Abstract
PURPOSE
Successful completion of chemotherapy is critical to improve breast cancer outcomes. Relative dose intensity (RDI), defined as the ratio of chemotherapy delivered to prescribed, is a measure of chemotherapy completion and is associated with cancer mortality. The effect of exercise and eating a healthy diet on RDI is unknown. We conducted a randomized trial of an exercise and nutrition intervention on RDI and pathologic complete response (pCR) in women diagnosed with breast cancer initiating chemotherapy.
METHODS
One hundred seventy-three women with stage I-III breast cancer were randomly assigned to usual care (UC; n = 86) or a home-based exercise and nutrition intervention with counseling sessions delivered by oncology-certified registered dietitians (n = 87). Chemotherapy dose adjustments and delays and pCR were abstracted from electronic medical records. T-tests and chi-square tests were used to examine the effect of the intervention versus UC on RDI and pCR.
RESULTS
Participants randomly assigned to intervention had greater improvements in exercise and diet quality compared with UC (P < .05). RDI was 92.9% ± 12.1% and 93.6% ± 11.1% for intervention and UC, respectively (P = .69); the proportion of patients in the intervention versus UC who achieved ≥85% RDI was 81% and 85%, respectively (P = .44). The proportion of patients who had at least one dose reduction and/or delay was 38% intervention and 36% UC (P = .80). Among 72 women who received neoadjuvant chemotherapy, women randomly assigned to intervention were more likely to have a pCR than those randomly assigned to UC (53% v 28%; P = .037).
CONCLUSION
Although a diet and exercise intervention did not affect RDI, the intervention was associated with a higher pCR in patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative and triple-negative breast cancer undergoing neoadjuvant chemotherapy.
INTRODUCTION
Advances in chemotherapy have contributed to decreased mortality in women with early-stage breast cancer.1 Completion of chemotherapy is critical to improve outcomes. Relative dose intensity (RDI), a measure of chemotherapy completion, is the ratio of the amount of chemotherapy delivered versus the amount initially prescribed, accounting for dose intensity and duration of drugs.2 An RDI <85% is a common clinical threshold whereby chemotherapy effectiveness and prognosis significantly worsen.3 Low RDI (<85%), primarily because of treatment side effects, is observed in 26% and 32% of women with breast cancer who receive conventional and dose-dense chemotherapy regimens, respectively.4
CONTEXT
Key Objective
Does exercise and eating a healthy diet improve chemotherapy completion and pathologic complete response (pCR) in women diagnosed with breast cancer and receiving chemotherapy.
Knowledge Generated
The intervention led to improvements in diet quality, physical activity levels, and pCR rates compared with usual care (UC). Chemotherapy completion rates were similar for women randomly assigned to intervention versus UC.
Relevance (K.D. Miller)
-
There are many reasons to encourage a healthy diet and regular exercise in women with breast cancer requiring chemotherapy. This study shows it is possible and may increase pCR. Nonpharmacologic interventions are worthy of great attention and appropriately powered trials.*
*Relevance section written by JCO Senior Deputy Editor, Kathy D. Miller, MD.
Higher levels of physical activity (PA) and better diet quality are associated with lower breast cancer mortality in observation studies.5-7 It is hypothesized that this occurs via improved metabolic and inflammatory biomarkers.8,9 Numerous organizations recommend a healthy diet, and aerobic and resistance exercise after completing cancer treatment.10-12 At diagnosis, many patients have low PA levels and suboptimal diets,13 and treatment often worsens these lifestyle behaviors.14
PA and optimal nutrition may improve RDI. Sarcopenia (low muscle mass) at diagnosis has been associated with lower RDI and higher overall mortality.15 Cancer and its treatment exacerbate muscle loss16; exercise interventions during treatment could preserve muscle and in turn improve RDI. Nutrition impact symptoms (NISs), such as fatigue, nausea, vomiting, oral mucositis, dysphagia, xerostomia, and decreased appetite, make it difficult to eat well, potentially affecting chemotherapy completion.17 It is estimated 80% of women with breast cancer have at least one NIS at 1 month after starting chemotherapy.18
Recent ASCO guidelines recommend exercise during active treatment on the basis of evidence that exercise maintains or improves cardiorespiratory fitness, strength, fatigue, and other patient-reported outcomes.19 However, there were little data evaluating the impact of exercise interventions on RDI to inform these guidelines. Exercise improved RDI in women with breast cancer in two trials,20,21 while several trials in other cancer populations did not show a significant impact.22 RDI was a secondary outcome in all these trials. One recent exercise trial with RDI as the primary end point in patients with colon cancer is being conducted.23 Dietary interventions during cancer treatment are limited and, to our knowledge, there are no published nutrition trials on RDI as a primary end point.
