Abstract
Purpose
Chronic inflammation, immune dysregulation, and oxidative stress are major drivers of age-related macular degeneration (AMD) pathogenesis. Lipopolysaccharide (LPS) is a potent proinflammatory toxin originating from gut bacteria. We assessed the association of a blood biomarker of LPS exposure with incident AMD.
Methods
The Alienor Study is a prospective population-based study, including 963 residents of Bordeaux (France), aged 73 years or more at baseline. Esterified 3-hydroxy fatty acids (3-OH FAs) were measured from blood samples as a proxy of LPS burden. AMD was graded from color retinal photographs and spectral domain optical coherence tomography, performed every two years from 2006 to 2017. Cox proportional hazards models were used to estimate associations of between esterified 3-OH FAs, using 722 eyes at risk for incident early AMD and 981 eyes at risk for incident advanced AMD.
Results
Higher esterified 3-OH FAs were associated with incident early AMD after adjusting for age and gender (hazard ratio [HR] = 1.21 for 1 standard deviation [SD] increase; 95% confidence interval [CI], 1.01–1.45; P = 0.04) but not with incident advanced AMD (HR = 1.03 for 1 SD increase; 95% CI, 0.73–1.45; P = 0.86). These associations remained stable after multivariate adjustment and imputation for missing covariates (early AMD HR = 1.22 for 1 SD increase; 95% CI, 1.01–1.46; P = 0.04; advanced AMD HR = 0.98 for 1 SD increase; 95% CI, 0.69–1.38; P = 0.91).
Conclusions
This study evidenced an association between higher esterified 3-OH FAs and incident early AMD, suggesting that exposure to LPS may be involved in the early pathophysiological processes of AMD.
Keywords: age-related macular degeneration, systemic inflammation, LPS, clinical (human) or epidemiologic studies: risk factor assessment
Age-related macular degeneration (AMD) is the leading cause of blindness among the elderly population in the developed world. Although large epidemiological studies have identified risk factors such as cigarette smoking, nutritional factors, cardiovascular diseases, and genetic markers, the underlying pathological mechanisms are not fully understood.1,2 Contributing factors in AMD pathogenesis are oxidative stress, along with immune dysregulation and chronic inflammation, particularly persisting low-grade inflammation.3,4
In recent years, a link between gut microbiota and the retina has been described as the so-called “gut-retina axis.”5,6 The human gut microbiota consists of trillions symbiotic micro-organisms living in a highly dynamic ecosystem.7 Besides facilitating digestion, the gut microbiota serve a variety of metabolic and supportive functions essential for physiological homeostasis, including maintenance of the intestinal barrier, participation in the intestinal immune system, and generation of biologically active metabolites.
Diet and age are known to influence the composition of the gut microbiota. In particular, high-fat diets promote endotoxin-producing and sulphate-reducing bacteria, associated with mucus layer degradation, low-grade inflammation, and insulin-resistance.8,9 Recent studies also showed that stress, alcohol, radiation, and lack of physical activities have an impact on gut microbiota.10–12 Alterations in the gut microbiota impair the integrity of intestinal barrier, leading to increased intestinal permeability. This increased permeability allows antigens, lipopolysaccharides (LPS), pathogens, and other proinflammatory substances to pass through intestinal barrier into circulation.
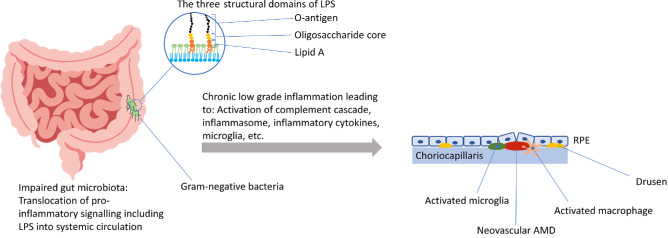
As a component of the outer membrane of Gram-negative bacteria, LPS is naturally found in the human gut microbiota. LPS is a large glycolipid consisting of three structural domains: a core oligosaccharide, an O-antigen polysaccharide and Lipid A (Fig. 1).13,14 Two molecules of 3-hydroxy (3-OH) fatty acids (FAs) are esterified to the glucosamine backbone of Lipid A, and two are attached in amide linkage.15 Lipid A acts as the pathogen-associated molecular pattern of LPS. When LPS concentrations increase, it acts as a potent immune activator and TLR4 ligand.16–18 Its inflammatory potential has been implicated in the development of multiple disease states, including insulin resistance, obesity, augmented cardiovascular risk, and fatty liver disease.19–22
Figure 1.
The “gut-retina axis” in AMD: Potential mechanisms that link impaired gut microbiota and LPS with the development and progression of AMD.
