Abstract
Background:
Studies across the globe generally reported increased mortality risks associated with particulate matter with aerodynamic diameter () exposure with large heterogeneity in the magnitude of reported associations and the shape of concentration-response functions (CRFs). We aimed to evaluate the impact of key study design factors (including confounders, applied exposure model, population age, and outcome definition) on effect estimates by harmonizing analyses on three previously published large studies in Canada [Mortality-Air Pollution Associations in Low Exposure Environments (MAPLE), 1991–2016], the United States (Medicare, 2000–2016), and Europe [Effects of Low-Level Air Pollution: A Study in Europe (ELAPSE), 2000–2016] as much as possible.
Methods:
We harmonized the study populations to individuals years of age, applied the same satellite-derived exposure estimates, and selected the same sets of potential confounders and the same outcome. We evaluated whether differences in previously published effect estimates across cohorts were reduced after harmonization among these factors. Additional analyses were conducted to assess the influence of key design features on estimated risks, including adjusted covariates and exposure assessment method. A combined CRF was assessed with meta-analysis based on the extended shape-constrained health impact function (eSCHIF).
Results:
More than 81 million participants were included, contributing 692 million person-years of follow-up. Hazard ratios and 95% confidence intervals (CIs) for all-cause mortality associated with a increase in were 1.039 (1.032, 1.046) in MAPLE, 1.025 (1.021, 1.029) in Medicare, and 1.041 (1.014, 1.069) in ELAPSE. Applying a harmonized analytical approach marginally reduced difference in the observed associations across the three studies. Magnitude of the association was affected by the adjusted covariates, exposure assessment methodology, age of the population, and marginally by outcome definition. Shape of the CRFs differed across cohorts but generally showed associations down to the lowest observed levels. A common CRF suggested a monotonically increased risk down to the lowest exposure level. https://doi.org/10.1289/EHP12141
Introduction
Ambient particulate matter (PM) pollution has been identified as a leading risk factor for global disease burden.1 Evaluating the risks attributable to PM with aerodynamic diameter () exposure and potential benefits related to the reduction in concentrations depends upon understanding both the magnitude and shape of the concentration-response functions (CRFs) of the association between and mortality. Studies across the globe generally reported positive associations between long-term exposure and increased mortality risks with substantial variations in the magnitude of associations.2 In addition, different shapes of CRFs have been reported.2 Investigators have speculated that variability in the observed associations could be related to differences in the study population, levels of exposure, composition of the mixture, exposure assessment methodology, or statistical models applied across studies. The influence of these factors has, however, not been systematically investigated.
Recently, three studies were conducted to investigate the health effects of low-level air pollution exposure in very large populations in Canada, the United States, and Europe. These are the Mortality-Air Pollution Associations in Low Exposure Environments (MAPLE) study by Brauer et al. in Canada,3 the Medicare study by Dominici et al. in the United States,4 and the Effects of Low-Level Air Pollution: A Study in Europe (ELAPSE) by Brunekreef et al. in Europe.5 All three studies reported statistically significantly positive associations between long-term exposure and mortality risks in the nationally representative administrative cohorts. Specifically, hazard ratios (HRs) and 95% confidence intervals (CIs) associated with a increase in exposure and all-cause or natural-cause mortality were 1.041 (1.036, 1.046) in the Canadian Census Health and Environment Cohorts (CanCHEC) in MAPLE,3 1.032 (1.025, 1.040) in the Medicare cohort,4 and 1.058 (1.022, 1.095) in the six ELAPSE administrative cohorts based on a meta-analytical estimate.5 Despite the close HRs with largely overlapping CIs, the three studies differed in many aspects, including levels and potentially the composition of exposure, as well as other key study design elements. One such difference was the age of the study populations: the Medicare cohort only included a population years of age, whereas MAPLE and ELAPSE included adults and years of age, respectively. In addition, the Medicare cohort investigated all-cause mortality, whereas MAPLE and ELAPSE analyzed mortality from natural causes, excluding accidental mortality and suicide. The three studies further applied different exposure assessment methodologies at different spatial resolutions ( in ELAPSE and in the North American studies).6–8 Furthermore, adjustments for different sets of potential confounders were dependent on data availability, and different statistical model specifications were used across the three studies. Harmonizing key study design elements across studies and comparing with the original estimates offer an opportunity to investigate sources of heterogeneity, relevant for future meta-analysis of studies.
In the present study, we assessed linear associations and shape of CRFs between long-term exposure and mortality by harmonizing analyses across MAPLE, Medicare, and ELAPSE as much as possible. We aimed to evaluate the impact of key study design factors (including confounders, applied exposure model, population age, and outcome definition) on effect estimates by the contrasts between and within studies.
Methods
The primary harmonized analyses conducted in all three studies involved analyzing associations between time-varying satellite-derived exposure estimates8,9 and all-cause mortality in adults years of age, adjusting for common potential confounders. The choice of population age and mortality end point was limited by the Medicare cohort, which only had data on all-cause mortality in a population years of age. In the Canadian and European studies, we additionally evaluated the influence of population age and outcome definition by performing analyses in the full cohort ( years of age in MAPLE and years of age in ELAPSE) and for natural mortality. We intentionally maintained some consistencies in our methodology with previously published studies rather than a fully harmonized approach to enable comparisons not only between studies but also within individual studies.
Study Populations and Mortality Outcomes
CanCHEC cohort.
The “stacked” CanCHEC cohort was created in MAPLE, which comprised three Census-based cohorts enrolled in the year 1991 ( respondents), 1996 ( respondents), and 2001 ( respondents), with duplicate records removed ( unique participants). Noninstitutionalized residents of Canada who responded to the long form census questionnaire were linked to vital statistics (including cause of death) and tax records from census day to 31 December 2016.3 Linkage was approved by Statistics Canada and is governed by the Directive on Microdata Linkage. All cohort participants were 25 years old or older at baseline.
Medicare cohort.
The Medicare cohort is an open cohort of Medicare enrollees 65 years old or older obtained from the Centers for Medicare and Medicaid Services. This cohort includes Medicare enrollees from 2000 to 2016 and includes demographic information on age, sex, race/ethnicity, date of death (all cause), and residential ZIP code.4 A unique patient ID is assigned to each enrollee to allow for tracking over time. Medicare enrollees entered the cohort in 2000 if enrolled before 2000 or upon their enrollment after 2000. After enrollment, each enrollee was followed annually until the year of their death or until 31 December 2016.
ELAPSE cohorts.
ELAPSE data included six European cohorts for which harmonized exposure estimates could be linked, comprised of five nationwide cohorts (i.e., Belgium, Denmark, the Netherlands, Norway, and Switzerland) and one citywide cohort (i.e., Rome, Italy).5 Participants 30 years old or older were enrolled between 2000 and 2008 based on data from census or population registries and followed-up through 2011–2016 ( participants). Identification of underlying causes of death for deceased individuals was based on linkage to mortality registries. The six cohorts were analyzed separately in national secure environments followed by random-effect meta-analyses.
In the present study, all-cause mortality (including accidental/trauma mortality) was primarily evaluated because the Medicare study included only information on all-cause mortality. Analyses were restricted to study populations 65 years old and older, again due to the age restriction of the Medicare cohort. Participants were followed up until death, emigration, loss to follow-up for other reasons, or end of the study, whichever came first.
Exposure Assessment and Assignment
Annual concentrations were estimated at a spatial resolution covering North America (data version V4.NA.02.MAPLE; Washington University in St. Louis Atmospheric Composition Analysis Group) and Europe (data version V4.EU.02).10 The methodology for exposure assessment and validation has been described in detail elsewhere.8,9 Briefly, concentrations were estimated by incorporating information from remote-sensing-based aerosol optical depth (AOD), chemical transport modeling, land use information, and ground-level measurements. In North America, surfaces were first simulated by a chemical transport model (GEOS-Chem) incorporating satellite AOD over the period 1989–2016. The estimates were then refined by applying geographically weighted regression incorporating ground-based measurement data. Relying on information on interannual variation from ground-based measurements of , PM with aerodynamic diameter (), and total suspended particulate matter (TSP), the estimates were back-casted to produce estimates for the 1981–1988 period, resulting in modeled annual surfaces across North America from 1981 to 2016. In Europe, annual surfaces were estimated for 2000–2016 based on ground-level measurements from the European Environment Agency Air Quality e-Reporting system.9 Evaluation against ground-based monitoring in North America (, , ) and Europe (, , ) indicated strong agreement.3,9 This set of exposure estimates was previously applied in the MAPLE study.3
To facilitate within-study comparisons, exposure assignment was conducted consistently with previously published reports from these studies, resulting in less harmonization.3–5 Annual exposures were estimated for study participants based on residential postal codes in MAPLE, residential ZIP code in Medicare, and exact residential addresses in ELAPSE. In Canadian urban areas, a residential postal code centroid is typically within of a person’s home, whereas in rural areas, the location for a given postal code is typically accurate within about .11 ZIP codes in the United States span in size from a single building to large areas that cut across states and have an average population of 30,000 people.12 exposures were represented as time-varying exposure incorporating residential mobility in most cohorts. In the Dutch and Danish cohorts within ELAPSE, residential mobility was not incorporated,5 consistent with previous analyses. The Dutch cohort had 5 years of follow-up only, suggesting mobility was likely not a critical concern in exposure misclassification. In MAPLE, a 10-year moving average of with a 1-year lag was assigned to a given person-year based on previous research that this approach yielded stronger associations with mortality.13,14 For example, the average of exposures from 1981 to 1990 would be assigned as the exposure for a participant in 1991. In ELAPSE and Medicare, annual average concentrations were assigned to individuals for that calendar year.
Potential Confounders
We harmonized the choice of confounder variables across cohorts as much as possible, considering different data availability, spatial scale, and contexts. We chose common confounder variables that cover the same concepts, accepting that the definition may differ across cohorts.
