Abstract
Adequate and timely delivery of iron is essential for brain development. The uptake of transferrin-bound (Tf) iron into the brain peaks at the time of myelination, whereas the recently discovered H-ferritin (FTH1) transport of iron into the brain continues to increase beyond the peak in myelination. Here we interrogate the impact of dietary iron deficiency on the uptake of FTH1-and Tf-bound iron. In the present study, we used C57BL/6J male and female mice at a developing (post-natal day (PND) 15) and adult age (PND 85). In developing mice, iron deficiency results in increased iron delivery from both FTH1 and Tf for both males and females. The amount of iron uptake from FTH1 was higher than the Tf and this difference between the iron delivery was much greater in females. In contrast, in the adult model, iron deficiency was associated with increased brain iron uptake by both FTH1 and Tf but only in the males. There was no increased uptake from either protein in the females. Moreover, transferrin receptor expression on the microvasculature as well as whole brain iron, and H and L ferritin levels revealed the male brains became iron deficient but not the female brains. Lastly, under normal dietary conditions, 55Fe uptake was higher in the developing group from both delivery proteins than in the adult group. These results indicate that there are differences in iron acquisition between the developing and adult brain for FTH1 and Tf during nutritional iron deficiency and demonstrate a level of regulation of brain iron uptake that is age and sex-dependent.
Keywords: Brain, Iron, Transferrin, H-ferritin, iron deficiency
Graphical Abstract
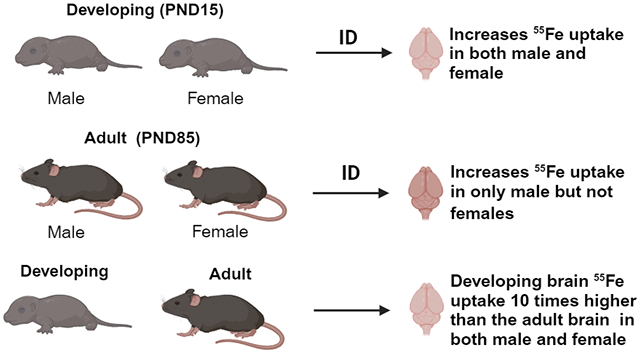
Developmental iron deficiency (ID) increased the brain 55Fe uptake in both males and females compared to the control mice. In adult mice, ID increases iron uptake in males but not females. The uptake of 55Fe was 10 times higher in the developing mice compared to the adult mice regardless of the sex and diet. Overall, our data concludes sex and diet effect of iron uptake into the brain during development and in developed brains.
1. Introduction
Iron is essential for proper central nervous system metabolism through its role in brain growth, myelin formation, and the synthesis of neurotransmitters.1,2 These processes are age and sex dependent and we have previously shown that brain iron uptake is also age and sex-dependent.3-6 Iron deficiency anemia, especially during development, critically impacts neurological and cognitive function to the extent that the deficits persist into adulthood.7,8 In adults, iron deficiency also altered cognitive function9 but few studies have been carried out to confirm if iron deficiency throughout nondevelopment periods of life is correlated with changes in behavior, cognition, brain function, and responses to iron therapy.7
Dietary iron enters the blood via transport through enterocytes and is released as ferric iron.10 In the blood, apo-transferrin binds to the ferric iron and delivers it to the targeted tissues as transferrin-bound iron.11,12 Brain iron transport studies have traditionally focused on transferrin-bound iron as the classical iron transporter to the brain.13,14 Transferrin-bound iron binds to the transferrin receptor1(TfR1) on the apical side of endothelial cells in the blood-brain barrier and is endocytosed and iron is transported to the brain parenchyma as a free iron via the iron exporter ferroportin1/hephaestin (FPN1/HEPH)15 or as a Tf-iron complex.16,17 Our laboratory has recently shown that H-ferritin (FTH1) also transports iron to the brain.16,18,19 Under physiological conditions ferritin stores approximately 2000 iron atoms.20 Our previous study showed that FTH1-bound iron was a major source of iron to the brain during development, and its uptake is sex-dependent.21 Ferritin typically consists of 24 subunits with different ratios of H- and L-ferritin, but these ratios are organ- and cell-specific.22 For example, in the brain, FTH1 is found mainly in neurons, whereas L-ferritin is the predominant form in microglial cells. Oligodendrocytes and astrocytes contain both H and L subunits.23,24 FTH1 has ferroxidase activity which converts ferrous iron to ferric iron.25 Our studies have focused on FTH1 and its role in iron delivery rather than its more traditional role of iron storage.
In the current study, we address the relative roles of FTH1 and Tf in brain iron delivery and regulation during iron deficiency. This study provides a model for addressing the considerable public health debate as to whether or not to provide iron supplementation to children and even adults due to the lack of knowledge regarding iron regulation uptake into the brain. The first model we interrogated is iron deficiency during development while the brain is growing. Secondly, we studied iron deficiency in young adults when the brain is more developed. Females are more likely to be iron deficient than males. Therefore, we included sex as a variable in these two models of iron deficiency. The data we have generated may inform strategies of dietary iron supplementation and its relative efficacy.
2. Material and Methods
2.1. Animal experiment-C57BL/6J mice and diets
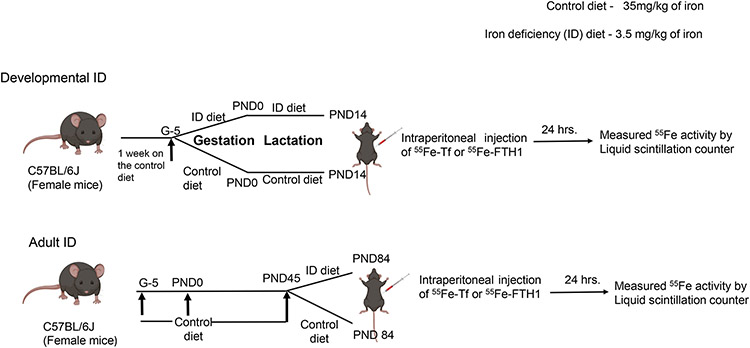
Male and female C57BL/6J mice were procured from the Jackson laboratory. Experimental protocols and procedures were approved by the IACUC, Penn State University (#PROTO201900998). Diet-induced iron deficiency was performed as described previously.26 Briefly, female breeders were fed a 35mg/kg iron diet (AIN93G-control diet) for seven days. We have found that this diet is adequate for pregnancy outcomes but does not allow for increased iron stores in the dam. Afterward, mice mated and pregnancy was confirmed by the presence of a vaginal plug. At gestational (G) day 5, the pregnant dams either continued on the control diet for control group (iron 35mg/kg diet, AIN-93G) or were switched to an iron-deficient diet for ID group (iron 3mg/kg diet #AIN-93) until the pups reached Post-natal Day (PND) 15. At PND14, the mice were injected with either 55Fe-Tf or 55Fe-FTH1 and tissues harvested after 24 hours. For the adult ID model, pregnant dams and pups were fed with control diet until PND45. At PND45 the mice were divided into two groups; a control group which continued on the same diet (iron 35mg/kg diet, AIN-93G) and an ID group which was fed an ID diet (iron 3mg/kg diet #AIN-93) until PND84. At PND84 mice were injected with 55Fe-Tf or 55Fe-FTH1 and tissues harvested after 24 hours (Figure1).
