Abstract
Purpose
To investigate gait kinematics during single- and dual-task walking in glaucoma patients compared with healthy controls.
Methods
Nineteen glaucoma patients (10 females, 9 males) and 30 healthy controls (17 females, 13 males) participated in this cross-sectional study. Spatiotemporal gait parameters (e.g., stride length, velocity, minimum toe clearance [MTC]) were assessed using inertial measurement units (sampling frequency 100 Hz) during single-task walking and dual-task walking at a comfortable velocity. During dual-task walking, participants walked and concurrently performed different cognitive tasks in a random order: (i) reaction time task, (ii) N-Back-task, and (iii) letter fluency task with two difficulty levels, respectively. Repeated measures analyses of covariance (Group × Condition) were conducted to analyze the data.
Results
A significant effect of group was found for the coefficient of variation (CoV) of the MTC, F(1,39) = 4.504, P = 0.040, = 0.104, with higher values in glaucoma patients. Based on the effect sizes, a main effect of group was also found for the MTC, F(1,39) = 2.668, P = 0.110, = 0.064, and the MTCCoV dual-task costs, F(1,38) = 3.225, P = 0.08, = 0.078, which was lower and higher, respectively, in glaucoma patients.
Conclusions
The present study revealed a significantly higher MTC variability as well as medium effect sizes for a lower MTC and higher MTC dual-task costs in glaucoma patients compared with healthy controls, which might be related to a higher risk of falling owing to tripping.
Translational Relevance
The minimum toe clearance might mirror disease-related changes in walking performance and might have prognostic value for assessing fall risk in glaucoma patients.
Keywords: cognitive function, gait, overground walking
Introduction
Glaucoma defines a group of slowly progressing optic neuropathies that result in visual field loss.1 It is differentiated into angle-closure glaucoma1,2 and the more frequent open-angle glaucoma.3 Glaucoma is considered the leading cause of irreversible blindness.4 In 2020, the projected number of people affected by glaucoma ranged between 40 and 80 years was 76 million, increasing to 111.8 million by 2040.5
In the early stages of open-angle glaucoma, the visual field loss is almost unnoticeable,2 but its extent increases with disease duration.1,5,6 Given that sensory input, and in particular visual information, is a prerequisite for the optimal control of human locomotion, visual field loss might impair gait performance and increase the risk of falling.7,8 However, although gait and balance performance measures were associated with fall rates in glaucoma patients, they could not sufficiently explain why people with greater visual field loss have a higher risk of falling.9 There are various studies that have already reported the effect of glaucoma on gait performance.8–12 They found, for instance, that glaucoma severity, operationalized via integrated visual field sensitivity, was associated with a wider base of support and higher gait variability measures during overground walking.12 Because gait variability measures, such as stride time variability, have been shown to be predictors of falls,13 they might be a valuable screening tool for the progression of the disease and the fall risk assessment of glaucoma patients.
In addition to these studies investigating the associations between visual impairment and gait measures in glaucoma patients, other investigators have examined the effect of glaucoma on walking performance in comparison with healthy controls.11,14 For instance, Lee et al.14 found that glaucoma patients walked slower and exhibited reduced cadence, gait cycle time, and stance time during normal walking and with obstacles compared with healthy controls. In contrast, Gomes et al.11 did not report any differences in spatiotemporal gait parameters compared with healthy controls, although glaucoma patients showed a reduced performance during the timed up and go test. Nevertheless, these studies have only recorded gait parameters during single-task but not motor-cognitive dual-task walking (i.e., walking + cognitive task). This is of particular importance, given that daily activities often require multitasking and a lower motor-cognitive dual-task walking performance is related to a higher risk of falling.15 Furthermore, a recently published review highlighted that glaucoma is frequently accompanied by cognitive dysfunction,16 which might impair motor-cognitive dual-task walking performance. In this regard, the type of the concurrently performed cognitive task during walking seems to have distinct effects on gait measures, such as velocity, with larger cognitive-motor interference when using working memory tasks.17 Moreover, these studies have not quantified the minimum toe clearance (MTC), which describes the minimal vertical toe to ground distance during the mid-swing phase.18 If the MTC is close to zero, there is a high likelihood of stumbling, which has been related to an increased risk of falling.19 Moreover, the variability of MTC seems a promising parameter, given that it was able to differentiate between various populations (e.g., young and elderly, fallers and non-fallers).18,19
Therefore, the present study quantified spatiotemporal gait parameters not only during single-task walking, but also during motor-cognitive dual-task walking in glaucoma patients compared with healthy controls. To investigate the impact of the type and difficulty of the concurrently performed cognitive task on gait measures, three different tasks were applied (i.e., reaction time task, N-Back-task, and letter fluency task) with two difficulty levels, respectively. Considering its importance for the occurrence of falls, the MTC and its variability were determined besides the classical spatiotemporal gait parameters (e.g., gait velocity and step length). It was hypothesized that the glaucoma patients show a reduced gait performance, especially during motor-cognitive dual-task walking, and that the MTC as well as its variability is different between glaucoma patients and healthy controls.
