Abstract
Ribosomal proteins (Rps) are essential for viability. Genetic mutations affecting Rp genes were first discovered in Drosophila, where they represent a major class of haploinsufficient mutations. One mutant copy gives rise to the dominant “Minute” phenotype, characterized by slow growth and small, thin bristles. Wild-type (WT) and Minute cells compete in mosaics, that is, Rp+/− are preferentially lost when their neighbors are of the wild-type genotype. Many features of Rp gene haploinsufficiency (i.e. Rp+/− phenotypes) are mediated by a transcriptional program. In Drosophila, reduced translation and slow growth are under the control of Xrp1, a bZip-domain transcription factor induced in Rp mutant cells that leads ultimately to the phosphorylation of eIF2α and consequently inhibition of most translation. Rp mutant phenotypes are also mediated transcriptionally in yeast and in mammals. In mammals, the Impaired Ribosome Biogenesis Checkpoint activates p53. Recent findings link Rp mutant phenotypes to other cellular stresses, including the DNA damage response and endoplasmic reticulum stress. We suggest that cell competition results from nonautonomous inputs to stress responses, bringing decisions between adaptive and apoptotic outcomes under the influence of nearby cells. In Drosophila, cell competition eliminates aneuploid cells in which loss of chromosome leads to Rp gene haploinsufficiency. The effects of Rp gene mutations on the whole organism, in Minute flies or in humans with Diamond-Blackfan Anemia, may be inevitable consequences of pathways that are useful in eliminating individual cells from mosaics. Alternatively, apparently deleterious whole organism phenotypes might be adaptive, preventing even more detrimental outcomes. In mammals, for example, p53 activation appears to suppress oncogenic effects of Rp gene haploinsufficiency.
Keywords: ribosomal protein, Minute mutation, cell competition, stress response, Xrp1, p53, protein translation, cell communication, cell nonautonomy, genetic mosaic
In this review, Kiparaki and Baker explore insights gained from studying mutations affecting Ribosomal protein (Rp) genes. First identified in Drosophila, Rp mutations are well studied and cause Diamond Blackfan Anemia in humans. Many features of mutant Rp phenotypes are transcriptionally mediated, including reduced translation and slow growth controlled by Xrp1 in Drosophila and activation of p53 in mammals. Cells with reduced Rp dosage suffer cell competition, a non-autonomous reduction in cell viability near to wild type cells.
Minute mutations and their cell competition in Drosophila
One hundred years ago, the Morgan Drosophila lab at Columbia University described a set of mutations dominantly reducing bristle size, which they called “Minute” mutations (Bridges and Morgan 1923). The dominant Minute phenotype, which also includes developmental delay and reduced fertility and viability, was found to be associated with dozens of independent loci, later found to correspond almost entirely to the ribosomal protein (Rp) genes (Fig. 1a, b). The homozygous M/M genotypes are lethal (Kongsuwan et al. 1985; Andersson et al. 1994; Marygold et al. 2007). Prior to their molecular identification, it was thought that Minute mutations might affect systemic growth signals, because growth and maturation are under hormonal control. In 1975, Morata and Ripoll studied genetic mosaics to demonstrate that Minute genotypes in fact affected cell division rate cell-autonomously (Morata and Ripoll 1975). They also described a further, nonautonomous effect they called “cell competition”. Specifically, they documented that Minute cells (cells heterozygous for Rp gene mutants, i.e. Rp+/− cells), in addition to being slow-growing, are selectively eliminated from mosaic imaginal discs and replaced by neighboring normal cells (Rp+/+ cells) (Fig. 1c) (Morata and Ripoll 1975). This is interesting because the flies entirely heterozygous for mutations in Rp genes (Rp+/− or “Minute” flies) are viable, with near-normal size (Morata 2021). The cell nonautonomous influence of wild-type (WT) cells on Rp+/− cells measurably reduced their growth, whereas the growth of the nearby WT cells seemed to increase (Simpson 1979; Simpson and Morata 1981). It is important to note that the cell-autonomous growth differences between WT and Rp+/− cells also affect their representation in mosaic tissues (Martin et al. 2009).
Fig. 1.
The “Minute” phenotype of Drosophila melanogaster. a) Mutations in many Rp genes were first recovered as mutations causing a dominant reduction in the length and thickness of bristles on the adult body. Here, for example, the dorsal thoraces of wild-type and M(3)95C heterozygous flies (heterozygous for a mutation of the RpS3 gene) are similar in size, but the M(3)95C/+ bristles are shorter and thinner. b) Rp gene mutations also cause a dominant developmental delay, illustrated here as a delay of ∼40 h in the emergence of M(3)67C/+ adults (heterozygous for a mutation in the RpS17 gene) compared to the wild-type controls (these data are for female flies; males exhibit a similar delay). c) Cartoon illustrating how the fate of a single Rp+/− imaginal disc cell (orange) depends on its neighbors. On the left, an Rp+/− cell exposed to Rp+/+ neighbors undergoes apoptosis, whereas the rate of apoptosis is much lower for Rp+/− cells surrounded by other Rp+/− cells (right). Selective apoptosis results in the competitive elimination of Rp+/− regions form mosaics and their replacement by wild-type, Rp+/+ cells. Created with BioRender.
Multiple observations support the idea that cell competition represents a specific, active process, not just the passive effect of intrinsic differences in growth rate. One is the observation that cells do not compete across boundaries between compartments, the blocks of differently specified cells that construct the Drosophila imaginal discs, even when growing at different rates (Garcia-Bellido et al. 1973; Simpson 1979; Simpson and Morata 1981). The importance of relative differences is also highlighted by the observation that Rp+/− cells are no longer out-competed by WT cells during starvation (Simpson 1979). Although starved Rp+/− cells are even more slowly growing, apparently they are less distinct from starved Rp+/+ cells, highlighting also the notion that cell competition depends on the relative cellular fitness. It was later found that cells with heterozygous mutations in Rp genes (Rp+/− cells) undergo apoptosis when surrounded by wild-type (WT) cells (Moreno et al. 2002). Rp+/− cell death is predominantly localized at the boundaries, adjacent to Rp+/+ cells (Li and Baker 2007). Alterations in cell survival due to the proximity of other cells is a further indication of the active nature of cell competition.
Besides Rp mutations, other genetic differences are also now known to lead to cell competitions, not only in Drosophila, but also in mammals (Baker 2017; Maruyama and Fujita 2017). One apparently conserved example, described first in Drosophila, is competition between cells that express different levels of the Myc transcription factor that are too modest to have much effect on growth or development by themselves. Cells that have an extra copy of the Myc genomic locus, or use the tubulin promoter to drive modest transcription of a dMyc transgene, become “super-competitors” that are able to eliminate the nearby normal cells (de la Cova et al. 2004; Moreno and Basler 2004). During Myc driven super-competition, winners shift their metabolism, not only to outgrow but also to eliminate adjacent WT cells (de la Cova et al. 2014; Banreti and Meier 2020). Competition between cells expressing different levels of Myc has also been observed in mammals, in embryonic and adult tissues (Claveria et al. 2013; Sancho et al. 2013; Villa del Campo et al. 2014; Ellis et al. 2019). Super-competition is also reported between WT cells and cells with increased activity of Yorkie (either due to mutations in Hippo pathway or expressing higher levels of Yki), Wg, or JAK/STAT activity (Tyler et al. 2007; Neto-Silva et al. 2010; Vincent et al. 2011; Rodrigues et al. 2012). Differences in Hippo pathway can also lead to cell competition in mammals (Hashimoto and Sasaki 2019; Moya et al. 2019). The fact that Myc and other genes implicated in super-competition are oncogenic in different types of cancers supported the notion that cell competition might contribute to tumor development in mammals, as has now been demonstrated in several examples (Suijkerbuijk et al. 2016; Di Giacomo et al. 2017; Patel et al. 2017; Liu et al. 2019; Madan et al. 2019; Moya et al. 2019).
In other contexts, cell competition has a tumor-suppressive role. For example, cells with mutations in genes that are involved in apicobasal polarity (e.g. scribble, discs large) are eliminated from the Drosophila tissues when they are adjacent to WT cells, but in the absence of wild-type cells, tissues comprised entirely of polarity deficient cells form large tumors (Brumby and Richardson 2003; Igaki et al. 2009; Menendez et al. 2010; Tamori et al. 2010). Whether elimination is truly a consequence of proximity to WT cells is currently controversial. Recent study has suggested a role for systemic signals (de Vreede et al. 2022). In mammals, cells that have loss of Scribble and are cocultured with WT cells undergo p53-dependent apoptosis (Wagstaff et al. 2016). Accordingly, competition of Scribble mutant cells might be a conserved phenomenon, although the role for p53 appears unique to mammals.
There is considerable interest in the molecular mechanisms of cell competition, the physiological consequences of cell competition, and how it may contribute to diseases such as cancer, or be exploited in regenerative medicine. Many of the studies aimed at understanding cell competition mechanisms have also proven informative regarding cell-autonomous aspects of the Minute phenotype. In this review, we will discuss the molecular basis of both the Minute phenotype, and of cell competition of Minute cells, primarily in the Drosophila context but bringing in findings from Rp mutations in yeast, nematodes, zebrafish, mouse, and humans when it is informative to do so. We hope that a deep dive into the relationship of cell competition to one particular mutant syndrome will reveal concepts that may be applicable to other genotypes also.
Genes required for the Drosophila Minute phenotype and for cell competition
We and others have described genetic screens that particularly identified two genes that contribute to both the Minute phenotype and to the competition on Minute mutant cells in Drosophila (Fig. 2) (Tyler et al. 2007; Lee et al. 2016, 2018; Baillon et al. 2018; Kale et al. 2018; Boulan et al. 2019; Ji et al. 2019). One gene encodes RpS12, a Rp of the small subunit and an essential protein. Unusually, rpS12 does not belong to the Minute class of Rp gene loci, because loss of one rpS12 gene copy does not lead to the dominant Minute phenotype, unlike haploinsufficiency for 66 of the 79 Rp loci (Marygold et al. 2007). A genetic screen recovered a missense point mutation of rpS12 that substitutes glycine 97 with aspartic acid (Tyler et al. 2007; Kale et al. 2018). This rpS12G97D allele does not affect the viability of homozygously mutant flies, but prevents the competitive elimination of Rp+/− cells mutated at other, dominant Minute Rp gene loci. Further studies conclude that, in addition to its essential role in the ribosome, the RpS12 protein has a second function as a sensor of Rp imbalance, and that this second role helps initiate the Minute phenotype. That is, haploinsufficiency for any of the 66 Rp genes that lead to a Minute phenotype appears to result in an increased, or novel, RpS12 activity that is responsible for aspects of the Minute phenotype (Kale et al. 2018). While the molecular basis of this signaling is not yet known, it is clear that it serves to activate expression of the second gene discovered to mediate the Minute phenotype, encoding the transcription factor Xrp1 (Lee et al. 2018; Boulan et al. 2019; Ji et al. 2019).
Fig. 2.