Figure 1: Experimental Model timeline.
For developmental ID, at gestational day 5, the pregnant dams were fed either a control diet (iron 35mg/kg diet, AIN-93G) or an iron-deficient diet (iron 3mg/kg diet #AIN-93) until the pups reached P14. At PND14 pups were injected IP with 55Fe-FTH1 and 55Fe-Tf and measured 55Fe activity in the brain and liver after 24 hours.
For the adult ID model, pups were weaned onto the control diet until PND45. At P45 the mice were divided into two groups; 1 ID group fed with ID diet (iron 3mg/kg diet #AIN-93) and a control group fed with control diet (iron 35mg/kg diet, AIN-93G) until P85. At P84 mice were injected IP with 55Fe-FTH1 and 55Fe-Tf and measured 55Fe activity in the brain and liver after 24 hours.
A total of 48 animals were utilized for control and ID groups in developing age 55Fe uptake study (n=6 for group in both sexes). For adult age experiments, 102 animals were used for Control and ID groups (n=5-6 for brain 55Fe uptake, n=5 for whole brain iron, n=9 for brain microvasculature (BMV) and parenchyma in both sexes).
2.2. Recombinant H-Ferritin
Wild-type human FTH1 containing a poly-His tag was subcloned into pET30a(+), to be produced in BL21 Escherichia coli.16 Isopropyl-β-D-thio-galactoside (IPTG) was used to induce expression. Following this, bacteria were lysed, and FTH1 protein was purified on a nickel column using standard techniques (GE Healthcare Bio-Sciences). Transferrin was purchased commercially (Sigma).
2.3. Radiolabeled iron preparation
55Fe (Perkin Elmer) was complexed with 1 mM nitrilotriacetic acid (NTA), 6 mM ferric chloride (FeCl3), and 0.5 M sodium bicarbonate (NaHCO3) at a ratio of 100 μL NTA: 6.7 μL FeCl3:23.3 μL NaHCO3 : 50 μCi 55FeCl3 to form the 55Fe-NTA complex19. After complexing, 55Fe-NTA was incubated with apo-Tf (Sigma) or FTH1 for 30 min to allow for iron loading. Unbound iron was separated from the total complex using PD midiTrap-G25 columns following the manufacturer’s instructions (GE Healthcare Bio-Sciences).
2.4. 55Fe uptake studies
Mice received a single intraperitoneal injection of 3.4 mg/kg body weight 55Fe-Tf or 55Fe-FTH1 with 50 μCi of 55FeCl3. Twenty-four hours after injection, blood was drawn and mice were transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4). Brains were collected, weighed immediately, and solubilized using 1 mL Solvable (Perkin Elmer) according to the manufacturer’s instructions. After solubilization, a 10 mL Hionic-Fluor scintillation cocktail (Perkin Elmer) was added. Samples were counted using the Hidex 300 SL (LabLogic) for three minutes each. Blank tube values were subtracted from the final counts to correct for background counts.
2.5. Isolation of brain microvasculature
Brain microvasculature isolation was performed as described previously.4 The brains were placed into a hand homogenizer unit containing 2 mL microvessel buffer (0.147 M NaCl, 0.4 mM KCl, 0.3 mM CaCl2, 0.12 mM MgCL2, 15 mM HEPES, 0.5% BSA, 5 mM glucose). Brain homogenate was transferred into a microcentrifuge tube and centrifuged at 1000 × g for 10 min at 4°C. The pellet was resuspended in 1 mL 1.015 g/mL Percoll. The resuspended pellet was layered on 3 mL of 1.05 g/mL Percoll, then centrifuged at 15,000 × g for 30 min at 4°C. The gradient was pierced by a needle and the microvessel-containing layer was collected. The microvessel fraction was passed through a 100-μm mesh filter. The remaining isolate was centrifuged at 1000 × g for 10 min at 4°C for final microvessel recovery. The final microvessel pellet was then resuspended in RIPA buffer containing 1X protease inhibitor cocktail (Sigma).
2.6. Immunoblotting
Immunoblotting was performed as described previously.16 Briefly, isolated brain microvasculature was homogenized in RIPA lysis buffer containing protease inhibitor cocktail (Cell signaling technology, USA) and phosphatase inhibitor cocktail (Sigma Aldrich, USA). After incubating on ice for 20 min, the homogenate was sonicated, centrifuged at 12000 rpm for 10 min at 4°C. The protein content of the supernatant was estimated using the micro-BCA kit method. An equal amount of protein (30μg) was fractionated on 4-20% SDS-gels under reducing conditions and transblotted onto PVDF membranes. The blots were blocked with 5% fat-free dry milk and probed for FTH1 (Cell Signaling Technology, 1:1000, 4393S), FTL (Abcam, 1:1000, ab69090), TfR1(Santa Cruz Biotechnology; 1:250, sc-65882), TIM-2 (Abcam, 1:1000, ab86480), or beta-actin (Sigma, 1:1000, A5441). Corresponding secondary antibody conjugated to HRP was used (1:5000, GE Amersham), and bands were visualized using ECL reagents (PerkinElmer) on an Amersham Imager 600 (GE Amersham). The images were quantified using ImageJ software (NIH, Bethesda, MD, U.S.A.) and normalized to respective loading control, the β-actin.
2.7. Hematology
The hemoglobin and hematocrit were measured using a Heska HT5 Veterinarian Hematology Analyzer (Heska Antech Company, Loveland, CO, U.S.A).
2.8. Serum iron and TIBC
Serum iron and transferrin iron binding capacity was performed according to manufactures protocols (Pointe Scientific, USA, # I7504-60).
2.9. Brain iron analysis
Tissue Acid digestion performed as described previously27. Briefly, wet brains were digested overnight with nitric acid (2 mL) and, 30% hydrogen peroxide (1 mL) at 60° C. Iron concentration was determined by ICP-AES against a standard (MERK-multi-element standard # 111355). Results were expressed as μg /g of wet tissue weight.