In the eye, proinflammatory signaling via LPS occurs in microglia, perivascular macrophages, dendritic cells, photoreceptors, and retinal pigment epithelium (RPE) cells and has been implicated in retinopathy of prematurity, diabetic retinopathy, and AMD (Fig. 1).5,23–27 Retinal explants stimulated by LPS showed a significant neuroinflammatory response characterized by upregulation of several cytokines and neuronal degeneration.27 Also, increased neuronal apoptosis and evidence of microglial activation after LPS exposure have been reported.28
Thus there is a substantial body of evidence pointing to the involvement of LPS-induced low-grade inflammation in the development of AMD. The mentioned in vitro studies are great models of disease, identifying pathways and links between exposure and disease outcome. Still, data from prospective human studies are needed to analyze long-term effects of chronic exposure.
Thus, in this study we assessed the association of a blood biomarker of LPS exposure with incident early and advanced AMD in the framework of a population-based cohort study of French older adults.
Methods
The Alienor Study (Antioxydants, Lipides Essentiels, Nutrition et maladies OculaiRes) is an ongoing prospective population-based study initiated in 2006 to assess the associations of age-related eye diseases with nutritional factors (in particular antioxidants, macular pigment, and fatty acids) and other risk factors (genetic susceptibility, environmental, and vascular factors, etc.).2
Study Cohort
Subjects of the Alienor Study were initially recruited from the Three City (3C) Study cohort of Bordeaux (France), which enrolled randomly selected individuals from electoral rolls from 1999 to 2001.29 Both the 3C Study cohort and the Alienor Study cohort have been described in detail elsewhere.2,29,30
The Alienor Study included 963 residents of Bordeaux, aged 73 years or more at baseline (2006–2008). Participants underwent biennial examinations at the Department of Ophthalmology of the University Hospital of Bordeaux during the study period from 2006 to 2017 (four follow-up visits, http://www.alienor-study.com/langue-english-1.html, accessed on September 8, 2023). Of the 963 Alienor participants, 624 were reexamined at the first follow-up (2009–2010), 614 at the second follow-up visit (2011–2012), 513 at the third follow-up visit (2013–2015), and 435 at the fourth follow-up visit (2015–2017).
The Alienor Study followed the tenets of the Declaration of Helsinki and was approved by the Ethical Committee of Bordeaux (Comité de Protection des Personnes Sud-Ouest et Outre-Mer III, code 2006/10) in May 2006. Informed consent of all participants of the Alienor Study was obtained after explanation of the nature and possible consequences of the study.
Imaging and AMD Grading
Each Alienor Study visit included 45° nonmydriatic color retinal photographs using a high-resolution digital nonmydriatic retinograph (TRC NW6S; Topcon, Japan). From the first follow-up visit (2009-2011), also spectral-domain optical coherence tomography (SD-OCT) examinations of the macula and the optic nerve were performed (Spectralis; Heidelberg Engineering, Heidelberg, Germany). All SD-OCT assessments were performed by the same experienced technician. For the macular thickness acquisition, the following conditions were used: resolution mode: high speed; scan angle: 20°; number of B-scans: 19; pattern size: 20 × 15 degrees; and distance between B-scans: 236 µm. A single horizontal and vertical B-scan image (1536 A-scans) centered on the fovea was also performed.
As previously described, the AMD grading scheme followed the International Classification and Grading System by Bird et al., modified for drusen size, location and area according to the Multi-Ethnic Study of Atherosclerosis.2,31,32 Grading was performed from retinal photographs in duplicate by two trained graders and inconsistencies between the two graders were adjudicated by a retina specialist.2,30 Early signs of AMD included large drusen (>125 microns in diameter), reticular pseudodrusen and pigmentary abnormalities (areas of hyperpigmentation and/or hypopigmentation (without visibility of choroidal vessels)). Neovascular AMD included serous or hemorrhagic detachment of the RPE or sensory retina, subretinal or sub-RPE hemorrhages, and fibrous scar tissue. Geographic atrophy was defined as a discrete area of retinal depigmentation, 175 µm in diameter or larger, characterized by a sharp border and the presence of visible choroidal vessels. In addition, SD-OCT macular scans were interpreted for signs of advanced AMD (retinal atrophy, subretinal fluid, subretinal tissue, pigment epithelium detachment, intra-retinal fluid).
Finally, presence of large drusen and/or pigmentary abnormalities and/or reticular pseudodrusen were defined as early AMD (in the absence of advanced AMD), which corresponds to the definition of intermediate AMD in more recent classifications.33 Classification of advanced AMD (atrophic or neovascular) was performed by a retina specialist, based on all available information (ophthalmological history and treatments, retinal photographs, SD-OCT scans).