A transcriptional stress response in rp mutant cells genetic screens and analyses have revealed that rp mutations activate expression of a transcription factor, Xrp1, by means of a special activity of the ribosomal protein RpS12 that is independently mutable from its essential function. Xrp1 activates PERK to phosphorylate eIF2α, and so suppresses most cellular translation. Xrp1 forms a heterodimer with the ubiquitous Irbp18 protein, constituting a sequence-specific transcription factor that regulates several hundred single copy genes, as well as some mobile elements. These include genes involved in the DDR as well as antioxidant genes implicated in the response to oxidative stress. It is presumed that these transcriptional targets, together or individually, lead to the Xrp1-dependent properties of Rp mutant cells, which include developmental delay, cell competition, JnK signaling, and defects in proteostasis and autophagy. In addition to Rp gene haploinsufficiency, Xrp1 is also activated by multiple other challenges, including endoplasmic reticulum (ER) stress, DNA damage, defective ribosome function, and oxidative stress. Thus, Xrp1 is a central player in a shared transcriptional response to multiple cellular stresses, at least some of which also lead to cell competition.
Mutations at the Xrp1 locus were recovered from at least four independent genetic screens, two targeting genes required for cell competition of Rp mutant cells (Lee et al. 2016; Baillon et al. 2018), one seeking genes required for the developmental delay caused by Rp depletion (Boulan et al. 2019), and one seeking modifiers of a amyotrophic lateral sclerosis (ALS) disease model in Drosophila (Mallik et al. 2018). Xrp1 had been described previously, and presumably received the name X-Ray induced P53-dependent #1, as the major transcriptional target of p53 following irradiation (Brodsky et al. 2004; Akdemir et al. 2007). Xrp1 encodes a AT-hook, bZip-domain protein that binds DNA as a heterodimer with Irbp18, another bZip protein that is the Drosophila C/EBP protein (Reinke et al. 2013; Francis et al. 2016). Xrp1/Irbp18 is also part of the protein complex binding to DNA sequences of the P element transposon (Francis et al. 2016).
In otherwise wild-type flies, Xrp1 null mutant animals are viable and morphologically normal, but Xrp1 is required in many aspects of Minute phenotype, including cell competition (Baillon et al. 2018; Lee et al. 2018). Xrp1 is also responsible for the reduced growth of Rp+/− imaginal disc cells, and contributes significantly to the developmental delay of Rp+/− flies, since the rate of development is substantially restored when Xrp1 is mutated (Lee et al. 2018; Boulan et al. 2019). Xrp1 is even responsible for the reduced translational rates in Rp+/− cells (Lee et al. 2018). Therefore, reduction in the rate of the bulk protein synthesis of the cell is not a direct effect of Rp haploinsufficiency, as might easily be predicted, but depends largely on a regulatory response coordinated by the Xrp1 transcription factor (Lee et al. 2018; Ji et al. 2019). Importantly, Xrp1 protein is almost undetectable in imaginal discs from the wild type, but mRNA levels are elevated and protein expressed in Rp+/− cells, downstream of the RpS12 activity that occurs (Lee et al. 2018).
How RpS12 is responsible for Xrp1 induction in Rp mutant cells is not yet certain. It could be that RpS12 in the ribosomal small subunit (SSU) particularly contributes to translational control of Xrp1 expression. If this is the case, the only way that extra copies of the rpS12 gene locus could enhance Xrp1 expression, developmental delay, and elimination of Rp mutant cells, would seem to be if two pools of SSU normally exist, one with and one without RpS12, so that rpS12 expression levels could affect the proportions of the two SSU species (Kale et al. 2018). This is potentially an example of the specialized ribosome hypothesis, which posits that all ribosomes may not share identical compositions, with structural variation contributing to functional specificity (Genuth and Barna 2018). Alternatively, RpS12 might be present in all SSU but have an additional function outside the ribosome that promotes Xrp1 expression (Kale et al. 2018). Many such extra-ribosomal functions have been described for other ribosomal proteins, including transcriptional and post-transcriptional regulation (Warner and McIntosh 2009).
Xrp1 expression, and cell competition, also result from multiple other genetic insults, besides mutating Rp genes. This includes reduction in rRNA transcription (by knock-down of TAF1B) (Kiparaki et al. 2022), impaired ribosome function (by knock-down of multiple initiation, elongation, and recycling factors) (Kiparaki et al. 2022), mutation in Helicase at 25E (Hel25E) (Ochi et al. 2021), inhibition of proteasome activity (Langton et al. 2021; Kumar and Baker 2022), depletion of the E3 ubiquitin ligase mahj/DCAF1 (Langton et al. 2021; Kumar and Baker 2022), and endoplasmic reticulum (ER) stress (Langton et al. 2021; Ochi et al. 2021; Kiparaki et al. 2022). Thus, Xrp1 appears to be a sensor of multiple insults that thereby share a common transcriptional response leading to reduced translation and growth and elimination of affected cells by cell competition (Kiparaki et al. 2022; Kumar and Baker 2022) (Fig. 2).
Gene regulation by Xrp1
Xrp1 has been confirmed to be a sequence-specific transcription factor, which focusses attention on the transcriptome of Minute cells (Kiparaki et al. 2022). RNA-seq indeed reveals a transcriptional signature of Minute wing imaginal discs, comprising several hundred genes with altered mRNA accumulation (Kucinski et al. 2017). More than 80% of these mRNA changes depend on Xrp1 and RpS12 function (Lee et al. 2018; Ji et al. 2019). These Xrp1-dependent transcriptional changes are likely to contribute to the Minute phenotype. One strong candidate is Dilp8, which is transcriptionally upregulated ∼10 × in the Rp+/− genotypes, and is likely contributing to the developmental delay of Minute larvae (Lee et al. 2018; Boulan et al. 2019; Ji et al. 2019). Which specific aspects of the transcriptional response lead to reduced translation, cellular growth, and cell competition is not yet certain. Overall, the Minute transcriptional response is enriched in DNA repair genes and antioxidant genes (Kucinski et al. 2017; Lee et al. 2018). There is no clear evidence for DNA damage or oxidative stress as yet (Ferrus 1975; Gladstone et al. 2012; Kucinski et al. 2017). The gene expression signatures possibly reflect shared roles of Xrp1 in other processes. That is, Xrp1 is induced as the major transcriptional target of p53 following DNA damage (Brodsky et al. 2004), and is thought to play a role in the DNA damage response (DDR), based on increased frequency of loss of heterozygosity in Xrp1 mutants following irradiation (Akdemir et al. 2007). The preponderance of DNA repair genes among Xrp1 targets may be related primarily to this aspect of Xrp1 function. Similarly, Xrp1 is induced by overexpression of Nrf2, the master regulator of oxidative stress (Langton et al. 2021), and so might play a role in oxidative stress. Certainly, Xrp1 mediates induction of antioxidant genes following ER stress (Brown et al. 2021). ER stress is tightly linked to oxidative stress, because oxidation of cysteine residues to form disulfides within the ER requires proper redox state (Cullinan et al. 2003; Harding et al. 2003; Tu and Weissman 2004). How these DNA repair-like and oxidative stress-like transcriptional programs contribute to reduced translation and growth, or to cell competition, remains to be determined.
Xrp1 regulates transposable element transcription
Transposable elements (TE) represent another potentially important target of Xrp1. Xrp1, as a heterodimer with Irbp18, binds to inverted repeats of the P element and appears to facilitate DNA repair after transposase cleavage, since it is required for full P element activity (Francis et al. 2016). Xrp1/Irbp18 is also the major transcriptional regulator of the retroelement Copia, whose transcription is elevated ∼10 × in Rp mutant wing discs in an Xrp1-dependent manner (Kiparaki et al. 2022). Increased TE expression and mobility are linked to age-associated phenotypes and to pathological conditions (Goodier 2016; Sun et al. 2018; Burns 2020). They are mutagenic, and also the main vectors of horizontal genetic information transfer, which are speculated to occur in neurodegenerative diseases and within cancer microenvironments (Schaack et al. 2010; Brettschneider et al. 2015; Kawamura et al. 2017; Chang and Dubnau 2019). p53 normally restrains retrotransposons both in Drosophila and in mammalian cancers (Goodier 2016; Kastenhuber and Lowe 2017; Tiwari et al. 2018, 2020). It is also speculated that somatic transpositions could be part of cellular diversity and neuronal plasticity mechanisms (Bourque et al. 2018), contributing to mosaicism and cell competition.
Xrp1 and pathways of neurodegenerative disease
Xrp1 is also known as a suppressor of a Drosophila model of ALS. Increased expression of Xrp1 mediates the toxic neuronal and muscular effects of mutations in the cabeza gene that encodes a FUS homolog implicated in the genesis of ALS (Mallik et al. 2018; Catinozzi et al. 2020). A hexanucleotide repeat expansion of the C9orf72 gene is the most common cause of familial ALS. Xrp1 and two of its targets shared in Rp+/− cells, Arc1 and Gadd45, are upregulated in a C9orf72 dipeptide repeat ALS Drosophila model (Xu et al. 2019). Interestingly, knocking down either Gadd45 or Arc1 was sufficient to ameliorate the neurodegenerative phenotypes of this model, but the potential role of Xrp1, which could be the most upstream regulator of this response, was not investigated in this study (Xu et al. 2019). Additionally, a recent study reports that the C9orf72 dipeptide activates a p53 transcriptional program driving neurodegeneration in multiple models, including Drosophila (Maor-Nof et al. 2021). These two studies strongly suggest that Xrp1 could drive a significant part of the C9orf72 neurodegenerative phenotype.
The suppression of translation in Minute mutants
As might be expected, global translation is reduced in Rp mutant cells, in Drosophila as in mice (Boring et al. 1989; Oliver et al. 2004). Our analysis had revealed that Xrp1 is responsible for the decreased rate of global protein synthesis in Rp+/− cells (Lee et al. 2018). Subsequently, at least four groups identified the basis of reduced translation in Drosophila as phosphorylation of the translation factor eIF2α (Baumgartner et al. 2021; Ochi et al. 2021; Recasens-Alvarez et al. 2021; Kiparaki et al. 2022). Phosphorylation of eIF2α is a well-known mechanism of translation initiation that globally inhibits cap-dependent translation initiation in response to ER stress, amino-acid starvation, and, in mammals, infection with certain viruses, and heme deficiency (Farrell et al. 1977; Wek 2018; Wang and Proud 2022). The kinase phosphorylating eIF2α in Minute cells is PERK, a transmembrane kinase that is best known for its activation by ER stress (Ochi et al. 2021; Kiparaki et al. 2022). Accordingly, depletion of PERK, which has negligible effect on wild-type wing discs, restores normal levels of eIF2α activity and overall translation, and the same is seen upon overexpression of PPP1R15, the sole eIF2α phosphatase known in Drosophila (Ochi et al. 2021; Kiparaki et al. 2022). It is remarkable that the overall reduction in translation that is a typical of Rp mutant cells is due, not to a reduction in ribosome numbers, which are not affected by Xrp1 mutations (Kiparaki et al. 2022), but to regulation of a translation initiation factor by a transcription factor.