Methods
Study Design
The data presented in this article were recorded during the baseline measurements of a longitudinal study examining the effects of a multimodal versus unimodal exercise intervention on visual, motor, and cognitive performance measures as well as structural and functional brain adaptations in glaucoma patients and healthy control subjects (German Clinical Trial Register, ID: DRKS00022519/05.08.2020, https://drks.de/search/de/trial/DRKS00022519). Study procedures were approved by the Ethics Committee of the University Medical Faculty Magdeburg (32/18) and performed in accordance with the tenets of the Declaration of Helsinki. All participants signed the informed consent form before participating in this study.
Participants
A sample size calculation for a repeated measures analysis of variance with two groups (glaucoma patients and healthy controls) and seven conditions (single-task and three dual-task conditions with two difficulty levels, respectively) was performed with G*Power (version 3.1).20 Because Lee et al.14 have found a large effect size for the lower gait velocity in glaucoma patients compared with healthy controls and that Gomes et al.11 have not found such a difference, a medium effect size (f = 0.25) was assumed. The significance level was set at 0.05 with a power of 0.95 and a correlation among the repeated measures of 0.5. According to the calculation, a total sample size of 26 participants (13 participants per group) was required.
Participants were recruited at the Department of Ophthalmology at the University Hospital Magdeburg, via local ophthalmologists and the national patient network. Inclusion criteria were as follows: 60 years of age or older; diagnosis of open-angle glaucoma (only for the glaucoma group); ability to walk at least 6 minutes without walking support. Exclusion criteria were (i) any eye disease affecting the indices of visual functions (e.g., cataract [except incipient stage], ocular trauma history and ocular surgeries [except glaucoma or cataract surgery]), (ii) neurological diseases, and (iii) diseases that limit the physical performance of the participants, including orthopedic diseases including arthrosis (grade II or higher), musculoskeletal impairments, tendinitis, tenosynovitis, myositis, prosthesis in the lower extremities, joint replacements, neurological disorders, rheumatism, cardiovascular disorders, stroke, and heart related disease.
A total of 49 participants, 19 with glaucoma (glaucoma group; 9 males and 10 females; mean age, 70.7 ± 5.9 years; mean height, 168.7 ± 8.5 cm; mean body mass, 73.0 ± 16.7 kg) and 30 aged-matched healthy participants (control group; 13 males and 17 females; mean age, 70.9 ± 5.1 years; mean height 168.3 ± 10.4 cm; mean body mass, 75.3 ± 17.8 kg) volunteered for this study (see Table 1 for ophthalmological details).
Table 1.
Comparison of Ophthalmological Results for the Control and Glaucoma Group
| Controls (n = 30) | Glaucoma (n = 19) | Difference | |
|---|---|---|---|
| Median | Range | Median | Range | P Value | |
| MD_right | 0.59 | 5.09 | −0.67 | 25.97 | 0.015 |
| MD_left | 0.15 | 5.92 | −1.09 | 22.00 | 0.009 |
| Mean ± SD | Mean ± SD | ||
| BCVA | −0.1 ± 0.08 | −0.07 ± 0.13 | 0.236 |
| pRNFL_right | 91 ± 12.29 | 77 ± 12.71 | <0.001 |
| pRNFL_left | 89 ± 10.81 | 76 ± 14.94 | <0.001 |
Typical traits of glaucoma (reduction of visual field sensitivities (MD); reduction of peripapillary retinal nerve fiber layer (pRNFL) thickness) were evident for the glaucoma group. BCVA, best-corrected visual acuity [logMAR]; pRNFL_right/left, peripapillary retinal nerve fiber layer (pRNFL) thickness [µm]; MD_right/left, mean deviation [db]; SD, standard devition.
General Procedures
Several tests were performed over a period of 2 consecutive days (at the same time of day) including the single- and dual-task gait analyses, the Trail Walking Test,21 and a visuo-motor reaction time task using the interactive system Speed Court (GlobalSpeed GmbH, Hemsbach, Germany).22
At the beginning of day 1, each subject underwent the following procedure: (i) signing the informed consent, (ii) documenting their physical activity with the German version of the Freiburger Questionnaire on Physical activity,23 (iii) measuring anthropometric data, (iv) assessing static postural control using the MFT S3 check (MFT Bodyteamwork GmbH, Kirchberg, Austria),24 (v) single-task walking, and (vi) dual-task walking. On day 2, the participants underwent the following procedure: (i) single task walking and (ii) dual-task walking. The dual-task gait tests were conducted in a randomized order. Participants performed three different cognitive tasks (i.e., reaction time task, N-Back-task, letter fluency task). In this regard, one of the cognitive tasks was performed on the first day and the others on day 2. Because of the corona pandemic, participants were instructed to wear an FFP2 mask during all laboratory visits.
Gait Analysis
Participants walked with comfort velocity forth and back over a 10-m track for 180 seconds in each condition. Spatiotemporal gait parameters were assessed using three inertial measurement units (XSENS MTw Awinda, Movella, Delft, Netherlands; sampling frequency 100 Hz). The inertial measurement units were placed proximal on each foot and the sternum. The outcome variables of interest were stride length (m), gait velocity (m/s), MTC (cm), and their respective coefficient of variation (CoV = 100 × standard deviation/mean). The gait parameters were calculated using the algorithm developed by Hamacher et al.25. The collected data were processed in MATLAB (MathWorks, Version R2020b, Natick, MA).