2.10. ELISA
Serum FTH1 ELISA was performed according to the manufacturer’s protocols (Life Span Biosciences, USA, # LS-F22534).
Statistical analysis
All data were presented as the mean ± SD. Statistical analysis was employed with the GraphPad Prism version 9.0 software. Comparison of differences between two or multiple groups by Student's t- test and two- way ANOVA respectively. P < 0.05 was considered statistically significant.
3. Results
3.1. Developmental ID is associated with increased brain uptake of 55Fe-FTH1 and 55Fe-Tf in male and female mice
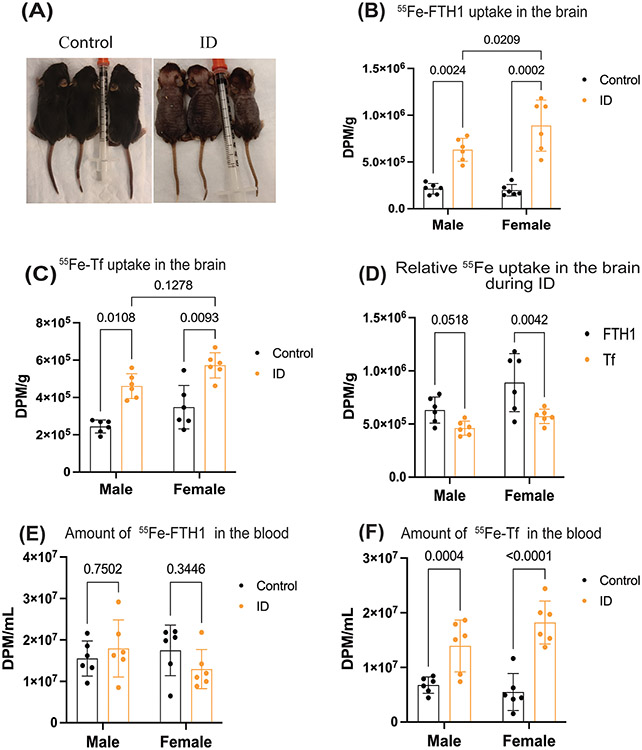
In the developmental model, iron deficiency was confirmed by differences in weight, color, and amount of hair between the two groups (Figure 2A, Supplementary Figure 1A). Collecting blood for hemoglobin analysis was prevented due to the small size of the animals, however the brain weights in control and ID pups were measured. The ID pups brain weights were significantly lower compared to the control group (supplementary figure1B). Subsequently, we measured the 55Fe uptake in brain from FTH1 or Tf. To measure iron uptake into the brain, we loaded either 3.5mg/kg of FTH1 or Tf with 50μCi of 55Fe and injected the radiolabeled proteins intraperitoneally. After 24 hours, the animals were perfused with 0.1M PBS and brains collected to measure iron uptake. Brain uptake of 55Fe-FTH1 and 55Fe-Tf increased significantly in the ID group in both sexes compared to corresponding control male and female mice (Figure, 2B, C). Furthermore, we measured the interaction between the diet and sex, there was a significant difference in uptake of 55Fe-FTH1 in males and females (Figure 2B) but there is no statistically significant increase in the uptake of 55Fe-Tf in female mice compared to the males (Figure 2B). Next, we measured the difference between the amount of iron delivered by each of the proteins. More 55Fe was taken into the brain during ID by FTH1- than Tf-55Fe in both sexes (Figure 2D). Additionally, we measured the 55Fe levels in the blood at the end of the experiment. There was no difference in the 55Fe-FTH1 between the control or ID in either male and females (Figure 2E) but 55Fe-Tf amounts were significantly higher in the ID blood compared to the control group in both sexes (Figure 2F).
Figure 2. Developmental ID is associated with increased brain uptake of 55Fe-FTH1 and 55Fe-Tf in male and female.
The pregnant mice were placed on control or ID diets. At PND14, 55Fe-Tf or 55Fe-FTH1 was injected intraperitoneally into the pups. After 24 hours 55Fe uptake into the brain was measured in a liquid scintillation counter, results are expressed in DPM/g. (A) Illustrates the images of the control and ID of PND14 pups. (B) illustrates the 55Fe-FTH1 uptake in the brains of male and female mice. (C), 55Fe-TF uptake in the brain. D) demonstrates the difference between the uptake of 55Fe-FTH1 and 55Fe-Tf in male and female mice brains ID groups. E and F) demonstrating the amount of 55Fe-FTH1 and 55Fe-Tf in blood respectively. 55Fe activity was measured through liquid scintillation and results are expressed as DPM/mL. n=6 for all conditions, the data were presented mean± S.D. and two-way-ANOVA was used for the statistical significance.
3.2. Iron deficient diet-induced iron deficiency in the adult male and female mice
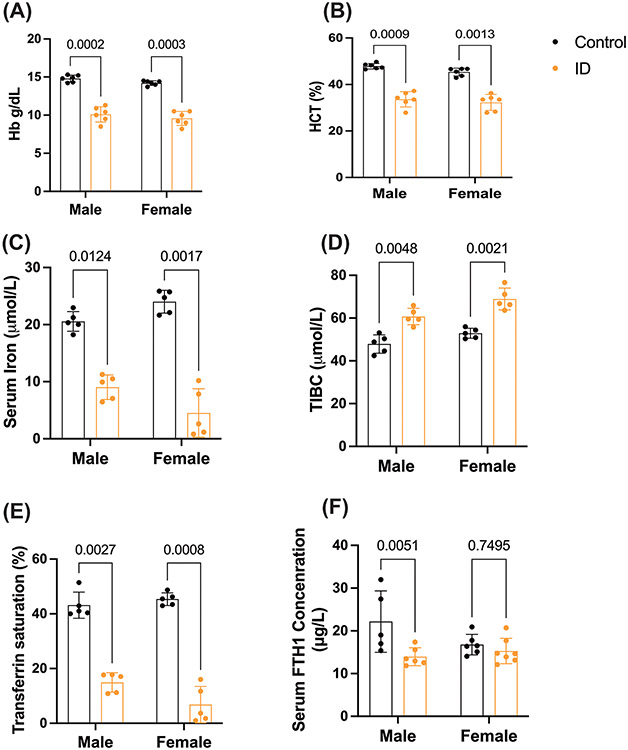
To demonstrate iron deficiency in the adult ID model, we measured the body weights and hematological parameters in the ID and control diet groups of adult male and female mice. The body weights of ID diet animals were decreased significantly compared to the control diet in both sexes (supplementary figure 2A). The hemoglobin and hematocrit (HCT) levels were significantly decreased in the ID diet group compared to the control diet group in both male and female mice (Figure 3A, B). We further determined the serum non-heme iron, total iron binding capacity (TIBC), Tf saturation, and FTH1 concentration in both adult male and female control and ID groups. The TIBC was significantly increased and, Tf saturation and serum non-heme iron levels were significantly decreased in the males and females in the ID group compared to the control group (Figure 3 C, D, E). In addition to serum iron, we also measured the serum FTH1 levels male and female mice during ID, in male mice FTH1 levels were significantly lower than the controls, while, the FTH1 levels in the control and ID female mice groups were not altered (Figure 3F).