Consensus on the role of eIF2α phosphorylation in reducing translation in Minute cells is not yet accompanied by understanding of the mechanisms of eIF2α phosphorylation, or of the relationship between eIF2α phosphorylation, reduced translation, and cell competition. Although RNA-seq analysis shows that Xrp1 alters mRNA levels of PERK and other ER stress proteins, it is uncertain whether these changes are sufficient to explain the altered PERK activity observed (Kiparaki et al. 2022). Two groups have demonstrated reduced proteasomal and autophagic flux in Rp mutants, leading to accumulation of ubiquitinylated protein aggregates and autophagosomes (Baumgartner et al. 2021; Recasens-Alvarez et al. 2021). The protein aggregates and autophagosome accumulations seen in mutants affecting the SSU are downstream of Xrp1 activity and could contribute to the Xrp1-dependent PERK activation in those cells (Kiparaki et al. 2022). Interestingly, RpL27+/− mutant cells do not show the same increase in protein aggregation over WT cells, even though Xrp1 and PERK are still activated (Kiparaki et al. 2022). This suggests that protein aggregation might not be equally prevalent in all Minute mutants. Although no obvious distinction has previously been made between Drosophila phenotypes resulting from mutations affecting the SSU or large subunit (LSU) (Marygold et al. 2007), responses to defects in subunit biogenesis differ in yeast (Cheng et al. 2019). PERK is a transmembrane protein whose regulatory domain within the ER interacts with luminal chaperones and unfolded proteins, so it is unlikely PERK is directly activated by proteotoxic stress in the cytoplasm. It is possible that PERK could be activated indirectly, because cytoplasmic and luminal proteins compete for proteasomal destruction (Nishitoh et al. 2002). All in all, the molecular steps between Xrp1 expression and PERK activation remain to be established.
There is also debate over the contribution of eIF2α phosphorylation to cell competition. It was shown several years ago that differences in translation and growth between cells do not necessarily stimulate competition. Wild-type cells are not significantly affected by neighboring cells growing more rapidly due to CycD/Cdk4 activity, or due to activation of the PI3K pathway (de la Cova et al. 2004). Similarly, cells experiencing reduced global translation due to overexpression of 4E-BP are not eliminated by competition with nearby WT cells, showing that reduced global translation is not sufficient for cell competition (Baumgartner et al. 2021). Consistent with this, cells depleted for translation factors including eIF4G, eIF5A, and eEF2 are not eliminated by competition from nearby wild-type cells as long as they are prevented from expressing Xrp1, even though they exhibit significantly reduced global translation (Kiparaki et al. 2022). These findings indicate that lowered translation does not seem to be sufficient for cell competition. Accordingly, depletion of PPP1R15 is not sufficient to induce cell competition in the absence of Xrp1 (Kiparaki et al. 2022). There is uncertainty, however, whether lowered translation might be necessary for cell competition, despite not being sufficient. This should be testable by restoring global translation rate to Rp imaginal disc cells by PERK depletion or by overexpression of PPP1R15. One group concluded that PERK depletion could suppress cell competition of Rp mutant cells, consistent with a contribution of reduced translation to cell competition (Ochi et al. 2021). However, we found that mutating PERK did not prevent elimination of Rp mutant cells, indicating that other aspects of Xrp1 function must be required (Kiparaki et al. 2022). This was in agreement with Xrp1 being necessary for competition of cells having defects in other steps of translation (such as initiation, elongation, etc.), whose elimination was not regulated by PERK and PPP1R15 (Kiparaki et al. 2022). The reasons for these contrasting results, and the contribution of changes in translation to cell competition, remain to be resolved.
The molecular mechanism of competitive cell interactions
The transcriptome changes wrought by Xrp1 in Rp mutant cells number in the hundreds of genes, and the changes in translation efficiency due to eIF2α phosphorylation are likely to be numerous also. The exact nature of the critical difference(s) between wild-type and Rp mutant cells that trigger local elimination of the latter are uncertain, although there are many theories. It has been proposed that WT and Rp mutant cells compete for Dpp signaling (Moreno et al. 2002), and are induced to express different isoforms of the putative Ca channel flower (Rhiner et al. 2010). It was proposed, and also disputed, that apoptotic corpse engulfment pathways somehow enhance apoptosis in Rp mutant cells (Li and Baker 2007; Lolo et al. 2012). Rp mutant cells are proposed to activate genes that also function in innate-immune pathways (Meyer et al. 2014). It is proposed that activity of Nrf2, which is the transcriptional master regulator of the oxidative stress response, is a trigger for elimination of Rp mutant cells, even though oxidative stress was not detected (Kucinski et al. 2017). Another hypothesis is that Rp cells near to WT cells activate autophagy, which is pro-apoptotic in the context of chronic Jnk signaling that all Rp mutant cells experience (Nagata et al. 2019). All these suggestions require more investigation. It should also be pointed out that most of these hypotheses do not specify whether or how a specific recognition of Rp mutant cells by WT cells takes place. In the case of WT cells undergoing elimination by super-competitor cells with elevated Myc, a cell competition mechanism thought to be distinct from that between WT and Rp mutant cells, the current model is that imbalances in expression of secreted innate-immune regulators and transmembrane receptors leads to local, pro-apoptotic innate-immune signals, without specific recognition of out-competed cells (Alpar et al. 2018). There is also the possibility that mechanical stress, due to differential growth of WT and Rp mutant cells, may contribute to cell elimination (Matamoro-Vidal and Levayer 2019). One potential insight into the mechanism of elimination is that, unlike most apoptotic processes in Drosophila development, which depend predominantly on a single initiator caspase, Dronc, competitive apoptosis of Rp mutant cells involves little Dronc activity and can also be initiated by the little-known caspase Dream/Strica (Kale et al. 2015). Unfortunately, this does rather little to clarify the mechanism of competitive cell death for now, since little is known regarding mechanisms of Dream/Strica activation.
“Minute” phenotypes and pathways in other eukaryotes
As might be expected, Rp genes are essential, and often have haploinsufficient mutant phenotypes, in organisms from yeast to mice and man. Unexpectedly, clear homologs of Xrp1 are difficult to find outside Dipteran insects, and are very divergent even there (Blanco et al. 2020). The rapid evolution does not indicate genetic drift due to lack of selective value, but instead occurs because Drosophila Xrp1 is evolving at a rapid rate under the strong influence of positive selection for evolutionary change (Blanco et al. 2020). It is interesting that Xrp1 interacts with at least two TEs, facilitating both P element transposition and transcription of Copia elements, because evolutionary arms races with pathogens are one possible cause of such rapid evolution (Francis et al. 2016; Kiparaki et al. 2022).
The difficulty recognizing Xrp1 homologs raises the question of whether Rp mutant phenotypes are different in other organisms, or mediated by divergent or distinct transcription factors. Remarkably, evidence is emerging that Rp mutant phenotypes in yeast, zebrafish, and mice do also depend on transcription, as seen for Drosophila. In yeast, acute RP depletion results in activation of the ribosome assembly stress response, rapidly interrupting RP gene transcription by depleting a transcription factor IFH1 from RP gene promoters (Albert et al. 2019; Tye et al. 2019). Chronic RP reduction, due to deletion of one paralog of the many duplicated pairs of RP genes in yeast, leads to reduced growth and changes in ribosome profiles that are almost entirely explained by changes in mRNA abundance, not by translation efficiency (Cheng et al. 2019). Zebrafish haploinsufficient for rp gene mutants show a cancer predisposition with ageing that is related to activation of the transcription factor p53 in rp mutants, and its inactivation in tumors (Amsterdam et al. 2004; MacInnes et al. 2008). In humans, mutations in many RP genes lead to Diamond-Blackfan Anemia, characterized by erythropoietic defects, as well as reduced growth, delayed maturity, skeletal malformations, and increased cancer predisposition (Vlachos et al. 2012, 2018; Ulirsch et al. 2018). As in zebrafish, p53 is activated in human and mouse cells heterozygous for RP mutations, and is responsible for aspects of the phenotype in Rp+/− mutant mice. The case can be made that mammalian p53 carries out functions similar to those of Xrp1, although there are also similarities to the bZip-domain protein DDIT3/CHOP, as explained further below.
Xrp1, P53, and DDIT3/CHOP
p53 is unrelated to Xrp1 by sequence and structure. The proteins are related functionally, however, because Xrp1 is a p53 target in the Drosophila DDR (Brodsky et al. 2004), and is believed to play a role in DDR because loss of heterozygosity following irradiation increases in the absence of Xrp1 (Akdemir et al. 2007). Xrp1 transcription is also increased after perturbation of the spindle-assembly checkpoint (Baillon et al. 2018). P53 is not required for Rp+/− phenotypes in Drosophila (Kale et al. 2015), but mammalian p53, by contrast, is part of the impaired ribosome biogenesis checkpoint (IRBC). Whenever mammalian ribosome biogenesis is disrupted, the 5S RNP, a component of the 60S large ribosomal subunit that comprises the 5S rRNA, RpL5, and RpL11, binds and inhibits HDM2 (the main ubiquitin ligase for p53 in humans: MDM2 in mice), resulting in p53 stabilization (Fig. 2a) (Zhang and Lu 2009; Bursać et al. 2012; Donati et al. 2013; Pelletier et al. 2020) (Gentillela et al. 2017; Pelletier et al. 2018). Recent studies have linked the reduced translation rates in Rps6+/− mouse cells to p53, comparable to the role of Xrp1 in the reduced translation rates of Rp+/− cells in Drosophila (Tiu et al. 2021). The IRBC is activated not only by Rp mutations but also by other nucleolar stresses, and by expression of oncogenes such as Myc, even though oncogenes enhance ribosome biogenesis (Fig. 2a) (Derenzini et al. 2017; Morcelle et al. 2019). We suggested that the mammalian IRBC may be replaced in Drosophila by a pathway in which RpS12 replaces RpL5/RpL11, activating Xrp1 in place of p53 (Fig. 3) (Baker et al. 2019).
Fig. 3.
Comparing the DNA damage response and IRBC in mammals and in Drosophila. a) In mammals, DNA damage activates p53, leading to adaptive responses and/or apoptosis. P53 is also activated by Rp haploisufficiency, through the Impaired Ribosome Biogenesis Checkpoint. Although it is not yet demonstrated that mosaic DNA damage, or mosaic Rp mutation, lead to cell competition in mammals, differences in p53 activity levels between mammalian cells do lead to competitive elimination of cells in many contexts with relatively higher p53 activity. b) Drosophila DNA damage activates p53, leading to adaptive responses and/or apoptosis. P53 also activates Xrp1 transcription, which contributes to adaptive responses to irradiation. Rp mutations do not activate p53 in Drosophila imaginal discs, but instead activate expression of Xrp1, a p53 target, which is required for cell competition.
