Abstract
Enteric and parasitic infections such as soil-transmitted helminths cause considerable mortality and morbidity in low- and middle-income settings. Earthen household floors are common in many of these settings and could serve as a reservoir for enteric and parasitic pathogens, which can easily be transmitted to new hosts through direct or indirect contact. We conducted a systematic review and meta-analysis to establish whether and to what extent improved household floors decrease the odds of enteric and parasitic infections among occupants compared with occupants living in households with unimproved floors. Following the PRISMA guidelines, we comprehensively searched four electronic databases for studies in low- and middle-income settings measuring household flooring as an exposure and self-reported diarrhoea or any type of enteric or intestinal-parasitic infection as an outcome. Metadata from eligible studies were extracted and transposed on to a study database before being imported into the R software platform for analysis. Study quality was assessed using an adapted version of the Newcastle-Ottawa Quality Assessment Scale. In total 110 studies were eligible for inclusion in the systematic review, of which 65 were eligible for inclusion in the meta-analysis after applying study quality cut-offs. Random-effects meta-analysis suggested that households with improved floors had 0.75 times (95CI: 0.67–0.83) the odds of infection with any type of enteric or parasitic infection compared with household with unimproved floors. Improved floors gave a pooled protective OR of 0.68 (95CI: 0.58–0.8) for helminthic infections and 0.82 OR (95CI: 0.75–0.9) for bacterial or protozoan infections. Overall study quality was poor and there is an urgent need for high-quality experimental studies investigating this relationship. Nevertheless, this study indicates that household flooring may meaningfully contribute towards a substantial portion of the burden of disease for enteric and parasitic infections in low- and middle-income settings.
Background
Enteric and parasitic infections remain amongst the most common diseases in low- and middle-income settings [1, 2]. Sub-Saharan Africa alone experienced an estimated 330,000 child deaths in 2015 due to diarrhoea-related illness [3]. Known risk factors for enteric infections and parasitic infections such as soil-transmitted helminths include inadequate access to basic levels of water, sanitation and hygiene (WASH), the absence or low coverage of vaccination or preventative chemotherapy programmes, and lower socio-economic status [4].
Ensuring community-wide access to at least basic levels of WASH is a core component of disease control programmes targeting soil-transmitted helminths and diarrhoeal diseases [5]. These interventions aim to control infection risk through reducing exposure to pathogens in the domestic environment. Observational studies typically indicate a relationship between household access to basic WASH services and reduced rates of enteric and parasitic infections and diarrhoea. However, recent large-scale, high-fidelity randomised controlled trials in Bangladesh, Zimbabwe, and Kenya evaluating the impact of different combinations of WASH interventions on childhood diarrhoea and stunting demonstrated either a modest effect or no effect at all on the incidence of diarrhoea [6–8]. These findings suggest that residual pathways for transmission of enteric infections can persist even when access to basic WASH services is ensured.
Hypothosised alternative pathways for infection include contaminated food [9–11], fomites [12], and the presence of animal faeces within the domestic environment [13]. The role of domestic floors, on which pre-school aged children spend a large proportion of their time, is increasingly being investigated as a potentially critical domain on which enteric pathogens are surviving and being transmitted to new hosts [14]. Household floors made from rudimentary or natural materials including sand, soil, clay, and animal dung are of particular interest as they can be difficult to clean and may remain damp, presenting a convenient surface on which pathogens can survive and proliferate. Studies that have conducted environmental sampling from within homes with these floor types have found reservoirs of bacterial, protozoan and parasitic pathogens [15–17].
Despite rapid improvements in the number of houses being built with improved materials across the globe, 12% of households in Latin America and the Caribbean and over half of all houses in Sub-Saharan Africa are made from unimproved materials [18, 19]. Given these vast numbers there is an urgent need to review the available evidence on the relationship between household flooring and enteric and parasitic infections. Despite an absence of experimental studies there is a significant body of observational literature examining this link. This systematic review and meta-analysis aims to establish whether, and to quantify to what extent, domestic flooring acts as an independent risk factor for enteric and parasitic infections in low- and middle-income countries.
Methods
Search strategy and screening
The study is in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines. The review protocol has been published [20] and is registered in PROSPERO (CRD42019156437). The PRISMA checklist is available in supplementary materials (S1 Checklist).
We searched EMBASE, MEDLINE, Web of Science, and Google Scholar with no language restrictions for articles published from 1980 onwards. Medical Subject Headings (MESH) search terms were used when searching PubMed/Medline. Search terms used included keywords referring to ‘household flooring’, ‘floor’, ‘dirt’, ‘earthen’, ‘cement’, ‘wood’, ‘tile’, ‘concrete’, ‘hard’, ‘solid’, ‘enteric’, ‘diarrhea’, ‘diarrhoea’, ‘soil-transmitted’, ‘helminth’, ‘worm’, ‘intestinal parasite’, ‘giardia’, ‘entamoeba’. Only studies taking place in low- or middle-income countries (LMIC) were eligible for full screening. Studies including child, adult and all-age populations were eligible for inclusion.
The primary outcome was laboratory-confirmed presence of any enteric or parasitic pathogen using microscopy, serology or molecular methods. The secondary outcomes were (1) presence of any type of helminth infection; (2) presence of a hookworm infection; (3) presence of any type of enteric bacterial or protozoan infection; (4) self-reported, caregiver-reported, or clinical record of diarrhoea taking place within the previous two weeks (S1 Table).
The exposure was type of household floor, which we dichotomized as earthen, mud, sand, dirt, clay, soil, sticks, bamboo floors (referred to in this study as “unimproved floors”) versus cement, concrete, tile, ceramic, parquet, vinyl, carpet, wooden floors (referred to in this study as “improved” floors). Studies that compared floor types in groupings incompatible with the above dichotomization were excluded from the meta-analysis component of this study to ensure comparability of exposure measurements between included studies. Wooden floors were included in the “improved” floor category, as most studies that referenced wood grouped it in this way.
The initial database search and article deduplication was carried out by BS in November 2019. follow-up searches and deduplications were conducted in September 2022 and August 2023 by HL to bring results up-to-date. Title and abstract screening were conducted in duplicate by HL and BS with discordant decisions resolved through mutual agreement by reviewers. Full article screening was conducted by HL.
Data extraction and quality assessment
Data extraction was conducted by HL using a data capture sheet with pre-specified criteria [20]. Where possible, adjusted measures of effect were extracted. If adjusted analysis was not completed, unadjusted measures of effect were taken instead. If no measure of effect was provided, raw numbers were extracted and used to calculate crude odds ratios and 95% confidence intervals. Where additional meta-data were required, authors were contacted directly.
Study quality was assessed by HL using an adapted version of the Newcastle-Ottawa Quality Assessment Scale for observational studies (S1 File). Studies scoring 50% or less of available points were deemed to have a high risk of bias (cross sectional studies: <6 points; cohort and case control studies <8 points) and those scoring above 50% were considered to have a low risk of bias. A quality control check on bias scoring was undertaken by BS on 10% of included studies with concordance of scoring assessed using Cohen’s Kappa coefficient. Discordant scores were resolved by mutual agreement. Only studies with low-risk of bias were retained for the meta-analysis.
Data analysis and synthesis
Extracted data was pooled using the tidyverse package on the R software platform (version 4.1.3) [21, 22]. Meta regression, forest plots, and funnel plots were performed using the meta package on the R platform [23]. Pooled results were reported using odds ratios and 95% confidence intervals. Studies reporting prevalence ratios were transformed to odds ratios. Longitudinal studies reporting incidence rate ratios were pooled separately with the intention of carrying out a distinct analysis; however, there were insufficient to carry out this separate analysis so these were excluded from the meta-analysis component of the study. Meta regression was undertaken for each outcome using random-effects models to account for heterogeneity between studies.
Heterogeneity was assessed using the I2 statistic with the following classifications applied: 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 75%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity [24]. Publication bias was assessed by examining funnel plots and more formally using Egger’s test statistic.
Results
Description of studies
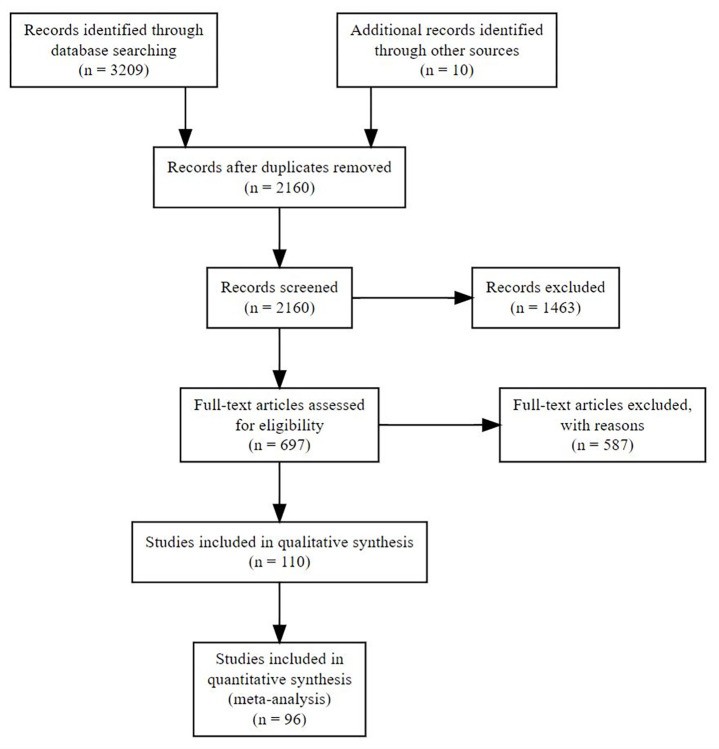
In total, searches returned 3209 articles, of which 1049 were identified as being duplicates and removed. The titles and abstracts of the remaining 2160 articles were screened, of which 697 were identified as being suitable for full article screening. Following article screening 110 [25–133] studies were marked as being eligible for inclusion in the qualitative synthesis (extracted study data is available in supplementary materials; S1 Data). Due to the insufficient number of studies reporting incidence risk ratios or hazard ratios (n = 5) [127–131] these studies were excluded from the meta-analysis component of this study. A further 10 studies included in the qualitative synthesis were excluded from the meta-analysis due to incompatible exposure groupings (grouping wooden floors with earthen and other unimproved floor types) [28, 42, 45, 53, 71, 72, 98, 103, 117, 125]. We contacted 45 authors requesting additional information, of which 11 responded. Based on the information provided nine studies became eligible for inclusion. The final number of studies eligible for inclusion in the meta-analysis was 96, containing a total of 144 separate analyses (Fig 1).
