal S., et al. , Sex differences in lung imaging and SARS-CoV-2 antibody responses in a COVID-19 golden syrian hamster model. mBio 12, e00974–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Condor Capcha J. M., et al. , Generation of SARS-CoV-2 spike pseudotyped virus for viral entry and neutralization assays: A 1-week protocol. Front. Cardiovasc Med. 7, 618651 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irion C. I., et al. , Col4a3-/- mice on Balb/C background have less severe cardiorespiratory phenotype and SGLT2 over-expression compared to 129x1/SvJ and C57Bl/6 backgrounds. Int. J. Mol. Sci. 23, 6674 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.