DDIT3/CHOP came to attention as a potential functional correspondent of Xrp1 through studies of the Drosophila C/EBP protein Irbp18, the only known heterodimer partner of Drosophila Xrp1 (Reinke et al. 2013; Francis et al. 2016). Xrp1 functions in Minute genotypes also depend on Irbp18 and are thought to be mediated by the Xrp1/Irbp18 heterodimer (Blanco et al. 2020). Mammalian heterodimer partners of C/EBP are therefore candidates to replace Xrp1 functionally in mammals. Accordingly, DDIT3/CHOP is a C/EBP partner that is also induced after irradiation, and promotes cell death, reminiscent of Xrp1 (Luethy et al. 1990; Yang et al. 2017). Ectopic hDDIT3 expression in Drosophila leads to a phenotype similar to that of Xrp1 overexpression, and shows some dependency on Irbp18 (Blanco et al. 2020). In mammals, DDIT3/CHOP particularly couples ER stress to apoptosis (Zinszner et al. 1998; Marciniak et al. 2004; Yamaguchi and Wang 2004; Ohoka et al. 2005; Li et al. 2014; Tian et al. 2019). DDIT3/CHOP protein expression is induced by Atf4, a transcription factor whose expression is enhanced by ER stress, because Atf4 is encoded by one of the few transcripts whose translation is enhanced when eIF2α is phosphorylated (Palam et al. 2011). In Drosophila, Xrp1 protein expression is also induced by ER stress and eIF2α phosphorylation. This was discovered both by the Igaki group, who found that ER stress leads to cell competition, which they then found to be Xrp1-dependent (Ochi et al. 2021), and also by the Ryoo group, who were inspired by the possibility that Xrp1 and DDIT3/CHOP might share similar functions to discover that Xrp1 was responsible for Atf4-independent transcriptional responses to ER stress in Drosophila (Brown et al. 2021). In the course of assessing whether eIF2α phosphorylation lies upstream or downstream of Xrp1 in the cell competition pathway, we and others additionally found that eIF2α phosphorylation stimulated Xrp1 expression, and cell competition (Langton et al. 2021; Kiparaki et al. 2022). All in all, somewhat related pathways of ER stress can be drawn for Drosophila and for mammals, whereby ER stress is coupled to cell death by Xrp1 in flies and by DDIT3/CHOP in mammals (Fig. 4). Accordingly, it is plausible that multiple other stresses besides Rp mutations, especially those leading to eIF2α phosphorylation, might promote cell competition if they occur in sporadic somatic cells, as has already been shown is the case for cells experiencing ER stress (Ochi et al. 2021; Kiparaki et al. 2022).
Fig. 4.
Aspects of the ER stress response in mammals and in Drosophila. a) In mammals, ER stress leads to eIF2α phosphorylation by PERK. This inhibits most cap-dependent translation, but paradoxically enhances translation of a select subset of mRNAs, containing unusual 5′-untranslated region (5'-UTR) structures. One of these encodes Atf4, a transcription factor controlling multiple aspects of the ER stress response. Atf4 also activates transcription of DDIT3/CHOP, a bZip-domain protein similar to Xrp1. DDIT3/CHOP is particularly involved in inducing apoptosis in response to ER stress. b) ER stress in Drosophila activates expression of Xrp1 protein independently of Atf4, perhaps because some Xrp1 mRNAs contain 5′-UTR structures that are typical of transcripts translated when eIF2α is phosphorylated. Xrp1 can induce expression of genes that contribute to ER stress adaptation and is a potent inducer of apoptosis.
Optimizing stress responses through cell competition?
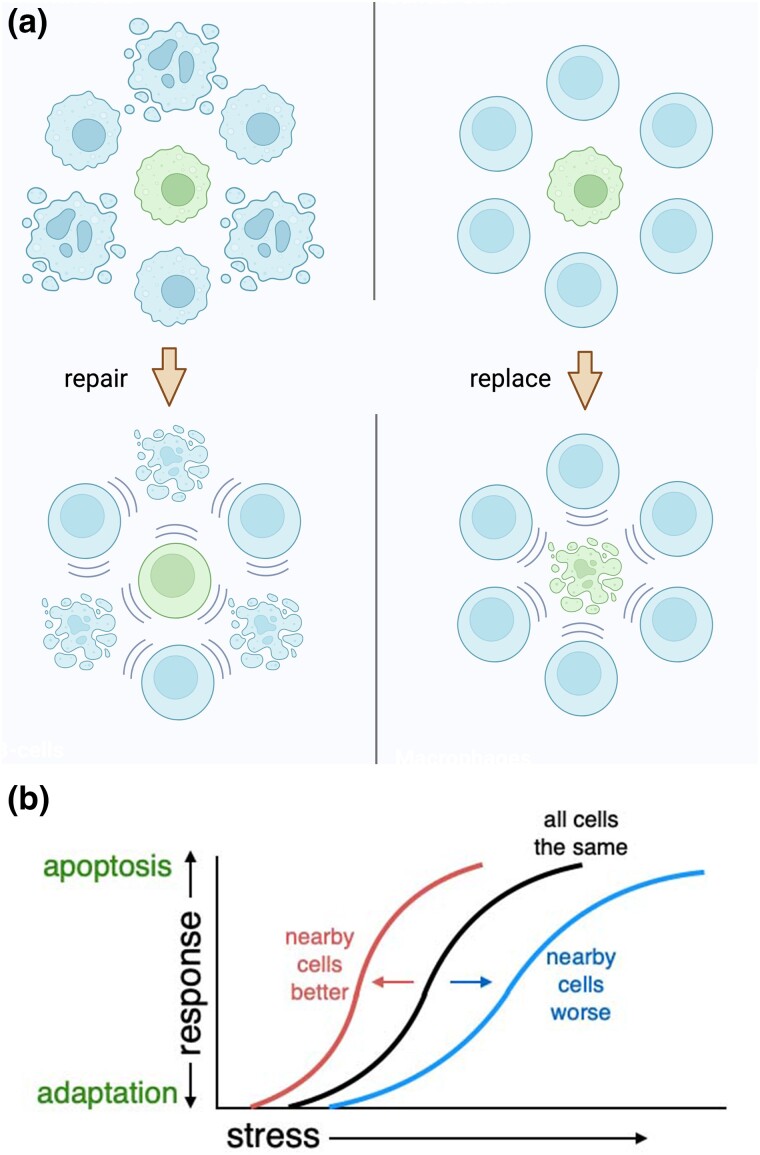
These recent studies reveal that Minute cells can be considered to exhibit a transcriptional stress response that is responsible for multiple aspects of the Minute phenotype, mediated by the bZip-domain transcription factor, Xrp1. The ribosome assembly stress is related to the unfolded protein response, the DDR, and other stress responses. One feature of many stress responses, including the unfolded protein response and the DDR, is their bifunctional role in either promoting cell adaptation and repair in the face of stress, or promoting cell death (Roos and Kaina 2013; Sano and Reed 2013; Green and Levine 2014; Navarro-Yepes et al. 2014). It is usually believed that stress responses effectively calculate, within each cell, the degree of stress-induced damage. This determines whether it is better to attempt to repair the damage and protect against ongoing stress or to eliminate the severely damaged cells by apoptosis. Removing the most damaged cells is expected economize on resources that could better be marshaled elsewhere, and perhaps to minimize transformation or other deleterious effects of highly damaged cells. It seems evident that a purely cell-autonomous system can only crudely optimize the decision to repair or replace. If damage is variable between cells, then a cell with any particular level of damage might either be the least damaged cell in the tissue, which it would presumably be imperative to preserve, or the most damaged, which might best be removed and replaced. Accordingly, a greater optimization of resources should be achieved if the status of other, nearby cells is factored into the repair/replace decision. Eliminating and replacing damaged cells should be a more attractive proposition when less-damaged replacements will be available nearby. By contrast, protecting and repairing damaged cells might be the only viable option when the status of other nearby cells is equally bad or worse. We propose that cell competition provides just such a mechanism to adjust the outcome of stress responses to the status of nearby cells (Fig. 5). The familiar image of genetically identical Rp+/− cells largely surviving en masse but predisposed to apoptotic death near to Rp+/+ cells may represent a bias of stress responses toward eliminating Rp+/− cells when more healthy cells are available nearby as a source of replacements.
Fig. 5.
Model for optimizing stress responses. a) The cartoon illustrates the fate of cells exposed to differing amounts of stress. In both the left and right panels, the central cell experiences identical stress levels. On the left, all the surrounding cells are at least as badly affected, or worse. In this situation, it would be most advantageous to preserve the less-damaged central cell, and repair it to the extent possible, as a resource for repopulating the tissue after removal of more severely damaged cells. On the right, the central cell is more stressed than it neighbors. Under these circumstances, it might be preferable to remove this cell, using the neighbors to provide a pool of replacements. Created with BioRender. b) The decision to repair or replace damaged cells could become context-dependent if the probability of apoptosis is affected both by the cell-autonomous stress level and by the status of the neighbors that are potential replacements. Such a system could result in progressive elimination of a more-stressed population in a mosaic, as seen in cell competition.
It may be interesting in this regard to compare the phenotypes of Rp gene mutations in organisms where cell competition is or is not possible. In the yeast Saccharomyces cerevisiae, for example, a unicellular organism where no selective advantage accrues from programmed cell death, no p53 gene or apoptosis pathway are found. Interestingly, RP gene mutations protect yeast from ER stress and extend replicative lifespan (which is not to say that these yeast strains are healthy in other respects) (Chiocchetti et al. 2007; Steffen et al. 2012). In Drosophila and in mammals, Rp mutations delay growth and increase mortality (Lambertsson 1998; Ulirsch et al. 2018). It could be that these deleterious consequences of Rp gene mutations in whole animals are collateral damage of the capacity to remove and therefore replace individual stressed and damaged cells by cell competition. It is notable, therefore, that like in yeast, rp mutations also extend lifespan in Caenorhabditis elegans, a multicellular animal where replacing apoptotic cells is not generally possible because of the inflexible cell lineage (Hansen et al. 2007). It will be interesting to see whether Rp mutation are generally more deleterious, on a whole animal basis, in multicellular organisms with regulative development where cell competition in response to mosaic mutation is possible, and less deleterious in unicellular organisms or those with mosaic development where death of individual damaged cells is less likely to be adaptive for the organism.
Physiological functions of cell competition
This review has not yet explicitly addressed how cell competition is advantageous for the organism. In Drosophila, multiple specific advantages of cell competition have been suggested. These include ensuring reproducible organ size in the face of variations of cell growth (Simpson and Morata 1981; de la Cova et al. 2004), eliminating spontaneous developmental defects, and thereby extending longevity (Merino et al. 2015), and eliminating preneoplastic cells before tumors can form (Brumby and Richardson 2003; Tamori et al. 2010). It is not certain whether Rp mutant cells are eliminated by cell competition in mammals (Oliver et al. 2004), but competitive elimination of cells expressing lower Myc levels is proposed to maintain pluripotency in early mouse embryogenesis(Díaz-Díaz et al. 2017), as well as shown to maintain epidermal function (Ellis et al. 2019).
Now that the pathway eliminating Rp+/− mutant cells is partially known, its contribution to these processes can be investigated in Drosophila. Xrp1 mutant adults do not exhibit obvious developmental defects, or shortened lifespan (Lee et al. 2018; Mallik et al. 2018), suggesting that this cell competition pathway may not be relevant to the removal of developmentally aberrant cells in the same way as has been suggested for other cell competition genes (Merino et al. 2015). Recently, however, Rp gene loci and the Xrp1 pathway have been found to help remove aneuploid cells (Ji et al. 2021).
A role of cell competition in removing aneuploid cells was first suggested based on the evidence that aneuploid cells resulting from DNA damage are eliminated from Drosophila imaginal discs, the presence of Rp loci all over the genome, and the known competitive elimination of Rp haploinsufficient cells (Titen and Golic 2008; McNamee and Brodsky 2009). Consistent with the predictions, cells with heterozygous chromosomal deletions are eliminated during development when the deletions included a Rp gene, but only in mosaics with WT cells, and depending on the rpS12 and Xrp1 genes, implicating cell competition (Ji et al. 2021). By contrast, cells heterozygous for chromosomal deletions that did not affect any Rp genes were generally able to proliferate and survive to differentiate adult tissues. The one exception encountered was that cells haploinsufficient for the eIF2γ gene were also lost by cell competition. The explanation may be that haploinsufficiency for eIF2γ, encoding a component of the eIF2 translation initiation factor, reduces eIF2 function much as phosphorylation of eIF2α does, and activates Xrp1 and cell competition by the same mechanism (Ji et al. 2021). Thus, the copy number of Rp genes, and possibly a few select other genes encoding proteins that act in the same pathway like eIF2γ, are spread across the genome and serve as sensors for some examples of aneuploidy. Eliminating aneuploid cells is likely to be a beneficial function of cell competition (Ji et al. 2021; Baker and Montagna 2022).