In CanCHEC,3 information on individual level covariates was collected at each census, including age (5-year age group), sex, immigrant status (yes, no), indigenous identity [defined as persons who identify as North American Indian and/or Métis and/or Inuit, and/or reported being a Treaty Indian or a Registered Indian and/or being a member of an Indian Band or First Nation (yes, no)], visible minority status [defined as “persons, other than Indigenous persons, who were not white in race or color” (yes, no)], household income in quintiles, educational attainment (high school graduation or below, high school, postsecondary nonuniversity, or university), marital status (never married/not common law, common-law, married, or separated/divorced/widowed), and employment status (employed, unemployed, or not in the labor force). A composite index was calculated at the census tract level (i.e., neighborhood of 2,500–8,000 people) in cities, and the census subdivision level (i.e., rural municipality) outside of cities to capture neighborhood-level marginalization. It contains four dimensions of marginalization: material deprivation (e.g., proportion of population with low education, low income), residential instability (e.g., proportion of dwellings that are not owned, proportion of multiunit housing), dependency (e.g., ratio of seniors and youth to working aged population), and ethnic concentration (e.g., proportion of recent immigrants and self-reported visible minorities). Airshed was defined by the Canadian Air Quality Management System on the basis of similar air-quality characteristics or dispersion patterns,15 which subdivides the country into six large geographic areas.
In Medicare,4 information on age, sex, race/ethnicity (White, Black, Asian, Hispanic, or North American Native), Medicaid eligibility (eligible, ineligible), and ZIP code was obtained from the Centers for Medicare & Medicaid Services. Information about Medicare beneficiary race and ethnicity was obtained from the Social Security Administration, which collects race and ethnicity data at the time of application for a Social Security Number.16 ZIP code level socioeconomic status (SES) was updated every year, including median household income, median house value, proportion of residents in poverty, proportion of residents that own their house, and proportion of residents with a high school diploma. Indicator for four Census geographic regions was created: Northeast, South, Midwest, and West.4
In the Belgian cohort,17 information on individual level covariates was collected at baseline (year 2001), including age, sex, marital status (single, married/cohabiting, divorced/separated, or widowed), country of origin (local, foreign), educational level (no/primary, secondary, or tertiary), and employment status (employed/self-employed, unemployed, homemaker, or retired). Area-level SES indicators were available at both neighborhood (sections, ) and regional levels (arrondissements, ), consisting of mean household net taxable income in euros in year 2011, percentage of working age population unemployed in year 2011, percentage of population with no/primary education in year 2011, and percentage of non-Western migrants in year 2001. Large area indicator was created for three regions: Brussels-Capital Region, Flemish Region, and Walloon Region.
In the Danish cohort,18 individual-level demographics were collected at baseline (year 2000), including age, sex, country of origin (Danish origin, immigrants/descendants from Western country of origin, or immigrants/descendants from non-Western country of origin), equivalized disposable household income in deciles, and employment status (employed, unemployed, or sick/cash support/student/pension/others). Area-level SES indicators for year 2001 were obtained at the parish () and municipality () levels, including mean equivalized disposable household income, percentage of population 15 years old and above unemployed, and percentage of population 30 years old and above with the highest educational attainment as primary level education. Large area indicator was created for five regions: Capital Region of Denmark, Region Zealand, Region of Southern Denmark, Central Denmark Region, and Region of Northern Denmark.
In the Dutch cohort,19 individual-level data were collected at baseline (year 2008), including age, sex, country of origin (Dutch, Western, non-Western, Morocco, Turkey, Suriname, or Antilles Netherlands), marital status (single, married, divorced/separated, or widowed), and household income in deciles. Area-level data were collected in year 2006 and available at both neighborhood (“wijk,” ) and regional levels [Coördinatiecommissie Regionaal Onderzoeksprogramma (COROP) areas, ]. Area-level SES indicators consisted of composite SES score (calculated based on education, income, and paid occupation), mean income per income recipient, number of people with income support per 1,000 inhabitants 15–64 years of age, and percentage of non-Western immigrants. Large area indicator was created for 12 provinces.
In the Norwegian cohort,20 individual-level data were available from baseline (year 2001) on age, sex, marital status (single, married, divorced/separated, or widowed), educational level (no/primary/lower secondary, upper secondary, college/university), employment status (employed/self-employed, unemployed, or retired), and household income in quartiles. Area-level SES indicators were available at both neighborhood (“delområde,” ) and county () levels, including these percentages among the 30–60-year-old cohort participants in year 2001: percentage of individuals with household income of median income after tax, percentage of individuals with educational attainment equal or below lower secondary, percentage of individuals unemployed. Large area indicator was created for five regions (“landsdel”): Northern Norway, Trøndelag, Western Norway, Southern Norway, and Eastern Norway.
In the Rome cohort,21 individual-level data were available from baseline (year 2001) on age, sex, marital status (single, married, separated/divorced, or widowed), educational level (primary or below, junior high school, high school, university), employment status [top qualified nonmanual employed (i.e., managers, university and high school professors, researchers), other nonmanual employed, manual labor employed, other employed (i.e., armed forces and retail sales), housewife, unemployed, retired, or others], and place of birth (Rome, other). SES indicators for year 2001 were available either at census tract () or district level (), on income in deciles (census tract), percentage of educational attainment equal or above university degree (district), percentage of educational attainment equal or below primary school (district), percentage of working age population unemployed (district), and socioeconomic position index (derived based on education, occupation, house ownership, family composition, crowding, and immigrant status) in quintiles (census tract). Regional level data were not necessary for the Rome study area.
In the Swiss cohort,22 individual-level data were available from baseline (year 2001) on age, sex, marital status (single, married, divorced/separated, or widowed), educational level (compulsory education or less, upper secondary level education, or tertiary level education), mother tongue (German and Rhaeto-Romansch, French, Italian, or other language), nationality (Swiss, non-Swiss), and employment status (employed/self-employed, unemployed, homemaker, or retired). Area-level SES indicators were available for year 2001 at both postcode () and canton () levels, including the Swiss neighborhood index of socioeconomic position (calculated based on median rent per square meter, proportion households headed by a person with primary education or less, proportion headed by a person in manual or unskilled occupation, and the mean number of persons per room),23 proportion of unemployed population 20–65 year of age, proportion of adults with compulsory or less education, and proportion of adults with tertiary education or higher. Large area indicator was created for seven regions at the Nomenclature of Territorial Units for Statistics (NUTS)-2 level.24
Statistical Analysis
Main analyses.
Cox proportional hazards regression models were applied in MAPLE and ELAPSE. As per the models applied in the published reports,3,5 age was included as the time axis in ELAPSE for better adjustment for potential confounding by age,25 whereas year of follow-up was included as the time axis in MAPLE. MAPLE analyses were further stratified by the three CanCHEC census enrollment years. In Medicare, a Cox regression-equivalent and more computationally efficient Poisson formulation was applied because of the size of the cohort.4 The Poisson regression model was fit with the count of all-cause deaths at the given follow-up year, calendar year, and ZIP code as the outcome, and the corresponding total person-time as the offset term. Time-varying exposure was included in the models as a linear function in all cohorts.
We specified four common models with increasing control for potential confounders. Model 1 included identical covariates available across cohorts—individual level age at follow-up, sex, and follow-up year. Model 2 further adjusted for selected covariates representing individual level SES and ethnicity—income quintiles, immigration status, visible minority status, and indigenous identity in CanCHEC; Medicaid eligibility and race in Medicare; educational level and country origin in the Belgian cohort; income deciles and country origin in the Danish and Dutch cohorts; income quartiles in the Norwegian cohort; educational level in the Roman cohort; educational level, nationality, and mother tongue in the Swiss cohort. Model 3 further adjusted for all available area-level SES covariates in each cohort as described above. Model 4 added large regional indicators as described above to account for residual spatial variation. We considered Model 4 as the main model, as it provided the maximum adjustment for potential confounding using the available information (Table S1). Participants with missing exposure or incomplete information on Model 4 covariates were excluded from all analyses. We conducted a random-effects meta-analysis across all eight cohorts to quantitatively evaluate whether harmonization reduced between-study variance in the magnitude of HRs.
Additional analyses.
In MAPLE and ELAPSE, we performed additional analyses in the full study populations ( years of age in MAPLE and years of age in ELAPSE), and for natural mortality, defined by the International Classification of Diseases, ninth revision (ICD-9) or tenth revision (ICD-10) codes: ICD-9: 1–779; ICD-10: A00–R99. We performed additional adjustment for potential individual level confounders that were not included in the main models, including educational level, marital status, and employment status. These additional analyses allowed comparison between the current study and the previously published reports3,5 and, thus, allowed more detailed evaluation of factors influencing the magnitude of observed associations. Statistical significance was determined based on a 95% CI of the effect estimate that did not include unity.
Characterizing the magnitude and shape of the –mortality association.
We modeled the shape of the CRFs based on the main Model 4, using the extended shape constrained health impact function (eSCHIF).3 The eSCHIF has been proposed as a biologically plausible function for modeling concentration–mortality associations for benefits analysis. The methodology has been described in detail before.26 Briefly, we first fit a Cox model (Cox-equivalent Poisson in Medicare) where exposure was included as a restricted cubic spline (RCS) function with . We selected RCS to flexibly model the association between and mortality.27 The RCS has the form
| (1) |
for knots, and
for K knot concentrations (). The K–1 unknown parameters () were estimated within the Cox survival model framework by including [, , …] as K–1 variables in the survival model. To each of the eight cohorts, we fit the RCS model in addition to other covariates using defined at the 5th, 23rd, 41st, 59th, 77th, and 95th percentiles of the respective exposure distributions. For each cohort, we extracted the parameter K–1 estimates and their respective covariance matrix, in addition to the values. Assuming the parameter estimates are multivariate normally distributed, we then simulated 1,000 sets of parameter values with mean given by the parameter estimates and dispersion given by the estimated covariance matrix. We then generated 1,000 sets of RCS predictions over the cohort-specific concentration range defined by their 2.5 to 97.5 exposure percentiles at increments using Equation 1.
A counterfactual concentration, , was incorporated into the RCS fit for each of the 1,000 sets of predictions by dividing the RCS prediction at any concentration greater than by the RCS prediction at . Based on this adjustment, the RCS prediction at the counterfactual equals unity for each of the 1,000 series of predictions. In this paper, we set the counterfactual at the fifth percentile of the cohort-specific exposure distributions.