Cognitive Tasks
The cognitive tasks included a reaction time task, N-Back-task, and a letter fluency task. Participants randomly performed the single cognitive task while sitting as well as concurrently during walking (motor-cognitive dual-task), for each cognitive task, respectively, to calculate the cognitive dual-task costs. Each cognitive task, irrespective if performed in the single- or dual-task condition, was performed with two levels of difficulty in a fixed order (easy to severe) and lasted 180 seconds, respectively. Thus, each task consisted of four trials. For the reaction time task and the modified N-Back-task, participants held a computer mouse in each hand, which were fixed with straps. Only the computer mouse in the dominant hand was useable. Further, all acoustic stimuli were played through a speaker placed in the center of the room.
During the reaction time task,26,27 participants were listening to a series of high and low sounds and had to click as fast as possible, when the high sound was played. Task difficulty was manipulated by using different interstimulus intervals (ISIs), easy (ISI of 3 seconds, 60 stimuli) and severe (ISI of 2 seconds, 90 stimuli).
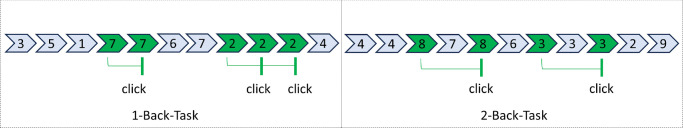
When performing the modified version of the N-Back-task (1-Back and 2-Back) to assess working memory,28,29 participants were instructed to listen to a series of numbers between one and nine. The numbers were electronically presented in a random order using Adobe Animate (Adobe Systems Software Ireland Limited, Dublin, Ireland) with an ISI of 2 seconds (90 stimuli, 30 responses). To perform the 1-Back-task (level of difficulty: easy) participants had to click the computer mouse as soon as possible, when the announced number was repeated (e.g., 1-1; see Fig. 1). The 2-Back-task (level of difficulty: severe) required the participants to click the mouse as fast as possible, when the first and third numbers were equal in a sequence (e.g., 1-2-1; see Fig. 1). Only correct responses were included in the analysis.
Figure 1.
N-Back-task with two levels of difficulty (easy = 1-Back-task and severe = 2-Back-task).
During the modified letter fluency task, which is assumed to assess inhibition, working memory, and switching,30 participants were given 180 seconds to name as many words as possible with a certain letter. In the easy level of difficulty, the letter was changed after 90 seconds. In the severe difficulty level, participants were given two letters and they had to produce as many words as possible, while switching between the letters (e.g., A, B: apple, bridge, answer). During the letter fluency task, participants responses were recorded with a microphone for later analysis. Words were excluded from the analysis if they started with a letter other than the one stated by the investigator or if there were repetitions of the same word with different endings. Proper words were defined as written in the German dictionary (Duden31) and newspaper, including first names.
Motor and Cognitive Dual-Task Costs
All these walking and cognitive tasks were performed as a single- and dual-task, respectively, to calculate the dual-task costs (DTCs) as follows:
| (1) |
| (2) |
ST and DT are the single- and dual-task performances, respectively. Equation (1) was used when a higher measurement value reflected a better performance. Otherwise, when a lower measurement value reflected a better performance, Equation (2) was used. Hence, positive values indicate higher DTCs, and negative values reflect a better dual-task performance compared with the single-task performance.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Science (SPSS Statistics Version 28.0, IBM Corp., New York, NY). Normal distribution was checked using the Shapiro-Wilk test. Differences between the groups in the anthropometric data were checked with Student's t-tests.
Because previous studies have shown analysis of covariance (ANCOVA) to be robust against moderate violation of normality,32 nonparametric tests were not used to check for differences. Therefore, a two-way repeated measures ANCOVA with the factors group (glaucoma patients versus healthy controls) and condition (single-task and dual-task conditions) was conducted to compare the data of the groups. Given that the number of males and females differed between groups and that body weight as well as height have an impact on gait parameters, they were used as covariates in the ANCOVA. In case of significant main effects or interaction effects, Bonferroni-corrected post hoc tests were conducted. Differences were considered significant when the P value was 0.05 or less. If a violation of sphericity was detected, the Greenhouse-Geisser correction was used. Data are presented as means ± standard deviations (SD) and mean differences with 95% confidence intervals (CI). The effect sizes (partial eta squared [) were interpreted according to Lakens33: small ( = 0.01), medium ( = 0.06), and large ( = 0.14).
Results
Owing to invalid gait data and drop-outs, data from six participants (four of the control group and two of the glaucoma group) were not completely included in the analyses. To increase transparency, the number of analyzed cases for the respective parameter is shown in Tables 2, 3, and 4. In the following discussion, only those results that differed significantly between groups or where the group difference exceeded a mean effect size ( = 0.06) are listed. An overview of all parameters including the P-values and effect sizes is presented in Tables 2, 3, and 4 and in Figure 2.
Table 2.