Incidence of early AMD was defined as the eye progressing from no AMD at baseline eye examination (2006–2008) to early AMD at any time-point during the study period (2006–2017). Incidence of advanced AMD was defined as the eye progressing from no or early AMD at baseline eye examination (2006–2008) to advanced AMD at any time-point during the study period. The date of occurrence of early/advanced AMD was calculated as the midpoint of the interval between the last visit without early/advanced AMD and the first visit with early/advanced AMD.
Assessment of LPS-Type Endotoxins
All plasma measurements, including LPS-type endotoxins were determined from blood samples collected at the 3C baseline visit (1999–2001) and stored at −80 °C until determination.
As direct measurement of LPS exposure is prone to technical and measurement biases,34–37 esterified 3-hydroxy fatty acids (3-OH FAs) in plasma were measured as a proxy of total plasma LPS burden using liquid chromatography tandem mass spectrometry (LC-tandem MS). This method has been established and previously described by Pais de Barros and colleagues.38,39 Briefly, the difference between the amounts of 3-OH FAs in plasma after strong acidic hydrolysis and the amounts of free (i.e., non-LPS) 3-OH FAs without strong acidic hydrolysis is calculated. The resulting circulating plasma esterified 3-OH FA levels are a proxy of total plasma LPS burden.
Other Variables
Polygenic risk score: Genotyping was performed by the French Centre National de Génotypage at 3C study baseline (1999–2001) using Illumina Human610-Quad BeadChips.40 The present genetic risk score is based on the weighted risk score published by Fritsche and colleagues and was described in detailed elsewhere.30,41
Mediterranean diet score: The Mediterranean diet score was calculated using a 148-items validated food frequency questionnaire (FFQ) administered at home by dieticians and the MEDI-LITE score developed by Sofi et al.42 As previously described, this score includes the frequency of consumption of nine components: High consumption of fruits, vegetables, legumes, cereals, fish and olive oil, moderate consumption of dairy products and alcohol, and low consumption of meat were defined as beneficial in this score.43 Consumption frequency thresholds were estimated by Sofi and colleagues from literature.42 The final score indicates the degree of adherence to the Mediterranean diet, ranging from 0 (poor adherence) to 18 (highest adherence).
Statistical Analyses
Associations of plasma esterified 3-OH FAs with AMD were estimated using Cox proportional hazards models with age as time scale and delayed entry. Using age as time scale adjusts automatically for the confounding effect of age and thus allows for a more appropriate adjustment of age of the elderly than the classical Cox models based on the time from entry in the study.44
The individual eye was used as the unit of analysis. To take into account intra-individual correlation between eyes of one participant, the “cluster” term was included in the using Cox proportional hazards models in R (R Foundation for Statistical Computing, Vienna, Austria).
Imputation was performed using multivariate imputation by chained equations (MICE), which assumes that missing data were Missing At Random (MAR). The missing values were imputed based on the observed values for a given individual and the relations observed in the data for other participants.45 For the fraction of missing/imputed data see Supplementary Table S1. A total of 10 cycles was performed to obtain the final imputed dataset for missing potential confounders. Multivariate adjustment was performed after imputation. Model 1 was adjusted for gender, model 2 was further adjusted for smoking status, plasma high-density lipoprotein (HDL) cholesterol, plasma low-density lipoprotein (LDL) cholesterol, plasma triglycerides, diet quality and AMD polygenic risk score and model 3 was further adjusted for number of drugs used, anti-inflammatory medication and lipid-lowering medication. Variables retained in model 2 were factors associated with the incidence of AMD and/or with LPS, in our study or in the literature.
R software version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses.
Results
Characteristics of the Studied Sample
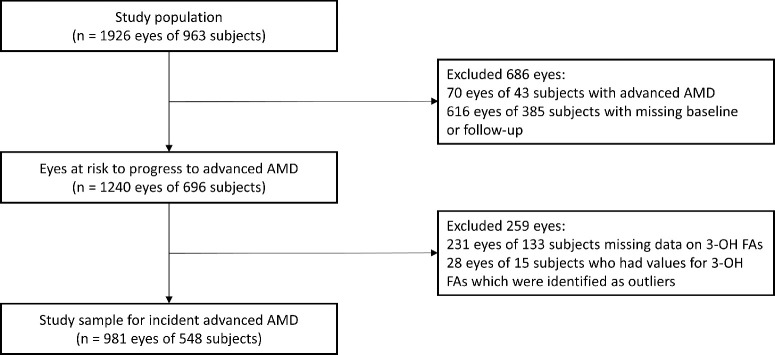
Of 1926 eyes of 963 participants, which were included in the Alienor Study, 70 eyes of 43 participants had already developed advanced AMD at baseline. In addition, 616 eyes of 385 participants had no gradable baseline and/or no gradable follow-up examination, leaving 1240 eyes of 696 participants at risk to develop incident advanced AMD (Fig. 2). Furthermore, 231 eyes of 133 participants were excluded because of missing data for plasma esterified 3-OH FA levels. Using Tukey's interquartile range criterion, we identified an additional 28 eyes of 15 participants as outliers (Supplementary Fig. S1). Therefore 981 eyes of 548 participants were included for further analyses of incident advanced AMD. For the sub-cohort of incident early AMD, the same study sample as for incident advanced AMD was used, after exclusion of participants with early AMD at baseline. Thus, of 981 eyes of 548 participants, 259 eyes of 194 participants who already had early AMD at baseline were excluded, leaving 722 eyes of 446 participants for the analyses of incident early AMD.