The best fitting shape of the association estimated by splines may not be entirely suitable for risk assessment due to potential oscillations over short concentration ranges. We thus remove this “wiggly” behavior using an algebraic function of the form
with representing the range in concentrations. Here, we extend the shape constrained health impact function, , with the additional term, , that allows more complex shapes and potentially negative associations. We do this since the RCS predictions have not been restricted to be monotonically increasing.
The unknown parameters (, , , , , and ) are determined such that they maximize the log-likelihood for each of the 1,000 series separately.3 The eSCHIF plot is graphically summarized by the mean of the 1,000 eSCHIF predictions at each concentration, and CI is provided by the 2.5 and 97.5 percentiles among the 1,000 eSCHIF curves.
We then used the following procedure to fit a common eSCHIF function to the eight cohort-specific mean eSCHIF predictions between their respective 2.5 and 97.5 exposure percentiles.28 Let represent the logarithm of the mean of the 1,000 RCS predictions for the sth of S studies at the jth of concentrations. Further, let represent the variance of the among the 1,000 predictions. Consider the multivariate meta-analysis model,
where is normally distributed with zero mean and variance , is normally distributed with zero mean and variance , and is set at the minimum concentration among all cohorts. Note, we assigned the same sampling variance to each of the values of since we have generated “pseudo-data” for each cohort and we need to reflect this data generation in the multivariate meta-analysis model. Here, represents the between cohort variation in the not explained by the eSCHIF model.
We estimated the unknown parameters (, , , , , and ) by first simulating a large number N (say 10,000) of values from the following distributions,
and given these sets of values, calculate two variables representing the two terms in the eSCHIF model not including the leading parameters and . These two variables are then included in the rma.mv routine in the R package metafor (version 4.2.0; R Development Core Team) with the following specifications: no intercept, cohort as a random effect, and . For each of the N runs, we extracted the estimate of , , and their corresponding 2 by 2 covariance matrices .
Finally, we formed an ensemble of the N models by weighting each of the N model predictions over the range in concentration by the corresponding model likelihood value.29 This approach incorporates the sampling uncertainty in and in addition to the uncertainty in the shapes defined by the values of , , , and . All analyses were performed in R software.
Results
Table 1 and Table S2 present the demographics and average exposure levels in each cohort for the population years of age and the full population. More than 106 million participants were included in the current analysis, contributing more than 970 million person-years of follow-up. exposure levels varied by cohort.
Table 1.
Characteristics of the study populations years of age in Medicare, CanCHEC, and ELAPSE cohorts.
| Medicare | Stacked CanCHEC | ELAPSE | ||||||
|---|---|---|---|---|---|---|---|---|
| Belgian | Danish | Dutch | Norwegian | Roman | Swiss | |||
| Year of enrollment | 2000–2016 | 1991, 1996, 2001 | 2001 | 2000 | 2008 | 2001 | 2001 | 2001 |
| Follow-up | ||||||||
| Start-end | 2000–2016 | 1991–2016 | 2001–2011 | 2000–2015 | 2008–2012 | 2001–2016 | 2001–2015 | 2001–2014 |
| Persons at risk | ||||||||
| 74,493,754 | 942,600a | 1,291,067 | 697,359 | 2,314,101 | 551,962 | 359,715 | 945,846 | |
| Person-years at risk | ||||||||
| 636,817,430 | 9,106,200a | 10,299,384 | 6,835,701 | 10,351,266 | 5,674,821 | 3,571,859 | 9,499,911 | |
| All-cause mortality | ||||||||
| person-years | 40.5 | 51.9 | 54.5 | 72.3 | 46.4 | 68.0 | 53.3 | 54.5 |
| Natural mortalityb | ||||||||
| person-years | — | 50.3 | 52.1 | 70.0 | 45.1 | 65.0 | 51.0 | 52.4 |
| Sex (%) | ||||||||
| Males | 44.6 | 42.9 | 45.8 | 41.9 | 43.4 | 41.2 | 41.6 | 42.0 |
| Females | 55.4 | 57.1 | 54.2 | 58.1 | 56.6 | 58.8 | 58.4 | 58.0 |
| Baseline age (years) | ||||||||
| exposure during follow-up () | ||||||||
| 5th−95th percentile | 5.0–14.5 | 3.7–12.0 | 12.3–19.3 | 6.5–13.1 | 12.3–18.1 | 4.2–10.3 | 14.8–22.4 | 10.3–21.7 |
| Individual-level SES and ethnicity | ||||||||
| Country of origin/ immigration status | ||||||||
| Local/nonimmigrant | — | 74.0 | 99.2 | 96.7 | 87.9 | — | — | — |
| Foreign/immigrant | — | 26.0 | 0.8 | 3.3 | 12.1 | — | — | — |
| Nationality (%) | ||||||||
| Swiss | — | — | — | — | — | — | — | 93.1 |
| Non-Swiss | — | — | — | — | — | — | — | 6.9 |
| Mother tongue (%) | ||||||||
| German and Rhaeto-Romansch | — | — | — | — | — | — | — | 69.9 |
| French | — | — | — | — | — | — | — | 20.8 |
| Italian | — | — | — | — | — | — | — | 7.3 |
| Other | — | — | — | — | — | — | — | 2.1 |
| Race (%) | ||||||||
| White | 84.1 | — | — | — | — | — | — | — |
| Black | 8.9 | — | — | — | — | — | — | — |
| Asian | 1.8 | — | — | — | — | — | — | — |
| Hispanic | 2.0 | — | — | — | — | — | — | — |
| North American Native | 0.3 | — | — | — | — | — | — | — |
| Visible minority status (%) | ||||||||
| Yes | — | 4.9 | — | — | — | — | — | — |
| No | — | 95.1 | — | — | — | — | — | — |
| Indigenous identity (%) | ||||||||
| Yes | — | 1.1 | — | — | — | — | — | — |
| No | — | 98.9 | — | — | — | — | — | — |
| Medicaid eligibility (%) | ||||||||
| Eligible | 11.6 | — | — | — | — | — | — | — |
| Ineligible | 88.4 | — | — | — | — | — | — | — |
| (Household) income in local currency | ||||||||
| Levels | — | Quintiles | — | Deciles | Deciles | Quartiles | — | — |
| Educational level (%) | ||||||||
| Primary or below | — | 55.2 | 50.7 | — | — | 48.9 | 51.0 | 41.5 |
| Secondary | — | 37.2 | 38.8 | — | — | 40.4 | 37.9c | 45.5 |
| Tertiary | — | 7.5 | 10.5 | — | — | 10.7 | 11.1 | 13.0 |
| Marital status (%) | ||||||||
| Single | — | 5.2 | 6.1 | — | 5.4 | 7.4 | 7.0 | 7.3 |
| Married | — | 66.7 | 57.9 | — | 58.5 | 52.3 | 57.4 | 58.7 |
| Divorced/separated | — | 6.0 | 4.6 | — | 7.3 | 6.3 | 3.4 | 6.0 |
| Widowed | — | 21.7 | 31.4 | — | 28.7 | 34.0 | 32.2 | 28.0 |
| Employment status (%) | ||||||||
| Employed | — | 11.1 | 0.6 | 4.5 | — | 6.1 | 3.7 | 5.4 |
| Unemployed | — | 0.5 | 0 | 0.1 | — | 0.1 | 0.1 | 0.1 |
| Homemaker | — | — | 2.0 | — | — | — | 30.1 | 0 |
| Retired | — | 88.3d | 97.4 | 95.4e | — | 93.8 | 58.2 | 94.5 |
Note: —, no data; CanCHEC, Canadian Census Health and Environment Cohorts; ELAPSE, Effects of Low-Level Air Pollution: A Study in Europe; , particulate matter with aerodynamic diameter ; SD, standard deviation.
Rounded to nearest 100 for confidentiality.
Natural mortality was defined by the International Classification of Diseases, 9th revision (ICD-9) or 10th revision (ICD-10) codes: ICD-9: 1–779; ICD-10: A00–R99.
Grouped junior high school and high school.
Employment status has three classes in CanCHEC: employed, unemployed, and not in the labor force (i.e., persons who left on disability, had retired, or had never worked).
Employment status has three classes in the Danish cohort: employed, unemployed, and pensioner (i.e., retired, sick, cash support, student, pension, or others).
Linear Associations
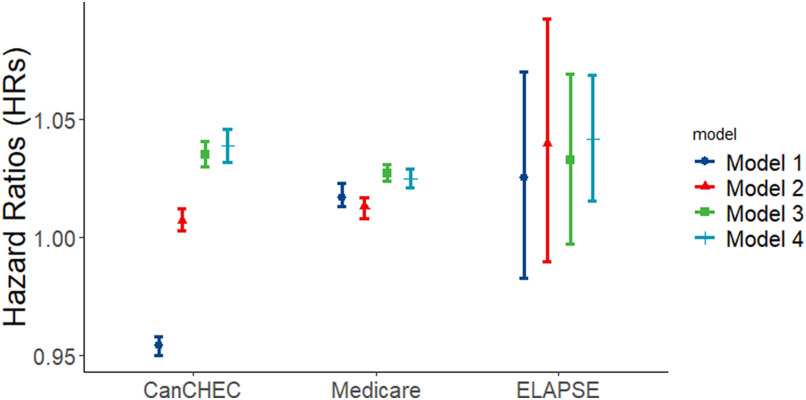
Figure 1 and Table 2 show the linear associations between exposure and mortality with increasing adjustment for covariates. In the minimally adjusted Model 1, associations were negative in CanCHEC, yet positive in Medicare and ELAPSE. In the fully adjusted main Model 4, HRs increased in all three studies. Adjustment for SES at individual- (Model 1 to Model 2) and area-level (Model 2 to Model 3) both influenced HRs, while the relative importance differed by cohort. Adjustment for individual SES had a relatively large impact in the stacked CanCHEC cohort, whereas it was less important in Medicare and ELAPSE. SES covers multiple dimensions, including income, education, occupation, and employment status. For harmonization, we restricted the individual SES indicators included in the main analyses for MAPLE and ELAPSE. We, however, did not observe much deviation in HRs after additionally adjusting for other individual SES indicators available in MAPLE and ELAPSE (additional Models 2, 3, and 4 in Table 2). In most cohorts, HRs derived from Model 4 and Model 3 were similar, suggesting that adjusting for individual- and area-level SES reduced the need to adjust for regional indicators. However, in the ELAPSE Belgian and Swiss cohorts, further adjustment for regional indicators of the country increased HRs moderately.