Fig 1. PRISMA flow diagram.
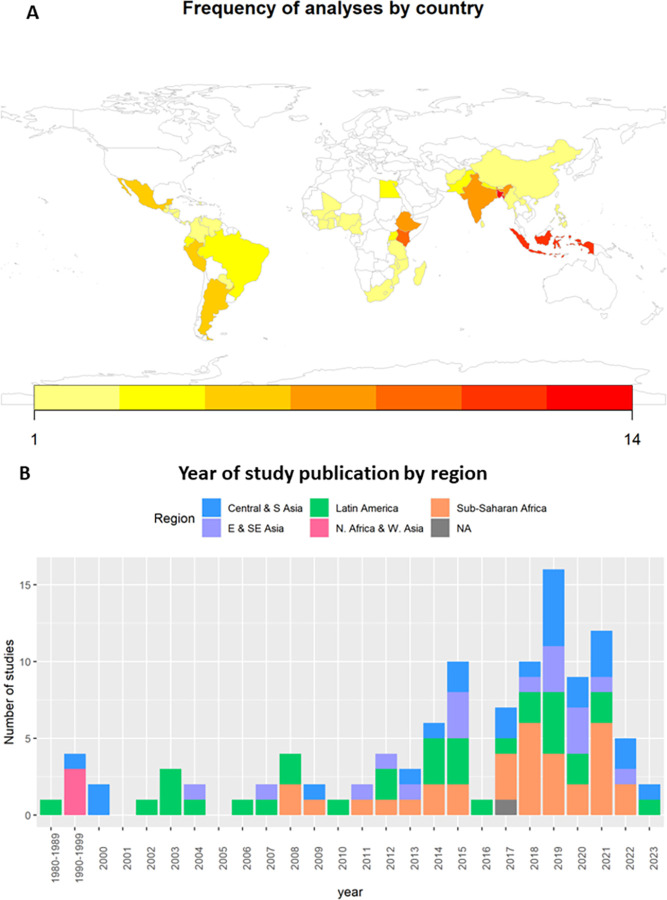
All reviewed studies were observational; of which the majority were cross-sectional studies (94/110), followed by cohort studies (10/110) and case-control studies (6/110). Most studies were conducted in either Sub-Saharan Africa (33/110) or Latin America (32/110). Central and South Asia had 24 studies, East and Southeast Asia had 17 studies, and North Africa and West Asia had 3 studies (Fig 2).
Fig 2. Study metadata.
A) frequency of analyses by country, and B) year of study publication by SDG region. Map was created using R Project for Statistical Computing v. 3.3.1 (https://www.R-project.org) and packages rworldmap v. 1.3–6 (https://cran.r-project.org/web/packages/rworldmap/index.html).
Qualitative synthesis—Categorisation of household flooring
Studies mostly conceptualised flooring dichotomously (104/110) with households grouped according to what studies considered to be improved versus unimproved types of flooring. The majority of studies (87/110) listed the specific flooring materials included in each group (e.g. cement, wood, earthen). Some studies (23/110) provided labels for their flooring categories (e.g. covered, sealed, man-made, natural). Of these 12/23 defined the material types that fit into their categories and 11/23 provided no information on what materials were included within the categories (S2 Table). While category names varied between studies, the specific flooring materials that were grouped together were mostly uniform. In studies where they were referenced, clay, dirt, dung, sawdust, straw, earth, sand and soil floor types were always included in the same grouping. Likewise, in studies where they were referenced, brick, carpet, cement, ceramic tiles, concrete and vinyl were always included in the same grouping. There was more heterogeneity in how bamboo and other wood-based floor types (planks/sticks/timber/wood) were classified. For wood-based floors 12/21 studies grouped them with cement or tile floors, 4/21 studies grouped them with soil-based floors, and 5/21 assigned them to a third “rudimentary” category that also included bamboo and stone. Out of the seven studies that referenced bamboo as a flooring material, four grouped it with soil-based floors and three assigned it to the “rudimentary” category.
Most studies (102/110) did not provide detail on the area within the home that was evaluated to determine the overall domestic floor type. Of those that provided clarification, two classified a dwelling to have an earthen floor if any part of the dwelling had an earthen floor; one classified dwellings to have an improved floor if at least one part of the dwelling had a floor material that was deemed to be improved; one used the floor type of the bedroom as a proxy-indicator for the whole dwelling; one specified that whatever was the most common flooring material should be used; and one specified that the categorisation should be dwellings with exclusively improved floors VS exclusively unimproved floors. Studies that used data from Demographic Health surveys (DHS) or Multiple-Indicator Cluster Surveys (MICS) used the most common floor type as a proxy for overall flooring type of the dwelling, but did not necessarily explicitly state this within the manuscript text.
Meta-analysis–Study populations, exposure, and outcomes
The majority of analyses eligible for inclusion in the meta-regression were conducted exclusively with children <18 years of age (100/144). The remaining 44/144 analyses were conducted either exclusively with adults or with populations comprised of both adults and children. Among the 144 analyses eligible for inclusion, 66 were categorized as being at high risk of bias, while 78 were deemed low risk. In total 69/144 analyses were adjusted, while among studies categorized as being at low risk of bias, 63/78 were adjusted. Cement floors were the most common flooring material to be explicitly referenced among improved flooring materials (96/144) followed by wood floors (36/144). Among unimproved floors, earthen (also including dirt, soil, and sand) was referenced in the great majority of analyses (119/144) (S1 Fig).
Pathogen-specific infections were identified as outcomes in 126/144 of the analyses eligible for inclusion in the meta regression (S3 Table). The remaining 18 analyses measured diarrhoea as an outcome. The majority of studies conducted analyses using a singular pathogen infection as an outcome (78/126), while 48/126 measured the presence of multiple pathogens to denote the presence of a generic infection. Overall, helminth species were specified in 95/126 analyses, with 60/126 analyses measuring A. lumbricoides infections and 46/126 measuring hookworms. Protozoan and bacterial infections were recorded as outcomes in 48/126 studies, with giardia infections measured in 26 analyses and cryptosporidium measured in 14. Viral infections were only recorded in two analysis and these were grouped with bacterial, helminthic, and protozoan infections to denote presence of a generic enteric infection.