Figure 2.
Flow chart showing selection of participants for analyses.
Among the 548 included participants, mean age at baseline examination was 79.9 years (± 4.5), of which 352 (64.2%) were female (Table 1). Included participants tended to be slightly younger and have higher LDL cholesterol (3.67 ± 0.82 vs. 3.55 ± 0.89) than the nonincluded participants of the Alienor Study. No significant difference was observed for other sociodemographic, lifestyle, medical, or genetic data. During a median follow-up time (censoring time)46 of 7.3 years (range, 0.7 to 10.4), 195 (27.0%) eyes developed incident early AMD and 72 (7.3%) eyes incident advanced AMD.
Table 1.
Baseline Sociodemographic, Lifestyle, Medical, and Genetic Characteristics According to Participants Included and Non-Included in the Statistical Analyses: Alienor Study, 2006–2017
| Characteristics | Included (n = 548) | Non-Included (n = 415) | P Value |
|---|---|---|---|
| Age (yr), mean ± SD | 79.86 ± 4.46 | 80.57 ± 4.38 | 0.01 |
| Gender | 0.10 | ||
| Men | 196 (35.77%) | 171 (41.20%) | |
| Women | 352 (64.23%) | 244 (58.80%) | |
| Smoking (pack-year) | n = 546 | n = 405 | 0.12 |
| Never smoker | 363 (66.48%) | 251 (61.98%) | |
| <20 years | 100 (18.32%) | 78 (19.26%) | |
| ≥20 years | 83 (15.20%) | 76 (18.76%) | |
| Mediterranean diet score, mean ± SD | 10.59 ± 2.03 | 10.39 ± 2.23 | 0.18 |
| Number of drugs used | 4.00 ± 2.53 | 4.13 ± 2.64 | 0.45 |
| Anti-inflammatory medication | 84 (15.33) | 75 (18.07) | 0.29 |
| Lipid-lowering medication | 190 (34.67) | 119 (28.67) | 0.06 |
| Plasma lipids (mmol/L) | |||
| Total cholesterol | 5.83 ± 0.94 | 5.71 ± 1.03 | 0.09 |
| LDL cholesterol | 3.67 ± 0.82 | 3.55 ± 0.89 | 0.03 |
| HDL cholesterol | 1.60 ± 0.39 | 1.58 ± 0.41 | 0.55 |
| Triglycerides | 1.20 ± 0.54 | 1.28 ± 0.69 | 0.07 |
| Genetic risk score | 0.28 ± 1.20 | 0.37 ± 1.20 | 0.31 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Multivariate Associations Between Esterified 3-OH FAs in Plasma and the Incidence of AMD
Associations of esterified 3-OH FAs in plasma with incident early and advanced AMD are shown in Table 2. After adjustment for age (as the timescale) and gender, higher plasma esterified 3-OH FAs was significantly associated with a higher risk of incident early AMD (hazard ratio [HR] for 1 standard deviation [SD] increase = 1.21; 95% confidence interval [CI], 1.01–1.45; P = 0.04). No significant association was observed between plasma esterified 3-OH FAs and incident advanced AMD (HR = 1.03 for 1 SD increase; 95% CI, 0.73–1.45; P = 0.86).
Table 2.
Associations between Esterified 3-OH FAs and Incidence of AMD: Alienor Study, 2006–2017
| Early AMD | Advanced AMD | |||
|---|---|---|---|---|
| No. of Eyes | Mean ± SD for 3-OH FAs | No. of Eyes | Mean ± SD for 3-OH FAs | |
| Non-Incident AMD | 527 | 254.6 ± 72.0 | 909 | 256.5 ± 74.0 |
| Incident AMD | 195 | 266.2 ± 77.5 | 72 | 256.9 ± 80.4 |
| HR (95% CI) | P Values | HR (95% CI) | P Values | |
| Model 1* | 1.21 (1.01–1.45) | 0.04 | 1.03 (0.73–1.45) | 0.86 |
| Model 2† | 1.22 (1.01–1.46) | 0.04 | 0.98 (0.69–1.38) | 0.91 |
| Model 3‡ | 1.21 (1.01–1.46) | 0.04 | 1.00 (0.71–1.40) | 0.98 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation; HR, hazard ratio.