In humans, aneuploidy is responsible for birth defects and miscarriages, and is a hallmark of ageing, cancer and neurodegeneration (Sheltzer and Amon 2011; Lopez-Otin et al. 2013; Yurov et al. 2019; Ben-David and Amon 2020). Removing sporadic aneuploid cells should therefore be advantageous in humans (Baker and Montagna 2022). Eliminating aneuploid cells from mammalian embryos exhibiting mosaic aneuploidy, which are surprisingly common, is likely to reduce miscarriage and birth defects, for example (Hook 1981; van Echten-Arends et al. 2011; Bazrgar et al. 2013; Greco et al. 2015). Mouse p53 is shown to mediate the elimination of aneuploid embryonic cells before, during and after implantation (Singla et al. 2020), consistent with the role of P53 activity differences in cell competition between cells in multiple other mammalian tissues (Bondar and Medzhitov 2010; Dejosez et al. 2013; Wagstaff et al. 2016; Fernandez-Antoran et al. 2019). It is also hypothesized that aneuploidy can sometimes be adaptive, where various stresses (e.g. ER stress, heat) lead cells to rearrange their chromosomes in order to adapt and survive (Beaupere et al. 2018; Chunduri and Storchova 2019).
Adaptive value of the Minute phenotype
If much of the effect of Rp gene haploinsufficiency reflects a transcriptional response, the question arises why such an apparently deleterious response has evolved. The findings in Drosophila raise the possibility that Xrp1 activity in Minute cells is adaptive because it enables cell competition to eliminate aneuploid cells, and cells with some other stresses. In this view, reduced translation and growth retardation would be adaptive for the organism when they occur only in sporadic cells, because they enable cell competition (Lee et al. 2018). It is possible that reduced translation and growth retardation serve no useful purpose when the whole animal is of the Rp mutant genotype, but represent the unavoidable price to be paid for the cell competition pathway. If is true of humans, Diamond-Blackfan Anemia could also be an unfortunate consequence of pathways that are adaptive when activated only in sporadic Rp mutant cells, but collateral damage when expressed in the whole organism.
An alternative hypothesis is that the consequences of Rp mutations on the whole animal are in fact adaptive, and that the deleterious appearance of reduced translation and growth is only superficial. This idea is encouraged by recent findings concerning mahj/Dcaf1, another mutant genotype that is eliminated by Xrp1-dependent competition. Although clones of mahj/Dcaf1 cells survive better in the absence of Xrp1, codepletion of mahj and Xrp1 from some tissues is lethal for the organism (Kumar and Baker 2022). Thus, Xrp1 function might be adaptive for mahj mutant cells, in addition to facilitating their elimination by cell competition (Kumar and Baker 2022).
There is, as yet, little evidence that the Minute phenotype is advantageous in Drosophila, but the IRBC may be adaptive in mammals. Mutating p53 prolongs survival of Rps6+/− embryos from E5.5 to E12.5, and suppresses morphological defects in mice with Rpl24 mutations, consistent with the notion that the IRBC is deleterious for whole animals (Panić et al. 2006; Barkić et al. 2009). On the other hand, mutating p53 increases embryonic lethality of Rpl24Bst/+ mice, indicating that IRBC is protective overall for survival of this genotype (Barkić et al. 2009). It may be worth noting that p53 also protects Drosophila cells expressing elevated Myc, which are at a disadvantage in mosaics and eliminated unless p53 is expressed (de la Cova et al. 2014).
It can also be argued that mammalian IRBC is adaptive through tumor suppression. Rp point mutations are recurrent in some cancers, indicating an oncogenic role (Sulima et al. 2017). Initially it was thought, based on cancer predisposition of Rp mutant zebrafish, that Rp mutations promote cancer because chronic p53 activation due to IRBC creates a growth advantage for p53 mutant cells, which are then oncogenic (Amsterdam et al. 2004; MacInnes et al. 2008). More recent evidence points in another direction. A mouse mutant of MDM2, C305F, blocks the IRBC and prevents p53 activation. Instead of preventing tumorigenesis in a Myc overexpression mouse model, MDM2(C305F) accelerates it, indicating that the IRBC in fact protects against an oncogenic consequence of Myc overexpression (Macias et al. 2010; Liu et al. 2017). Further evidence comes from RPL5 mutations found in human tumor sequences. RpL5 is one of the HDM2-inactivating proteins that normally activates IRBC; this is prevented by the specific tumor alleles. These RPL5-mutant tumors generally contain WT p53 alleles (Oršolić et al. 2020). If tumors containing MDM2 or RPL5 mutations that do not activate IRBC and create no selection for secondary p53 mutations are tumorigenic, this argues strongly that RP mutations are intrinsically oncogenic. They do not need p53 mutations to cause cancer, rather the p53 activation normally induced by IRBC is tumor-suppressive.
Only a small proportion of overall cancers contain RP point mutant drivers, but the potential significance of tumor suppression by the IRBC is greatly enhanced by the possibility that RP genes are important sensors of aneuploidy in humans, as well as in Drosophila. A study of gene expression and proteomic changes in immortalized human retinal pigmented epithelium cell lines that lost one or another chromosome suggested that the main effect of monosomy is haploinsufficiency for RP genes (Chunduri et al. 2021). The same authors propose that human cancers that lose p53 tend to have chromosome losses rather than chromosome gains, consistent with the idea that chromosome loss may be oncogenic through RP gene haploinsufficiency, which is suppressed by p53 activation (Chunduri et al. 2021).
The oncogenic mechanism implied for Rp mutations is not yet clear although models have been suggested (Sulima et al. 2017, 2019; Girardi et al. 2018). One can imagine changes in translation that occur as a direct consequence of altered Rp levels, accumulation or turnover of Rp left unused when another Rp is limiting, or accumulation or turnover of unused rRNA or rRNA-derived species. A 10–30% reduction in ribosomal subunits occurs in Drosophila Rp+/− mutants (Lee et al. 2018; Kiparaki et al. 2022). This might be sufficient to affect translation of specific messages. There is little evidence of this in yeast, however, where ribosome profiling shows that changes in translation after reduced RP gene transcription are mostly explained by changing mRNA abundance, not changes in translation efficiency (Cheng et al. 2019). It may seem surprising that a 50% reduction in Rp gene transcription leads only to 10–30% reduction in ribosome number. Ribosomes are required for cell growth and so reduced ribosome numbers slow changes in cell volume and mass, compensating for ribosome numbers (Kiparaki et al. 2022). Longstanding analyses of cellular resource allocation lead to a similar conclusion (Maaloe 1979; Scott et al. 2014; Metzl-Raz et al. 2017; Shore and Albert 2022). Basically, cell growth requires Rp synthesis for ribosome biogenesis as well as expression of many other proteins. Because total protein synthesis capacity is finite, cells cannot double synthesis of the Rp proteins to compensate for haploinsufficiency in one Rp gene without reducing translation of other proteins required for growth. Conversely, if cells do not compensate for diminished ribosome biogenesis in Rp mutant heterozygotes, then the growth-related translation products cannot be utilized. Accordingly, cells find an intermediate state where ribosome numbers are partially compensated, while also partially reducing resources devoted to translating other growth-related genes.
Interestingly, gene expression changes typical of mutations affecting the yeast LSU differ from those affecting the SSU (Cheng et al. 2019). In particular, mutations affecting SSU biogenesis tend to promote accumulation of mRNA encoding Rp and ribosome biogenesis factors, suggesting a compensatory response, whereas mutations affecting LSU biogenesis tend to promote accumulation of mRNA encoding proteasome and autophagy functions, suggesting an adaptive response to enable turnover of unused ribosome components. The mRNAs encoding Rp and translation factors are reduced in Drosophila Rp mutants lacking Xrp1 (Ji et al. 2019), and the same is true in monosomic human cells lacking p53 (Chunduri et al. 2021). Thus, Xrp1 in Drosophila, and p53 in human cells, regulate ribosome biogenesis mRNAs oppositely to yeast deficient for SSU RP genes.
Unused ribosome components, or the consequences of their turnover, present a further molecular mechanism by which Rp mutations affect cells. In yeast, acute depletion of RP, or rRNA biogenesis leads to aggregation of orphan Rp (Albert et al. 2019; Tye et al. 2019). This has been proposed to occur in Drosophila (Baumgartner et al. 2021; Recasens-Alvarez et al. 2021) but the protein aggregates detected so far, as well as the reduced autophagic flux and proteasome activity reported, occur downstream of Xrp1, and apparently not as a direct effect of orphan Rp (Kiparaki et al. 2022). It is also interesting that, in Drosophila, decreased autophagic flux and increased proteotoxic stress have been reported predominantly from Minute mutations affecting the SSU (Nagata et al. 2019; Baumgartner et al. 2021; Recasens-Alvarez et al. 2021; Kiparaki et al. 2022). The dependence of Xrp1 expression on RpS12, however, does indicate a role for at least this Rp in triggering the Minute phenotype. Although the subject of little investigation so far, rRNA turnover is also likely to increase in Rp mutants, with potential effects on activity of the exosome, which is thought to be generally responsible for turnover of unused rRNA (Sinturel et al. 2017).
Concluding remarks
Recent advances are transforming our understanding of the Minute syndrome in Drosophila, caused by haploinsufficiency for Rp genes, both with respect to the cell-autonomous consequences for translation and growth, and the nonautonomous process of cell competition. It has been remarkable and unexpected to find that many aspects of the Minute phenotype in Drosophila depend on a transcriptional stress response. Evidence is emerging for similar conclusions in yeast and mammals. In the latter case, mediated at least in part by p53, rather than by Xrp1 as in Drosophila. Connections between the Minute phenotype and other stress responses including ER stress, the DDR, and oxidative stress make it possible to envisage cell competition as the consequence of nonautonomous inputs into cell death and survival outputs of stress responses. It is now demonstrated in Drosophila that cell competition can play an important role removing sporadic segmental aneuploid cells, individual cells experiencing ER stress, and perhaps cells experiencing other related stresses. Many details remain to be resolved, for example including how PERK is activated in Rp mutant cells, and the molecular nature of the interactions presumed to occur between competing cells. An important question remains whether an adaptive contribution of cell competition to maintaining optimal tissue constitution during development is sufficient to offset the apparently deleterious consequences of Minute mutations for nonmosaic flies, or whether the nonmosaic Minute phenotype could also be beneficial for another reason. Given the potential significance of Rp protein haploinsufficiency to human cancer, arising either through Rp point mutants or through chromosome monosomies, the question of what molecular mechanisms result directly from Rp haploinsufficiency, and how they may be suppressed by Xrp1 or p53 activity respectively, may be an important question for the future.
Acknowledgements
We thank Drs Julie Secombe, Amit Kumar, and Eleni Tsakiri for comments on the manuscript. The authors declare no conflicts of interest.
Contributor Information
Marianthi Kiparaki, Institute for Fundamental Biomedical Research, Biomedical Sciences Research Center “Alexander Fleming”, Vari 16672, Greece.