Meta-analysis–Model results
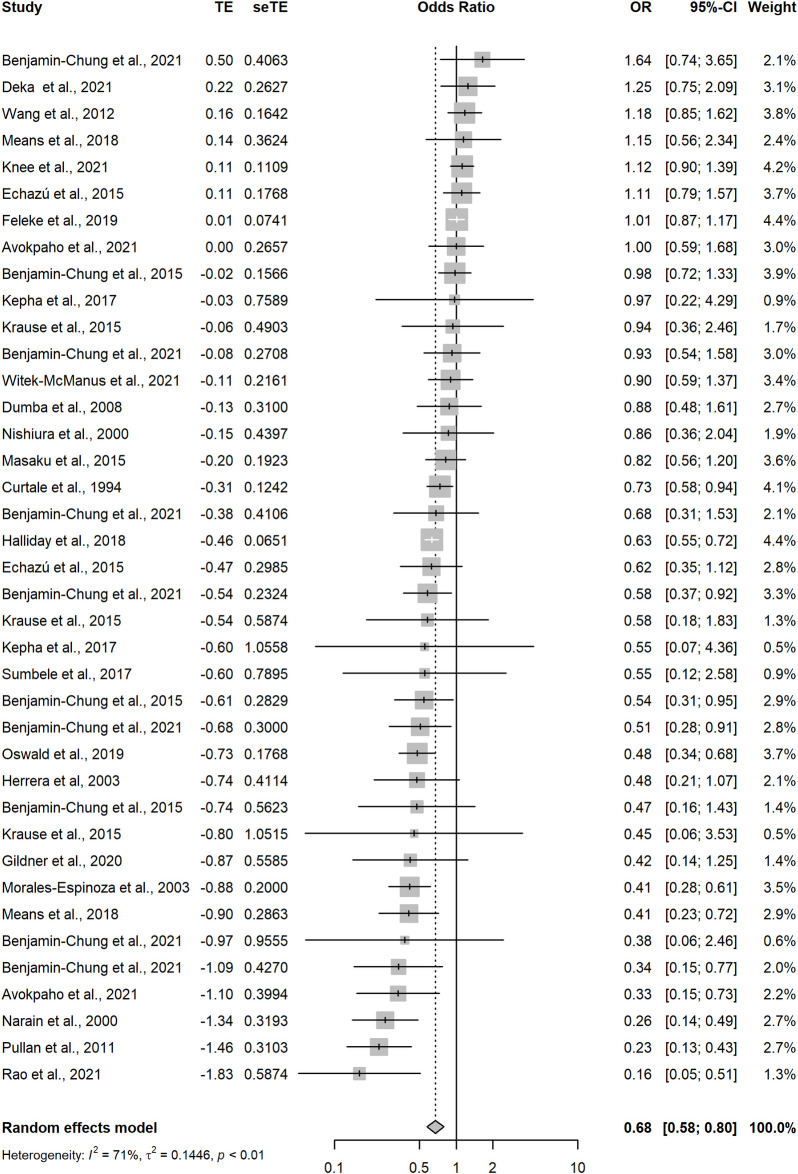
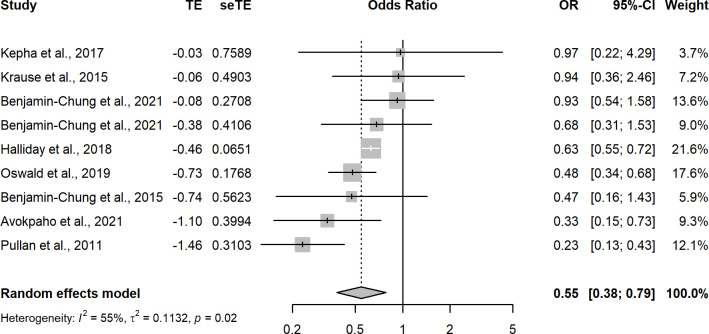
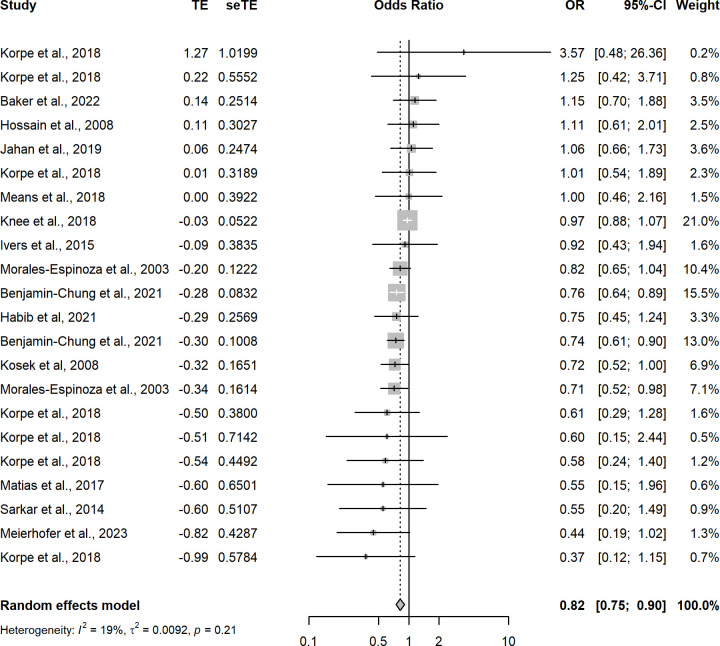
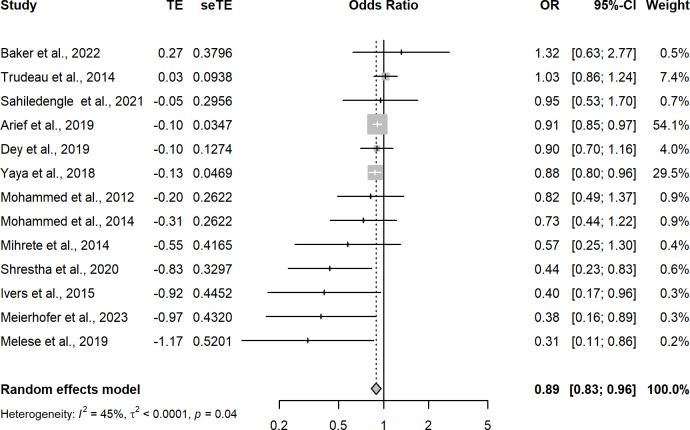
Random-effects meta-regression of the 65 analyses with a low risk of bias that measured the association between improved household flooring and presence of at least one enteric or parasitic infection resulted in a pooled OR of 0.74 (95CI: 0.67–0.83) (Fig 3 and Table 1). This indicates that individuals with an improved household floor had 0.74 the odds of having a parasitic or enteric infection compared with individuals living in a dwelling with an unimproved floor. The I2 was 63%—indicating possible substantial heterogeneity in effect estimates between studies. A model including analyses that measured only helminthic infections (n = 39) produced a pooled OR of 0.68 (95CI: 0.58–0.80) with an I2 of 71%, again indicating possible substantial heterogeneity in effect estimates between studies (Fig 4). Hookworm-only, low-risk of bias analyses (n = 9) produced a pooled OR of 0.53 (95CI: 0.36–0.77) with an I2 55% (Fig 5). Sub-analysis of analyses measuring only bacteria and/or protozoan infections (n = 22) resulted in a pooled OR of 0.82 (95CI: 0.75–0.90) and an I2 of 19% indicating low-levels of heterogeneity between analyses (Fig 6). Analyses that included diarrhoea as an outcome (n = 13) had a pooled OR of 0.89 (95CI:0.83–0.96) and an I2 of 45% (Fig 7).
Fig 3. Pooled effect of an improved household floor (VS unimproved floor) on odds of any type of enteric or parasitic infection in any age group (low risk of bias studies only).
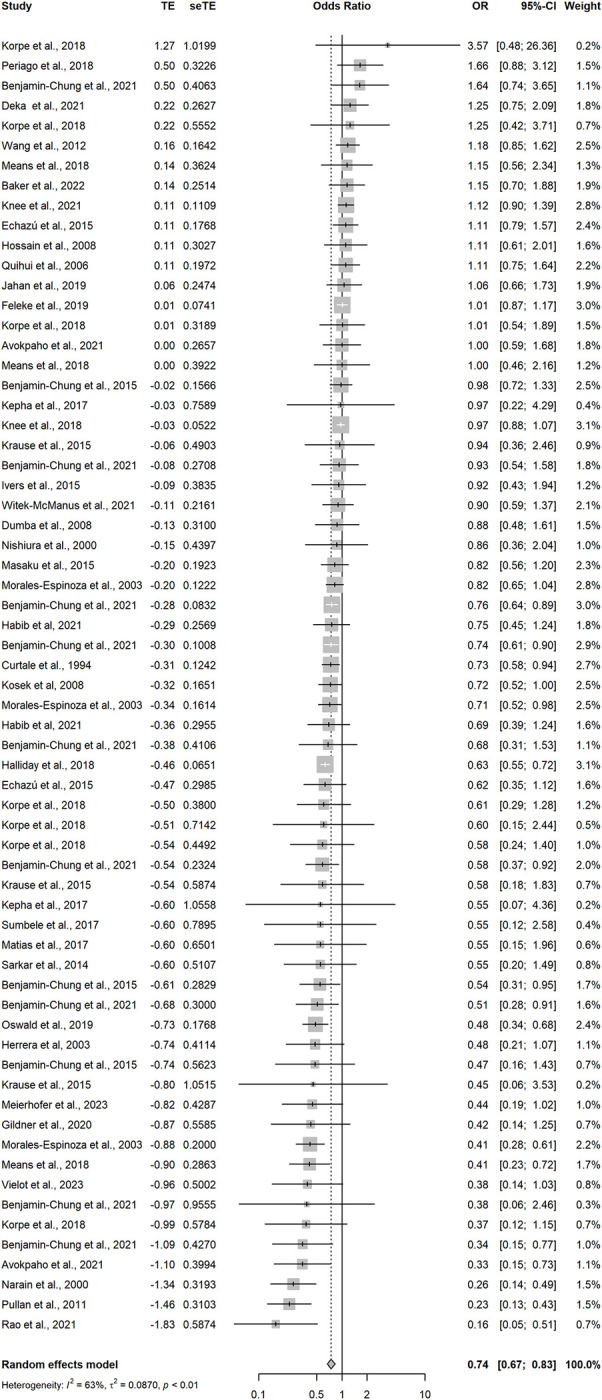
Table 1. Random-effects meta-regression model outputs (studies with low-risk of bias only) showing pooled effect of improved flooring (versus unimproved flooring) on outcomes of interest.
| Outcome grouping | Region | N* | OR | Lower 95ci | Upper 95ci | I2 | Egger p value |
|---|---|---|---|---|---|---|---|
| All pathogens | All regions | 65 | 0.74 | 0.67 | 0.83 | 0.63 | 0.02 |
| Diarrhoea exclusive | All regions | 13 | 0.89 | 0.83 | 0.96 | 0.45 | 0.04 |
| Helminth exclusive | All regions | 39 | 0.68 | 0.58 | 0.80 | 0.71 | 0.07 |
| Hookworm exclusive | All regions | 9 | 0.55 | 0.38 | 0.79 | 0.55 | NA# |
| Protozoa or bacteria exclusive | All regions | 22 | 0.82 | 0.75 | 0.90 | 0.19 | 0.25 |
| All pathogens | Central & S Asia | 18 | 0.73 | 0.57 | 0.92 | 0.52 | 0.43 |
| All pathogens | Latin America | 18 | 0.71 | 0.56 | 0.89 | 0.54 | 0.15 |
| All pathogens | SS Africa | 27 | 0.75 | 0.64 | 0.89 | 0.72 | 0.22 |
*Including only studies with low risk of bias. Table with outputs from models including (1) all studies and (2) studies with low and medium risk of bias can be found in S4 Table in the supplementary materials
#Too few studies (<10) in this analyses grouping to perform egger test.
Fig 4. Pooled effect of an improved household floor (VS unimproved floor) on odds of any type of helminthic infection in any age group (low risk of bias studies only).
Fig 5. Pooled effect of an improved household floor (VS unimproved floor) on odds of hookworm infections in any age group (low risk of bias studies only).
Fig 6. Pooled effect of an improved household floor (VS unimproved floor) on odds of any type of bacterial or protozoan infection in any age group (low risk of bias studies only).