Model 1, HR was estimated using Cox proportional model adjusted for gender. HR for 1 SD increase.
Model 2, HR was estimated using Cox proportional model adjusted for gender, smoking, HDL cholesterol, LDL cholesterol, triglycerides, diet quality and AMD polygenic risk score. HR for 1 SD increase.
Model 3, HR was estimated using Cox proportional model adjusted for gender, smoking, HDL cholesterol, LDL cholesterol, triglycerides, diet quality, AMD polygenic risk score, number of drugs used, anti-inflammatory medication and lipid-lowering medication. HR for 1 SD increase.
The association between plasma esterified 3-OH FAs and incident early AMD remained stable after multivariate adjustment and imputation for missing covariates (HR = 1.22 for 1 SD increase; 95% CI, 1.01–1.46; P = 0.036). As shown in Figure 3, the hazard of incident early AMD increased with esterified 3-OH FAs in plasma, after multivariate adjustment. The hazard function is represented using p-spline with 4 degrees of freedom, showing a continuous increase of the association between plasma esterified 3-OH FAs and incident early AMD to about the mid of the fourth quartile, followed by a steeper increase afterward.
Figure 3.
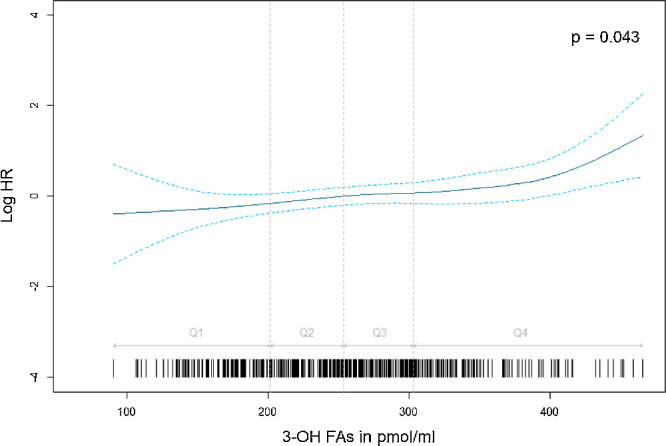
Association between 3-OH FAs and incidence of early AMD by eye adjusted for age, gender, smoking status, HDL cholesterol, LDL cholesterol, triglycerides, genetic risk score, and Mediterranean diet score. Data from the Alienor Study 2006–2017 (n = 722). All quantitative variables were modelled using p-spline with four degrees of freedom in the Cox model.
In multivariable analyses, esterified 3-OH FAs in plasma were not associated with incident advanced AMD (HR = 0.98 for 1 SD increase; 95% CI, 0.69–1.38; P = 0.91).
Finally, we performed sensitivity analyses by excluding participants with less than one year of follow-up (12 participants for the analysis of advanced AMD and seven participants for the analysis of early AMD). The results were similar to the main analyses (data not shown).
Discussion
In this cohort of French older adults, higher baseline levels of 3-OH FAs, a proxy of plasma LPS exposure, were significantly associated with the incidence of early AMD. Because this gut endotoxin has proinflammatory properties, these results add to the evidence regarding the role of inflammation in AMD and further document emerging evidence of the gut-retina axis.
To our knowledge, this prospective population-based study is the first to investigate the relationship between plasma endotoxin exposure and the incidence of AMD.
However, many in vitro and animal studies underline the influence of plasma endotoxin exposure on AMD: Using primary human RPE cultures and ARPE19 cells, Leung et al.47 demonstrated that LPS induced RPE-cytotoxicity via proinflammatory autocrine signaling of interleukin-6 and interleukin-8, implying the role of LPS in the progression of macular pathologies.
In neovascular in vivo AMD models, both significant reduction of choroidal neovascularization (CNV) size with LPS treatment and exacerbation of CNV have been described. Specifically, Feng et al.48 described promotion of CNV lesions after intravitreal LPS pretreatment (10 µg; 10 ng/mL) one day before laser irradiation via TLR4 activation and subsequent upregulation of the expression of CXC chemokine receptors 4 and 7 in choroid-retinal endothelial cells. In line with this observation, also an enhancement of proinflammatory microglia/macrophages status by intravenously injected LPS (1 mg/kg) was observed to correlate with larger CNV leakage areas in the early onset of laser-induced CNV.25 By contrast, it has been shown that low-dose (20 µg) intraperitoneal LPS treatment before laser-induced CNV treatment suppresses CNV via interleukin-10 secretion by peritoneal macrophages in mice.24 Examining the contribution of macrophage subtypes in CNV formation, Zandi et al.49 observed that the M1 macrophage transforming cytokine cocktail (INF-γ and LPS) injected into the vitreal cavity reduced CNV size and leakage. Taken together, the proinflammatory or anti-inflammatory direction of the immune response in vivo seems to depend on the various LPS treatment regimens (before or after CNV induction, location and dosage of injection) used in each individual study. These regimens activate different signaling pathways determining the responsiveness and polarization of microglia/macrophages and thus explaining the variability of experimental outcome. However, all of these studies underline the immune modulatory influence of LPS on changes associated with AMD.