If exercise and a high-quality diet improve RDI, they could improve breast cancer prognosis. Pathologic complete response (pCR), defined as disappearance of all invasive cancer in the breast after completion of neoadjuvant chemotherapy, is an important prognostic measure.24 We are not aware of any trials of exercise and/or diet on pCR in patients with breast cancer.
Given high rates of chemotherapy side effects and suboptimal chemotherapy completion rates, we examined the effect of a home-based exercise and nutrition intervention versus usual care (UC) on RDI in women diagnosed with breast cancer initiating chemotherapy. A secondary end point was pCR in those receiving neoadjuvant chemotherapy.
METHODS
Study Design
The Lifestyle, Exercise, and Nutrition Early After Breast Cancer (LEANer) study was a two-arm randomized trial comparing a nutrition and exercise intervention versus UC on RDI (primary aim) and pCR (secondary aim for neoadjuvant chemotherapy patients) at the postchemotherapy time point.25 This study was approved by the Yale and the Dana-Farber/Harvard Cancer Center Institutional Review Boards. All participants signed informed consent. LEANer is registered at ClinicalTrials.gov (identifier: NCT03314688).
Eligibility Criteria
Eligible participants were women newly diagnosed with stage I-III breast cancer, receiving chemotherapy, willing to be randomly assigned, able to walk, <150 min/wk of moderate- to vigorous-intensity PA, eating <7 fruits or vegetables daily, and able to understand instructions in English. Women were ineligible if they had received their second chemotherapy cycle, pregnant or intending to become pregnant, had experienced a stroke or myocardial infarction in the past year, had dementia, had major psychiatric disease, or were participating in a weight loss program.
Recruitment
Women were recruited between February 2018 and July 2021 through the Smilow Cancer Hospital Network at Yale-New Haven Hospital and the Dana-Farber Cancer Institute. Preliminary eligibility was assessed using the electronic medical record (EMR). After chemotherapy prescription was confirmed and oncologist approval was obtained, participants were screened. If eligible and interested in participating, informed consent was obtained.
Randomization
After completing baseline questionnaires, participants were randomly assigned to intervention or UC. Randomization was stratified by human epidermal growth factor receptor 2 (HER2) status (HER2-positive or HER2-negative [HER2–]), hormone receptor (HR) status (HR-positive [HR+] or HR-negative), and number of chemotherapy cycles (four cycles or >4) with computer-generated randomization lists for each stratum using the permutation method with variable block sizes.
RDI
Data were abstracted from the EMR and treating team after each chemotherapy cycle including date and dose of each drug administered, and reason for any dose adjustments and/or dose delays. RDI was expressed as a percentage of actual chemotherapy dose intensity divided by prescribed dose intensity on the basis of standard formulas. The prescribed dose intensity was calculated as
where BSA refers to body surface area (m2). The actual dose intensity was calculated as
RDI was calculated for each drug separately and then averaged across all chemotherapy drugs for each patient.4 Categorical outcomes for completion were created including RDI <85% or ≥85%, dose reduction, dose delay >5 days, and combination of dose reduction, skip, earlier termination, and/or delays. Dose discontinuation because of allergies to drugs was included in RDI calculation but not included in the other categorical outcomes. Reasons for chemotherapy dose reductions and delays were documented.
pCR
For women receiving neoadjuvant chemotherapy, the pathology report was obtained after surgery. pCR was recorded as yes if there was no residual invasive disease noted in the pathologic specimen (ypT0N0 or ypTisN0).
Questionnaires
Participants completed self-report questionnaires on demographics and medical history at baseline and after completing the last chemotherapy treatment. Disease stage, surgery, chemotherapy, weight and height at the first and last chemotherapy was obtained from the EMR. An interviewer-administered PA questionnaire assessed type, frequency, and duration of activities, over the past 3 months.26 Dietary intake over the past 3 months was assessed via a validated self-administered food frequency questionnaire.27 At baseline and end of chemotherapy, participants completed a 9-symptom Patient-Reported Outcomes-Common Terminology Criteria for Adverse Events (PRO-CTCAE) questionnaire experienced in the past 4 weeks. Postchemotherapy scores were adjusted for baseline scores.28
Intervention
Intervention Goals
The goal was for participants to adopt a set of dietary and PA guidelines.10-12 The Healthy Eating Index-2015 was used as a measure of diet quality.29 Adherence to the PA guidelines was defined as ≥150 min/wk of moderate- to vigorous-intensity PA or 75 min/wk of vigorous-intensity PA and twice-weekly resistance training.10
Intervention Delivery and Duration
The exercise and nutrition counseling intervention was delivered individually via a combination of in-person, telephone, or video per participant preference and COVID-19 restrictions. The intervention included four weekly sessions in the first month, two biweekly sessions for months 2 and 3, and monthly sessions thereafter. The number of intervention sessions delivered during chemotherapy varied on the basis of chemotherapy duration.