Figure 1.
Associations between exposure (per ) and all-cause mortality derived from the minimally adjusted Model 1 to the fully adjusted main Model 4 in the harmonized analyses for all-cause mortality in population years of age in CanCHEC (1991–2016), Medicare (2000–2016), and ELAPSE (2000–2016). Numeric effect estimates are shown in Table 2. Model 1 is adjusted for age, sex, and follow-up year; Model 2 is further adjusted for individual level SES and ethnicity; Model 3 is further adjusted for area-level SES; and Model 4 added large regional indicators. Note: CanCHEC, Canadian Census Health and Environment Cohorts; ELAPSE, Effects of Low-Level Air Pollution: A Study in Europe; , particulate matter with aerodynamic diameter ; SES, socioeconomic status.
Table 2.
Associations between exposure (per ) and mortality from all-causes and natural causes in the full cohort and the cohort. The second column (, all-cause) represents the main harmonized analyses.
| y | Full cohorts: y for ELAPSE cohorts; y for CanCHEC | |||
|---|---|---|---|---|
| All-cause [HR (95% CI)] | Natural causes [HR (95% CI)] | All-cause [HR (95% CI)] | Natural causes [HR (95% CI)] | |
| CanCHEC (1991–2016) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 0.954 (0.950, 0.958) | 0.954 (0.950, 0.958) | 0.948 (0.944, 0.951) | 0.954 (0.950, 0.958) |
| Model 2 | 1.007 (1.003, 1.012) | 1.009 (1.004, 1.013) | 1.014 (1.010, 1.019) | 1.020 (1.015, 1.024) |
| Model 3 | 1.035 (1.030, 1.041) | 1.036 (1.031, 1.041) | 1.029 (1.025, 1.034) | 1.035 (1.030, 1.040) |
| Model 4 | 1.039 (1.032, 1.046) | 1.042 (1.035, 1.049) | 1.039 (1.033, 1.045) | 1.046 (1.039, 1.053) |
| Model 2add | 1.009 (1.004, 1.013) | 1.010 (1.006, 1.015) | 1.015 (1.011, 1.019) | 1.021 (1.016, 1.025) |
| Model 3add | 1.027 (1.022, 1.032) | 1.028 (1.023, 1.033) | 1.019 (1.015, 1.024) | 1.026 (1.022, 1.031) |
| Model 4add | 1.037 (1.030, 1.044) | 1.040 (1.033, 1.047) | 1.037 (1.031, 1.044) | 1.045 (1.039, 1.052) |
| Medicare (2000–2016) | ||||
| ; ; | — | — | ||
| Model 1 | 1.017 (1.013, 1.023) | NA | NA | NA |
| Model 2 | 1.013 (1.008, 1.017) | NA | NA | NA |
| Model 3 | 1.027 (1.024, 1.031) | NA | NA | NA |
| Model 4 | 1.025 (1.021, 1.029) | NA | NA | NA |
| Model 2add | NA | NA | NA | NA |
| Model 3add | NA | NA | NA | NA |
| Model 4add | NA | NA | NA | NA |
| ELAPSE overall (i.e., pooled estimate from random-effects meta-analysis of 6 ELAPSE cohorts) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 1.026 (0.983, 1.070) | 1.022 (0.976, 1.070) | 1.053 (0.986, 1.123) | 1.053 (0.989, 1.121) |
| Model 2 | 1.040 (0.990, 1.093) | 1.038 (0.985, 1.093) | 1.071 (0.997, 1.151) | 1.072 (0.999, 1.151) |
| Model 3 | 1.033 (0.997, 1.070) | 1.032 (0.994, 1.071) | 1.050 (0.997, 1.105) | 1.051 (1.001, 1.104) |
| Model 4 | 1.042 (1.015, 1.069)a | 1.041 (1.014, 1.069)a | 1.064 (1.021, 1.108)a | 1.063 (1.022, 1.106)a |
| Model 2add | 1.036 (0.992, 1.081) | 1.033 (0.987, 1.082) | 1.054 (0.995, 1.117) | 1.056 (0.998, 1.117) |
| Model 3add | 1.030 (0.997, 1.063) | 1.029 (0.995, 1.064) | 1.038 (0.996, 1.082) | 1.040 (1.000, 1.082) |
| Model 4add | 1.037 (1.014, 1.061)b | 1.036 (1.012, 1.062)b | 1.050 (1.018, 1.082)b | 1.050 (1.020, 1.081)b |
| Belgian (2001–2011) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 0.959 (0.952, 0.966) | 0.963 (0.955, 0.970) | 0.979 (0.973, 0.985) | 0.985 (0.979, 0.992) |
| Model 2 | 0.977 (0.970, 0.984) | 0.983 (0.975, 0.990) | 0.998 (0.992, 1.004) | 1.006 (0.999, 1.012) |
| Model 3 | 0.974 (0.967, 0.982) | 0.981 (0.973, 0.988) | 0.990 (0.984, 0.996) | 0.998 (0.992, 1.005) |
| Model 4 | 1.006 (0.997, 1.014) | 1.005 (0.996, 1.013) | 1.028 (1.021, 1.036) | 1.028 (1.021, 1.036) |
| Model 2add | 0.973 (0.966, 0.981) | 0.979 (0.972, 0.987) | 0.982 (0.976, 0.988) | 0.990 (0.984, 0.997) |
| Model 3add | 0.973 (0.966, 0.980) | 0.979 (0.972, 0.987) | 0.979 (0.973, 0.985) | 0.988 (0.981, 0.994) |
| Model 4add | 1.005 (0.997, 1.013) | 1.004 (0.996, 1.012) | 1.021 (1.014, 1.028) | 1.022 (1.014, 1.029) |
| Danish (2000–2015) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 1.112 (1.102, 1.122) | 1.111 (1.100, 1.121) | 1.217 (1.208, 1.226) | 1.212 (1.203, 1.221) |
| Model 2 | 1.155 (1.145, 1.166) | 1.155 (1.145, 1.166) | 1.265 (1.256, 1.275) | 1.263 (1.253, 1.272) |
| Model 3 | 1.097 (1.084, 1.111) | 1.097 (1.083, 1.111) | 1.166 (1.155, 1.178) | 1.164 (1.152, 1.176) |
| Model 4 | 1.095 (1.080, 1.110) | 1.094 (1.079, 1.110) | 1.166 (1.153, 1.178) | 1.162 (1.149, 1.175) |
| Model 2add | 1.133 (1.122, 1.144) | 1.134 (1.123, 1.144) | 1.197 (1.189, 1.207) | 1.198 (1.189, 1.207) |
| Model 3add | 1.086 (1.073, 1.100) | 1.086 (1.072, 1.100) | 1.122 (1.110, 1.133) | 1.121 (1.109, 1.132) |
| Model 4add | 1.082 (1.067, 1.097) | 1.082 (1.067, 1.097) | 1.118 (1.106, 1.130) | 1.117 (1.104, 1.129) |
| Dutch (2008–2012) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 1.054 (1.044, 1.064) | 1.052 (1.042, 1.063) | 1.075 (1.066, 1.085) | 1.074 (1.064, 1.083) |
| Model 2 | 1.071 (1.060, 1.081) | 1.069 (1.059, 1.080) | 1.096 (1.086, 1.105) | 1.093 (1.084, 1.103) |
| Model 3 | 1.058 (1.047, 1.069) | 1.058 (1.048, 1.069) | 1.074 (1.065, 1.084) | 1.074 (1.064, 1.084) |
| Model 4 | 1.064 (1.047, 1.082) | 1.064 (1.047, 1.082) | 1.086 (1.071, 1.102) | 1.083 (1.068, 1.099) |
| Model 2add | 1.056 (1.046, 1.067) | 1.055 (1.045, 1.065) | 1.072 (1.063, 1.081) | 1.071 (1.062, 1.080) |
| Model 3add | 1.053 (1.041, 1.065) | 1.055 (1.043, 1.067) | 1.063 (1.052, 1.074) | 1.064 (1.053, 1.075) |
| Model 4add | 1.058 (1.041, 1.075) | 1.057 (1.040, 1.075) | 1.071 (1.056, 1.086) | 1.069 (1.054, 1.085) |
| Norwegian (2001–2016) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 1.017 (1.007, 1.026) | 1.017 (1.007, 1.026) | 1.036 (1.028, 1.045) | 1.032 (1.024, 1.041) |
| Model 2 | 1.014 (1.004, 1.023) | 1.014 (1.004, 1.023) | 1.044 (1.036, 1.053) | 1.040 (1.031, 1.049) |
| Model 3 | 1.039 (1.029, 1.049) | 1.037 (1.027, 1.048) | 1.057 (1.048, 1.066) | 1.053 (1.044, 1.063) |
| Model 4 | 1.034 (1.024, 1.045) | 1.034 (1.024, 1.045) | 1.053 (1.044, 1.063) | 1.051 (1.042, 1.061) |
| Model 2add | 1.024 (1.014, 1.033) | 1.024 (1.014, 1.034) | 1.044 (1.036, 1.053) | 1.041 (1.033, 1.050) |
| Model 3add | 1.035 (1.025, 1.045) | 1.034 (1.023, 1.044) | 1.045 (1.036, 1.054) | 1.043 (1.034, 1.052) |
| Model 4add | 1.029 (1.019, 1.039) | 1.029 (1.018, 1.040) | 1.042 (1.033, 1.051) | 1.040 (1.031, 1.050) |
| Roman (2001–2015) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 1.038 (1.018, 1.059) | 1.043 (1.022, 1.064) | 1.050 (1.033, 1.069) | 1.055 (1.037, 1.073) |
| Model 2 | 1.045 (1.024, 1.065) | 1.050 (1.029, 1.071) | 1.059 (1.041, 1.077) | 1.064 (1.045, 1.082) |
| Model 3 | 1.044 (1.022, 1.066) | 1.049 (1.027, 1.072) | 1.046 (1.027, 1.065) | 1.051 (1.031, 1.071) |
| Model 4 | NA | NA | NA | NA |
| Model 2add | 1.044 (1.023, 1.064) | 1.049 (1.028, 1.070) | 1.055 (1.037, 1.073) | 1.060 (1.041, 1.078) |
| Model 3add | 1.043 (1.021, 1.065) | 1.048 (1.026, 1.071) | 1.044 (1.025, 1.063) | 1.049 (1.030, 1.069) |
| Model 4add | NA | NA | NA | NA |
| Swiss (2001–2014) | ||||