Figure 3: Iron deficient diet-induced iron deficiency in the adult male and female mice.
Postnatal 45-day old mice (mice male and female) were placed on control or ID diets for 6 weeks, after that blood was collected and measured for hematological and iron parameters. (A, B), Hb and HCT levels were significantly reduced in the ID diet compared to the control diet. (C) Illustrates the Serum iron concentration. (D), Total iron binding capacity. (E), Transferrin saturation. (F), Serum FTH1 concentration. n=5-6 for all conditions, the data were presented mean± S.D. and two-way-ANOVA were used for the statistical significance.
3.3. The uptake of 55Fe-FTH1 and 55Fe-Tf into the brains of male and female mice are different in the Adult ID model
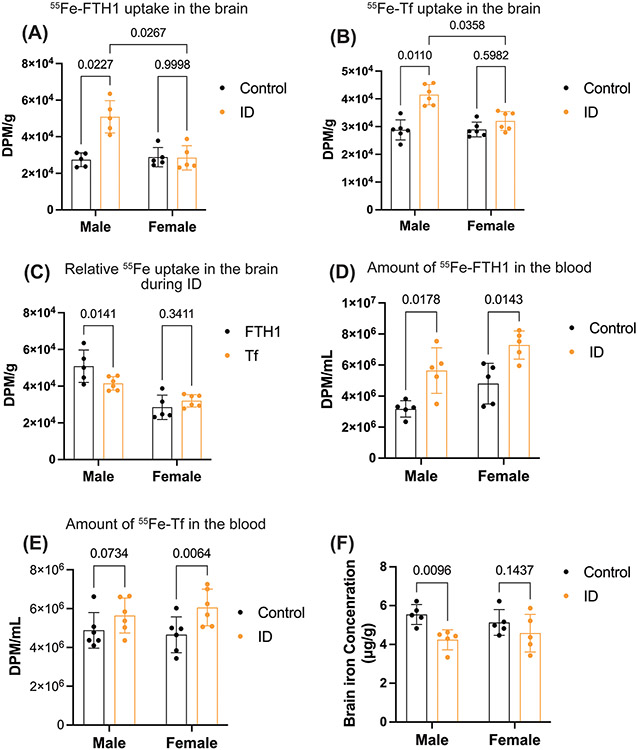
Following confirmation of ID in adult mice, we measured the brain weights of control and ID groups of male and female mice. The brain weights were not altered between control and ID groups in either sex (supplementary figure 2B). Next, we determined the 55Fe uptake into the brain. In the adult model of ID, 55Fe-FTH1 and 55Fe-Tf uptake into the brain was significantly higher in the ID group compared to the control group in the male mice brains but there are no ID induced differences in the 55Fe-FTH1or 55Fe-Tf uptake in brains of the female mice (Figure 4A and B). Furthermore, we measured sex -dependent differences in 55Fe uptake during ID. The 55Fe uptake from both Tf and FTH1 was greater in ID males compared to females (Figure 4A and B). Moreover, the FTH1 bound iron uptake was significantly higher than the Tf bound iron uptake in males but in females, there is no difference between the iron delivery from either protein (Figure 4C). We further measured whether whole brain iron concentration was altered during adult ID in male and female. For this study, we perfused the ID male and female mice with 0.1M PBS and brain tissue was isolated. The whole brain tissues were digested with nitric acid measured iron concentration by ICP-AES. The whole brain iron levels in the males were significantly decreased in the ID group compared to the control but not in the females (Figure 4D). Next, we measured the 55Fe levels in blood during adult ID in both male and female mice. The 55Fe-FTH1 levels were significantly higher in both male and female compared to the control during ID (Figure 4E). The 55Fe-Tf amount in the blood was significantly higher in the ID group compared to the control group in female mice but in males there is no significant difference (Figure 4F).
Figure 4: The uptake of 55Fe- FTH1 and 55Fe-Tf into the brains of male and female mice are different in the Adult ID model.
Young, adults (P45, mice male and female) were placed on control or ID diets for 6 weeks (until PND 84), and then 55Fe-Tf or 55Fe-FTH1 was injected intraperitoneally. After 24 hours the brains were harvested and 55Fe uptake was measured in a liquid scintillation counter. The results are expressed in DPM/g. For normal brain iron, A separate group of Young, adults (P45, mice male and female) from those used in the uptake studies were placed on control or ID diets for 6 weeks. At that point, the animals were perfused with 0.1N saline, and brains were collected the whole brain’s iron levels measured by ICP-AES. (A) illustrates the 55Fe-FTH1 uptake in the brains of male and female mice. (B), 55Fe-TF uptake in the brain C) demonstrate the difference between the uptake of 55Fe-FTH1 and 55Fe-Tf in male and female mice brains. (D) Whole brain iron levels in male and female mice's control and ID groups. E and F) illustrating the amount of 55Fe-FTH1 and 55Fe-Tf in blood respectively. 55Fe activity was measured through liquid scintillation and results are expressed as DPM/ mL. n=5-6 for all conditions, the data were presented as mean ± S.D., and two-way-ANOVA was used for the statistical significance.
3.4. Sex specific changes in iron transporter expression in brain microvasculature and parenchymal ferritin expression in the adult ID model.