Although our prospective study provides new insights into the association between incidence of AMD and a proxy of plasma LPS burden, limitations of this study have to be acknowledged.
Because to date no standardization of LPS quantification exists, and the widely used limulus amebocyte lysate assay only measures the reactive fraction of LPS, we applied a proxy method to estimate the LPS exposure by translating esterified 3-OH FAs assessed by LC-tandem MS into LPS exposure.38,50 However, in certain circumstances esterified 3-OH FA in plasma can also indicate the presence of lipid A without acyloxyacyl structures, which are essential for bioactive signaling.15 Thus the analysis of 3-OH FAs may have led to misclassification of the exposure itself, because LPS exposure may be different from 3-OH FAs. Furthermore, the assessment of exposure was based on a single blood draw, performed about eight years before the first ophthalmological examination. However, differential misclassification of 3-OH FAs in AMD and non-AMD cases seems unlikely, and thus misclassification of LPS exposure would tend to bias the associations with AMD toward the null.
Regarding potential confounders, we cannot rule out the possibility of residual confounding by factors that have not been evaluated or are suboptimally measured. In particular, the question arises whether the association between LPS and the incidence of early AMD is merely diet related. Indeed, healthy diet patterns such as the Mediterranean diet or prudent diet have been associated with low circulating 3-OH FAs.43 There is also evidence suggesting that high consumption of saturated fats increases postprandial LPS concentrations.51,52 However, our results were similar in the unadjusted and the fully adjusted model (which includes adjustment for Mediterranean diet and circulating lipids), suggesting that our results were not highly confounded by nutritional status. Nevertheless, residual confounding because of measurement error (in particular, the Mediterranean diet score is based on food questionnaires relying on the subjects’ memories, rendering this score prone to noise) or to other dimensions of diet that were not assessed in this study may be present.
Selection bias cannot be completely dismissed in our study, because participants included in this analysis were slightly younger and had higher LDL levels than nonparticipants.
Another limitation of our study was the small number of patients with advanced AMD, which might have induced insufficient statistical power for detecting existing associations. This may in part explain the absence of significant relationships with advanced AMD.
The strengths of the study include a large and well-defined cohort, a detailed standardized ophthalmologic examination, and long follow-up time. High-quality retinal imaging reviewed by independent retina specialists ensured negligible misclassification of AMD cases. Our analyses accounted for time to development of AMD in each individual eye and included a genetic risk score based on 49 single nucleotide polymorphisms associated with AMD.41 Dietary assessment was conducted according to the MEDI-LITE score.42 In addition, our findings are based on prospective follow-up, thus limiting reverse causation. However, only randomized clinical trials can prove the causal nature of the associations.
Conclusions
An association between higher plasma esterified 3-OH FAs and incident early AMD was observed in our cohort, suggesting that long-term proinflammatory exposure to LPS is involved in the early pathophysiological processes of AMD. This study adds to the available literature on LPS and AMD underlining the inflammatory component of AMD development. However, future studies are warranted to explore this complex relation and to better understand the underlying mechanisms.
Supplementary Material
Acknowledgments
Supported by German Research Foundation (DFG) grant PL 5077/1-1 (to PL). The ALIENOR Study was supported by Théa Pharma, Fondation Voir et Entendre, University of Bordeaux, Agence Nationale de la Recherche (ANR 2010-PRSP-011 VISA), CFSR Recherche (Club Francophone des Spécialistes de la Rétine) and the French Ministry of Health (PHRC, 2012, PHRC12_157 ECLAIR). Also supported by the FRAILOMIC Initiative (FP7-HEALTH-2012-Proposal No. 305483–2) and the ITMO Santé Publique—Alliance nationale pour les sciences de la vie et de la santé (AVIESAN) (ISP05 2014). Théa Pharma participated in the design of the Alienor Study, but none of the sponsors participated in the collection, management, statistical analysis, and interpretation of the data or in the preparation, review, or approval of the present manuscript.