| ; ; ; | ; ; ; | |||
| Model 1 | 0.982 (0.980, 0.983) | 0.956 (0.952, 0.961) | 0.975 (0.972, 0.979) | 0.977 (0.973, 0.980) |
| Model 2 | 0.989 (0.988, 0.991) | 0.968 (0.964, 0.972) | 0.989 (0.985, 0.992) | 0.990 (0.986, 0.994) |
| Model 3 | 0.992 (0.990, 0.993) | 0.975 (0.970, 0.980) | 0.975 (0.971, 0.979) | 0.977 (0.973, 0.981) |
| Model 4 | 1.014 (1.011, 1.016) | 1.008 (1.001, 1.014) | 1.010 (1.005, 1.015) | 1.011 (1.006, 1.017) |
| Model 2add | 0.991 (0.990, 0.993) | 0.970 (0.965, 0.974) | 0.988 (0.984, 0.992) | 0.988 (0.984, 0.993) |
| Model 3add | 0.996 (0.994, 0.998) | 0.981 (0.976, 0.986) | 0.983 (0.978, 0.987) | 0.984 (0.979, 0.988) |
| Model 4add | 1.011 (1.009, 1.013) | 1.005 (0.998, 1.011) | 1.007 (1.001, 1.012) | 1.008 (1.002, 1.013) |
Note: Model 1 adjusted for age at follow-up, sex, and follow-up year; Model 2 further adjusted for individual level SES and ethnicity; Model 3 further adjusted for area-level SES; and Model 4 added large regional indicators. Additional Model 2—Main model 2 further adjusted for individual level educational level, marital status, and employment status; Additional Model 3—Main model 3 further adjusted for individual level educational level, marital status, and employment status; Additional Model 4—Main model 4 further adjusted for individual level educational level, marital status, and employment status. Model specifications in each cohort are as follows. CanCHEC Model 1: cohort (strata), sex (strata), 5-year age groups (strata), follow-up year (time axis); Model 2: Model , immigration status, visible minority, indigenous identity; Model 3: Model composite SES index; Model 4: Model ; Model 2add: Model , marital status, employment status; Model 3add: Model , marital status, employment status; Model 4add: Model , marital status, employment status. Medicare Model 1: sex (strata), 5-year age groups (strata), follow-up year (strata); Model 2: Model (strata), Medicaid eligibility (strata); Model 3: Model , median house value, poverty rate, house-owing rate, high education rate; Model 4: Model . Belgian Model 1: age at follow-up (time axis), sex (strata), follow-up year (strata); Model 2: Model , education; Model 3: Model , unemployment rate, low education rate, non-Western ethnic rate at both neighborhood and regional levels; Model 4: Model ; Model 2add: Model , employment status; Model 3add: Model , employment status; Model 4add: Model , employment status. Danish Model 1: age at follow-up (time axis), sex (strata), follow-up year (strata); Model 2: Model , income; Model 3: Model , unemployment rate, low education rate at both neighborhood and regional levels; Model 4: Model ; Model 2add: Model ; Model 3add: Model ; Model 4add: Model . Dutch Model 1: age at follow-up (time axis), sex (strata), follow-up year (strata); Model 2: Model , income; Model 3: Model , income, unemployment rate, non-Western ethnic rate at both neighborhood and regional levels; Model 4: Model ; Model 2add: Model ; Model 3add: Model ; Model 4add: Model . Norwegian Model 1: age at follow-up (time axis), sex (strata), follow-up year (strata); Model 2: Model ; Model 3: Model , unemployment rate, low education rate at both neighborhood and regional levels; Model 4: Model ; Model 2add: Model , marital status, employment status; Model 3add: Model , marital status, employment status; Model 4add: Model , marital status, employment status. Roman Model 1: age at follow-up (time axis), sex (strata), follow-up year (strata); Model 2: Model ; Model 3: Model , unemployment rate, low education rate, high education rate, composite SES index at neighborhood level; Model 2add: Model , employment status; Model 3add: Model , employment status. Swiss Model 1: age at follow-up (time axis), sex (strata), follow-up year (strata); Model 2: Model , mother tongue, education; Model 3: Model , unemployment rate, low education rate, high education rate at both neighborhood and regional levels; Model 4: Model ; Model 2add: Model , employment status; Model 3add: Model , employment status; Model 4add: Model , employment status. —, no data; CanCHEC, Canadian Census Health and Environment Cohorts; CI, confidence interval; ELAPSE, Effects of Low-Level Air Pollution: A Study in Europe; HR, hazard ratio; NA, not applicable; , particulate matter with aerodynamic diameter ; PY, person-years; SES, socioeconomic status.
Meta-analytical estimate of main Model 4 results from Belgian, Danish, Dutch, Norwegian, and Swiss cohorts and main Model 3 result from the Roman cohort.
Meta-analytical estimate of additional Model 4 results from Belgian, Danish, Dutch, Norwegian, and Swiss cohorts and additional Model 3 result from the Roman cohort.
HRs were higher in the full population than in those years of age in ELAPSE cohorts, whereas similar HRs were observed for the two age groups in MAPLE (Table 2). Differences in HRs for mortality from all causes and natural causes was minor, which is consistent with the observation that the fraction of nonnatural deaths is low ( in MAPLE and ELAPSE in the population years of age).
Comparison with previously published individual reports.
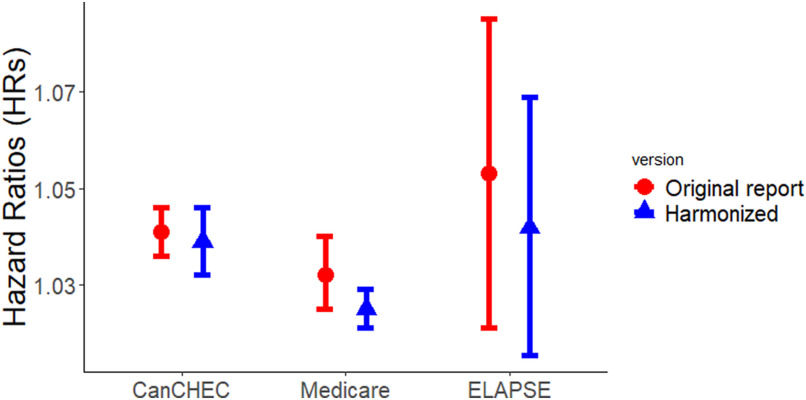
Figure 2 shows the associations between exposure and all-cause mortality derived from the fully adjusted main Model 4 in the year old populations compared to the effect estimates published in previous reports.3–5 We observed statistically significant positive associations in all studies with a similar magnitude and overlapping CIs. Variability in HRs across the three studies was only reduced slightly after performing the harmonized analytical approach, as indicated by the minor decrease in the estimate of tau-squared (Figure S1).
Figure 2.
Associations between exposure (per ) and mortality in the harmonized analyses and the original reports. Numeric effect estimates derived from the current harmonized analyses are shown in Table 2. Hazard ratios and 95% CIs associated with a increase in exposure were 1.041 (1.036, 1.046) for the stacked CanCHEC (1991–2016), 1.032 (1.025, 1.040) for Medicare (2000–2016), and 1.058 (1.022, 1.095) for the six ELAPSE cohorts (Belgian, 2001–2011; Danish, 2000–2015; Dutch, 2008–2012; Norwegian, 2001–2016; Roman, 2001–2015; Swiss, 2001–2014) in the respective original reports.3–5 HRs in the original reports were associated with different ages, exposure models applied, statistical analyses, and mortality definitions. Note: CanCHEC, Canadian Census Health and Environment Cohorts; CI, confidence interval; ELAPSE, Effects of Low-Level Air Pollution: A Study in Europe; HR, hazard ratio; , particulate matter with aerodynamic diameter .
Using the harmonized exposure model (i.e., MAPLE exposure) in the present analyses yielded comparable HRs to those obtained by applying the initial exposure estimates in the original Medicare and ELAPSE studies (Table S3). However, HRs differed substantially in individual ELAPSE cohorts with higher HRs estimated by the harmonized exposure in some cohorts (i.e., Belgian, Danish, Dutch), and lower HRs in others (i.e., Norwegian and Swiss).