Fig 7. Pooled effect of an improved household floor (VS unimproved floor) on odds of self-reported diarrhoea among any age group (low risk of bias studies only).
A separate sub-group analysis conducted by region showed negligible difference on the pooled effect estimates of household improved flooring on any enteric or parasitic infection between regions (Sub-Saharan Africa = 0.75 (95CI: 0.64–0.87) / Central and East Asia = 0.73 (95CI: 0.57–0.92) / Latin America = 0.71 (95CI: 0.56–0.89)). Funnel plots were also run for each outcome grouping and are presented in the supplementary materials (S2–S6 Figs).
Discussion
This study is the first of its kind to undertake a systematic review and meta-analysis examining the association between household flooring and enteric and parasitic infections. The meta-analysis demonstrated that in low- and middle-income settings improved household flooring is protective against enteric and parasitic infections when compared with unimproved household flooring. Improved flooring was found to be significantly protective against generic helminthic infections, hookworm-specific infections, generic enteric bacterial and protozoan infections, and diarrhoea. The largest pooled effect estimates were observed when including studies measuring hookworm infections or generic helminthic infections as outcomes. A sub-group analysis by region showed no meaningful difference in the protective effect of improved flooring between Sub-Saharan Africa, Central and East Asia, and Latin America. However, given that the protective effect of improved household flooring varied according to pathogen type, the epidemiological profile of a country or region will be an important consideration when evaluating the role of flooring in facilitating transmission of enteric and parasitic infections.
A limitation of this systematic review and meta-analysis is that it relies exclusively on results from observational studies, as to date, no experimental research evaluating the relationship between flooring and enteric or parasitic infections has been published in the literature. Results should therefore be treated with some caution as they are vulnerable to confounding and publication bias. To mitigate this risk, the analysis presented in this paper adopted a high threshold for study inclusion based on risk of bias score. Nevertheless, it is possible that residual confounding, particularly in relation to the role of socio-economic status (which could be independently associated with both exposure and outcome) may have substantially influenced these results, especially in studies that were not adjusted for these factors.
Results from this meta-analysis support findings from previous environmental sampling studies that have found high levels of bacterial contamination [15], as well as viruses, protozoa and helminths on earthen household floors [16, 17]. Among household members, infants and young children are potentially more likely to be exposed to these pathogens as they explore environments with their hands and commonly perform hand-to-mouth actions [14, 134, 135]. If infected, children are also more likely to experience severe disease compared with adults [1]. Future research should focus on conducting high-quality, randomised experimental studies that can evaluate the impact of improved flooring on child and adult health, isolated from potential confounders such as socio-economic status and access to WASH. Outcomes of interest could include enteric infections, soil-transmitted helminthiasis, and diarrhoea. Future research could also examine the persistence of different pathogens on improved floors such as cement and tiled surfaces, as only a handful of studies have so far examined this [15, 17]. Beyond physical health, future studies could also explore whether household flooring affects the psychological wellbeing of occupants, including both adults and children. and whether broader non-health outcomes such as economic activity and educational performance are linked with household flooring type.
There was some evidence for publication bias in the pooled model. This could be in part explained by the fact that many of the included studies were not designed to examine household flooring as a primary exposure of interest, but instead as part of a wider suite of covariates in a generic risk factor analysis. As a result, in some studies where household flooring was found to be insufficiently associated with the outcome it was dropped from the final multivariate model and its measure of effect was not reported, despite its initial inclusion. This phenomenon makes the availability of studies reporting a significant association between household flooring and health outcomes more likely when compared to studies that report no effect. To address this, authors were contacted to provide the adjusted measure of effect, and if no response was provided then the univariate measure of effect was used and a higher risk of bias score was applied to the study.
There was heterogeneity in how studies categorized and reported household flooring. Primarily this related to how wood and bamboo floors were classified, with some studies including them with cement-based and other “improved” floor types and other studies grouping them with earthen and other “unimproved” floor types. For future studies, adoption of a standardized classification system for floors would improve inter-study comparability. Currently, the DHS and MICS question modules are aligned on how to measure household flooring, and as such offer a validated and widely-adopted criteria for measuring floors that future studies should consider adopting. However, using a one-size-fits-all method for measuring and categorizing flooring comes at the risk of jeopardizing the internal validity of studies, as local variations in flooring types may not be captured by the DHS/MICS question.
Findings from this systematic review and meta-analysis indicate that household flooring should be considered an important domain of transmission for enteric and parasitic infections in low- and middle-income countries. The substantial number of households that reside in homes built with unimproved materials in low- and middle-income countries suggest that the collective cost in morbidity and mortality due to unimproved flooring could be considerable. However, the quality of available evidence is weak and as such there is an urgent need for high-quality experimental studies investigating the relationship between flooring and enteric and parasitic infections.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(CSV)
(CSV)
Acknowledgments
We would like to thank all authors who responded to requests for data or additional information on study methodologies.
Data Availability
The data used in this analysis is attached in supplementary materials.
Funding Statement
This work was supported by UK Research and Innovation (UKRI) Global Challenges Research Fund (GCRF) (grant number MR/T029811/1 to RP), as supported by Research England. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases. 2018;18(11):1211–28. doi: 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartorius B, Cano J, Simpson H, Tusting LS, Marczak LB, Miller-Petrie MK, et al. Prevalence and intensity of soil-transmitted helminth infections of children in sub-Saharan Africa, 2000–18: a geospatial analysis. The Lancet Global Health. 2021;9(1):e52–e60. doi: 10.1016/S2214-109X(20)30398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiner RC Jr, Graetz N, Casey DC, Troeger C, Garcia GM, Mosser JF, et al. Variation in childhood diarrheal morbidity and mortality in Africa, 2000–2015. New England Journal of Medicine. 2018;379(12):1128–38. doi: 10.1056/NEJMoa1716766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adu-Gyasi D, Asante KP, Frempong MT, Gyasi DK, Iddrisu LF, Ankrah L, et al. Epidemiology of soil transmitted Helminth infections in the middle-belt of Ghana, Africa. Parasite epidemiology and control. 2018;3(3):e00071. doi: 10.1016/j.parepi.2018.e00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ending the neglect to attain the Sustainable Development Goals: a global strategy on water, sanitation and hygiene to combat neglected tropical diseases, 2021–2030. Geneva: World Health Organization, 2021. [Google Scholar]
- 6.Humphrey JH, Mbuya MNN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. The Lancet Global Health. 2019;7(1):e132–e47. doi: 10.1016/S2214-109X(18)30374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. The Lancet Global Health. 2018;6(3):e316–e29. doi: 10.1016/S2214-109X(18)30005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. The Lancet Global Health. 2018;6(3):e302–e15. doi: 10.1016/S2214-109X(17)30490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larbi RT, Atiglo DY, Peterson MB, Biney AAE, Dodoo ND, Dodoo FN-A. Household food sources and diarrhoea incidence in poor urban communities, Accra Ghana. PLoS One. 2021;16(1):e0245466. doi: 10.1371/journal.pone.0245466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk MD, Angulo FJ, Havelaar AH, Black RE. Diarrhoeal disease in children due to contaminated food. Bull World Health Organ. 2017;95(3):233–4. Epub 20161125. doi: 10.2471/BLT.16.173229 ; PubMed Central PMCID: PMC5328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogbo FA, Agho K, Ogeleka P, Woolfenden S, Page A, Eastwood J, et al. Infant feeding practices and diarrhoea in sub-Saharan African countries with high diarrhoea mortality. PLoS One. 2017;12(2):e0171792. doi: 10.1371/journal.pone.0171792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penakalapati G, Swarthout J, Delahoy MJ, McAliley L, Wodnik B, Levy K, et al. Exposure to Animal Feces and Human Health: A Systematic Review and Proposed Research Priorities. Environ Sci Technol. 2017;51(20):11537–52. Epub 20171009. doi: 10.1021/acs.est.7b02811 ; PubMed Central PMCID: PMC5647569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delahoy MJ, Wodnik B, McAliley L, Penakalapati G, Swarthout J, Freeman MC, et al. Pathogens transmitted in animal feces in low- and middle-income countries. International Journal of Hygiene and Environmental Health. 2018;221(4):661–76. doi: 10.1016/j.ijheh.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong LH. Age-related changes to environmental exposure: variation in the frequency that young children place hands and objects in their mouths. J Expo Sci Environ Epidemiol. 2019;30:205–16. doi: 10.1038/s41370-019-0115-8 [DOI] [PubMed] [Google Scholar]