Nicholas E Baker, Department of Genetics, Albert Einstein College of Medicine, Bronx, NY 10461, USA; Developmental and Molecular Biology, Albert Einstein College of Medicine, Bronx, NY 10461, USA; Department of Visual Sciences and Ophthalmology, Albert Einstein College of Medicine, Bronx, NY 10461, USA.
Funding
Our research on Rp gene mutations and cell competition is supported by grants from the National Institutes of Health (NIH grants GM104213 and GM120451 to N.E.B.), and from the Hellenic Foundation for Research and Innovation (HFRI) (Grant Agreement No. 7306 to M.K.).
Literature cited
- Akdemir F, Christich A, Sogame N, Chapo J, Abrams JM. P53 directs focused genomic responses in Drosophila. Oncogene. 2007;26(36):5184–5193. doi: 10.1038/sj.onc.1210328. [DOI] [PubMed] [Google Scholar]
- Albert B, Kos-Braun IC, Henras AK, Dez C, Rueda MP, Zhang X, Gadal O, Kos M, Shore D. A ribosome assembly stress response regulates transcription to maintain proteome homeostasis. Elife. 2019;8:e45002. doi: 10.7554/eLife.45002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpar L, Bergantinos C, Johnston LA. Spatially restricted regulation of Spätzle/Toll signaling during cell competition. Dev Cell. 2018;46(6):706–719.e5. doi: 10.1016/j.devcel.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, Hopkins N. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2004;2(5):E139. doi: 10.1371/journal.pbio.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Saeboe-Larssen S, Lambertsson A, Merriam J, Jacobs-Lorena M. A Drosophila third chromosome Minute locus encodes a ribosomal protein. Genetics. 1994;137(2):513–520. doi: 10.1093/genetics/137.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillon L, Germani F, Rockel C, Hilchenbach J, Basler K. Xrp1 is a transcription factor required for cell competition-driven elimination of loser cells. Sci Rep. 2018;8(1):17712. doi: 10.1038/s41598-018-36277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE. Mechanisms of cell competition emerging from Drosophila studies. Curr Opin Cell Biol. 2017;48:40–46. doi: 10.1016/j.ceb.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Kiparaki M, Khan C. A potential link between p53, cell competition and ribosomopathy in mammals and in Drosophila. Dev Biol. 2019;446(1):17–19. doi: 10.1016/j.ydbio.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Montagna C. Reducing the aneuploid cell burden—cell competition and the ribosome connection. Dis Model Mech. 2022;15(11):dmm049673. doi: 10.1242/dmm.049673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banreti AR, Meier P. The NMDA receptor regulates competition of epithelial cells in the Drosophila wing. Nat Commun. 2020;11(1):2228. doi: 10.1038/s41467-020-16070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkić M, Crnomarković S, Grabušić K, Bogetić I, Panić L, Tamarut S, Cokarić M, Jerić I, Vidak S, Volarević S. The p53 tumor suppressor causes congenital malformations in Rpl24-deficient mice and promotes their survival. Mol Cell Biol. 2009;29(10):2489–2504. doi: 10.1128/MCB.01588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner ME, Dinan MP, Langton PF, Kucinski I, Piddini E. Proteotoxic stress is a driver of the loser status and cell competition. Nat Cell Biol. 2021;23(2):136–146. doi: 10.1038/s41556-020-00627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazrgar M, Gourabi H, Valojerdi MR, Yazdi PE, Baharvand H. Self-correction of chromosomal abnormalities in human preimplantation embryos and embryonic stem cells. Stem Cells Dev. 2013;22(17):2449–2456. doi: 10.1089/scd.2013.0053. [DOI] [PubMed] [Google Scholar]
- Beaupere C, Dinatto L, Wasko BM, Chen RB, VanValkenburg L, Kiflezghi MG, Lee MB, Promislow DEL, Dang W, Kaeberlein M, et al. Genetic screen identifies adaptive aneuploidy as a key mediator of ER stress resistance in yeast. Proc Natl Acad Sci U S A. 2018;115(38):9586–9591. doi: 10.1073/pnas.1804264115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U, Amon A. Context is everything: aneuploidy in cancer. Nat Rev Genet. 2020;21(1):44–62. doi: 10.1038/s41576-019-0171-x. [DOI] [PubMed] [Google Scholar]
- Blanco J, Cooper JC, Baker NE. Roles of C/EBP class bZip proteins in the growth and cell competition of Rp (‘Minute’) mutants in Drosophila. Elife. 2020;9:e50535. doi: 10.7554/eLife.50535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6(4):309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Sinervo B, Schubiger G. Experimental phenocopy of a Minute maternal-effect mutation alters blastoderm determination in embryos of Drosophila melanogaster. Dev Biol. 1989;132(2):343–354. doi: 10.1016/0012-1606(89)90231-5. [DOI] [PubMed] [Google Scholar]
- Boulan L, Andersen D, Colombani J, Boone E, Leopold P. Inter-organ growth coordination is mediated by the Xrp1-dilp8 axis in Drosophila. Dev Cell. 2019;49(5):811–818.e4. doi: 10.1016/j.devcel.2019.03.016. [DOI] [PubMed] [Google Scholar]
- Bourque G, Burns KH, Gehring M, Gorbunova V, Seluanov A, Hammell M, Imbeault M, Izsvák Z, Levin HL, Macfarlan TS, et al. Ten things you should know about transposable elements. Genome Biol. 2018;19(1):199. doi: 10.1186/s13059-018-1577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 2015;16(2):109–120. doi: 10.1038/nrn3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CB, Morgan TH. The third-chromosome group of mutant characters of Drosophila melanogaster. Carnegie Institute Publication. 1923;327:1–251. [Google Scholar]
- Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, Rio DC, Rubin GM. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24(3):1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B, Mitra S, Roach FD, Vasudevan D, Ryoo HD. The transcription factor Xrp1 is required for PERK-mediated antioxidant gene induction in Drosophila. Elife. 2021;10. doi: 10.7554/eLife.74047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. Scribble mutants cooperate with oncogenic Ras or notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22(21):5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KH. Our conflict with transposable elements and its implications for human disease. Annu Rev Pathol. 2020;15(1):51–70. doi: 10.1146/annurev-pathmechdis-012419-032633. [DOI] [PubMed] [Google Scholar]
- Bursać S, Brdovčak MC, Pfannkuchen M, Orsolić I, Golomb L, Zhu Y, Katz C, Daftuar L, Grabušić K, Vukelić I, et al. Mutual protection of ribosomal proteins L5 and L11 from degradation is essential for p53 activation upon ribosomal biogenesis stress. Proc Natl Acad Sci U S A. 2012;109(50):20467–20472. doi: 10.1073/pnas.1218535109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catinozzi M, Mallik M, Frickenhaus M, Been M, Sijlmans C, Kulshrestha D, Alexopoulos I, Weitkunat M, Schnorrer F, Storkebaum E. The Drosophila FUS ortholog cabeza promotes adult founder myoblast selection by Xrp1-dependent regulation of FGF signaling. PLoS Genet. 2020;16(4):e1008731. doi: 10.1371/journal.pgen.1008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YH, Dubnau J. The gypsy endogenous retrovirus drives non-cell-autonomous propagation in a Drosophila TDP-43 model of neurodegeneration. Curr Biol. 2019;29(19):3135–3152.e4. doi: 10.1016/j.cub.2019.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Mugler CF, Keskin A, Hodapp S, Chan LY-L, Weis K, Mertins P, Regev A, Jovanovic M, Brar GA. Small and large ribosomal subunit deficiencies lead to distinct gene expression signatures that reflect cellular growth rate. Mol Cell. 2019;73(1):36–47.e10. doi: 10.1016/j.molcel.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchetti A, Zhou J, Zhu H, Karl T, Haubenreisser O, Rinnerthaler M, Heeren G, Oender K, Bauer J, Hintner H, et al. Ribosomal proteins Rpl10 and Rps6 are potent regulators of yeast replicative life span. Exp Gerontol. 2007;42(4):275–286. doi: 10.1016/j.exger.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chunduri NK, Menges P, Zhang X, Wieland A, Gotsmann VL, Mardin BR, Buccitelli C, Korbel JO, Willmund F, Kschischo M, et al. Systems approaches identify the consequences of monosomy in somatic human cells. Nat Commun. 2021;12(1):5576. doi: 10.1038/s41467-021-25288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunduri NK, Storchova Z. The diverse consequences of aneuploidy. Nat Cell Biol. 2019;21(1):54–62. doi: 10.1038/s41556-018-0243-8. [DOI] [PubMed] [Google Scholar]
- Claveria C, Giovinazzo G, Sierra R, Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500(7460):39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejosez M, Ura H, Brandt VL, Zwaka TP. Safeguards for cell cooperation in mouse embryogenesis shown by genome-wide cheater screen. Science. 2013;341(6153):1511–1514. doi: 10.1126/science.1241628. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston L. Drosophila Myc regulates organ size by inducing cell competition. Cell. 2004;117(1):107–116. doi: 10.1016/S0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Senoo-Matsuda N, Ziosi M, Wu D, Bellosta P, Quinzii C, Johnston L. Supercompetitor status of Drosophila Myc cells requires p53 as a fitness sensor to reprogram metabolism and promote viability. Cell Metab. 2014;19(3):470–483. doi: 10.1016/j.cmet.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzini M, Montanaro L, Trere D. Ribosome biogenesis and cancer. Acta Histochem. 2017;119(3):190–197. doi: 10.1016/j.acthis.2017.01.009. [DOI] [PubMed] [Google Scholar]
- de Vreede G, Gerlach SU, Bilder D. Epithelial monitoring through ligand-receptor segregation ensures malignant cell elimination. Science. 2022;376(6590):297–301. doi: 10.1126/science.abl4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Díaz C, Fernandez de Manuel L, Jimenez-Carretero D, Montoya MC, Clavería C, Torres M. Pluripotency surveillance by Myc-driven competitive elimination of differentiating cells. Dev Cell. 2017;42(6):585–599.e4. doi: 10.1016/j.devcel.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Di Giacomo S, Sollazzo M, de Biase D, Ragazzi M, Bellosta P, Pession A, Grifoni D. Human cancer cells signal their competitive fitness through MYC activity. Sci Rep. 2017;7(1):12568. doi: 10.1038/s41598-017-13002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Peddigari S, Mercer CA, Thomas G. 5S ribosomal RNA is an essential component of a nascent ribosomal precursor complex that regulates the Hdm2-p53 checkpoint. Cell Rep. 2013;4(1):87–98. doi: 10.1016/j.celrep.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SJ, Gomez NC, Levorse J, Mertz AF, Ge Y, Fuchs E. Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature. 2019;569(7757):497–502. doi: 10.1038/s41586-019-1199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell PJ, Balkow K, Hunt T, Jackson RJ, Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Antoran D, Piedrafita G, Murai K, Ong SH, Herms A, Frezza C, Jones PH. Outcompeting p53-mutant cells in the normal esophagus by redox manipulation. Cell Stem Cell. 2019;25(3):329–341.e6. doi: 10.1016/j.stem.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrus A. Parameters of mitotic recombination in minute mutants of Drosophila melanogaster. Genetics. 1975;79(4):589–599. doi: 10.1093/genetics/79.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MJ, Roche S, Cho MJ, Beall E, Min B, Panganiban RP, Rio DC. Drosophila IRBP bZIP heterodimer binds P-element DNA and affects hybrid dysgenesis. Proc Natl Acad Sci U S A. 2016;113(46):13003–13008. doi: 10.1073/pnas.1613508113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973;245(147):251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Gentilella A, Morón-Duran FD, Fuentes P, Zweig-Rocha G, Riaño-Canalias F, Pelletier J, Ruiz M, Turón G, Castaño J, Tauler A, et al. Autogenous control of 5′TOP mRNA stability by 40S ribosomes. Mol Cell. 2017;67(1):55–70.e4. doi: 10.1016/j.molcel.