Shape of the CRF
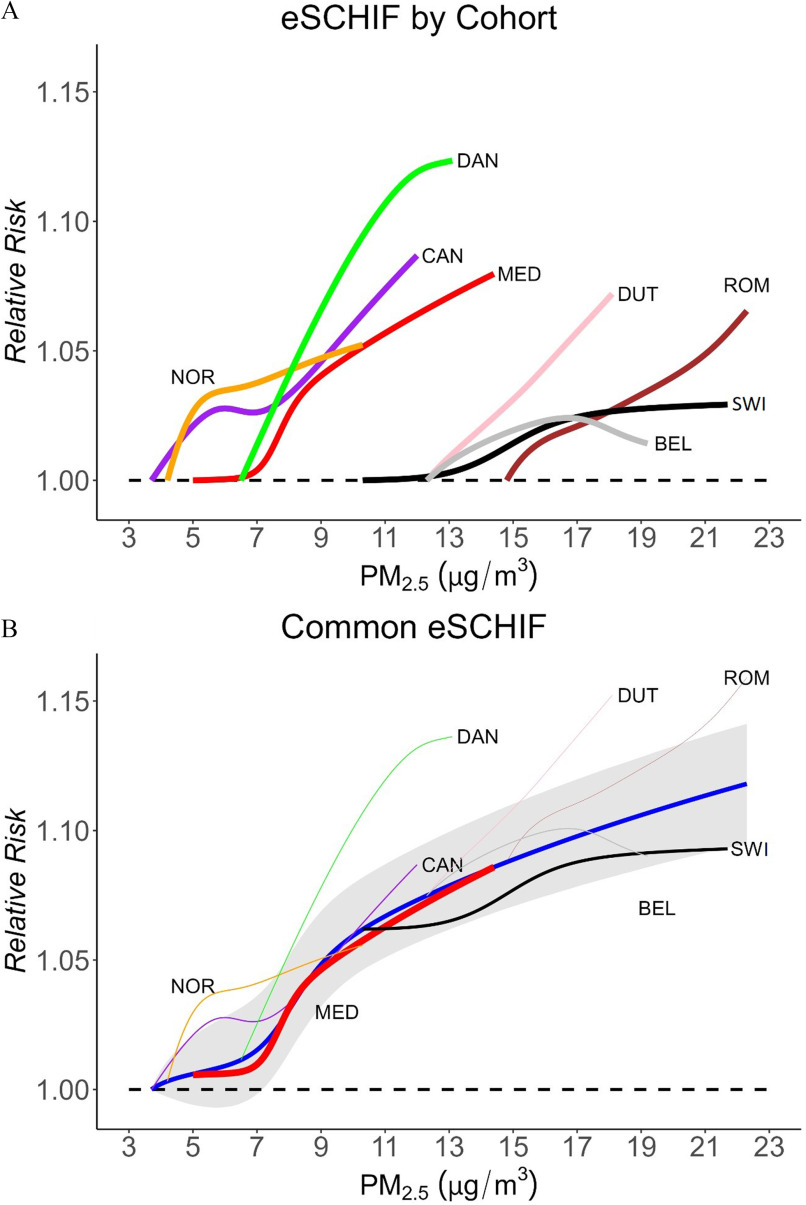
Figure 3A shows the mean eSCHIF fits of associations between exposure and all-cause mortality in populations years of age in eight individual cohorts. The individual curves with their own scaling are shown in Figure S2. The shape of the CRFs differed substantially across cohorts. In the CanCHEC and Roman cohorts, we observed a pattern of increasing spline predictions at lower concentrations followed by a flattening of predictions at median concentrations, with increasing predictions at higher concentrations. In the Dutch cohort, the concentration–mortality pattern was nearly linear. In the Danish and Norwegian cohorts, the curves exhibited a supralinear pattern, with a flattening out of the tail at high concentrations. In the Medicare and Swiss cohorts, the concentration–mortality pattern was similar to that of a sigmodal function with a slow increase at low concentrations followed by a steep increase in predictions over medium concentrations and a flatter increase at high concentrations. In the Belgian cohort, the curve sloped upward toward the mean and then downwards as concentrations increased further. The curves generally showed increased risk with higher concentrations starting with the lowest observed levels within each study population, except for the Medicare cohort that displayed a slight decrease in risk over its lowest concentrations followed by a sharp increase in risk over median concentrations.
Figure 3.
CRF for exposure and all-cause mortality in population years of age. (A) Mean of eSCHIF fits in eight individual cohorts with counterfactual levels equal to the fifth percentile of the cohort-specific exposure distributions (corresponding values specified in Table 1). (B) Common eSCHIF (blue solid line) and confidence interval (shaded area) combined by meta-analysis. In panel B, all curves share the common counterfactual level represented by the lowest fifth percentile of the cohort-specific exposure distributions across all cohorts (i.e., counterfactual level set to ). Each cohort only contributed information over its concentration range. The thickness of the individual lines indicated the weights each cohort contributed to in the meta-analysis: Medicare (0.444), Swiss (0.213), CanCHEC (0.084), Norway (0.080), Belgian (0.067), Danish (0.049), Dutch (0.045), Rome (0.017). Note: BEL, Belgian (2001–2011); CAN, CanCHEC (1991–2016); CanCHEC, Canadian Census Health and Environment Cohorts; CI, confidence interval; CRF, concentration-response function; DAN, Danish (2000–2015); DUT, Dutch (2008–2012); ELAPSE, Effects of Low-Level Air Pollution: A Study in Europe; eSCHIF, extended shape-constrained health impact function; HR, hazard ratio; MED, Medicare (2000–2016); NOR, Norwegian (2001–2016); , particulate matter with aerodynamic diameter ; ROM, Roman (2001–2015); SWI, Swiss (2001–2014).
Figure 3B shows a common eSCHIF created by a meta-analysis. The weight of each contributing cohort is shown as the thickness of the individual curves: Medicare (0.444), Swiss (0.213), CanCHEC (0.084), Norway (0.080), Belgian (0.067), Danish (0.049), Dutch (0.045), and Rome (0.017). The common eSCHIF curve increased monotonically from the lowest observed level (), with a steep slope observed from 7 to . From , the slope of curve started to flatten out. From , the curve was almost linear. The common curve showed an increased risk with wide uncertainty at lower concentrations () due to the variation in the sublinear Medicare cohort shape and the supralinear CanCHEC and Norwegian cohort shapes. The common eSCHIF was highly influenced by the Medicare cohort over the lower concentration ranges, whereas it was dominated by the Swiss cohort over the higher concentrations. We observed an increased mortality risk associated with exposure down to the lowest observed level in the common eSCHIF.
Discussion
Our findings indicate that employing a harmonized analytical approach led to a minor reduction in the variation among the observed associations among three large cohort studies conducted in Canada (MAPLE), the United States (Medicare), and Europe (ELAPSE), when contrasted with the findings reported in prior individual study publications. The magnitude of the observed association was influenced by the adjusted confounders, the age of the population, the applied exposure model, and to a slight extent, the definition of the outcome. The shape of the association differed across cohorts but generally showed associations down to the lowest observed levels. A common CRF indicated a monotonically increasing risk pattern, starting from the lowest exposure level.
Confounder Adjustment
We found that adjustment for a more complete list of potential confounders reduced the differences in the observed associations, suggested by the larger variability across studies in the minimally adjusted Model 1 HRs compared to the fully adjusted Model 4 HRs. Adjustment for SES influenced HRs differently across cohorts. This probably reflects different underlying associations between exposure and socioeconomic factors in different populations. For example, in Rome, the (more polluted) historic city center is predominantly inhabited by wealthier people because of the high housing costs,21 whereas in the United States, areas with low-income populations have been consistently exposed to higher air pollution levels than areas with high-income groups.30
Adjustment for regional indicators in addition to SES increased HRs moderately in the ELAPSE Belgian and Swiss cohorts. The Belgian cohort findings are consistent with a previous analysis conducted within the same cohort using both ELAPSE and alternative exposure models.17 The change in HRs with additional adjustment for large area indicators likely reflects broad-scale spatial variation in mortality risks due to factors other than exposure and adjusted (socioeconomic) covariates, such as geographic variations in diagnostic and therapeutic practices or cultural and health-related behaviors.31 In the North American settings, the spatial variations in health may also be related to climate differences across large geographical area. Previous analyses also adjusted for meteorological variables to account for large scale variations in health instead of/in addition to regional indicators.32,33 Climate difference is a less likely explanation for the large-scale variation in mortality patterns within the ELAPSE cohorts, as the climate is usually relatively homogeneous within moderately sized countries.
Exposure Assessment
In ELAPSE, applying the harmonized exposure model (i.e., MAPLE exposure) resulted in similar overall HR when compared to the original exposure estimates used. However, varying HRs were observed among individual ELAPSE cohorts. This finding is consistent with the previously reported time-independent analysis applying the harmonized and ELAPSE exposures.5 Since the harmonized and ELAPSE exposures were modeled over a large area in Europe, the models may tend to average out within-country variations in the relationship between air pollution and predictors across Europe, which could result in less accurate predictions at the national levels. As noted by the Atmospheric Composition Analysis Group that created the harmonized exposure surface for Europe (V4.EU.02),10,34 these exposure estimates are primarily intended to aid in large-scale studies. This might explain the overall similar but individually varying HRs for the six European cohorts. Jerrett et al. documented lower HRs for mortality in the American Cancer Society study when only remote sensing data were used to assess exposure.35 In our study, we found lower HRs in the Medicare and higher HRs in ELAPSE when we used harmonized exposure estimates. All exposure estimates in our studies used a combination of remote sensing and ground-level monitoring data, however.
The harmonized exposure was estimated at a spatial resolution, which is usually sufficient for because of the largely regional spatial distribution of with limited local variability, though the composition of mass may differ.36 For example, exposure estimates at a spatial resolution of may not adequately capture the spatial variation of traffic-related air pollution, which could introduce bias in the health effect estimates. As the composition of mass varies across study areas, this may contribute to the different observed effect estimates for .
The geocoding and exposure period assignments varied largely across studies, which may contribute to the remaining heterogeneity in the observed effect estimates. The assignment of exposure to the relatively coarse ZIP code level in Medicare may result in more measurement errors compared to the assignment to postal code level in MAPLE and residential address level in ELAPSE. However, the small fine-scale spatial variation of concentrations suggests that these measurement errors are likely small. For pollutants with strong local sources such as ultrafine particles, this would have been an important concern. Consistently, in the MAPLE study, HRs were not sensitive to exposure assignment at different spatial scales (1, 5, and ).13 In MAPLE, a 10-year moving average of exposure was adopted because it was previously documented that the longer moving average exposure window provided stronger associations with mortality.13,14 For example, HRs of nonaccidental mortality for a increase in were 1.04 (95% CI: 1.01, 1.06) and 1.11 (95% CI: 1.09, 1.13) for 3-year and 10-year exposure periods in 2001 CanCHEC.3 We were not able to renew exposure assignment for ELAPSE and Medicare to obtain similar 10-year moving averages, but this implies that weaker associations may be observed if a 1-year exposure window had been applied in MAPLE. The Dutch and Danish cohorts did not incorporate residential mobility in the exposure assessment. This approach is consistent with previous analyses within ELAPSE, which facilitated within-study comparison,5 though this may introduce measurement errors. The Dutch cohort had 5 years of follow-up only, suggesting that residential mobility was likely not a critical concern. The exposure estimates used in MAPLE before 1989 were based on backcasting, which may introduce measurement errors. However, this method involved creating prediction models using data from various sources and had been validated to accurately predict a large portion of measured .8
Age of Study Population
In ELAPSE, HRs were higher for the full population than for those years of age, while MAPLE showed similar HRs across age groups. We have no clear explanation of the different findings in ELAPSE and MAPLE. There is evidence that HRs in the elderly (e.g., years of age) tend to be lower than in a younger population because of competing risks for the elderly, a finding corroborated by the original ELAPSE report and several previous studies,5,21,37,38 notably with overlapping CIs. As the elderly had much higher mortality baseline risks than the younger populations, the absolute number of deaths attributable to may be higher in the elderly.