- 15.Exum NG, Olórtegui MP, Yori PP, Davis MF, Heaney CD, Kosek M, et al. Floors and Toilets: Association of Floors and Sanitation Practices with Fecal Contamination in Peruvian Amazon Peri-Urban Households. Environ Sci Technol. 2016;50(14):7373–81. Epub 20160708. doi: 10.1021/acs.est.6b01283 ; PubMed Central PMCID: PMC6400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capone D, Berendes D, Cumming O, Holcomb D, Knee J, Konstantinidis KT, et al. Impact of an Urban Sanitation Intervention on Enteric Pathogen Detection in Soils. bioRxiv. 2021:2021.04.02.438233. doi: 10.1021/acs.est.1c02168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mejia R, Seco-Hidalgo V, Garcia-Ramon D, Calderón E, Lopez A, Cooper PJ. Detection of enteric parasite DNA in household and bed dust samples: potential for infection transmission. Parasites & Vectors. 2020;13(1):141. doi: 10.1186/s13071-020-04012-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patricio Bouillon C. Room for Development: Housing Markets in Latin America and the Carribean. Inter-American Development Bank, 2022. [Google Scholar]
- 19.Tusting LS, Bisanzio D, Alabaster G, Cameron E, Cibulskis R, Davies M, et al. Mapping changes in housing in sub-Saharan Africa from 2000 to 2015. Nature. 2019;568(7752):391–4. doi: 10.1038/s41586-019-1050-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sartorius B, Legge H, Pullan R. Does suboptimal household flooring increase the risk of diarrhoea and intestinal parasite infection in low and middle income endemic settings? A systematic review and meta-analysis protocol. Systematic Reviews. 2020;9(1):113. doi: 10.1186/s13643-020-01384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2022. [Google Scholar]
- 22.Wickham et al. Welcome to the Tidyverse Journal of Open Source Software. 2019;4(43). 10.21105/joss.01686. [DOI] [Google Scholar]
- 23.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence Based Mental Health. 2019;22(4):153. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane 2022. [Google Scholar]
- 25.Yulianto E. Hubungan higiene sanitasi dengan kejadian penyakit cacingan pada siswa Sekolah Dasar Negeri Rowosari 01 Kecamatan Tembalang Kota Semarang tahun ajaran 2006/2007:. Universitas Negeri Semarang; 2007. [Google Scholar]
- 26.Yaya S, Hudani A, Udenigwe O, Shah V, Ekholuenetale M, Bishwajit G. Improving Water, Sanitation and Hygiene Practices, and Housing Quality to Prevent Diarrhea among Under-Five Children in Nigeria. Trop Med Infect Dis. 2018;3(2). Epub 20180412. doi: 10.3390/tropicalmed3020041 ; PubMed Central PMCID: PMC6073794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witek-McManus S, Simwanza J, Chisambi AB, Kepha S, Kamwendo Z, Mbwinja A, et al. Epidemiology of soil-transmitted helminths following sustained implementation of routine preventive chemotherapy: Demographics and baseline results of a cluster randomised trial in southern Malawi. PLoS Negl Trop Dis. 2021;15(5):e0009292. Epub 20210512. doi: 10.1371/journal.pntd.0009292 ; PubMed Central PMCID: PMC8224978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wihelmus O, Paun R. The Factors That Influence the Incidence of Infection of Intestinal Worms in Children Under Five with the Problem of Nutritional Stunting in the South Timor Timor District (TTS). Global Journal of Health Science. 2022;Vol. 14, No. 5 [Google Scholar]
- 29.Wang X, Zhang L, Luo R, Wang G, Chen Y, Medina A, et al. Soil-Transmitted Helminth Infections and Correlated Risk Factors in Preschool and School-Aged Children in Rural Southwest China. PLoS One. 2012;7(9):e45939. doi: 10.1371/journal.pone.0045939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vázquez FA, Ramírez DR, Echague G, Sosa L, Cabello MÁ, Samudio M, et al. [Prevalence and intensity of geohelminths infection characterizing the socio-cultural and environmental factors that affect the infection of school children, Paraguay, 2015]. Revista chilena de infectologia: organo oficial de la Sociedad Chilena de Infectologia. 2018;35(5):501–8. doi: 10.4067/s0716-10182018000500501 . [DOI] [PubMed] [Google Scholar]
- 31.Uma Maheswari R. A Study of the Impact of Mass Drug Administration (Dec and Albendazole) on the Prevalence of Soil-Transmitted Helminths Among Children Aged 5–14 Years in a Rural Population. Chennai: Madras Medical College; 2009. [Google Scholar]
- 32.Trudeau J, Aksan AM, Vásquez WF. Water system unreliability and diarrhea incidence among children in Guatemala. Int J Public Health. 2018;63(2):241–50. Epub 20171116. doi: 10.1007/s00038-017-1054-6 . [DOI] [PubMed] [Google Scholar]
- 33.Tabi ESB, Eyong EM, Akum EA, Löve J, Cumber SN. Soil-transmitted Helminth infection in the Tiko Health District, South West Region of Cameroon: a post-intervention survey on prevalence and intensity of infection among primary school children. Pan Afr Med J. 2018;30:74. Epub 20180529. doi: 10.11604/pamj.2018.30.74.15676 ; PubMed Central PMCID: PMC6191252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suriyasa P, Balgis B, Saptono R, Hapsari MI. Potable water source and the method of garbage disposal in lowering the risk of diarrhea. Medical Journal of Indonesia. 2004;13:119. [Google Scholar]
- 35.Suraweera O, Galgamuwa L., Wickramasinghe S., Iddawela D., & Nandasiri N. Soil-transmitted helminth infections, associated factors and nutritional status in an estate community in Sri Lanka. Sri Lankan Journal of Infectious Diseases. 2018;8 (2):100–14. [Google Scholar]
- 36.Sumbele IU, Nkemnji GB, Kimbi HK. Soil-transmitted helminths and plasmodium falciparum malaria among individuals living in different agroecosystems in two rural communities in the mount Cameroon area: a cross-sectional study. Infect Dis Poverty. 2017;6(1):67. Epub 20170316. doi: 10.1186/s40249-017-0266-6 ; PubMed Central PMCID: PMC5353792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sultana Y, Amin A, Sehrin S, Begum A. Occurrence Of Strongyloides Stercoralis In Slum Areas Of Dhaka, Bangladesh. Journal of the Asiatic Society of Bangladesh, Science. 2015;41:45–50. doi: 10.3329/jasbs.v41i1.46189 [DOI] [Google Scholar]
- 38.Soriano G. Prevalence of Soil Transmitted Helminths and Associate Transmission Factors among School Children in a Selected Barangay in Trece Martires City, Cavite. International Journal of Medical Sciences and Technology (IJMST). 2019. [Google Scholar]
- 39.Solano R L, Acuña G I, Barón MA, Morón de Salim A, Sánchez J A. Asociación entre pobreza e infestación parasitaria intestinal en preescolares, escolares y adolescentes del sur de valencia estado Carabobo-Venezuela. Kasmera. 2008;36(2):137–47. [Google Scholar]
- 40.Sinmegn Mihrete T, Asres Alemie G, Shimeka Teferra A. Determinants of childhood diarrhea among underfive children in Benishangul Gumuz Regional State, North West Ethiopia. BMC Pediatrics. 2014;14(1):102. doi: 10.1186/1471-2431-14-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrestha A, Six J, Dahal D, Marks S, Meierhofer R. Association of nutrition, water, sanitation and hygiene practices with children’s nutritional status, intestinal parasitic infections and diarrhoea in rural Nepal: a cross-sectional study. BMC Public Health. 2020;20(1):1241. Epub 20200815. doi: 10.1186/s12889-020-09302-3 ; PubMed Central PMCID: PMC7429949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semuel S, Maxsi I. Analisis Model Faktor Risiko Infeksi Cacing Gelang (Ascaris Lumbricoides) pada Murid SD di Distrik Arso Kabupaten Keerom Papua. Jurnal Buski. 2014;5(1). [Google Scholar]
- 43.Sarkar R, Kattula D, Francis MR, Ajjampur SS, Prabakaran AD, Jayavelu N, et al. Risk factors for cryptosporidiosis among children in a semi urban slum in southern India: a nested case-control study. Am J Trop Med Hyg. 2014;91(6):1128–37. Epub 20141020. doi: 10.4269/ajtmh.14-0304 ; PubMed Central PMCID: PMC4257634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahiledengle B, Kumie A, Atlaw D, Tekalegn Y, Woldeyohannes D, Zenbaba D, et al. The Role of Household Flooring on Childhood Diarrhea Among Children 0 to 23 Months of Age in Ethiopia: A Nationally Representative Cross-Sectional Study Using a Multi-Level Mixed Effect Analysis. Environ Health Insights. 2021;15:11786302211064423. Epub 20211212. doi: 10.1177/11786302211064423 ; PubMed Central PMCID: PMC8671690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saha A, Hayen A, Ali M, Rosewell A, Clemens J, Macintyre C, et al. Socioeconomic risk factors for cholera in different transmission settings: An analysis of the data of a cluster randomized trial in Bangladesh. Vaccine. 2017;35. doi: 10.1016/j.vaccine.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez-García R, Rodríguez-Guzmán LM, Sánchez-Maldonado MI, Gómez-Delgado A, Rivera-Cedillo R. [Prevalence and risk factors associated with intestinal parasitoses in pregnant women and their relation to the infant’s birth weight]. Ginecol Obstet Mex. 2002;70:338–43. . [PubMed] [Google Scholar]