Disclosure: P.P. Larsen, None; C. Féart, Laboratoire Lescuyer (F), Synadiet (C); J.-P. Pais de Barros, None; B.M.J. Merle, Théa Pharma (F, C); L. Gayraud, None; M.-N. Delyfer, Abbvie (C), Bayer (C), Horus Pharma (C), Novartis (C), Roche, (C), Théa(C); J.-F. Korobelnik, Allergan (C), Abbvie (C), Apellis (C), Bayer (C), Janssen (C), NanoRetina (C), Roche (C), Thea (C), Carl Zeiss Meditec (C); C. Delcourt, Allergan (C), Chauvin-Bausch+Lomb (C), Thea Pharma (C), Novartis (C)
References
- 1. Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY.. Age-related macular degeneration. Lancet. 2012; 379(9827): 1728–1738. [DOI] [PubMed] [Google Scholar]
- 2. Delcourt C, Korobelnik JF, Barberger-Gateau P, et al.. Nutrition and age-related eye diseases: the Alienor (Antioxydants, lipides essentiels, nutrition et maladies oculaires) study. J Nutr Health Aging. 2010; 14: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Datta S, Cano M, Ebrahimi K, Wang L, Handa JT.. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017; 60: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K.. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016; 73: 1765–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rinninella E, Mele MC, Merendino N, et al.. The role of diet, micronutrients and the gut microbiota in age-related macular degeneration: new perspectives from the gut–retina axis. Nutrients. 2018; 10: 1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowan S, Jiang S, Korem T, et al.. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci USA. 2017; 114(22): E4472–E4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ursell LK, Metcalf JL, Parfrey LW, Knight R.. Defining the human microbiome. Nutr Rev. 2012; 70(23): S38–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devkota S, Wang Y, Musch MW, et al.. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012; 487(7405): 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang C, Zhang M, Wang S, et al.. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010; 4: 232–241. [DOI] [PubMed] [Google Scholar]
- 10. Paulos CM, Wrzesinski C, Kaiser A, et al.. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007; 117: 2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazzon E, Cuzzocrea S.. Role of TNF-α in ileum tight junction alteration in mouse model of restraint stress. Am J Physiol - Gastrointest Liver Physiol. 2008; 294: 1268–1281. [DOI] [PubMed] [Google Scholar]
- 12. Enomoto N, Ikejima K, Yamashina S, et al.. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001; 25(6 suppl.): 51–54. [DOI] [PubMed] [Google Scholar]
- 13. Raetz CRH, Whitfield C.. Lipopolysaccharide endotoxins endotoxins as activators of innate immunity. Annu Rev Biochem. 2008; 71: 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertani B, Ruiz N.. Function and biogenesis of lipopolysaccharides. EcoSal Plus. 2018; 8(1): 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munford RS. Endotoxemia-menace, marker, or mistake? J Leukoc Biol. 2016; 100: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott AJ, Oyler BL, Goodlett DR, Ernst RK.. Lipid A structural modifications in extreme conditions and identification of unique modifying enzymes to define the Toll-like receptor 4 structure-activity relationship. Biochim Biophys Acta Mol Cell Biol Lipids. 2017; 1862: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Needham BD, Trent MS.. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol. 2013; 11: 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chassaing B, Gewirtz AT.. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol. 2014; 42: 49–53. [DOI] [PubMed] [Google Scholar]
- 19. Cani PD, Amar J, Iglesias MA, et al.. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007; 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- 20. Cani PD, Possemiers S, Van De Wiele T, et al.. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009; 58: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Witkowski M, Weeks TL, Hazen SL.. Gut microbiota and cardiovascular disease. Circ Res. 2020; 127: 553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI.. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444(7122): 1027–1031. [DOI] [PubMed] [Google Scholar]
- 23. Tremblay S, Favret S, Binet F, et al.. Systemic inflammatory stress provokes abnormal retinal vascular development. Invest Ophthalmol & Vis Sci. 2013; 54: 5611. [DOI] [PubMed] [Google Scholar]
- 24. Matsumura N, Kamei M, Tsujikawa M, Suzuki M, Xie P, Nishida K.. Low-dose lipopolysaccharide pretreatment suppresses choroidal neovascularization via IL-10 induction. PLoS One. 2012; 7(7): e39890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsioti I, Steiner BL, Escher P, Zinkernagel MS, Benz PM, Kokona D.. Systemic lipopolysaccharide exposure exacerbates choroidal neovascularization in mice [Published online ahead of print November 28, 2022]. Ocul Immunol Inflamm. [DOI] [PubMed] [Google Scholar]