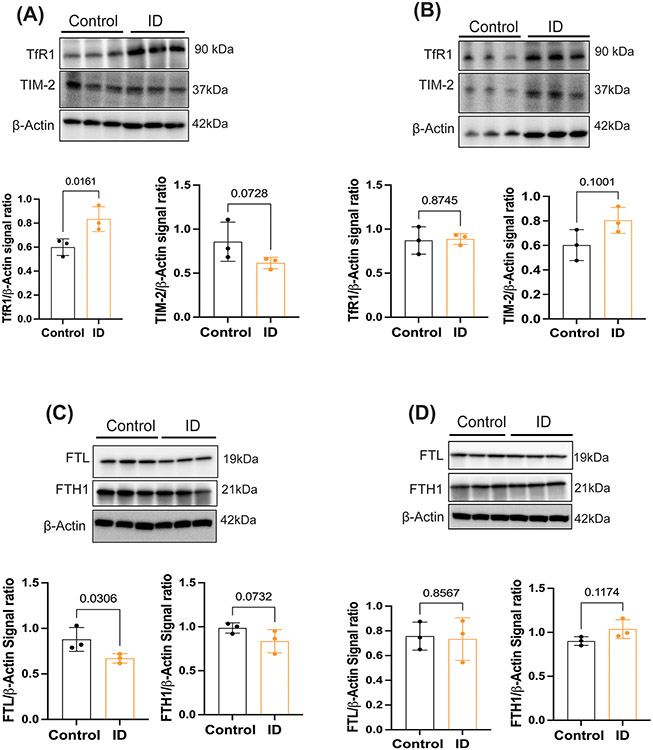
We next assessed whether diet-induced iron deficiency alters the parenchymal expression of H and L ferritin as well as brain microvasculature FTH1 and Tf receptor expressions. First, we isolated brain microvasculature and measured the TfR1 and T cell immunoglobulin-domain and mucin-domain-2 (TIM-2) receptor expression in both sexes. TfR1 expressions were significantly increased in the ID males (Figure 5A), but there is no significant difference between the control and ID conditions in females (Figure 5B). TIM-2 expression was not changed significantly between the control and ID groups in either of the sexes. In the brain, iron is stored in the ferritin and ID conditions increase the turnover of ferritin.24,28 Therefore, we further investigated the expression of the H and L ferritin in whole brain parenchyma of control and ID in male and female mice. In male mice, L and H ferritin expression levels decreased in the iron deficient group compared to the control mice (Figure 5C) but were unchanged in female mice during ID (Figure 5D).
Figure 5: Sex-specific changes in iron transporter expression in brain microvasculature and ferritin expression in the parenchyma of the adult ID model.
A separate group of young, adults (P45, male and female mice) from those used in the uptake studies were placed on control or ID diets for 6 weeks. At that point, the animals were perfused with 0.1N saline, brains were collected and brain microvasculature and parenchyma separated from the whole brain. (A) Representative immunoblots from males for TfR1 and TIM-2. Each band is a pooled sample of 3 animals and data normalized to actin (total 9 males per group), B) Representative immunoblot from females for TfR1 and TIM-2. Each band is a pooled sample of 3 animals and data normalized to actin (total 9 females per group), C) Illustrates the whole brain parenchyma L and H ferritin expression in males D) demonstrates the whole brain parenchyma L and H ferritin expression in females. Each band is a pooled sample of 3 animals and data normalized to actin (total 9 males per group) The data in each graph are presented mean± S.D., and a Student’s t-test was used for the statistical significance.
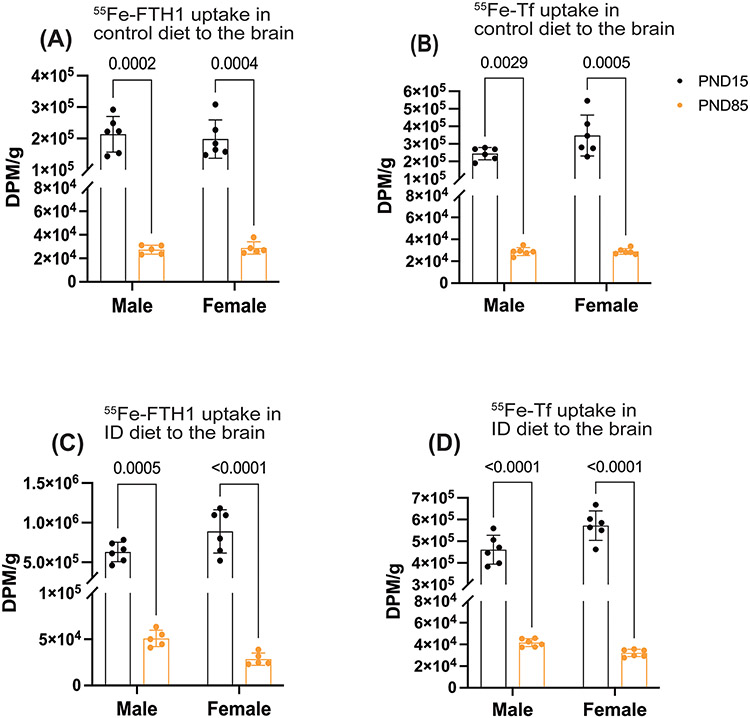
3.5. The uptake of 55Fe-FTH1 and 55Fe-Tf was greater in the developing mice compared to the adult mice’s brain
Based on the results presented in Figures 2 and 4, we further measured the FTH1- and Tf-bound iron uptake differences between the developing and adult brain of control and ID groups in males and females. First, we compared the 55Fe uptake between the developing and adult brains on control diets. The 55Fe uptake was significantly higher in developing group compared to the adults for both FTH1 and Tf (Figure 6A, B). Next, we measured the 55Fe uptake in ID conditions, The FTH1 and Tf bound 55Fe uptake was 10 times higher in the developing mice compared to the adult mice (Figure 6 C, D).
Figure 6: The uptake of FTH1-and Tf-55Fe was greater in the developing mice compared to the adult mice’s brain.
55Fe-FTH1 or 55Fe-TF was injected into the early-age (P15) and adult-age (P85) iron deficiency mice and measured the 55Fe activity between the early-age and adult-age brain. 55Fe activity was measured through liquid scintillation and results are expressed as DPM/g. A and B) illustrate the comparison of 55Fe-FTH1 and -Tf brain uptake between the P15 and P85 in the control diet (A) 55Fe-FTH1, (B) 55Fe-Tf uptake. C, and D) demonstrate the comparison of 55Fe-FTH1- and -Tf brain uptake between the P14 and P85 in ID conditions. (C) 55Fe-FTH1 and (D) 55Fe-Tf respectively. n=5-6 per group for all conditions, the data are presented mean± S.D. and two-way ANOVA was used for the statistical significance.