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuth NR, Barna M. Heterogeneity and specialized functions of translation machinery: from genes to organisms. Nat Rev Genet. 2018;19(7):431–452. doi: 10.1038/s41576-018-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi T, Vereecke S, Sulima SO, Khan Y, Fancello L, Briggs JW, Schwab C, de Beeck JO, Verbeeck J, Royaert J, et al. The T-cell leukemia-associated ribosomal RPL10 R98S mutation enhances JAK-STAT signaling. Leukemia. 2018;32(3):809–819. doi: 10.1038/leu.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone M, et al. A translation inhibitor identified in a Drosophila screen enhances the effect of ionizing radiation and taxol in mammalian models of cancer. Dis Model Mech. 2012;5:342–350. doi: 10.1242/dmm.008722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodier JL. Restricting retrotransposons: a review. Mob DNA. 2016;7(1):16. doi: 10.1186/s13100-016-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157(1):65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee S-J, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sasaki H. Epiblast formation by TEAD-YAP-dependent expression of pluripotency factors and competitive elimination of unspecified cells. Dev Cell. 2019;50(2):139–154.e5. doi: 10.1016/j.devcel.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Hook EB. Prevalence of chromosome abnormalities during human gestation and implications for studies of environmental mutagens. Lancet. 1981;2(8239):169–172. doi: 10.1016/S0140-6736(81)90356-1. [DOI] [PubMed] [Google Scholar]
- Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16(3):458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Chuen J, Kiparaki M, Baker N. Cell competition removes segmental aneuploid cells from Drosophila imaginal disc-derived tissues based on ribosomal protein gene dose. Elife. 2021;10:e61172. doi: 10.7554/eLife.61172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Kiparaki M, Folgado V, Kumar A, Blanco J, Rimesso G, Chuen J, Liu Y, Zheng D, Baker NE. Drosophila Rps12 controls translation, growth, and cell competition through Xrp1. PLoS Genet. 2019;15(12):e1008513. doi: 10.1371/journal.pgen.1008513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale A, Ji Z, Kiparaki M, Blanco J, Rimesso G, Flibotte S, Baker NE. Ribosomal protein S12e has a distinct function in cell competition. Dev Cell. 2018;44(1):42–55.e4. doi: 10.1016/j.devcel.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale A, Li W, Lee CH, Baker NE. Apoptotic mechanisms during competition of ribosomal protein mutant cells: roles of the initiator caspases dronc and dream/strica. Cell Death Differ. 2015;22(8):1300–1312. doi: 10.1038/cdd.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber ER, Lowe SW. Putting p53 in context. Cell. 2017;170(6):1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Yamamoto Y, Sato TA, Ochiya T. Extracellular vesicles as trans-genomic agents: emerging roles in disease and evolution. Cancer Sci. 2017;108(5):824–830. doi: 10.1111/cas.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiparaki M, Khan C, Folgado-Marco V, Chuen J, Moulos P, Baker NE. The transcription factor Xrp1 orchestrates both reduced translation and cell competition upon defective ribosome assembly or function. Elife. 2022;11:e71705. doi: 10.7554/eLife.71705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan K, Yu Q, Vincent A, Frisardi MC, Rosbash M, Lengyel JA, Merriam J. A Drosophila Minute gene encodes a ribosomal protein. Nature. 1985;317(6037):555–558. doi: 10.1038/317555a0. [DOI] [PubMed] [Google Scholar]
- Kucinski I, Dinan M, Kolahgar G, Piddini E. Chronic activation of JNK JAK/STAT and oxidative stress signalling causes the loser cell status. Nat Commun. 2017;8(1):136. doi: 10.1038/s41467-017-00145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Baker NE. The CRL4 E3 ligase mahjong/DCAF1 controls cell competition through the transcription factor Xrp1, independently of polarity genes. Development. 2022;149(22). doi: 10.1242/dev.200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsson A. The Minute genes in Drosophila and their molecular functions. Adv Genet. 1998;38:69–134. doi: 10.1016/S0065-2660(08)60142-X. [DOI] [PubMed] [Google Scholar]
- Langton PF, Baumgartner ME, Logeay R, Piddini E. Xrp1 and Irbp18 trigger a feed-forward loop of proteotoxic stress to induce the loser status. PLoS Genet. 2021;17(12):e1009946. doi: 10.1371/journal.pgen.1009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-H, Kiparaki M, Blanco J, Folgado V, Ji Z, Kumar A, Rimesso G, Baker NE. A regulatory response to ribosomal protein mutations controls translation, growth, and cell competition. Dev Cell. 2018;46(4):456–469.e4. doi: 10.1016/j.devcel.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Rimesso G, Reynolds DM, Cai J, Baker NE. Whole-genome sequencing and iPLEX MassARRAY genotyping map an EMS-induced mutation affecting cell competition in Drosophila melanogaster. G3 (Bethesda). 2016;6(10):3207–3217. doi: 10.1534/g3.116.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129(6):1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai). 2014;46(8):629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- Liu S, Tackmann NR, Yang J, Zhang Y. Disruption of the RP-MDM2-p53 pathway accelerates APC loss-induced colorectal tumorigenesis. Oncogene. 2017;36(10):1374–1383. doi: 10.1038/onc.2016.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yee PP, Wei Y, Liu Z, Kawasawa YI, Li W. Differential YAP expression in glioma cells induces cell competition and promotes tumorigenesis. J Cell Sci. 2019;132(5). doi: 10.1242/jcs.225714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolo FN, Casas-Tinto S, Moreno E. Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell Rep. 2012;2(3):526–539. doi: 10.1016/j.celrep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethy JD, Fargnoli J, Park JS, Fornace AJ, Jr, Holbrook NJ. Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J Biol Chem. 1990;265(27):16521–16526. doi: 10.1016/S0021-9258(17)46254-5. [DOI] [PubMed] [Google Scholar]
- Maaloe O. Regulation of the protein synthesis machinery—ribosomes, tRNA, factors, and so on. In: Goldberger RF, editor. Biological Regulation and Development. Boston: (MA: ): Springer; 1979. p. 487–542. [Google Scholar]
- Macias E, Jin A, Deisenroth C, Bhat K, Mao H, Lindström MS, Zhang Y. An ARF-independent c-MYC-activated tumor suppression pathway mediated by ribosomal protein-Mdm2 interaction. Cancer Cell. 2010;18(3):231–243. doi: 10.1016/j.ccr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes AW, Amsterdam A, Whittaker CA, Hopkins N, Lees JA. Loss of p53 synthesis in zebrafish tumors with ribosomal protein gene mutations. Proc Natl Acad Sci U S A. 2008;105(30):10408–10413. doi: 10.1073/pnas.0805036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan E, Pelham CJ, Nagane M, Parker TM, Canas-Marques R, Fazio K, Shaik K, Yuan Y, Henriques V, Galzerano A, et al. Flower isoforms promote competitive growth in cancer. Nature. 2019;572(7768):260–264. doi: 10.1038/s41586-019-1429-3. [DOI] [PubMed] [Google Scholar]
- Mallik M, Catinozzi M, Hug CB, Zhang L, Wagner M, Bussmann J, Bittern J, Mersmann S, Klämbt C, Drexler HCA, et al. Xrp1 genetically interacts with the ALS-associated FUS orthologue caz and mediates its toxicity. J Cell Biol. 2018;217(11):3947–3964. doi: 10.1083/jcb.201802151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor-Nof M, Shipony Z, Lopez-Gonzalez R, Nakayama L, Zhang Y-J, Couthouis J, Blum JA, Castruita PA, Linares GR, Ruan K, et al. P53 is a central regulator driving neurodegeneration caused by C9orf72 poly(PR). Cell. 2021;184(3):689–708.e20. doi: 10.1016/j.cell.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FA, Herrera SC, Morata G. Cell competition, growth and size control in the Drosophila wing imaginal disc. Development. 2009;136(22):3747–3756. doi: 10.1242/dev.038406. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Fujita Y. Cell competition in mammals—novel homeostatic machinery for embryonic development and cancer prevention. Curr Opin Cell Biol. 2017;48:106–112. doi: 10.1016/j.ceb.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Roote J, Reuter G, Lambertsson A, Ashburner M, Millburn GH, Harrison PM, Yu Z, Kenmochi N, Kaufman TC, et al. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 2007;8(10):R216. doi: 10.1186/gb-2007-8-10-r216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoro-Vidal A, Levayer R. Multiple influences of mechanical forces on cell competition. Curr Biol. 2019;29(15):R762–R774. doi: 10.1016/j.cub.2019.06.030. [DOI] [PubMed] [Google Scholar]
- McNamee LM, Brodsky MH. p53-independent apoptosis limits DNA damage-induced aneuploidy. Genetics. 2009;182(2):423–435. doi: 10.1534/genetics.109.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J, Perez-Garijo A, Calleja M, Morata G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci U S A. 2010;107(33):14651–14656. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino M, Rhiner C, Lopez-Gay J, Buechel D, Hauert B, Moreno E. Elimination of unfit cells maintains tissue health and prolongs lifespan. Cell. 2015;160(3):461–476. doi: 10.1016/j.cell.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzl-Raz E, Kafri M, Yaakov G, Soifer I, Gurvich Y, Barkai N. Principles of cellular resource allocation revealed by condition-dependent proteome profiling. Elife. 2017;6. doi: 10.7554/eLife.28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SN, Amoyel M, Bergantiños C, de la Cova C, Schertel C, Basler K, Johnston LA. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science. 2014;346(6214):1258236. doi: 10.1126/science.1258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G. Cell competition: a historical perspective. Dev Biol. 2021;476:33–40. doi: 10.1016/j.ydbio.2021.02.012. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev Biol. 1975;42(2):211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Morcelle C, Menoyo S, Morón-Duran FD, Tauler A, Kozma SC, Thomas G, Gentilella A. Oncogenic MYC induces the impaired ribosome biogenesis checkpoint and stabilizes p53 independent of increased ribosome content. Cancer Res. 2019;79(17):4348–4359. doi: 10.1158/0008-5472.CAN-18-2718. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. Dmyc transforms cells into super-competitors. Cell. 2004;117(1):117–129. doi: 10.1016/S0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416(6882):755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Moya IM, Castaldo SA, Van den Mooter L, Soheily S, Sansores-Garcia L, Jacobs J, Mannaerts I, Xie J, Verboven E, Hillen H, et al. Peritumoral activation of the Hippo pathway effectors YAP and TAZ suppresses liver cancer in mice. Science. 2019;366(6468):1029–1034. doi: 10.1126/science.aaw9886. [DOI] [PubMed] [Google Scholar]
- Nagata R, Nakamura M, Sanaki Y, Igaki T. Cell competition is driven by autophagy. Dev Cell. 2019;51(1):99–112.e4. doi: 10.1016/j.devcel.2019.08.018. [DOI] [PubMed] [Google Scholar]