Means ± Standard Deviations of the Gait Data of Glaucoma Patients and Healthy Controls as well as the Outcomes of the ANCOVA
| Repeated Measures ANCOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Single -Task Walking | Letter Fluency Task (easy) | Letter Fluency Task (severe) | Reaction Time Task (easy) | Reaction Time Task (severe) | N-Back Task (easy) | N-Back Task (severe) | Condition × Group | Group | Condition |
| MTC (cm) | ||||||||||
| Control (N = 27) | 2.543± 0.718 | 2.078± 0.564 | 1.999± 0.559 | 2.311± 0.634 | 2.220± 0.650 | 2.286± 0.650 | 2.152± 0.656 | |||
| F3.19, 124.46 = 0.902P = 0.447, = 0.023 | F1,39 = 2.569P = 0.117, = 0.062 | F3.19, 124.46 = 0.249P = 0.873, = 0.006 | ||||||||
| Glaucoma (N = 17) | 2.311± 0.370 | 1.765± 0.429 | 1.624± 0.416 | 2.131± 0.399 | 2.025± 0.379 | 2.055± 0.326 | 1.909± 0.351 | |||
| MTCCoV (%) | ||||||||||
| Control(N = 27) | 30.934± 6.896 | 31.497± 8.582 | 32.524± 10.124 | 29.103± 6.339 | 29.782± 6.747 | 30.982± 8.529 | 31.459± 8.831 | |||
| F2.68,104.35 = 2.604P = 0.062, 0.063 | F1,39 = 4.947 P = 0.032, = 0.113 | F2.68,104.35 = 0.451P = 0.695, = 0.011 | ||||||||
| Glaucoma (N = 17) | 34.637± 6.979 | 40.780± 13.452 | 42.040± 10.873 | 32.873± 5.437 | 35.171± 6.784 | 34.945± 7.635 | 36.572± 9.693 | |||
| Stride length (cm) | ||||||||||
| Control (N = 28) | 1.324± 0.103 | 1.222± 0.110 | 1.215± 0.113 | 1.271± 0.115 | 1.273± 0.106 | 1.273± 0.103 | 1.251± 0.107 | |||
| F3.18,127.11 = 0.692P = 0.567, = 0.017 | F1,40 = 0.104P = 0.748, = 0.003 | F3.18,127.11 = 0.597P = 0.627, = 0.015 | ||||||||
| Glaucoma (N = 17) | 1.319± 0.132 | 1.205± 0.142 | 1.195± 0.148 | 1.282± 0.119 | 1.289± 0.120 | 1.265± 0.140 | 1.240± 0.141 | |||
| Stride lengthCoV (%) | ||||||||||
| Control(N = 28) | 14.861± 2.778 | 13.667± 2.359 | 13.343± 2.953 | 13.111± 2.317 | 13.745± 2.642 | 13.311± 2.769 | 13.241± 2.851 | |||
| F4.47,178.81 = 0.517P = 0.743, = 0.013 | F1,40 = 0.177P = 0.676, = 0.004 | F4.47,178.81 = 3.048 P = 0.015, = 0.071 | ||||||||
| Glaucoma (N = 17) | 15.236± 2.460 | 13.532± 2.582 | 13.417± 2.492 | 13.906± 2.752 | 13.805± 2.695 | 13.329± 2.180 | 13.283± 2.308 | |||
| Gait velocity (m/s) | ||||||||||
| Control(N = 28) | 1.264± 0.110 | 1.081± 0.154 | 1.062± 0.156 | 1.195± 0.145 | 1.190± 0.139 | 1.187± 0.133 | 1.145± 0.140 | |||
| F2.74,109.58 = 0.571P = 0.620, = 0.014 | F1,40 = 0.018P = 0.894, = 0.000 | F2.74,109.58 = 0.566P = 0.623, = 0.014 | ||||||||
| Glaucoma (N =17) | 1.244± 0.173 | 1.044± 0.204 | 1.010± 0.245 | 1.197± 0.156 | 1.212± 0.166 | 1.176± 0.181 | 1.131± 0.173 | |||
| Gait velocityCoV (%) | ||||||||||
| Control (N = 28) | 16.904± 2.986 | 15.786± 2.593 | 15.346± 3.019 | 14.970± 2.741 | 15.599± 3.094 | 15.017± 3.049 | 15.072± 3.059 | |||
| F3.93,157.24 = 0.792P = 0.530, = 0.019 | F1,40 = 0.360P = 0.552, = 0.009 | F3.93,157.24 = 1.317P = 0.266, = 0.032 | ||||||||
| Glaucoma (N = 17) | 17.488± 2.892 | 16.337± 3.335 | 15.977± 2.671 | 15.534± 3.070 | 15.290± 2.841 | 15.085± 2.508 | 15.187± 2.647 | |||
Significant differences and differences based on effect sizes are presented in bold.
Table 3.