Definition of Mortality Endpoint
We observed minor differences in HRs for mortality from all causes and natural causes, suggesting that the definition of overall mortality is not a crucial factor for difference in the observed associations across cohorts. This finding supports the interchangeable usage of both mortality end points in previous systematic reviews and comparison between studies.2,39 Analyzing natural-cause mortality may prevent noise introduced in the analysis, as most nonnatural mortality is not associated with air pollution exposure. Recently, some studies have suggested a possible etiological link between long-term air pollution exposure and suicides, as well as psychiatric disorder mortality,40 some of which may be coded as nonnatural mortality (accidents related to substance abuse, etc.).
Shape of the CRF
Shape of the CRF differed largely across cohorts, which likely reflects that different settings have different populations and mixtures at the same mass level. For example, reflects urban populations in Canada but may be indicative of suburban or even rural populations in the Netherlands. Sources and composition of mass also vary across regions,10,41 which in turn results in different associations with mortality.42,43
In the common eSCHIF, we observed an increased mortality risk associated with exposure down to the lowest observed level. This is consistent with findings from an ensemble CRF between exposure and natural-cause mortality estimated from 41 cohorts in 16 countries using an earlier version of the SCHIF methodology.28 Some of the current cohorts were included in the previous analysis as well, including the ELAPSE Dutch cohort with a different follow-up period.
Methodology applied for producing the CRF may affect the shape of the curve. In the ELAPSE report,5 CRF curves produced by the spline functions tended to be less smooth than the more parametric SCHIF function (an earlier version of the currently applied eSCHIF function). The shape of the curve reported for the Medicare cohort in the original paper/report deviated from the current analyses.4,32 In the previous report, the HR increased monotonically from the lowest exposure, though with a higher slope from about . Here, the spline predictions indicated a slightly decreased association over the lower exposure levels; however, the exposure models were different between these methods. The Belgian and Roman curves also deviated from the previous report,5,20 but the different populations included in the analyses make it difficult to compare. In CanCHEC, a similar flattening of the CRF was observed in the middle concentration range, which could possibly be explained by the regional difference in the air pollution mixture.3 For assessment of the shape of the CRF, the nonparametric nature of the splines may be an advantage compared to approaches borrowing information from assumptions about the shape of the function. For health impact assessment, the biologically more plausible SCHIF functions are more attractive than the spline functions.28,44 However, the methods generally resulted in the same conclusion about the shape of the CRF, that is the absence of evidence for a level below which no associations were found.
Strengths, Limitations, and Implications
In this study, we were able to partially harmonize analyses across three very large studies in the United States, Canada, and Europe by using the same exposure model and, to the extent possible, the same covariates and statistical model. We were able to evaluate the strength and direction of key influential study design factors on low-level effect estimates by the contrasts between and within studies. These findings would assist in evaluating the risks attributable to exposure in evidence synthesis and likely be important for supporting public health policy and burden of illness assessments.
We intentionally kept some design decisions previously justified in the individual studies to allow comparisons not only between but also within studies. This resulted in remaining heterogeneity across studies in the exposure period assignment, the definition of covariates adjusted for, and the way that time was represented in the models. We discussed the potential influence in the respective sections above. In addition, we were not able to account for differences in levels and composition of exposure, availability of information on potential confounders, characteristics of study populations, and availability of residential address spatial resolution. Despite the substantial differences that remained across studies, we observed very comparable effect estimates of exposure.
All cohorts included in the present study are administrative cohorts and are therefore most representative of the general population. However, we were not able to adjust for individual lifestyle factors such as smoking because of the lack of information in these cohorts. Using indirect adjustment approaches, we previously documented that HRs were not substantially affected by missing data on individual lifestyle, including smoking.3,5,32 There is an implicit assumption that lack of adjustment for individual level confounders such as smoking would lead to an overestimation of air pollution risks, although this assumption has been previously refuted.45 In order for an individual level variable Z (e.g., smoking) to confound the relationship between X (e.g., air pollution) and Y (e.g., mortality), the variable Z must be a predictor of X conditional on all of the other covariates that are included in the model.46 If there is an association between air pollution and lifestyle (e.g., smoking), it is likely related to socioeconomic factors, so the potential confounding by lifestyle may have been partially accounted for by the adjustment for individual and area-level SES in the model. In Medicare and a Dutch national health survey,32,47 smoking was found to be only weakly correlated with air pollution exposure, conditional on the other covariates included in the model. Even if there is residual confounding by behavioral factors, it can result in bias either toward or away from the null, depending on the direction of associations between air pollution exposure and lifestyle risk factors. In the MAPLE and ELAPSE reports,5,14 we previously documented smaller effect estimates in the administrative cohorts compared to cohorts that had individual lifestyle data available. In a recent systematic review of the association between and mortality, the meta-analytical effect estimate was not affected by excluding administrative cohorts that did not have individual lifestyle data available. HRs associated with a increase in exposure were 1.08 (1.06, 1.09) for all studies and 1.08 (1.05, 1.10) for the studies with individual lifestyle data only.2
Conclusions
The magnitude of the association was affected by the adjusted confounders, exposure model applied, age of the population, and marginally by outcome definition. Applying a harmonized analytical approach marginally reduced the difference in the observed associations across the three studies. A common CRF suggested a monotonically increasing risk starting from the lowest exposure level.
Supplementary Material
Acknowledgments
F. Dominici, M. Brauer, and B. Brunekreef are the principal investigators (PIs) of the Medicare, MAPLE, and ELAPSE projects, respectively. J. Chen, D. Braun, T. Christidis, M. Cork, S. Rodopoulou, E. Samoli, M. Stafoggia, K. Wolf, X. Wu, W. Yuchi, R.T. Burnett, G. Hoek, F. Dominici, M. Brauer, and B. Brunekreef equally contributed to the study concept. J. Chen and R.T. Burnett pooled results from all cohorts and performed the linear and nonlinear meta-analyses. J. Chen wrote the first draft of the manuscript. S. Rodopoulou, E. Samoli, M. Cork, T. Christidis, W. Yuchi, M. Stafoggia, X. Wu, and R.T. Burnett developed the statistical codes and provided key methodological support. D. Braun, T. Christidis, M. Cork, M. Stafoggia, X. Wu, W. Yuchi, M. Bauwelinck, Y.H. Lim, B. Oftedal, M. Strak, D. Vienneau, and J. Zhang analyzed cohort-specific data. The data were accessed and verified by T. Christidis, W. Yuchi, and R.T. Burnett for the stacked CanCHEC cohort; D. Braun, M. Cork, and X. Wu for the Medicare cohort; M. Bauwelinck and M. Stafoggia for the Belgian cohort; J. Zhang, Y.H. Lim, and Z.J. Andersen for the Danish cohort; M. Strak, J.O. Klompmaker, and N.A.H. Janssen for the Dutch cohort; B. Oftedal and D.T. Kristoffersen for the Norwegian cohort; M. Stafoggia and G. Hoek for the Rome cohort; and D. Vienneau and K. de Hoogh for the Swiss cohort. All authors contributed to clinical or epidemiological interpretations of the study findings and critical revision of the article for important intellectual content. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors had final responsibility for the decision to submit for publication.
Research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers.
The exposure data used in this work are publicly available via the Washington University in St. Louis Atmospheric Composition Analysis Group (https://sites.wustl.edu/acag/datasets/surface-pm2-5/#V4.NA.02.MAPLE). The cohort data could not be shared among the named authors, nor can the data be shared externally due to strict national data protection regulations and restrictions in the data-use agreements.