- 47.Richards L, Delgado C, Goy M, Liang S, Periago M. Prevalence of intestinal parasites and related risk factors in rural localities from Pampa del Indio, Chaco, Argentina. UF Journal of Undergraduate Research. 2019;21. doi: 10.32473/ufjur.v21i1.107939 [DOI] [Google Scholar]
- 48.Reyes J. Factores sociodemográficos y su relación con parasitosis intestinal en niños de la Escuela Marieta de Veintimilla del Barrio Motupe de Loja: Universidad Nacional de Loja; 2019. [Google Scholar]
- 49.Rao G, Blackstock A, Derado G, Cuéllar V, Juliao P, Alvarez M, et al. An evaluation of water, sanitation, and hygiene status and household assets and their associations with soil-transmitted helminthiasis and reported diarrhea in Nueva Santa Rosa, Guatemala. Journal of Water, Sanitation and Hygiene for Development. 2021;11. doi: 10.2166/washdev.2021.160 [DOI] [Google Scholar]
- 50.Quintero K, Durán C, Duri D, Medina F, Garcia J, Hidalgo G, et al. Household social determinants of ascariasis and trichuriasis in North Central Venezuela. International Health. 2012;4(2):103–10. doi: 10.1016/j.inhe.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 51.Quihui L, Valencia ME, Crompton DW, Phillips S, Hagan P, Morales G, et al. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health. 2006;6:225. Epub 20060906. doi: 10.1186/1471-2458-6-225 ; PubMed Central PMCID: PMC1584408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pullan RL, Kabatereine NB, Bukirwa H, Staedke SG, Brooker S. Heterogeneities and consequences of Plasmodium species and hookworm coinfection: a population based study in Uganda. J Infect Dis. 2011;203(3):406–17. Epub 20101227. doi: 10.1093/infdis/jiq063 ; PubMed Central PMCID: PMC3038339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prado MS, Strina A, Barreto ML, Oliveira-Assis AM, xfa cia, et al. Risk Factors for Infection with Giardia duodenalis in Pre-School Children in the City of Salvador, Brazil. Epidemiology and Infection. 2003;131(2):899–906. doi: 10.1017/s0950268803001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Periago MV, García R, Astudillo OG, Cabrera M, Abril MC. Prevalence of intestinal parasites and the absence of soil-transmitted helminths in Añatuya, Santiago del Estero, Argentina. Parasit Vectors. 2018;11(1):638. Epub 20181214. doi: 10.1186/s13071-018-3232-7 ; PubMed Central PMCID: PMC6295026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page C. HIV-1 and Helminth Co-Infection in Adults in Kenya Yale Medicine Thesis Digital Library. 368.: Yale; 2009. [Google Scholar]
- 56.Oswald WE, Halliday KE, McHaro C, Witek-McManus S, Kepha S, Gichuki PM, et al. Domains of transmission and association of community, school, and household sanitation with soil-transmitted helminth infections among children in coastal Kenya. PLoS Negl Trop Dis. 2019;13(11):e0007488. Epub 20191125. doi: 10.1371/journal.pntd.0007488 ; PubMed Central PMCID: PMC6901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nuryanti NM, Subrata IM. Soil Transmitted Helminths Infection in Elementary School Students in Highland and Lowland Areas of Gianyar Regency. KEMAS: Jurnal Kesehatan Masyarakat. 2018;(Vol 13, No 3 (2018)):323–30. [Google Scholar]
- 58.Nath TC, Eom KS, Choe S, Mukutmoni M, Khanum H, Bhuiyan JU, et al. An update of intestinal helminth infections among urban slum communities in Bangladesh. IJID Reg. 2022;5:1–7. Epub 20220818. doi: 10.1016/j.ijregi.2022.08.004 ; PubMed Central PMCID: PMC9465421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narain K, Rajguru SK, Mahanta J. Prevalence of Trichuris trichiura in relation to socio-economic & behavioural determinants of exposure to infection in rural Assam. Indian J Med Res. 2000;112:140–6. . [PubMed] [Google Scholar]
- 60.Mukutmoni M, Khanum H. Prevalence and risk factors of intestinal Helminthiasis among the children of Begun Bari slum, Tejgaon, Dhaka. Bangladesh Journal of Zoology. 2018;45(2):123–9. doi: 10.3329/bjz.v45i2.35707 [DOI] [Google Scholar]
- 61.Mosawi SH, Zarghona Z, Dalimi A, Jokelainen P, Safa AH, Mohammadi MR, et al. Particularly neglected in countries with other challenges: High Toxoplasma gondii seroprevalence in pregnant women in Kabul, Afghanistan, while a low proportion know about the parasite. PLoS One. 2019;14(10):e0223585. Epub 20191010. doi: 10.1371/journal.pone.0223585 ; PubMed Central PMCID: PMC6786618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morales-Espinoza EM, Sánchez-Pérez HJ, García-Gil M, Vargas-Morales G, Méndez-Sánchez JD, Pérez-Ramírez M. Intestinal parasites in children, in highly deprived areas in the border region of Chiapas, Mexico. Salud publica de Mexico. 2003;45 5:379–88. doi: 10.1590/s0036-36342003000500008 [DOI] [PubMed] [Google Scholar]
- 63.Mohammed S, Tilahun M, Tamiru D. Morbidity and Associated Factors of Diarrheal Diseases Among Under Five Children in Arba-Minch District, Southern Ethiopia, 2012. Science Journal of Public Health. 2013;1:102. [Google Scholar]
- 64.Mohammed S, Tamiru D. The Burden of Diarrheal Diseases among Children under Five Years of Age in Arba Minch District, Southern Ethiopia, and Associated Risk Factors: A Cross-Sectional Study. Int Sch Res Notices. 2014;2014:654901. Epub 20141118. doi: 10.1155/2014/654901 ; PubMed Central PMCID: PMC4897213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammed S. The Occurrence of Childhood Diarhea and Its Home Management among Mothers of Under-Five Years Children in Arba Minch Zuria, Southern Ethiopia. Science Journal of Public Health. 2013;1:135. doi: 10.11648/j.sjph.20130103.15 [DOI] [Google Scholar]
- 66.Melese B, Paulos W, Astawesegn FH, Gelgelu TB. Prevalence of diarrheal diseases and associated factors among under-five children in Dale District, Sidama zone, Southern Ethiopia: a cross-sectional study. BMC Public Health. 2019;19(1):1235. Epub 20190906. doi: 10.1186/s12889-019-7579-2 ; PubMed Central PMCID: PMC6729095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Means AR, van Lieshout L, Brienen E, Yuhas K, Hughes JP, Ndungu P, et al. Combined effectiveness of anthelmintic chemotherapy and WASH among HIV-infected adults. PLOS Neglected Tropical Diseases. 2018;12(1):e0005955. doi: 10.1371/journal.pntd.0005955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mationg ML, Tallo V, Williams G, Gordon C, Clements A, McManus D, et al. The control of soil-transmitted helminthiases in the Philippines: the story continues. Infectious Diseases of Poverty. 2021;10. doi: 10.1186/s40249-021-00870-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matias WR, Teng JE, Hilaire IJ, Harris JB, Franke MF, Ivers LC. Household and Individual Risk Factors for Cholera among Cholera Vaccine Recipients in Rural Haiti. Am J Trop Med Hyg. 2017;97(2):436–42. Epub 20170719. doi: 10.4269/ajtmh.16-0407 ; PubMed Central PMCID: PMC5544067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masaku J, Madigu N, Okoyo C, Njenga SM. Current status of Schistosoma mansoni and the factors associated with infection two years following mass drug administration programme among primary school children in Mwea irrigation scheme: A cross-sectional study. BMC Public Health. 2015;15:739. Epub 20150801. doi: 10.1186/s12889-015-1991-z ; PubMed Central PMCID: PMC4522152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mario S, Achmad Fickry F, Rostika F, Mohammad Z, Nur Alam F, Samwilson S, editors. Analysis on Incidents of Helminthiasis Based on Home Sanitation of Elementary-School Children in Seluma Regency. Proceedings of the 2nd Sriwijaya International Conference of Public Health (SICPH 2019); 2020. 2020/06/19: Atlantis Press. [Google Scholar]
- 72.Londoño-Franco Á L, Loaiza-Herrera J, Lora-Suárez FM, Gómez-Marín JE. [Blastocystis sp. frequency and sources among children from 0 to 5 years of age attending public day care centers in Calarcá, Colombia]. Biomedica. 2014;34(2):218–27. doi: 10.1590/s0120-41572014000200008 . [DOI] [PubMed] [Google Scholar]
- 73.Liu C, Luo R, Yi H, Zhang L, Li S, Bai Y, et al. Soil-Transmitted Helminths in Southwestern China: A Cross-Sectional Study of Links to Cognitive Ability, Nutrition, and School Performance among Children. PLoS Negl Trop Dis. 2015;9(6):e0003877. Epub 20150625. doi: 10.1371/journal.pntd.0003877 ; PubMed Central PMCID: PMC4481344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin A, Arnold BF, Afreen S, Goto R, Huda TMN, Haque R, et al. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am J Trop Med Hyg. 2013;89(1):130–7. Epub 20130429. doi: 10.4269/ajtmh.12-0629 ; PubMed Central PMCID: PMC3748469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kusmi H, Irawati N, Kadri H. Hubungan Sanitasi Lingkungan Rumah dengan Kejadian Askariasis dan Trikuriasis pada Siswa SD N 29 Purus Padang. Jurnal Kesehatan Andalas. 2015;4. [Google Scholar]
- 76.Kurscheid J, Laksono B, Park MJ, Clements ACA, Sadler R, McCarthy JS, et al. Epidemiology of soil-transmitted helminth infections in Semarang, Central Java, Indonesia. PLoS Negl Trop Dis. 2020;14(12):e0008907. Epub 20201228. doi: 10.1371/journal.pntd.0008907 ; PubMed Central PMCID: PMC7793285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krause RJ, Koski KG, Pons E, Sinisterra O, Scott ME. Ascaris and hookworm transmission in preschool children in rural Panama: role of subsistence agricultural activities. Parasitology. 2016;143(8):1043–54. doi: 10.1017/S0031182016000366 . [DOI] [PubMed] [Google Scholar]