- 26. Qin X, Zou H.. The role of lipopolysaccharides in diabetic retinopathy. BMC Ophthalmol. 2022; 22: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghosh F, Abdshill H, Arnér K, Voss U, Taylor L.. Retinal neuroinflammatory induced neuronal degeneration - Role of toll-like receptor-4 and relationship with gliosis. Exp Eye Res. 2018; 169(November 2017): 99–110. [DOI] [PubMed] [Google Scholar]
- 28. Bauer PM, Zalis MC, Abdshill H, Deierborg T, Johansson F, Englund-Johansson U.. Inflamed in vitro retina: cytotoxic neuroinflammation and galectin-3 expression. PLoS One. 2016; 11(9): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alpérovitch A, Amouyel P, Dartigues JF, et al.. Vascular factors and risk of dementia: design of the three-city study and baseline characteristics of the study population. Neuroepidemiology. 2003; 22: 316–325. [DOI] [PubMed] [Google Scholar]
- 30. Merle BMJ, Cougnard-Grégoire A, Korobelnik JF, et al.. Plasma lutein, a nutritional biomarker for development of advanced age-related macular degeneration: the Alienor Study. Nutrients. 2021; 13: 2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein R, Klein BEKK, Knudtson MD, et al.. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006; 113: 373–380. [DOI] [PubMed] [Google Scholar]
- 32. Bird AC, Bressler NM, Bressler SB, et al.. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995; 39: 367–374. [DOI] [PubMed] [Google Scholar]
- 33. Ferris FL, Wilkinson CP, Bird A, et al.. Clinical classification of age-related macular degeneration. Ophthalmology. 2013; 120: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen J. The detection and interpretation of endotoxaemia. Intensive Care Med Suppl. 2000; 26: 51–56. [DOI] [PubMed] [Google Scholar]
- 35. Chen L, Mozier N.. Comparison of Limulus amebocyte lysate test methods for endotoxin measurement in protein solutions. J Pharm Biomed Anal. 2013; 80: 180–185. [DOI] [PubMed] [Google Scholar]
- 36. Dheda S, Min H, Vesey D, Hawley C, Johnson DW, Fahim M.. Establishing a stable platform for the measurement of blood endotoxin levels in the dialysis population. Diagnosis. 2020; 8: 249–256. [DOI] [PubMed] [Google Scholar]
- 37. Boutagy NE, McMillan RP, Frisard MI, Hulver MW.. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie. 2016; 124(3): 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pais de Barros JP, Gautier T, Sali W, et al.. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. J Lipid Res. 2015; 56: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weil D, Pais de Barros JP, Mourey G, et al.. Circulating levels of 3-hydroxymyristate, a direct quantification of endotoxaemia in noninfected cirrhotic patients. Liver Int. 2019; 39: 106–114. [DOI] [PubMed] [Google Scholar]
- 40. Lambert JC, Heath S, Even G, et al.. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009; 41: 1094–1099. [DOI] [PubMed] [Google Scholar]
- 41. Fritsche LG, Igl W, Bailey JNC, et al.. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016; 48: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sofi F, Dinu M, Pagliai G, Marcucci R, Casini A.. Validation of a literature-based adherence score to Mediterranean diet: the MEDI-LITE score. Int J Food Sci Nutr. 2017; 68: 757–762. [DOI] [PubMed] [Google Scholar]
- 43. André P, Pais de Barros JP, MJ Merle B, et al.. Mediterranean diet and prudent diet are both associated with low circulating esterified 3-hydroxy fatty acids, a proxy of LPS burden, among older adults. Am J Clin Nutr. 2021; 114: 1080–1091. [DOI] [PubMed] [Google Scholar]
- 44. Lamarca R, Alonso J, Gómez G, Muñoz Á.. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998; 53(5): 337–343. [DOI] [PubMed] [Google Scholar]
- 45. Azur MJ, Stuart EA, Frangakis C, Leaf PJ.. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011; 20: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Betensky RA. Measures of follow-up in time-to-event studies: why provide them and what should they be? Clin Trials. 2015; 12: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leung KW, Barnstable CJ, Tombran-Tink J.. Bacterial endotoxin activates retinal pigment epithelial cells and induces their degeneration through {IL}-6 and {IL}-8 autocrine signaling. Mol Immunol. 2009; 46: 1374–1386. [DOI] [PubMed] [Google Scholar]
- 48. Feng YF, Guo H, Yuan F, Shen MQ.. Lipopolysaccharide promotes choroidal neovascularization by up-regulation of CXCR4 and CXCR7 expression in choroid endothelial cell. PLoS One. 2015; 10(8): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zandi S, Nakao S, Chun KH, et al.. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 2015; 10: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gnauck A, Lentle RG, Kruger MC.. Chasing a ghost? Issues with the determination of circulating levels of endotoxin in human blood. Crit Rev Clin Lab Sci. 2016; 53: 197–215. [DOI] [PubMed] [Google Scholar]
- 51. Laugerette F, Vors C, Géloën A, et al.. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem. 2011; 22: 53–59. [DOI] [PubMed] [Google Scholar]
- 52. Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E.. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009; 50: 90–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.