Dear Editor,
Coring is described as the impalement of the pieces of rubber stopper in the needle and its introduction in the vial with the impending risk of getting injected intravenously into the body, posing a constant threat to the patient's health. The incidence varies between 3.0% and 97%.[1] There is always a risk of wear and tear, abrasion and fragmentation of rubber particles, which can gain access to the vials and remain unnoticed due to their tiny size, masked by the medication's labels and its opacity.
We present a case of coring while loading Inj. propofol (Hyprovan 200 mg/20 ml; Celon Labs, Telangana, India) from the vial with the help of a 10-ml syringe (Romojet™, Romsons Juniors, India) with a 21G sharp needle for induction in a case of laparoscopic cholecystectomy in a 39-year-old lady weighing 58 kg. Before administering the drug, a black particulate contaminant was noticed in the vial [Figure 1a], so the entire drug in the syringe and the vial were discarded, and a fresh vial was selected.
Figure 1.
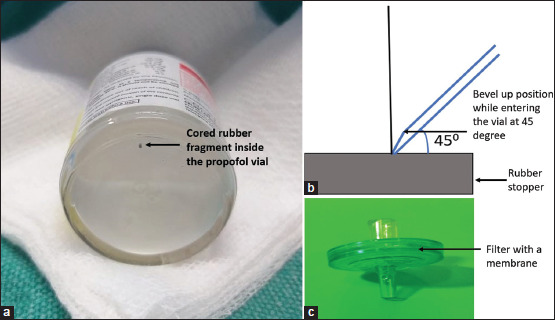
(a) Cored rubber particle in the vial. (b) Angle of entry at 45° for needle puncture with the bevel up while puncturing. (c) Use of interface-like spikes/filters during drug aspiration
Coring exposes the patient to an increased health risk, especially with multidose vials. Repeated puncture of these vials and size of the particulate larger than 6–8 µm in diameter can lead to phlebitis, pulmonary embolism, granulomas, latex allergy and mechanical blockade of the angiocath.[2,3] The factors which primarily affect coring include the rubber stopper's material, diaphragm's thickness, type of needle tip (blunt vs. sharp bevelled), penetration angle, needle bevel position and multidose vials.[1,2,3] The factors include the inferior elastomeric quality of the stopper, diminished resealability and reduced tensile strength.[2,3] Wani et al.[4] reported that fragmentation happened in 40.2% of the vials where a blunt cannula tip was used, in contrast to only 4.8% of vials being penetrated with sharp bevelled needles. Chotikawanich et al.[2] conducted an experimental study with a multidose vial of propofol. The authors punctured the rubber stopper with various needle sizes (18G, 20G, 21G) at different angles (45° and 90°). They concluded that coring increased with the larger size needle regardless of the angle. Rase et al.[3] tested the two-needle penetration method with varying sizes (18G, 20G, 22G). The first penetration method encompassed a 45° entry angle with the pressure applied along it, and the second method included the pressure applied in a direction perpendicular to the surface of the stopper. The authors observed increased incidence with a needle size of more than 22G with a bevel-down position; the fragmentation was maximum with the second penetration method regardless of the needle sizes. Heckar et al.[5] reported that the size of the cored particle varied in length between 29 and 244 µm and in width between 14 and 83 µm when using 18G and 23G needles, respectively.
To conclude, the mitigation strategy is suggested are meticulous inspection of the vial, changing the angle of entry to 45° [Figure 1b], sharp bevelled needles of not less than 22G, syringe attachments with interface-like spikes/filters [Figure 1c], single-use vials and promoting closed drug delivery system with prefilled syringes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient consented to her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hruska JL, Saasouh W, Alhamda MS. Coring revisited: A case report and literature review. Cureus. 2022;14:e29750. doi: 10.7759/cureus.29750. doi:10.7759/cureus. 29750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chotikawanich T, Kammee T, Khantee S. The impact of needle size and angle on rubber coring after multiple puncturing of multi-dose propofol vial rubber stoppers. Heliyon. 2022;8:e09389. doi: 10.1016/j.heliyon.2022.e09389. doi:10.1016/j.heliyon. 2022.e09389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rase M, Hanlon M, Ho L, Duriez D, Zhao C. Vial coring and fragmentation incidence after angled penetration of rubber stoppers with single-use hypodermic needles. Pharm Technol Hosp Pharm. 2021;6:20210004. doi:10.1515/pthp-2021-0004. [Google Scholar]
- 4.Wani T, Wadhwa A, Tobias JD. The incidence of coring with blunt versus sharp needles. J Clin Anesth. 2014;26:152–4. doi: 10.1016/j.jclinane.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Heckar A, Di Maro A, Liechti E, Klenke FM. Avoiding unconscious injection of vial-derived rubber particles during intra-articular drug administration. Osteoarthr Cartil Open. 2021;3:100164. doi: 10.1016/j.ocarto.2021.100164. doi:10.1016/j.ocarto. 2021.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]