- 78.Kosek M, Yori PP, Pan WK, Olortegui MP, Gilman RH, Perez J, et al. Epidemiology of highly endemic multiply antibiotic-resistant shigellosis in children in the Peruvian Amazon. Pediatrics. 2008;122(3):e541–9. Epub 20080818. doi: 10.1542/peds.2008-0458 ; PubMed Central PMCID: PMC6204332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korpe PS, Valencia C, Haque R, Mahfuz M, McGrath M, Houpt E, et al. Epidemiology and Risk Factors for Cryptosporidiosis in Children From 8 Low-income Sites: Results From the MAL-ED Study. Clin Infect Dis. 2018;67(11):1660–9. doi: 10.1093/cid/ciy355 ; PubMed Central PMCID: PMC6233690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knee J, Sumner T, Adriano Z, Berendes D, de Bruijn E, Schmidt WP, et al. Risk factors for childhood enteric infection in urban Maputo, Mozambique: A cross-sectional study. PLoS Negl Trop Dis. 2018;12(11):e0006956. Epub 20181112. doi: 10.1371/journal.pntd.0006956 ; PubMed Central PMCID: PMC6258421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knee J, Sumner T, Adriano Z, Anderson C, Bush F, Capone D, et al. Effects of an urban sanitation intervention on childhood enteric infection and diarrhea in Maputo, Mozambique: A controlled before-and-after trial. Elife. 2021;10. Epub 20210409. doi: 10.7554/eLife.62278 ; PubMed Central PMCID: PMC8121544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kepha S, Mwandawiro CS, Anderson RM, Pullan RL, Nuwaha F, Cano J, et al. Impact of single annual treatment and four-monthly treatment for hookworm and Ascaris lumbricoides, and factors associated with residual infection among Kenyan school children. Infect Dis Poverty. 2017;6(1):30. Epub 20170209. doi: 10.1186/s40249-017-0244-z ; PubMed Central PMCID: PMC5299645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaliappan S, Ramanujam K, Manuel M, Farzana J, Janagaraj V, Laxmanan S, et al. Soil-transmitted helminth infections after mass drug administration for lymphatic filariasis in rural southern India. Trop Med Int Health. 2022;27(1):81–91. Epub 20211116. doi: 10.1111/tmi.13697 . [DOI] [PubMed] [Google Scholar]
- 84.Jahan Y, Moriyama M, Hossain S, Rahman M, Ferdous F, Ahmed S, et al. Relation of childhood diarrheal morbidity with the type of tube well used and associated factors of Shigella sonnei diarrhea in rural Bangladesh site of the Global Enteric Multicenter Study. Tropical Medicine and Health. 2019;47. doi: 10.1186/s41182-019-0158-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacobsen KH, Ribeiro PS, Quist BK, Rydbeck BV. Prevalence of intestinal parasites in young Quichua children in the highlands of rural Ecuador. J Health Popul Nutr. 2007;25(4):399–405. ; PubMed Central PMCID: PMC2754013. [PMC free article] [PubMed] [Google Scholar]
- 86.Ivers L, Hilaire I, Teng J, Almazor C, Jerome J, Ternier R, et al. Effectiveness of reactive oral cholera vaccination in rural Haiti: A case-control study and bias-indicator analysis. The Lancet Global health. 2015;3:e162–8. doi: 10.1016/S2214-109X(14)70368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hossain MJ, Saha D, Antonio M, Nasrin D, Blackwelder WC, Ikumapayi UN, et al. Cryptosporidium infection in rural Gambian children: Epidemiology and risk factors. PLoS Negl Trop Dis. 2019;13(7):e0007607. Epub 20190726. doi: 10.1371/journal.pntd.0007607 ; PubMed Central PMCID: PMC6685629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holland CV, Taren DL, Crompton DW, Nesheim MC, Sanjur D, Barbeau I, et al. Intestinal helminthiases in relation to the socioeconomic environment of Panamanian children. Soc Sci Med. 1988;26(2):209–13. doi: 10.1016/0277-9536(88)90241-9 . [DOI] [PubMed] [Google Scholar]
- 89.Herrera J, Marcos L, Terashima A, Alvarez H, Samalvides F, Gotuzzo E. [Factors associated with strongyloides stercoralis infection in an endemic area in Peru]. Rev Gastroenterol Peru. 2006;26(4):357–62. . [PubMed] [Google Scholar]
- 90.Han K, Wai K, Aye K, Kyaw K, Maung W, Oo T. Emerging neglected helminthiasis and determinants of multiple helminth infections in flood-prone township in Myanmar. Tropical Medicine and Health. 2019;47. doi: 10.1186/s41182-018-0133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halliday KE, Oswald WE, McHaro C, Beaumont E, Gichuki PM, Kepha S, et al. Community-level epidemiology of soil-transmitted helminths in the context of school-based deworming: Baseline results of a cluster randomised trial on the coast of Kenya. PLoS Negl Trop Dis. 2019;13(8):e0007427. Epub 2019/08/10. doi: 10.1371/journal.pntd.0007427 ; PubMed Central PMCID: PMC6719894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hall A, Conway DJ, Anwar KS, Rahman ML. Strongyloides stercoralis in an urban slum community in Bangladesh: factors independently associated with infection. Trans R Soc Trop Med Hyg. 1994;88(5):527–30. doi: 10.1016/0035-9203(94)90146-5 . [DOI] [PubMed] [Google Scholar]
- 93.Habib A, Andrianonimiadana L, Rakotondrainipiana M, Andriantsalama P, Randriamparany R, Randremanana RV, et al. High prevalence of intestinal parasite infestations among stunted and control children aged 2 to 5 years old in two neighborhoods of Antananarivo, Madagascar. PLoS Negl Trop Dis. 2021;15(4):e0009333. Epub 20210420. doi: 10.1371/journal.pntd.0009333 ; PubMed Central PMCID: PMC8087024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gildner TE, Cepon-Robins TJ, Liebert MA, Urlacher SS, Schrock JM, Harrington CJ, et al. Market integration and soil-transmitted helminth infection among the Shuar of Amazonian Ecuador. PLoS One. 2020;15(7):e0236924. Epub 20200731. doi: 10.1371/journal.pone.0236924 ; PubMed Central PMCID: PMC7394393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geus D, Sifft K, Habarugira F, Mugisha J, Mukampunga C, Ndoli J, et al. Co-infections with Plasmodium, Ascaris and Giardia among Rwandan schoolchildren. Tropical Medicine & International Health. 2019;24. doi: 10.1111/tmi.13206 [DOI] [PubMed] [Google Scholar]
- 96.Gamboa MI, Zonta ML, Navone GT. Distribución de geohelmintos y situación socio-ambiental en dos provincias argentinas con diferente fisonomía biogeográfica. 2011. [Google Scholar]
- 97.Gabrie JA, Rueda MM, Canales M, Gyorkos TW, Sanchez AL. School hygiene and deworming are key protective factors for reduced transmission of soil-transmitted helminths among schoolchildren in Honduras. Parasites & Vectors. 2014;7. doi: 10.1186/1756-3305-7-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forrer A, Vounatsou P, Sayasone S, Vonghachack Y, Bouakhasith D, Utzinger J, et al. Risk profiling of hookworm infection and intensity in southern Lao People’s Democratic Republic using Bayesian models. PLoS Negl Trop Dis. 2015;9(3):e0003486. Epub 20150330. doi: 10.1371/journal.pntd.0003486 ; PubMed Central PMCID: PMC4378892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feleke BE, Beyene MB, Feleke TE, Jember TH, Abera B. Intestinal parasitic infection among household contacts of primary cases, a comparative cross-sectional study. PLoS One. 2019;14(10):e0221190. doi: 10.1371/journal.pone.0221190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Errea RA, Vasquez-Rios G, Calderon ML, Siu D, Duque KR, Juarez LH, et al. Soil-Transmitted Helminthiasis in Children from a Rural Community Taking Part in a Periodic Deworming Program in the Peruvian Amazon. Am J Trop Med Hyg. 2019;101(3):636–40. doi: 10.4269/ajtmh.18-1011 ; PubMed Central PMCID: PMC6726937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Echazú A, Bonanno D, Juarez M, Cajal SP, Heredia V, Caropresi S, et al. Effect of Poor Access to Water and Sanitation As Risk Factors for Soil-Transmitted Helminth Infection: Selectiveness by the Infective Route. PLOS Neglected Tropical Diseases. 2015;9(9):e0004111. doi: 10.1371/journal.pntd.0004111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dumba R, Kaddu JB, Wabwire Mangen F. Intestinal helminths in Luweero district, Uganda. Afr Health Sci. 2008;8(2):90–6. ; PubMed Central PMCID: PMC2584320. [PMC free article] [PubMed] [Google Scholar]
- 103.Dukpa T, Dorji N, Thinley S, Wangchuk, Tshering K, Gyem K, et al. Soil-Transmitted Helminth infections reduction in Bhutan: A report of 29 years of deworming. PLoS One. 2020;15(1):e0227273. Epub 20200103. doi: 10.1371/journal.pone.0227273 ; PubMed Central PMCID: PMC6941809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dita S, Eti K,????? M. FAKTOR YANG BERHUBUNGAN DENGAN PENYAKIT KECACINGAN PADA ANAK SEKOLAH DASAR DI KELURAHAN LEGOK KOTA JAMBI TAHUN 2020. Jurnal Endurance: Kajian Ilmiah Problema Kesehatan. 2021;6 (1) [Google Scholar]
- 105.Dey NC, Parvez M, Islam MR, Mistry SK, Levine DI. Effectiveness of a community-based water, sanitation, and hygiene (WASH) intervention in reduction of diarrhoea among under-five children: Evidence from a repeated cross-sectional study (2007–2015) in rural Bangladesh. Int J Hyg Environ Health. 2019;222(8):1098–108. Epub 20190820. doi: 10.1016/j.ijheh.2019.08.006 . [DOI] [PubMed] [Google Scholar]