4. Discussion
Our previous in vitro and in vivo studies have shown that FTH1 and Tf delivered iron to the brain,16,18,19 including during development.21 In the current study, we investigated the role of FTH1 and Tf in brain iron delivery during iron deficiency in developing and adult age, and the regulatory signals regarding brain iron status. This is the first demonstration of the relative ability of FTH1and Tf to transport iron to the brain and how this transport is altered by diet, age and sex. The results of this study also demonstrated that FTH1- and Tf-bound iron acquisition by the brain is significantly higher in developing mouse brain compared to the adult brain. Our Tf-mediated iron uptake data is consistent with previous studies demonstrating that uptake of iron peaks during development and then decreases in adult brain.29,30
Iron is essential for developing brains because neural cells require constant and timely access to iron, especially during peak energy demand, such as mitochondria- genesis, synaptogenesis, and myelination.1,29 Iron deficiency during development results in lower brain weights and impaired formation of myelin.31-35 A key question, however, is, during periods of iron deficiency, does brain iron uptake increase or does the brain develop within the confines of iron deficiency? In the present study, the brain weights of ID pups were significantly lower and FTH1 and Tf bound iron uptake were significantly higher compared to control male and female pups. These data strongly support the notion that there are signals in the developing ID brain to increase iron uptake and those signals positively impact both iron delivery proteins. In newborns, the serum ferritin concentration was significantly higher than the mothers36 but the subunit composition of serum ferritin remains unclear in newborns. Of note, was that the amount of 55Fe delivered from FTH1 was significantly higher than delivered by Tf and the difference in FTH1 uptake compared to Tf is sex dependent. This report is consistent with our previous study that FTH1 transports more iron compared to the Tf in the normal developing brain and the uptake patterns were sex dependent.21
The mechanism for brain iron acquisition has traditionally been ascribed to Tf bound iron uptake via the TfR1 on the blood brain barrier endothelial cells.15 This study and previous work from our laboratory have established a significant role for FTH1 in brain iron delivery as well.15,18 The receptor for FTH1 is TIM-2 although FTH1 can also reportedly bind to the TfR1.17,37 TfR1 expression on the endothelial cells is regulated by the amount of brain iron38 but the regulation of expression TIM-2 is not known. Indeed, in this study we demonstrated the TIM-2 was not increased in the brain microvasculature when the brain was iron deficient and TfR1 was increased. We have also demonstrated the endothelial cells are not a passive conduit for iron movement into the brain but rather can serve as a reservoir and iron release can be induced.17,39 The mechanism of iron release into the brain involves the iron export protein ferroportin17,40 and recently we demonstrated in a cell culture model of the blood brain barrier both FTH1 and Tf bound iron transport from endothelial cells via exosomes.16
In the brain, the concentration of iron is highest at birth (PND 0), decreases through PND 0 to 22, and again increases from weaning (PND23) to PND 60 similar level to that at birth.41-43 During the first week of postnatal development, brain iron is stored predominantly in ferritin and at this stage the brain grows rapidly, and the level of brain activity increases, which creates a high demand for iron41,42 However, in gestational and lactational ID models, the brain iron levels were significantly lowered compared to control at PND7.44,45 As a result, ferritin-iron stores were relatively low at PND1444 and the brain presumably sends signals to the endothelial cells to import more iron from blood. This concept is evident in studies on diet-induced developing iron deficient animals where cross-fostering to iron normal dams can replete brain iron levels.26,46,47 The present study is consistent with the findings of the PND 15 model and show increased iron uptake from Tf and FTH1 in the iron deficient PND15 animals. Our adult ID study differs from previous studies that ID started at PND 2148,49 but in our animals ID was not started until PND45. Thus, our data suggest once the set point of brain iron concentration is reached the brain can undergo iron deficiency at a later age but only in males. Moreover, the adult onset iron deficiency can signal for increased iron delivery from both Tf and FTH1. The identity of the signals to increase brain iron uptake during development are not known but we have demonstrated the role of holo-Tf and apo-Tf ratios in regulating Tf uptake in the adult brain.19 It is reasonable to assume that these ratios also drive brain iron uptake during development. The data further imply that female brains have better ability to retain iron than males. This a notion supported by our previous iron uptake work where we showed that in adult wild type mice 59Fe-Tf brain uptake was initially similar in both males and females at 24 hours. However, after 5 days, males lose significantly more 59Fe compared to the females while female mice 59Fe levels remained stable.4
In the present study, the adult ID was associated with increased brain iron uptake in the males from both Tf and FTH1 but there was no increase in iron delivery in the adult female brain from either protein. This important finding reflects the male brain becomes iron-deficient but the female brain does not; despite all the systemic parameters demonstrating significant iron deficiency in the females. Thus, there are two key elements to this finding; 1) Previous studies proposed that a “set point” for brain iron concentrations is met by accumulating iron during the weaning period, which is based on the brain metabolic activity.41,50-52 Alterations in iron availability during developmental period are addressed by both males and females by increasing iron uptake from both iron delivery proteins. 2) The regulation of brain iron uptake stems from the brain and is not influenced by the systemic iron deficiency. In our adult model, both males and females became systemically iron deficient but only the male brains became iron-deficient. This iron deficiency in the males was met by increased delivery of iron from both proteins. There was no increase in brain iron delivery in the females. Our recent in vitro and in vivo studies demonstrated that the regulation of brain iron acquisition is modulated by release of iron from the endothelial cells of the BBB in response to the ratio of brain apo/holo Tf and also this regulation is sex dependent.17,19,53 Furthermore, in the brain, the ratio of apo/holo transferrin depends upon the brain iron concentration. Lower brain iron concentration increased the ratio, which further increase the iron transport into the brain by modulating FPN1/HEPH axis.53 The ratio of apo/holo Tf in our previous study did not increase FTH1 suggesting there is another signal in the iron deficient brain for increasing FTH1. Together these results, indicate that brain iron levels regulate the iron uptake into the brain, not systemic iron level and there is a sex difference in iron uptake/release regulation, the data also suggest that brain iron retention is better in females than males during ID.
A limitation of the current study is that we were unable to measure the concentrations of FTH1 and Tf in developing mice blood. Consequently, we are unable to determine the specific activity of the circulating FTH1 and Tf and thus the absolute (hot +cold) iron uptake mediated by FTH1 and Tf.
This study indicates that FTH1-bound iron plays a highly significant role in transporting iron to brain compared to the Tf bound iron during diet-induced ID in developing and adult brain and this delivery mode can be upregulated by diet, but the upregulation is sex and age dependent. These studies further suggest that not only is brain iron uptake regulated but the regulatory mechanisms are different for Tf and FTH1 and there are sex differences in the engagement of these regulatory mechanisms. These data potentially address two significant public health issues regarding brain iron supplementation during development and adult age. Firstly, the brain appears aware of its iron requirement during development and sends signals to increase iron acquisition to meet the metabolic demands of development. Secondly, it is clear that brain iron uptake is tightly regulated and the regulation is independent, at least in the adult model, of the peripheral iron status. Overall, these data provide compelling evidence for a sex and diet effect of iron uptake into the brain during development and in developed brains.