- Navarro-Yepes J, Burns M, Anandhan A, Khalimonchuk O, del Razo LM, Quintanilla-Vega B, Pappa A, Panayiotidis MI, Franco R. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid Redox Signal. 2014;21(1):66–85. doi: 10.1089/ars.2014.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto-Silva RM, de Beco S, Johnston LA. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell. 2010;19(4):507–520. doi: 10.1016/j.devcel.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16(11):1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi N, Nakamura M, Nagata R, Wakasa N, Nakano R, Igaki T. Cell competition is driven by Xrp1-mediated phosphorylation of eukaryotic initiation factor 2alpha. PLoS Genet. 2021;17(12):e1009958. doi: 10.1371/journal.pgen.1009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24(6):1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver ER, Saunders TL, Tarle SA, Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131(16):3907–3920. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oršolić I, Bursać S, Jurada D, Drmić Hofman I, Dembić Z, Bartek J, Mihalek I, Volarević S. Cancer-associated mutations in the ribosomal protein L5 gene dysregulate the HDM2/p53-mediated ribosome biogenesis checkpoint. Oncogene. 2020;39(17):3443–3457. doi: 10.1038/s41388-020-1231-6. [DOI] [PubMed] [Google Scholar]
- Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286(13):10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panić L, Tamarut S, Sticker-Jantscheff M, Barkić M, Solter D, Uzelac M, Grabušić K, Volarević S. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol Cell Biol. 2006;26(23):8880–8891. doi: 10.1128/MCB.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Shah HS, Shrivastava N. c-Myc-dependent cell competition in human cancer cells. J Cell Biochem. 2017;118(7):1782–1791. doi: 10.1002/jcb.25846. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Riaño-Canalias F, Almacellas E, Mauvezin C, Samino S, Feu S, Menoyo S, Domostegui A, Garcia-Cajide M, Salazar R, et al. Nucleotide depletion reveals the impaired ribosome biogenesis checkpoint as a barrier against DNA damage. EMBO J. 2020;39(13):e103838. doi: 10.15252/embj.2019103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Thomas G, Volarevic S. Ribosome biogenesis in cancer: new players and therapeutic avenues. Nat Rev Cancer. 2018;18(1):51–63. doi: 10.1038/nrc.2017.104. [DOI] [PubMed] [Google Scholar]
- Recasens-Alvarez C, Alexandre C, Kirkpatrick J, Nojima H, Huels DJ, Snijders AP, Vincent J-P. Ribosomopathy-associated mutations cause proteotoxic stress that is alleviated by TOR inhibition. Nat Cell Biol. 2021;23(2):127–135. doi: 10.1038/s41556-020-00626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke AW, Baek J, Ashenberg O, Keating AE. Networks of bZIP protein-protein interactions diversified over a billion years of evolution. Science. 2013;340(6133):730–734. doi: 10.1126/science.1233465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiner C, López-Gay JM, Soldini D, Casas-Tinto S, Martín FA, Lombardía L, Moreno E. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev Cell. 2010;18(6):985–998. doi: 10.1016/j.devcel.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Rodrigues AB, Zoranovic T, Ayala-Camargo A, Grewal S, Reyes-Robles T, Krasny M, Wu DC, Johnston LA, Bach EA. Activated STAT regulates growth and induces competitive interactions independently of Myc, Yorkie, wingless and ribosome biogenesis. Development. 2012;139(21):4051–4061. doi: 10.1242/dev.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332(2):237–248. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Sancho M, Di-Gregorio A, George N, Pozzi S, Sánchez J, Pernaute B, Rodríguez T. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev Cell. 2013;26(1):19–30. doi: 10.1016/j.devcel.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaack S, Gilbert C, Feschotte C. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol Evol. 2010;25(9):537–546. doi: 10.1016/j.tree.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M, Klumpp S, Mateescu EM, Hwa T. Emergence of robust growth laws from optimal regulation of ribosome synthesis. Mol Syst Biol. 2014;10(8):747. doi: 10.15252/msb.20145379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Amon A. The aneuploidy paradox: costs and benefits of an incorrect karyotype. Trends Genet. 2011;27(11):446–453. doi: 10.1016/j.tig.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D, Albert B. Ribosome biogenesis and the cellular energy economy. Curr Biol. 2022;32(12):R611–R617. doi: 10.1016/j.cub.2022.04.083. [DOI] [PubMed] [Google Scholar]
- Simpson P. Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev Biol. 1979;69(1):182–193. doi: 10.1016/0012-1606(79)90284-7. [DOI] [PubMed] [Google Scholar]
- Simpson P, Morata G. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev Biol. 1981;85(2):299–308. doi: 10.1016/0012-1606(81)90261-X. [DOI] [PubMed] [Google Scholar]
- Singla S, Iwamoto-Stohl LK, Zhu M, Zernicka-Goetz M. Autophagy-mediated apoptosis eliminates aneuploid cells in a mouse model of chromosome mosaicism. Nat Commun. 2020;11(1):2958. doi: 10.1038/s41467-020-16796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinturel F, Gerber A, Mauvoisin D, Wang J, Gatfield D, Stubblefield JJ, Green CB, Gachon F, Schibler U. Diurnal oscillations in liver mass and cell size accompany ribosome assembly cycles. Cell. 2017;169(4):651–663.e14. doi: 10.1016/j.cell.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KK, McCormick MA, Pham KM, MacKay VL, Delaney JR, Murakami CJ, Kaeberlein M, Kennedy BK. Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics. 2012;191(1):107–118. doi: 10.1534/genetics.111.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijkerbuijk SJ, Kolahgar G, Kucinski I, Piddini E. Cell competition drives the growth of intestinal adenomas in Drosophila. Curr Biol. 2016;26(4):428–438. doi: 10.1016/j.cub.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulima SO, Hofman IJF, De Keersmaecker K, Dinman JD. How ribosomes translate cancer. Cancer Discov. 2017;7(10):1069–1087. doi: 10.1158/2159-8290.CD-17-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulima SO, Kampen KR, De Keersmaecker K. Cancer biogenesis in ribosomopathies. Cells. 2019;8(3):229. doi: 10.3390/cells8030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Samimi H, Gamez M, Zare H, Frost B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci. 2018;21(8):1038–1048. doi: 10.1038/s41593-018-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Bialucha CU, Tian A-G, Kajita M, Huang Y-C, Norman M, Harrison N, Poulton J, Ivanovitch K, Disch L, et al. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 2010;8(7):e1000422. doi: 10.1371/journal.pbio.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Li Y, Kang P, Wang Z, Yue F, Jiao P, Yang N, Qin S, Yao S. Endoplasmic reticulum stress-dependent autophagy inhibits glycated high-density lipoprotein-induced macrophage apoptosis by inhibiting CHOP pathway. J Cell Mol Med. 2019;23(4):2954–2969. doi: 10.1111/jcmm.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titen SW, Golic KG. Telomere loss provokes multiple pathways to apoptosis and produces genomic instability in Drosophila melanogaster. Genetics. 2008;180(4):1821–1832. doi: 10.1534/genetics.108.093625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiu GC, Kerr CH, Forester CM, Krishnarao PS, Rosenblatt HD, Raj N, Lantz TC, Zhulyn O, Bowen ME, Shokat L, et al. A p53-dependent translational program directs tissue-selective phenotypes in a model of ribosomopathies. Dev Cell. 2021;56(14):2089–2102.e11. doi: 10.1016/j.devcel.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari B, Jones AE, Abrams JM. Transposons, p53 and genome security. Trends Genet. 2018;34(11):846–855. doi: 10.1016/j.tig.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari B, Jones AE, Caillet CJ, Das S, Royer SK, Abrams JM. P53 directly represses human LINE1 transposons. Genes Dev. 2020;34(21–22):1439–1451. doi: 10.1101/gad.343186.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164(3):341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye BW, Commins N, Ryazanova LV, Wühr M, Springer M, Pincus D, Churchman LS. Proteotoxicity from aberrant ribosome biogenesis compromises cell fitness. Elife. 2019;8:e43002. doi: 10.7554/eLife.43002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler DM, Li W, Zhuo N, Pellock B, Baker NE. Genes affecting cell competition in Drosophila. Genetics. 2007;175(2):643–657. doi: 10.1534/genetics.106.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulirsch JC, Verboon JM, Kazerounian S, Guo MH, Yuan D, Ludwig LS, Handsaker RE, Abdulhay NJ, Fiorini C, Genovese G, et al. The genetic landscape of Diamond-Blackfan anemia. Am J Hum Genet. 2018;103(6):930–947. doi: 10.1016/j.ajhg.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, Repping S. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update. 2011;17(5):620–627. doi: 10.1093/humupd/dmr014. [DOI] [PubMed] [Google Scholar]
- Villa del Campo C, Claveria C, Sierra R, Torres M. Cell competition promotes phenotypically silent cardiomyocyte replacement in the mammalian heart. Cell Rep. 2014;8(6):1741–1751. doi: 10.1016/j.celrep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Kolahgar G, Gagliardi M, Piddini E. Steep differences in wingless signaling trigger Myc-independent competitive cell interactions. Dev Cell. 2011;21(2):366–374. doi: 10.1016/j.devcel.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A, Rosenberg PS, Atsidaftos E, Alter BP, Lipton JM. Incidence of neoplasia in diamond blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood. 2012;119(16):3815–3819. doi: 10.1182/blood-2011-08-375972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A, Rosenberg PS, Atsidaftos E, Kang J, Onel K, Sharaf RN, Alter BP, Lipton JM. Increased risk of colon cancer and osteogenic sarcoma in Diamond-Blackfan anemia. Blood. 2018;132(20):2205–2208. doi: 10.1182/blood-2018-05-848937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff L, Goschorska M, Kozyrska K, Duclos G, Kucinski I, Chessel A, Hampton-O’Neil L, Bradshaw CR, Allen GE, Rawlins EL, et al. Mechanical cell competition kills cells via induction of lethal p53 levels. Nat Commun. 2016;7(1):11373. doi: 10.1038/ncomms11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Proud CG. The role of eIF2 phosphorylation in cell and organismal physiology: new roles for well-known actors. Biochem J. 2022;479(10):1059–1082. doi: 10.1042/BCJ20220068. [DOI] [PubMed] [Google Scholar]
- Warner JR, McIntosh KB. How common are extraribosomal functions of ribosomal proteins? Mol Cell. 2009;34(1):3–11. doi: 10.1016/j.molcel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC. Role of eIF2alpha kinases in translational control and adaptation to cellular stress. Cold Spring Harb Perspect Biol. 2018;10(7):a032870. doi: 10.1101/cshperspect.a032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Bao P, Jiang X, Wang H, Qin M, Wang R, Wang T, Yang Y, Lorenzini I, Liao L, et al. Reactivation of nonsense-mediated mRNA decay protects against C9orf72 dipeptide-repeat neurotoxicity. Brain. 2019;142(5):1349–1364. doi: 10.1093/brain/awz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279(44):45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu L, Naik I, Braunstein Z, Zhong J, Ren B. Transcription factor C/EBP homologous protein in health and diseases. Front Immunol. 2017;8:1612. doi: 10.3389/fimmu.2017.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, Vorsanova SG, Iourov IY. Chromosome instability in the neurodegenerating brain. Front Genet. 2019;10:892. doi: 10.3389/fgene.2019.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16(5):369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]