- 106.Deka S, Barua D, Bahurupi Y, Kalita D. Assessment of the Prevalence of Soil-Transmitted Helminth Infections and Associated Risk Factors among School-Aged Children in a Flood-Affected Area of Northeast India. Am J Trop Med Hyg. 2021;105(2):480–9. Epub 20210706. doi: 10.4269/ajtmh.20-1238 ; PubMed Central PMCID: PMC8437162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de la Luz Galván-Ramírez M, Madriz-Elisondo AL, Ramírez CGT, de Jesús Romero Rameño J, de la OCDA, López MAC. Enteroparasitism and Risk Factors Associated with Clinical Manifestations in Children and Adults of Jalisco State in Western Mexico. Osong Public Health Res Perspect. 2019;10(1):39–48. doi: 10.24171/j.phrp.2019.10.1.08 ; PubMed Central PMCID: PMC6396823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Curtale F, Pezzotti P, Saad YS, Aloi A. An analysis of individual, household, and environmental risk factors for intestinal helminth infection among children in Qena Governorate, Upper Egypt. J Trop Pediatr. 1999;45(1):14–7. doi: 10.1093/tropej/45.1.14 . [DOI] [PubMed] [Google Scholar]
- 109.Cociancic P, Torrusio SE, Zonta ML, Navone GT. Risk factors for intestinal parasitoses among children and youth of Buenos Aires, Argentina. One Health. 2020;9:100116. Epub 20191128. doi: 10.1016/j.onehlt.2019.100116 ; PubMed Central PMCID: PMC6909185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chege NM, Ondigo BN, Onyambu FG, Kattam AM, Lagat N, Irungu T, et al. The prevalence of intestinal parasites and associated risk factors in school-going children from informal settlements in Nakuru town, Kenya. Malawi Med J. 2020;32(2):80–6. doi: 10.4314/mmj.v32i2.5 ; PubMed Central PMCID: PMC8788588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chege N. Prevalence, Molecular Characterization and Risk Factors Associated With Intestinal Parasites among School Going Children from Informal Settlements of Nakuru Town, Kenya 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Candela E, Goizueta C, Periago MV, Muñoz-Antoli C. Prevalence of intestinal parasites and molecular characterization of Giardia intestinalis, Blastocystis spp. and Entamoeba histolytica in the village of Fortín Mbororé (Puerto Iguazú, Misiones, Argentina). Parasit Vectors. 2021;14(1):510. Epub 20211001. doi: 10.1186/s13071-021-04968-z ; PubMed Central PMCID: PMC8485468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bhuiyan MAI, Jhinu ZN, Jaliluzzaman, Mukutmoni M. Personal hygiene practices and socio-economic conditions as influential factors for intestinal parasitic infection in Dhaka city dwellers. Bangladesh Journal of Zoology. 2019;47(1):129–36. doi: 10.3329/bjz.v47i1.42028 [DOI] [Google Scholar]
- 114.Benjamin-Chung J, Nazneen A, Halder AK, Haque R, Siddique A, Uddin MS, et al. The Interaction of Deworming, Improved Sanitation, and Household Flooring with Soil-Transmitted Helminth Infection in Rural Bangladesh. PLoS Negl Trop Dis. 2015;9(12):e0004256. Epub 20151201. doi: 10.1371/journal.pntd.0004256 ; PubMed Central PMCID: PMC4666415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benjamin-Chung J, Crider YS, Mertens A, Ercumen A, Pickering AJ, Lin A, et al. Household finished flooring and soil-transmitted helminth and Giardia infections among children in rural Bangladesh and Kenya: a prospective cohort study. Lancet Glob Health. 2021;9(3):e301–e8. doi: 10.1016/S2214-109X(20)30523-4 ; PubMed Central PMCID: PMC7900607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bauza V, Byrne DM, Trimmer JT, Lardizabal A, Atiim P, Asigbee MAK, et al. Child soil ingestion in rural Ghana—frequency, caregiver perceptions, relationship with household floor material and associations with child diarrhoea. Trop Med Int Health. 2018;23(5):558–69. Epub 20180410. doi: 10.1111/tmi.13050 . [DOI] [PubMed] [Google Scholar]
- 117.Baker KK, Mumma JAO, Simiyu S, Sewell D, Tsai K, Anderson JD, et al. Environmental and behavioural exposure pathways associated with diarrhoea and enteric pathogen detection in 5-month-old, periurban Kenyan infants: a cross-sectional study. BMJ Open. 2022;12(10):e059878. Epub 20221031. doi: 10.1136/bmjopen-2021-059878 ; PubMed Central PMCID: PMC9628658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Avokpaho E, Houngbégnon P, Accrombessi M, Atindégla E, Yard E, Rubin Means A, et al. Factors associated with soil-transmitted helminths infection in Benin: Findings from the DeWorm3 study. PLoS Negl Trop Dis. 2021;15(8):e0009646. Epub 20210817. doi: 10.1371/journal.pntd.0009646 ; PubMed Central PMCID: PMC8396766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Assaré RK, N’Tamon RN, Bellai LG, Koffi JA, Mathieu TI, Ouattara M, et al. Characteristics of persistent hotspots of Schistosoma mansoni in western Côte d’Ivoire. Parasit Vectors. 2020;13(1):337. Epub 20200702. doi: 10.1186/s13071-020-04188-x ; PubMed Central PMCID: PMC7333430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Armiyanti Y, Utami WS, Nurdian Y, Ng JAS, Hermansyah B. Hookworm infection and the risk factors among plantation workers in Jember, Indonesia. Annals of Tropical Medicine and Public Health. 2020. [Google Scholar]
- 121.Arief W, Arif P. Sanitation, drinking water access and diarrhea in Indonesia. Russian Journal of Agricultural and Socio-Economic Sciences. 2019. [Google Scholar]
- 122.Alves de Oliveira Serra MA, Chaves Cde S, Branco Coêlho ZC, de Castro Rodrigues NL, Martins Vale J, Teixeira MJ, et al. Comparison between Two Decades of Prevalence of Intestinal Parasitic Diseases and Risk Factors in a Brazilian Urban Centre. Interdiscip Perspect Infect Dis. 2015;2015:546705. Epub 20151125. doi: 10.1155/2015/546705 ; PubMed Central PMCID: PMC4673330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alelign T, Degarege A, Erko B. Soil-Transmitted Helminth Infections and Associated Risk Factors among Schoolchildren in Durbete Town, Northwestern Ethiopia. J Parasitol Res. 2015;2015:641602. Epub 20150616. doi: 10.1155/2015/641602 ; PubMed Central PMCID: PMC4487929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alarcón M, Iannacone J, Blanco Y. Parasitosis intestinal, factores de riesgo y seroprevalencia de toxocariosis en pobladores del parque industrial de Huaycán, Lima, Perú. Neotropical Helminthology. 2010;Vol. 4, N°. 1 17–36. [Google Scholar]
- 125.Ajjampur SSR, Kaliappan SP, Halliday KE, Palanisamy G, Farzana J, Manuel M, et al. Epidemiology of soil transmitted helminths and risk analysis of hookworm infections in the community: Results from the DeWorm3 Trial in southern India. PLoS Negl Trop Dis. 2021;15(4):e0009338. Epub 20210430. doi: 10.1371/journal.pntd.0009338 ; PubMed Central PMCID: PMC8184002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Afroz S, Debsarma S, Dutta S, Rhaman M, Mohsena M. Prevalence of helminthic infestations among Bangladeshi rural children and its trend since mid-seventies. IMC Journal of Medical Science. 2019;13. doi: 10.3329/imcjms.v13i1.42038 [DOI] [Google Scholar]
- 127.Rogawski ET, Bartelt LA, Platts-Mills JA, Seidman JC, Samie A, Havt A, et al. Determinants and Impact of Giardia Infection in the First 2 Years of Life in the MAL-ED Birth Cohort. J Pediatric Infect Dis Soc. 2017;6(2):153–60. doi: 10.1093/jpids/piw082 ; PubMed Central PMCID: PMC5907871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Srinivasan P, Lawa HR, Rosado JL, Al Mamun A, Khatun M, Santos JI, et al. Household and personal factors are sources of heterogenity in intestinal parasite clearance among Mexican children 6–15 months of age supplemented with vitamin A and zinc. Acta Trop. 2016;156:48–56. Epub 20160107. doi: 10.1016/j.actatropica.2015.12.001 . [DOI] [PubMed] [Google Scholar]
- 129.Mahmud MA, Chappell C, Hossain MM, Habib M, Dupont HL. Risk factors for development of first symptomatic Giardia infection among infants of a birth cohort in rural Egypt. Am J Trop Med Hyg. 1995;53(1):84–8. . [PubMed] [Google Scholar]
- 130.Musiime AK, Krezanoski PJ, Smith DL, Kilama M, Conrad MD, Otto G, et al. House design and risk of malaria, acute respiratory infection and gastrointestinal illness in Uganda: A cohort study. PLOS Global Public Health. 2022;2(3):e0000063–e. doi: 10.1371/journal.pgph.0000063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wright CE, Alamy ME, DuPont HL, Holguin AH, Hsi BP, Thacker SB, et al. The Role of Home Environment in Infant Diarrhea in Rural Egypt. American Journal of Epidemiology. 1991;134(8):887–94. doi: 10.1093/oxfordjournals.aje.a116163 [DOI] [PubMed] [Google Scholar]
- 132.Vielot NA, Zepeda O, Reyes Y, González F, Vinjé J, Becker-Dreps S, et al. Household Surveillance for Norovirus Gastroenteritis in a Nicaraguan Birth Cohort: A Nested Case—Control Analysis of Norovirus Risk Factors. Pathogens. 2023;12(3):505. doi: 10.3390/pathogens12030505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meierhofer R, Kunwar BM, Shrestha A. Changes in water treatment, hygiene practices, household floors, and child health in times of Covid-19: A longitudinal cross-sectional survey in Surkhet District, Nepal. Int J Hyg Environ Health. 2023;249:114138. Epub 20230214. doi: 10.1016/j.ijheh.2023.114138 ; PubMed Central PMCID: PMC9925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sosa-Moreno A, Lee GO, Van Engen A, Sun K, Uruchima J, Kwong LH, et al. Characterizing Behaviors Associated with Enteric Pathogen Exposure among Infants in Rural Ecuador through Structured Observations. The American Journal of Tropical Medicine and Hygiene. 2022;106(6):1747–56. doi: 10.4269/ajtmh.21-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zartarian VG FA, Leckie JO. Quantifying videotaped activity patterns: video translation software and training methodologies. J Expo Sci Environ Epidemiol. 1997;7:535–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(CSV)
(CSV)
Data Availability Statement
The data used in this analysis is attached in supplementary materials.