Means ± Standard Deviations for the Cognitive and Motor Dual Task Costs of Glaucoma Patients and Healthy Controls as well as the Outcomes of the ANCOVA
| Repeated Measures ANCOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition | Letter Fluency Task (n) (easy) | Letter Fluency Task (n) (severe) | Reaction Time Task (s) (easy) | Reaction Time Task (s) (severe) | N-Back Task (n) (easy) | N-Back Task (n) (severe) | Condition × Group | Group | Condition |
| Cognitive performance (%) | |||||||||
| Control(N = 28) | 4.006± 20.681 | 2.097± 18.255 | 4.635± 10.497 | 5.672± 7.869 | 2.141± 7.332 | −13.774± 78.047 | |||
| F1.47,60.43 = 1.724P = 0.193, = 0.040 | F1,41 = 0.271P = 0.605, = 0.007 | F1.47,60.43 = 0.526P = 0.539, = 0.013 | |||||||
| Glaucoma (N = 18) | 6.238± 17.746 | 3.407± 20.174 | −1.920± 10.544 | 3.702± 6.876 | −3.462± 11.994 | 9.035± 13.025 | |||
| MTC (%) | |||||||||
| Control(N = 26) | 17.671± 8.349 | 20.736± 8.526 | 7.849± 6.869 | 11.290± 11.144 | 8.171± 7.429 | 13.736± 9.733 | |||
| F2.58,97.93 = 2.647 P = 0.062, = 0.065 | F1,38 = 2.221P = 0.144, = 0.055 | F2.58,97.93 = 0.125P = 0.925, =0.003 | |||||||
| Glaucoma (N = 17) | 25.451± 12.096 | 31.394± 12.629 | 6.371± 9.572 | 11.174± 7.832 | 10.156± 5.063 | 16.482± 9.565 | |||
| MTCCoV (%) | |||||||||
| Control(N = 26) | −1.397± 18.134 | 0.781± 15.732 | −5.234± 12.208 | −3.119± 16.326 | −0.719± 12.426 | 0.693± 11.809 | |||
| F3.03,115.22 = 3.006 P = 0.033, = 0.073 | F1,38 = 2.770 P = 0.104, 0.068 | F3.03,115.22 = 0.941P = 0.424, = 0.024 | |||||||
| Glaucoma (N = 17) | 12.361± 19.368 | 16.442± 15.587 | −7.256± 11.753 | −0.843± 11.497 | 1.321± 10.654 | 4.371± 13.745 | |||
| Strid length (%) | |||||||||
| Control(N = 27) | 7.352± 4.865 | 7.893± 4.749 | 3.362± 3.790 | 3.101± 4.916 | 3.112± 3.677 | 4.646± 3.985 | |||
| F2.74,106.81 = 1.090P = 0.354, = 0.027 | F1,39 = 0.192P = 0.664, = 0.005 | F2.74,106.81 = 0.381P = 0.749, = 0.010 | |||||||
| Glaucoma (N = 17) | 8.899± 5.421 | 9.661± 6.443 | 2.423± 2.723 | 1.744± 2.598 | 3.076± 3.602 | 4.906± 5.885 | |||
| Stride lengthCoV (%) | |||||||||
| Control(N = 27) | −7.777± 12.596 | −11.620± 14.006 | −14.173± 14.603 | −10.578± 21.010 | −10.517± 13.900 | −11.923± 16.952 | |||
| F3.04,118.64 = 0.695P = 0.559, = 0.018 | F1,39 = 0.116P = 0.735, = 0.003 | F3.04,118.64 = 3.904 P = 0.010, = 0.091 | |||||||
| Glaucoma (N = 17) | −14.696± 14.225 | −15.575± 14.042 | −10.939± 9.339 | −11.777± 10.523 | −10.623± 9.914 | −11.341± 12.820 | |||
| Gait velocity (%) | |||||||||
| Control(N = 27) | 14.024± 10.082 | 15.598± 10.096 | 4.752± 6.962 | 4.975± 9.097 | 4.198± 7.314 | 7.365± 8.675 | |||
| F2.52,98.10 = 1.018P = 0.379, = 0.025 | F1,39 = 0.288P = 0.595, = 0.007 | F2.52,98.10 = 0.108P = 0.993, = 0.003 | |||||||
| Glaucoma (N = 17) | 16.220± 10.771 | 19.149± 14.464 | 2.875± 5.869 | 1.726± 6.529 | 3.324± 5.323 | 6.767± 9.672 | |||
| Gait velocityCoV (%) | |||||||||
| Control(N = 27) | −7.012± 11.200 | −10.689± 10.875 | −12.721± 11.003 | −9.476± 16.809 | −12.279± 13.979 | −12.279± 15.680 | |||
| F2.77,107.90 = 0.980P = 0.400, = 0.025 | F1,39 = 0.398P = 0.532, = 0.010 | F2.77,107.90 = 3.814 P = 0.014, = 0.089 | |||||||
| Glaucoma (N = 17) | −8.995± 13.613 | −10.785± 13.723 | −14.363± 8.729 | −16.027± 9.927 | −14.027± 8.972 | −13.512± 10.662 | |||
Significant differences and differences based on effect sizes are presented in bold.
Table 4.