Supplementary Material
Acknowledgements
The authors would like Dr. Todd Schell for use of the Hidex 300 SL (LabLogic), and the Health Physics Department at Penn State College of Medicine for radioisotope assistance. J.R.C and I.A.S would like to acknowledge funding received from the National Institute of Health (NIH–R01NS113912).
Footnotes
Conflict of interest
JRC is a founder and Chairman of the Board of Sidero Bioscience LLC., a company founded on patented technology to use H-ferritin as a dietary iron supplement. The remaining authors declared no conflict of interest.
Data Availability Statement
The data generated within this study is available upon request to the corresponding author.
Reference
- 1.Beard JL, Connor JR, Jones BC. Iron in the Brain. Nutrition Reviews. 2009;51(6):157–170. doi: 10.1111/j.1753-4887.1993.tb03096.x [DOI] [PubMed] [Google Scholar]
- 2.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739 [DOI] [PubMed] [Google Scholar]
- 3.Chiou B, Neely EB, Mcdevitt DS, Simpson IA, Connor JR. Transferrin and H-ferritin involvement in brain iron acquisition during postnatal development: impact of sex and genotype. J Neurochem. 2020;152(3):381–396. doi: 10.1111/jnc.14834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duck KA, Neely EB, Simpson IA, Connor JR. A role for sex and a common HFE gene variant in brain iron uptake. J Cereb Blood Flow Metab. 2018;38(3):540–548. doi: 10.1177/0271678X17701949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Research. 1993;610(1):127–134. doi: 10.1016/0006-8993(93)91225-H [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Li C, Zhang W, Wang W, Tang Y. Sex differences in the white matter and myelinated nerve fibers of Long-Evans rats. Brain Research. 2008;1216:16–23. doi: 10.1016/j.brainres.2008.03.052 [DOI] [PubMed] [Google Scholar]
- 7.Beard J. Iron Deficiency Alters Brain Development and Functioning. The Journal of Nutrition. 2003;133(5):1468S–1472S. doi: 10.1093/jn/133.5.1468S [DOI] [PubMed] [Google Scholar]
- 8.Georgieff MK. Long-term Brain and Behavioral Consequences of Early Iron Deficiency. Nutr Rev. 2011;69(Suppl 1):S43–S48. doi: 10.1111/j.1753-4887.2011.00432.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. The Lancet. 1996;348(9033):992–996. doi: 10.1016/S0140-6736(96)02341-0 [DOI] [PubMed] [Google Scholar]
- 10.Sharp P, Srai SK. Molecular mechanisms involved in intestinal iron absorption. World J Gastroenterol. 2007;13(35):4716–4724. doi: 10.3748/wjg.v13.i35.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondaiah P, Yaduvanshi PS, Sharp PA, Pullakhandam R. Iron and Zinc Homeostasis and Interactions: Does Enteric Zinc Excretion Cross-Talk with Intestinal Iron Absorption? Nutrients. 2019;11(8):1885. doi: 10.3390/nu11081885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iron transport proteins: Gateways of cellular and systemic iron homeostasis ∣ Elsevier Enhanced Reader. doi: 10.1074/jbc.R117.786632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochimica et Biophysica Acta (BBA) - General Subjects. 2012;1820(3):188–202. doi: 10.1016/j.bbagen.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 14.Moos T, Morgan EH. Transferrin and Transferrin Receptor Function in Brain Barrier Systems. :19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson IA, Ponnuru P, Klinger ME, et al. A novel model for brain iron uptake: introducing the concept of regulation. J Cereb Blood Flow Metab. 2015;35(1):48–57. doi: 10.1038/jcbfm.2014.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palsa K, Baringer SL, Shenoy G, Simpson IA, Connor JamesR. Exosomes are involved in iron transport from human blood-brain barrier endothelial cells and are modified by endothelial cell iron status. Journal of Biological Chemistry. Published online January 2023:102868. doi: 10.1016/j.jbc.2022.102868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiou B, Neal EH, Bowman AB, Lippmann ES, Simpson IA, Connor JR. Endothelial cells are critical regulators of iron transport in a model of the human blood–brain barrier. J Cereb Blood Flow Metab. 2019;39(11):2117–2131. doi: 10.1177/0271678X18783372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher J, Devraj K, Ingram J, et al. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. American Journal of Physiology-Cell Physiology. 2007;293(2):C641–C649. doi: 10.1152/ajpcell.00599.2006 [DOI] [PubMed] [Google Scholar]
- 19.Baringer SL, Neely EB, Palsa K, Simpson IA, Connor JR. Regulation of brain iron uptake by apo- and holo-transferrin is dependent on sex and delivery protein. Fluids Barriers CNS. 2022;19(1):49. doi: 10.1186/s12987-022-00345-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arosio P, Ingrassia R, Cavadini P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790(7):589–599. doi: 10.1016/j.bbagen.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 21.Chiou B, Neely EB, Mcdevitt DS, Simpson IA, Connor JR. Transferrin and H-ferritin involvement in brain iron acquisition during postnatal development: impact of sex and genotype. J Neurochem. 2020;152(3):381–396. doi: 10.1111/jnc.14834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1996;1275(3):161–203. doi: 10.1016/0005-2728(96)00022-9 [DOI] [PubMed] [Google Scholar]
- 23.Connor JR, Boeshore KL, Benkovic SA, Menzies SL. Isoforms of ferritin have a specific cellular distribution in the brain. Journal of Neuroscience Research. 1994;37(4):461–465. doi: 10.1002/jnr.490370405 [DOI] [PubMed] [Google Scholar]
- 24.Han J, Beard JL, Day JR, Connor JR. H and L Ferritin Subunit mRNA Expression Differs in Brains of Control and Iron-Deficient Rats. The Journal of Nutrition. 2002;132(9):2769–2774. doi: 10.1093/jn/132.9.2769 [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Chasteen ND. Ferroxidase activity of ferritin: effects of pH, buffer and Fe(II) and Fe(III) concentrations on Fe(II) autoxidation and ferroxidation. Biochem J. 1999;338(Pt 3):615–618. [PMC free article] [PubMed] [Google Scholar]
- 26.Unger EL, Hurst AR, Georgieff MK, et al. Behavior and Monoamine Deficits in Prenatal and Perinatal Iron Deficiency Are Not Corrected by Early Postnatal Moderate-Iron or High-Iron Diets in Rats. The Journal of Nutrition. 2012;142(11):2040–2049. doi: 10.3945/jn.112.162198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondaiah P, Palika R, Mashurabad P, Singh Yaduvanshi P, Sharp P, Pullakhandam R. Effect of zinc depletion/repletion on intestinal iron absorption and iron status in rats. The Journal of Nutritional Biochemistry. 2021;97:108800. doi: 10.1016/j.jnutbio.2021.108800 [DOI] [PubMed] [Google Scholar]