References
- 1.Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. The Lancet 396(10258):1223–1249, 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Hoek G. 2020. Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int 143:105974, PMID: , 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- 3.Brauer M, Brook JR, Christidis T, Chu Y, Crouse DL, Erickson A, et al. 2022. Mortality-Air Pollution Associations in Low Exposure Environments (MAPLE): Phase 2. Research Report 212. Boston, MA: Health Effects Institute. [PMC free article] [PubMed] [Google Scholar]
- 4.Dominici F, Zanobetti A, Schwartz J, Braun D, Sabath B, Wu X. 2022. Assessing adverse health effects of long-term exposure to low levels of ambient air pollution: implementation of causal inference methods. Res Rep Health Eff Inst 2022(211):1–56, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 5.Brunekreef B, Strak M, Chen J, Andersen ZJ, Atkinson R, Bauwelinck M, et al. 2021. Mortality and Morbidity Effects of Long-Term Exposure to Low-Level PM2.5, Black Carbon, NO2 and O3: An Analysis of European Cohorts. Research Report. Boston, MA: Health Effects Institute. [PMC free article] [PubMed] [Google Scholar]
- 6.de Hoogh K, Chen J, Gulliver J, Hoffmann B, Hertel O, Ketzel M, et al. 2018. Spatial PM2.5, NO2, O3 and BC models for Western Europe – evaluation of spatiotemporal stability. Environ Int 120:81–92, PMID: , 10.1016/j.envint.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 7.Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. 2019. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int 130:104909, PMID: , 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng J, Li C, Martin RV, van Donkelaar A, Hystad P, Brauer M. 2019. Estimated long-term (1981–2016) concentrations of ambient fine particulate matter across North America from chemical transport modeling, satellite remote sensing, and ground-based measurements. Environ Sci Technol 53(9):5071–5079, PMID: , 10.1021/acs.est.8b06875. [DOI] [PubMed] [Google Scholar]
- 9.Hammer MS, van Donkelaar A, Li C, Lyapustin A, Sayer AM, Hsu NC, et al. 2020. Global estimates and long-term trends of fine particulate matter concentrations (1998–2018). Environ Sci Technol 54(13):7879–7890, PMID: , 10.1021/acs.est.0c01764. [DOI] [PubMed] [Google Scholar]
- 10.van Donkelaar A, Martin RV, Li C, Burnett RT. 2019. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol 53(5):2595–2611, PMID: , 10.1021/acs.est.8b06392. [DOI] [PubMed] [Google Scholar]
- 11.Khan S, Pinault L, Tjepkema M, Wilkins R. 2018. Positional accuracy of geocoding from residential postal codes versus full street addresses. Health Rep 29(2):3–9, PMID: . [PubMed] [Google Scholar]
- 12.Krieger N, Waterman P, Chen JT, Soobader M-J, Subramanian SV, Carson R. 2002. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and us census–defined geographic areas—the public health disparities geocoding project. Am J Public Health 92(7):1100–1102, PMID: , 10.2105/ajph.92.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crouse DL, Erickson AC, Christidis T, Pinault L, van Donkelaar A, Li C, et al. 2020. Evaluating the sensitivity of PM2.5-mortality associations to the spatial and temporal scale of exposure assessment. Epidemiology 31(2):168–176, PMID: , 10.1097/EDE.0000000000001136. [DOI] [PubMed] [Google Scholar]
- 14.Brauer M, Brook JR, Christidis T, Chu Y, Crouse DL, Erickson A, et al. 2019. Mortality–Air Pollution Associations in Low-Exposure Environments (MAPLE): Phase 1. Research Report. Boston, MA: Health Effects Institute. [PMC free article] [PubMed] [Google Scholar]
- 15.Crouse DL, Philip S, van Donkelaar A, Martin RV, Jessiman B, Peters PA, et al. 2016. A new method to jointly estimate the mortality risk of long-term exposure to fine particulate matter and its components. Sci Rep 6:18916, PMID: , 10.1038/srep18916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filice CE, Joynt KE. 2017. Examining race and ethnicity information in Medicare administrative data. Med Care 55(12):e170–e176, PMID: , 10.1097/MLR.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 17.Bauwelinck M, Chen J, de Hoogh K, Katsouyanni K, Rodopoulou S, Samoli E, et al. 2022. Variability in the association between long-term exposure to ambient air pollution and mortality by exposure assessment method and covariate adjustment: a census-based country-wide cohort study. Sci Total Environ 804:150091, PMID: , 10.1016/j.scitotenv.2021.150091. [DOI] [PubMed] [Google Scholar]
- 18.So R, Andersen ZJ, Chen J, Stafoggia M, de Hoogh K, Katsouyanni K, et al. 2022. Long-term exposure to air pollution and mortality in a Danish nationwide administrative cohort study: beyond mortality from cardiopulmonary disease and lung cancer. Environ Int 164:107241, PMID: , 10.1016/j.envint.2022.107241. [DOI] [PubMed] [Google Scholar]
- 19.Klompmaker JO, Janssen N, Andersen ZJ, Atkinson R, Bauwelinck M, Chen J, et al. 2021. Comparison of associations between mortality and air pollution exposure estimated with a hybrid, a land-use regression and a dispersion model. Environ Int 146:106306, PMID: , 10.1016/j.envint.2020.106306. [DOI] [PubMed] [Google Scholar]
- 20.Stafoggia M, Oftedal B, Chen J, Rodopoulou S, Renzi M, Atkinson RW, et al. 2022. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet Health 6(1):e9–e18, PMID: , 10.1016/S2542-5196(21)00277-1. [DOI] [PubMed] [Google Scholar]
- 21.Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, et al. 2013. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect 121(3):324–331, PMID: , 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vienneau D, Stafoggia M, Rodopoulou S, Chen J, Atkinson RW, Bauwelinck M, et al. 2023. Association between exposure to multiple air pollutants, transportation noise and cause-specific mortality in adults in Switzerland. Environ Health 22(1):29, PMID: , 10.1186/s12940-023-00983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panczak R, Galobardes B, Voorpostel M, Spoerri A, Zwahlen M, Egger M, Swiss National Cohort and Swiss Household Panel. 2012. A Swiss neighbourhood index of socioeconomic position: development and association with mortality. J Epidemiol Community Health 66(12):1129–1136, PMID: , 10.1136/jech-2011-200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EUROSTAT. 2023. Glossary: Nomenclature of Territorial Units for Statistics (NUTS). https://ec.europa.eu/eurostat/web/nuts/nuts-maps [accessed 28 November 2023].
- 25.Samoli E, Rodopoulou S, Hvidtfeldt UA, Wolf K, Stafoggia M, Brunekreef B, et al. 2021. Modeling multi-level survival data in multi-center epidemiological cohort studies: applications from the ELAPSE project. Environ Int 147:106371, PMID: , 10.1016/j.envint.2020.106371. [DOI] [PubMed] [Google Scholar]
- 26.Weichenthal S, Pinault L, Christidis T, Burnett RT, Brook JR, Chu Y, et al. 2022. How low can you go? Air pollution affects mortality at very low levels. Sci Adv 8(39):eabo3381, PMID: , 10.1126/sciadv.abo3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell J, Frank E, Harrell FE. 2015. Multivariable modeling strategies. In: Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer International Publishing, 63–102. [Google Scholar]
- 28.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, et al. 2018. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA 115(38):9592–9597, PMID: , 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckland ST, Burnham KP, Augustin NH. 1997. Model selection: an integral part of inference. Biometrics 53(2):603–618, 10.2307/2533961. [DOI] [Google Scholar]
- 30.Jbaily A, Zhou X, Liu J, Lee T-H, Kamareddine L, Verguet S, et al. 2022. Air pollution exposure disparities across US population and income groups. Nature 601(7892):228–233, PMID: , 10.1038/s41586-021-04190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Hemelrijck WMJ, Willaert D, Gadeyne S. 2016. The geographic pattern of Belgian mortality: can socio-economic characteristics explain area differences? Arch Public Health 74(1):22, 10.1186/s13690-016-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. 2017. Air pollution and mortality in the Medicare population. N Engl J Med 376(26):2513–2522, PMID: , 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cakmak S, Hebbern C, Vanos J, Crouse DL, Burnett R. 2016. Ozone exposure and cardiovascular-related mortality in the Canadian census health and environment cohort (CANCHEC) by spatial synoptic classification zone. Environ Pollut 214:589–599, PMID: , 10.1016/j.envpol.2016.04.067. [DOI] [PubMed] [Google Scholar]
- 34.Atmospheric Composition Analysis Group. 2023. Surface PM2.5. St. Louis, MO: Washington University. https://sites.wustl.edu/acag/datasets/surface-pm2-5/ [accessed 28 November 2023]. [Google Scholar]
- 35.Jerrett M, Turner MC, Beckerman BS, Pope CA, van Donkelaar A, Martin RV, et al. 2017. Comparing the health effects of ambient particulate matter estimated using ground-based versus remote sensing exposure estimates. Environ Health Perspect 125(4):552–559, PMID: , 10.1289/EHP575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoek G, Meliefste K, Cyrys J, Lewné M, Bellander T, Brauer M, et al. 2002. Spatial variability of fine particle concentrations in three European areas. Atmospheric Environ 36(25):4077–4088, 10.1016/S1352-2310(02)00297-2. [DOI] [Google Scholar]
- 37.Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. 2014. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 383(9919):785–795, PMID: , 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 38.Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, et al. 2015. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian census health and environment cohort (CanCHEC). Environ Health Perspect 123(11):1180–1186, PMID: , 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huangfu P, Atkinson R. 2020. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: a systematic review and meta-analysis. Environ Int 144:105998, PMID: , 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braithwaite I, Zhang S, Kirkbride JB, Osborn DP, Hayes JF. 2019. Air pollution (particulate matter) exposure and associations with depression, anxiety, bipolar, psychosis and suicide risk: a systematic review and meta-analysis. Environ Health Perspect 127(12):126002, PMID: , 10.1289/EHP4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belis C, Karagulian F, Larsen BR, Hopke P. 2013. Critical review and meta-analysis of ambient particulate matter source apportionment using receptor models in Europe. Atmospheric Environ 69:94–108, 10.1016/j.atmosenv.2012.11.009. [DOI] [Google Scholar]
- 42.Chen J, Rodopoulou S, de Hoogh K, Strak M, Andersen Zorana J, Atkinson R, et al. 2021. Long-Term exposure to fine particle elemental components and natural and cause-specific mortality—a pooled analysis of eight European cohorts within the ELAPSE project. Environ Health Perspect 129(4):47009, PMID: , 10.1289/EHP8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Zhang Z, van Donkelaar A, Bai L, Martin RV, Lavigne E, et al. 2020. Understanding the joint impacts of fine particulate matter concentration and composition on the incidence and mortality of cardiovascular disease: a component-adjusted approach. Environ Sci Technol 54(7):4388–4399, PMID: , 10.1021/acs.est.9b06861. [DOI] [PubMed] [Google Scholar]
- 44.Nasari MM, Szyszkowicz M, Chen H, Crouse D, Turner MC, Jerrett M, et al. 2016. A class of non-linear exposure-response models suitable for health impact assessment applicable to large cohort studies of ambient air pollution. Air Qual Atmos Health 9(8):961–972, PMID: , 10.1007/s11869-016-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vodonos A, Awad YA, Schwartz J. 2018. The concentration-response between long-term PM(2.5) exposure and mortality; a meta-regression approach. Environ Res 166:677–689, PMID: , 10.1016/j.envres.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 46.Dominici F, Wang C, Crainiceanu C, Parmigiani G. 2008. Model selection and health effect estimation in environmental epidemiology. Epidemiology 19(4):558–560, PMID: , 10.1097/EDE.0b013e31817307dc. [DOI] [PubMed] [Google Scholar]
- 47.Strak M, Janssen N, Beelen R, Schmitz O, Karssenberg D, Houthuijs D, et al. 2017. Associations between lifestyle and air pollution exposure: potential for confounding in large administrative data cohorts. Environ Res 156:364–373, PMID: , 10.1016/j.envres.2017.03.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.