Means ± Standard Deviations for the Cognitive Performance Measures of Glaucoma Patients and Healthy Controls as well as the Outcomes of the ANCOVA
| Single Task | Dual Task | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Letter Fluency Task (n) (easy) | Letter Fluency Task (n) (severe) | Reaction Time Task (s) (easy) | Reaction Time Task (s) (severe) | N-Back Task (n) (easy) | N-Back Task (n) (severe) | Letter Fluency Task (n) (easy) | Letter Fluency Task (n) (severe) | Reaction Time Task (s) (easy) | Reaction Time Task (s) (severe) | N-Back Task (n) (easy) | N-Back Task (n) (severe) |
| Control (N = 28) | 37.71 ± 11.025 | 30.46 ± 10.171 | 0.952 ± 0.146 | 0.889 ± 0.117 | 29.46 ± 1.170 | 22.32 ± 5.787 | 35.68 ± 11.447 | 29.32 ± 9.452 | 1.004 ± 0.146 | 0.945 ± 0.118 | 28.79 ± 1.792 | 22.54 ± 5.029 |
| Glaucoma (N = 18) | 38.83 ± 9.051 | 32.44 ±9.513 | 1.000 ± 0.107 | 0.898 ± 0.096 | 28.78 ± 2.713 | 24.50 ± 3.930 | 36.44 ± 11.041 | 31.28 ± 10.621 | 0.988 ± 0.134 | 0.934 ± 0.097 | 29.50 ± 1.465 | 22.44 ± 5.216 |
| Repeated measures ANCOVA | ||||||||||||
| Condition × Group | Group | Condition | ||||||||||
| F2.30.94.250 = 0.287 | F1,41 = 0.112 | F2.30,94.25 = 0.894 | ||||||||||
| P = 0.781, = 0.007 | P = 0.740, = 0.003 | P = 0.425, = 0.021 | ||||||||||
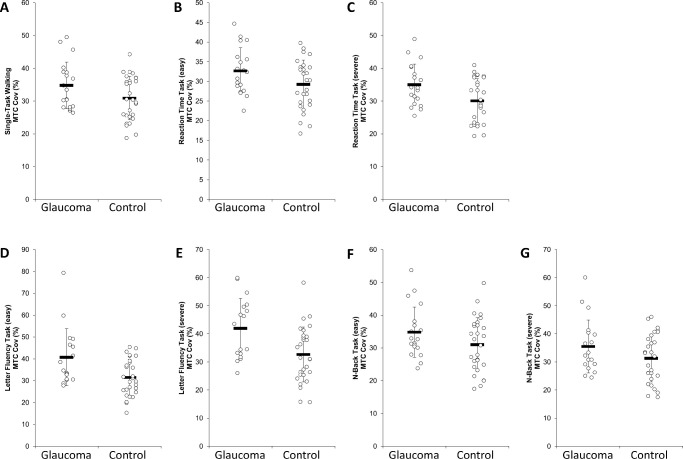
Figure 2.
Means, standard deviation and, individual data for the MTCCoV of glaucoma patients and healthy controls.
A significant group effect was found for the MTCCoV, F (1,39) = 4.974, P = 0.032, = 0.113, irrespective of the conditions (mean difference = 4.891; 95% CI, 0.455–9.328%) (Fig. 2). In addition, based on the effect size, a group x condition effect was found for the MTCCoV, F (2.68,104.35) = 2.604, P = 0.062, = 0.063. Moreover, based on the effect size, a main effect of group was found for the MTC, indicating that glaucoma patients had a lower MTC compared with the healthy controls, F (1,39) = 2.569, P = 0.117, = 0.062. Additionally, based on the effect size, a main effect of group was found for the DTC MTCCoV, F (1,38) = 2.770, P = 0.104, = 0.068, indicating that glaucoma patients had higher DTCs compared with healthy controls. Further, a significant group × condition effect was detected for DTC MTCCoV, F (3.03,115.22) = 3.005, P = 0.033, = 0.073, but the post hoc analyses indicated no differences between the groups (all P > 0.05). Furthermore, a significant condition effect was revealed for stride lengthCoV, F (4.47, 178.81) = 3.048, P = 0.015, = 0.071. Post hoc tests indicated that stride lengthCoV was higher in the single-task compared with each dual-task condition irrespective of the group (all P < 0.001). Moreover, a significant condition effect for DTC stride lengthCoV was found, F (3.04, 118.64) = 3.904, P = 0.010, = 0.091, but the post hoc analyses indicated no differences between the conditions (all P > 0.999). At last, a significant condition effect for DTC gait velocityCoV was detected, F (2.77,107.90) = 3.814, P = 0.014, = 0.089. Post hoc tests indicated no differences between the conditions (all P > 0.05).
Discussion
To the authors’ knowledge, the present study quantified spatiotemporal gait parameters including the MTC during single-task and motor-cognitive dual-task walking (with different tasks and difficulty levels) in glaucoma patients and healthy controls for the first time. The main findings are as follows. (i) The MTCCoV was significantly higher in glaucoma patients compared with healthy controls, irrespective of the testing conditions. Moreover, based on the effect sizes, (ii) the MTC was lower and (iii) the DTC MTCCoV was higher in glaucoma patients compared with the healthy controls. Further, (iv) stride lengthCoV was higher in the single-task compared with the dual-task conditions.