- 28.Pino JMV, da Luz MHM, Antunes HKM, Giampá SQ de C, Martins VR, Lee KS. Iron-Restricted Diet Affects Brain Ferritin Levels, Dopamine Metabolism and Cellular Prion Protein in a Region-Specific Manner. Frontiers in Molecular Neuroscience. 2017;10. Accessed July 3, 2023. https://www.frontiersin.org/articles/10.3389/fnmol.2017.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Developmental Brain Research. 1990;55(1):35–42. doi: 10.1016/0165-3806(90)90103-6 [DOI] [PubMed] [Google Scholar]
- 30.Moos T, Morgan EH. A Morphological Study of the Developmentally Regulated Transport of Iron into the Brain. Developmental Neuroscience. 2002;24(2-3):99–105. doi: 10.1159/000065702 [DOI] [PubMed] [Google Scholar]
- 31.Badaracco ME, Ortiz EH, Soto EF, Connor J, Pasquini JM. Effect of transferrin on hypomyelination induced by iron deficiency. Journal of Neuroscience Research. 2008;86(12):2663–2673. doi: 10.1002/jnr.21709 [DOI] [PubMed] [Google Scholar]
- 32.Lozoff B, Georgieff MK. Iron Deficiency and Brain Development. Seminars in Pediatric Neurology. 2006;13(3):158–165. doi: 10.1016/j.spen.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 Pt 2):S34–43; discussion S72-91. doi: 10.1301/nr.2006.may.s34-s43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guitart ME, Vence M, Correale J, Pasquini JM, Rosato-Siri MV. Ontogenetic oligodendrocyte maturation through gestational iron deprivation: The road not taken. Glia. 2019;67(9):1760–1774. doi: 10.1002/glia.23647 [DOI] [PubMed] [Google Scholar]
- 35.Markova V, Holm C, Pinborg AB, Thomsen LL, Moos T. Impairment of the Developing Human Brain in Iron Deficiency: Correlations to Findings in Experimental Animals and Prospects for Early Intervention Therapy. Pharmaceuticals (Basel). 2019;12(3):120. doi: 10.3390/ph12030120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milman N, Ibsen KK, Christensen JM. Serum Ferritin and Iron Status in Mothers and Newborn Infants. Acta Obstetricia et Gynecologica Scandinavica. 1987;66(3):205–211. doi: 10.3109/00016348709020748 [DOI] [PubMed] [Google Scholar]
- 37.Li L, Fang CJ, Ryan JC, et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. 2010;107(8):3505–3510. doi: 10.1073/pnas.0913192107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy RC, Kosman DJ. Iron transport across the blood-brain barrier; Development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci. 2015;72(4):709–727. doi: 10.1007/s00018-014-1771-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy RC, Kosman DJ. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front Mol Neurosci. 2015;8. doi: 10.3389/fnmol.2015.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You L, Yu PP, Dong T, et al. Astrocyte-derived hepcidin controls iron traffic at the blood-brain-barrier via regulating ferroportin 1 of microvascular endothelial cells. Cell Death Dis. 2022;13(8):1–12. doi: 10.1038/s41419-022-05043-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roskams AJI, Connor JR. Iron, Transferrin, and Ferritin in the Rat Brain During Development and Aging. Journal of Neurochemistry. 2002;63(2):709–716. doi: 10.1046/j.1471-4159.1994.63020709.x [DOI] [PubMed] [Google Scholar]
- 42.Holmes-Hampton GP, Chakrabarti M, Cockrell AL, et al. Changing Iron Content of the Mouse Brain during Development. Metallomics. 2012;4(8):761–770. doi: 10.1039/c2mt20086d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keen CL, Hurley LS. Developmental changes in concentrations of iron, copper, and zinc in mouse tissues. Mechanisms of Ageing and Development. 1980;13(2):161–176. doi: 10.1016/0047-6374(80)90059-7 [DOI] [PubMed] [Google Scholar]
- 44.Greminger AR, Lee DL, Shrager P, Mayer-Pröschel M. Gestational Iron Deficiency Differentially Alters the Structure and Function of White and Gray Matter Brain Regions of Developing Rats. The Journal of Nutrition. 2014;144(7):1058–1066. doi: 10.3945/jn.113.187732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moos T, Skjørringe T, Thomsen LL. Iron deficiency and iron treatment in the fetal developing brain – a pilot study introducing an experimental rat model. Reproductive Health. 2018;15(1):93. doi: 10.1186/s12978-018-0537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felt BT, Beard JL, Schallert T, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behavioural Brain Research. 2006;171(2):261–270. doi: 10.1016/j.bbr.2006.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early Postnatal Iron Repletion Overcomes Lasting Effects of Gestational Iron Deficiency in Rats. The Journal of Nutrition. 2007;137(5):1176–1182. doi: 10.1093/jn/137.5.1176 [DOI] [PubMed] [Google Scholar]
- 48.Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional Brain Iron, Ferritin and Transferrin Concentrations during Iron Deficiency and Iron Repletion in Developing Rats. The Journal of Nutrition. 1997;127(10):2030–2038. doi: 10.1093/jn/127.10.2030 [DOI] [PubMed] [Google Scholar]
- 49.Erikson KM, Jones BC, Beard JL. Iron Deficiency Alters Dopamine Transporter Functioning in Rat Striatum. The Journal of Nutrition. 2000;130(11):2831–2837. doi: 10.1093/jn/130.11.2831 [DOI] [PubMed] [Google Scholar]
- 50.Hare D, Ayton S, Bush A, Lei P. A delicate balance: Iron metabolism and diseases of the brain. Front Aging Neurosci. 2013;5:34. doi: 10.3389/fnagi.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaur D, Peng J, Chinta SJ, et al. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiology of Aging. 2007;28(6):907–913. doi: 10.1016/j.neurobiolaging.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 52.Ben-Shachar D, Ashkenazi R, Youdim MBH. Long-term consequence of early iron-deficiency on dopaminergic neurotransmission in rats. International Journal of Developmental Neuroscience. 1986;4(1):81–88. doi: 10.1016/0736-5748(86)90019-5 [DOI] [PubMed] [Google Scholar]
- 53.Baringer SL, Palsa K, Spiegelman VS, Simpson IA, Connor JR. Apo- and holo-transferrin differentially interact with hephaestin and ferroportin in a novel mechanism of cellular iron release regulation. J Biomed Sci. 2023;30(1):36. doi: 10.1186/s12929-023-00934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated within this study is available upon request to the corresponding author.