To the best of our knowledge, only two studies11,14 compared spatiotemporal gait parameters of glaucoma patients with those of healthy controls and they have found conflicting results. Whereas Lee et al.14 found an impaired single-task walking performance in glaucoma patients compared with healthy people (i.e., lower walking velocity, cadence, gait cycle time, and stance time), Gomes et al.11 did not observe any differences. Regarding gait velocity and step length, our results are in line with the outcome of Gomes et al.,11 who did not report differences between glaucoma patients and healthy controls during single-task walking. Furthermore, the findings from our present study also reported an absence of group differences, even during dual-task walking for these parameters. This outcome was unexpected, given that a lower dual-task gait performance in glaucoma patients was assumed owing to the visual7,8 and cognitive impairments16 associated with the disease. This discrepancy is likely due to the mainly mild stage of glaucoma progression in the present study and an absence of cognitive dysfunction. It should be noted, however, that significantly higher MTCCoV (P = 0.032, = 0.113) was evident in glaucoma patients, which has been shown to be associated with an increased risk of falling owing to tripping.19 These results are supported by the lower MTC (P = 0.117, = 0.062) and the higher DTC MTCCoV (P = 0.104, = 0.068) found in the glaucoma patients compared with healthy controls. Therefore, it seems of promise to investigate the prognostic value of the MTC for the assessment of fall risk in glaucoma patients.
Surprisingly, stride lengthCoV (P = 0.015, = 0.071) was significantly lower in the dual-task compared with the single-task condition. This was unexpected, given that performing a concurrent cognitive task while walking is often associated with higher gait variability.34 However, better walking performance during the dual-task compared with the single-task condition was also observed by other investigators,35 which was attributed to the synergy effects of the concurrent tasks. In this regard, it was speculated that the rhythm of steps and verbal answers is similar, which might positively influence gait performance during dual-task walking. Alternatively, this observation might be a result of the competition for attentional resources and task prioritization. Although it was suggested that posture is prioritized during motor-cognitive dual-task walking, there are also contrary findings. Therefore, it was suggested that task prioritization during motor-cognitive dual-task walking is strongly determined by the attentional resources, postural reserve, hazard estimation, and other intrinsic factors (e.g., expertise, skilled performance, mood, personality).36
The following limitations have to be considered in this study. (i) The single-task walking trials were always performed before the dual-task walking trials. However, given that potential sequential effects are systematic and stable, the group comparisons should not be affected. (ii) The measurements were conducted on 2 days, because the presented data belong to a longitudinal training study. However, this seems not relevant, given the reliability of gait data is very high.37 (iii) The participants were not asked about all possible comorbidities that might have influenced gait performance (e.g., diabetes) and (iv) they were examined in the laboratory, which might have affected their usual walking behavior. Moreover, the results might have been modulated by (v) the Hawthorne effect and (vi) prior falls leading to an altered gait performance.
In conclusion, the present study revealed a significantly higher MTCCoV as well as medium effect sizes for a lower MTC and higher MTCCoV DTCs in glaucoma patients compared with healthy controls, which might be related to a higher risk of falling owing to tripping.
Acknowledgments
Supported by funding of the German Research Foundation (DFG; #658883) to MBH (HO-2002/20-1) & LS (SCHE 1584/5-1).
Disclosure: C.W. Freitag, None; M. Behrens, None; T. Menrad, None; K.O. Al-Nosairy, None; F.H. Stolle, None; G.T. Prabhakaran, None; R. Beyer, None; H. Thieme, None; M.B. Hoffmann, None; L. Schega, None
References
- 1. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th edition. Br J Ophthalmol. 2021; 105(Suppl 1): 1–169. Available at: https://bjo.bmj.com/content/105/Suppl_1/1.long. [DOI] [PubMed] [Google Scholar]
- 2. Crabb DP, Smith ND, Glen FC, Burton R, Garway-Heath DF. How does glaucoma look? Patient perception of visual field loss. Ophthalmology. 2013; 120(6): 1120–1126. [DOI] [PubMed] [Google Scholar]
- 3. Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR.. The prevalence of glaucoma in the melbourne visual impairment project. Ophthalmology. 1998; 105(4): 733–739. [DOI] [PubMed] [Google Scholar]
- 4. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Global Health. 2021; 9(2): e144–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121(11): 2081–2090. [DOI] [PubMed] [Google Scholar]
- 6. Schuster AK, Erb C, Hoffmann EM, Dietlein T, Pfeiffer N.. The diagnosis and treatment of glaucoma. Deutsches Arzteblatt International. 2020; 117(13): 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture. 1997; 5(1): 54–69. [Google Scholar]
- 8. Jian-Yu E, Mihailovic A, Garzon C, et al.. Association between visual field damage and gait dysfunction in patients with glaucoma. JAMA Ophthalmol. 2021; 139(10): 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mihailovic A, de Luna RM, West SK, Friedman DS, Gitlin LN, Ramulu PY. Gait and balance as predictors and/or mediators of falls in glaucoma. Invest Ophthalmol Vis Sci. 2020; 61(3): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bicket AK, Mihailovic A, E J-Y, et al.. Gait in elderly glaucoma: impact of lighting conditions, changes in lighting, and fear of falling. Transl Vis Sci Technol. 2020; 9(13): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gomez HDA, Moreira BDS, Sampaio RF, et al.. Gait parameters, functional mobility and fall risk in individuals with early to moderate primary open angle glaucoma: a cross-sectional study. Braz J Phys Ther. 2018; 22(5): 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mihailovic A, Swenor BK, Friedman DS, West SK, Gitlin LN, Ramulu PY.. Gait Implications of visual field damage from glaucoma. Transl Vis Sci Technol. 2017; 6(3): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hausdorff JM, Rios DA, Edelberg HK.. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001; 82(8): 1050–1056. [DOI] [PubMed] [Google Scholar]
- 14. Lee H-S, Lee K-J, Kim J-L, Leem H-S, Shin H-J, Kwon HG.. Gait characteristics during crossing over obstacle in patients with glaucoma using insole foot pressure. Medicine. 2021; 100(32): e26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muir-Hunter SW, Wittwer JE.. Dual-task testing to predict falls in community-dwelling older adults: a systematic review. Physiotherapy. 2016; 102(1): 29–40. [DOI] [PubMed] [Google Scholar]
- 16. Arrigo A, Aragona E, Saladino A, et al.. Cognitive dysfunctions in glaucoma: an overview of morpho-functional mechanisms and the impact on higher-order visual function. Front Aging Neurosci. 2021; 13: 747050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leone C, Moumdjian L, Patti F, et al.. Comparing 16 different dual-tasking paradigms in individuals with multiple sclerosis and healthy controls: working memory tasks indicate cognitive-motor interference. Front Neurol. 2020; 11: 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrett RS, Mills PM, Begg RK.. A systematic review of the effect of ageing and falls history on minimum foot clearance characteristics during level walking. Gait Posture. 2010; 32(4): 429–435. [DOI] [PubMed] [Google Scholar]
- 19. Hamacher D, Hamacher D, Schega L.. Towards the importance of minimum toe clearance in level ground walking in a healthy elderly population. Gait Posture. 2014; 40(4): 727–729. [DOI] [PubMed] [Google Scholar]
- 20. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39(2): 175–191. [DOI] [PubMed] [Google Scholar]
- 21. Schott N. Trail Walking Test zur Erfassung der motorisch-kognitiven Interferenz bei älteren Erwachsenen. Entwicklung und Überprüfung der psychometrischen Eigenschaften des Verfahrens. Zeitschrift fur Gerontologie und Geriatrie. 2015; 48(8): 722–733. [DOI] [PubMed] [Google Scholar]
- 22. Bartels T, Proeger S, Brehme K, et al.. The SpeedCourt system in rehabilitation after reconstruction surgery of the anterior cruciate ligament (ACL). Arch Ortho Trauma Surg. 2016; 136(7): 957–966. [DOI] [PubMed] [Google Scholar]
- 23. Frey I, Berg A, Grathwohl D, Keul J.. Freiburger Fragebogen zur körperlichen Aktivität—Entwicklung, Prüfung und Anwendung. Sozial- und Praventivmedizin. 1999; 44(2): 55–64. Available at: https://link.springer.com/article/10.1007/bf01667127. [DOI] [PubMed] [Google Scholar]
- 24. Tilscher H, Gruber D, Lembert S, Raschner C.. Auswirkungen von Beeinträchtigungen am Bewegungsapparat auf das Ergebnis des S3-Körperstabilitätstests. Manuelle Medizin. 2007; 45(6): 409–414. Available at: https://link.springer.com/article/10.1007/s00337-007-0558-1. [Google Scholar]
- 25. Hamacher D, Hamacher D, Taylor WR, Singh NB, Schega L.. Towards clinical application: repetitive sensor position re-calibration for improved reliability of gait parameters. Gait Posture. 2014; 39(4): 1146–1148. [DOI] [PubMed] [Google Scholar]
- 26. Chu Y-H, Tang P-F, Peng Y-C, Chen H-Y.. Meta-analysis of type and complexity of a secondary task during walking on the prediction of elderly falls. Geriatr Gerontol Int. 2013; 13(2): 289–297. [DOI] [PubMed] [Google Scholar]
- 27. Faulkner KA, Redfern MS, Cauley JA, et al.. Multitasking: association between poorer performance and a history of recurrent falls. J Am Geriatr Soc. 2007; 55(4): 570–576. [DOI] [PubMed] [Google Scholar]
- 28. Owen AM, McMillan KM, Laird AR, Bullmore E.. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005; 25(1): 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hennah C, Ellis G, Doumas M.. Dual task walking in healthy aging: effects of narrow and wide walking paths. PLoS One. 2021; 16(12): e0261647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shao Z, Janse E, Visser K, Meyer AS.. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014; 5: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kunkel-Razum K, Stang C. Duden: Die deutsche Rechtschreibung. 27., völl. Neu bearb. Und erw. Aufl. Berlin: Dudenverlag; 2017. Der Duden in zwölf Bänden; Bd. 1. [Google Scholar]
- 32. Vickers AJ. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med Res Methodol. 2005; 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013; 4: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leone C, Feys P, Moumdjian L, D'Amico E, Zappia M, Patti F. Cognitive-motor dual-task interference: a systematic review of neural correlates. Neurosci Biobehav Rev. 2017; 75: 348–360. [DOI] [PubMed] [Google Scholar]
- 35. Beurskens R, Helmich I, Rein R, Bock O.. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol. 2014; 92(3): 122–128. [DOI] [PubMed] [Google Scholar]
- 36. Yogev-Seligmann G, Hausdorff JM, Giladi N.. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Movement Disord. 2012; 27(6): 765–770. [DOI] [PubMed] [Google Scholar]
- 37. Riva F, Bisi MC, Stagni R.. Gait variability and stability measures: minimum number of strides and within-session reliability. Comput Biol Med. 2014; 50: 9–13. [DOI] [PubMed] [Google Scholar]