Content of Counseling Sessions
The intervention was based on the LEAN study, which was adapted from the Diabetes Prevention Program and grounded in the social cognitive therapy, with further modifications for active treatment, including management of chemotherapy side effects.30 Registered dietitians (RDs) who were Certified Specialists in Oncology Nutrition by the Academy of Nutrition and Dietetics, with additional training in exercise science, led the 30-minute counseling sessions.
Nutrition
The nutrition counseling promoted a predominantly plant-based diet modified for texture and flavor as needed to support adequate macronutrient and micronutrient food intake and optimal glucose management. Recommendations included eating a combination of ≥5 fruits and/or vegetable servings/d, ≥25 g/d of fiber, <30 g/d of added sugars, ≤18 ounces/wk of red meat, limited consumption of processed foods, and alcohol consumption ≤1 drink per day.11
PA
The PA program relied on counseling sessions and home-based exercise including a progressive strength training program, with an emphasis on brisk walking and reaching a goal of ≥150 min/wk of moderate- to vigorous-intensity PA or 75 min/wk of vigorous-intensity PA and twice-weekly resistance training.
UC
The UC group had access to an RD and survivorship clinics at any time throughout treatment per usual clinical care, with referral at the discretion of the treating oncologist. At the end of the study, UC participants were offered an individualized counseling session with a study RD and received a copy of the study materials.
Statistical Considerations
Power and Sample Size Considerations
The sample size calculation was performed using PASS 12 (2013 NCSS, LLC, Kayesvile, UT). Our power calculation indicated 86 subjects per arm (n = 172) would achieve 90% power to detect a 0.05 (or 5%) difference in RDI between two arms at a significance level of 0.05, using a two-sided two-sample equal-variance t-test.
Statistical Analyses Plan
Patient characteristics were summarized by randomization groups. The primary end point of RDI was analyzed both as a continuous outcome and as a dichotomized outcome using an 85% cutoff. Hypotheses were tested according to intention-to-treat and statistical significance was defined as P < .05, two-sided. T-test was used to compare continuous outcomes by randomization group, while chi-square test was used for categorical outcomes. We explored effect modification of RDI by patient baseline characteristics and prognostic factors, including menopausal status at diagnosis, tumor HR status, baseline age (<65 v ≥65 years), BMI (<30 v ≥30 kg/m2), self-identified race and ethnicity, living with or without someone, and education level. All analyses were performed using SAS 9.4 (Cary, NC).
RESULTS
We screened 425 women via telephone; 173 women enrolled and were randomly assigned to intervention (n = 87) or UC (n = 86; Fig 1). Age, race, ethnicity, and tumor type did not differ for women enrolled versus those screened and not enrolled.
FIG 1.
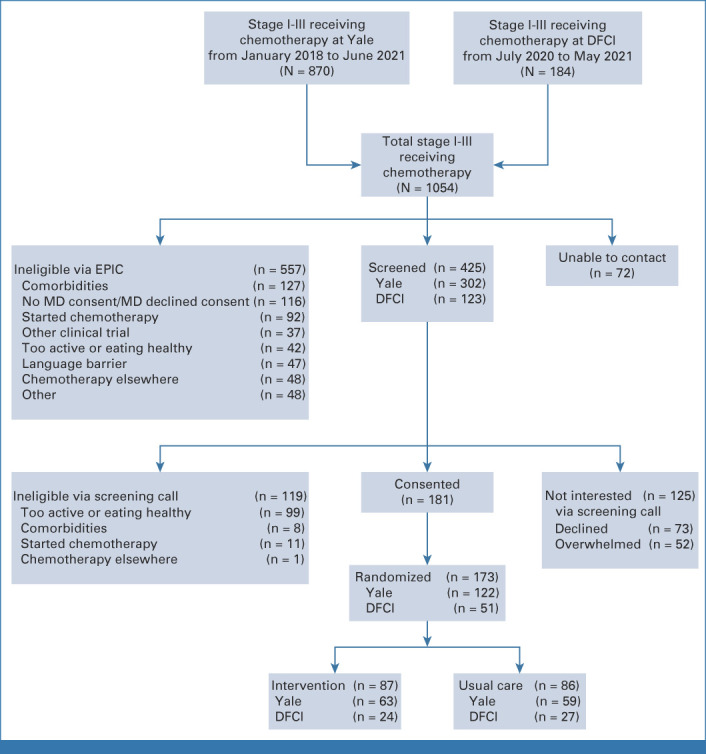
The LEANer study schema. DFCI, Dana-Farber Cancer Institute; EPIC, electronic portfolio of international credentials; LEANer, Lifestyle, Exercise, and Nutrition Early After Diagnosis; MD, medical doctor; pCR, pathologic complete response.
Baseline Characteristics
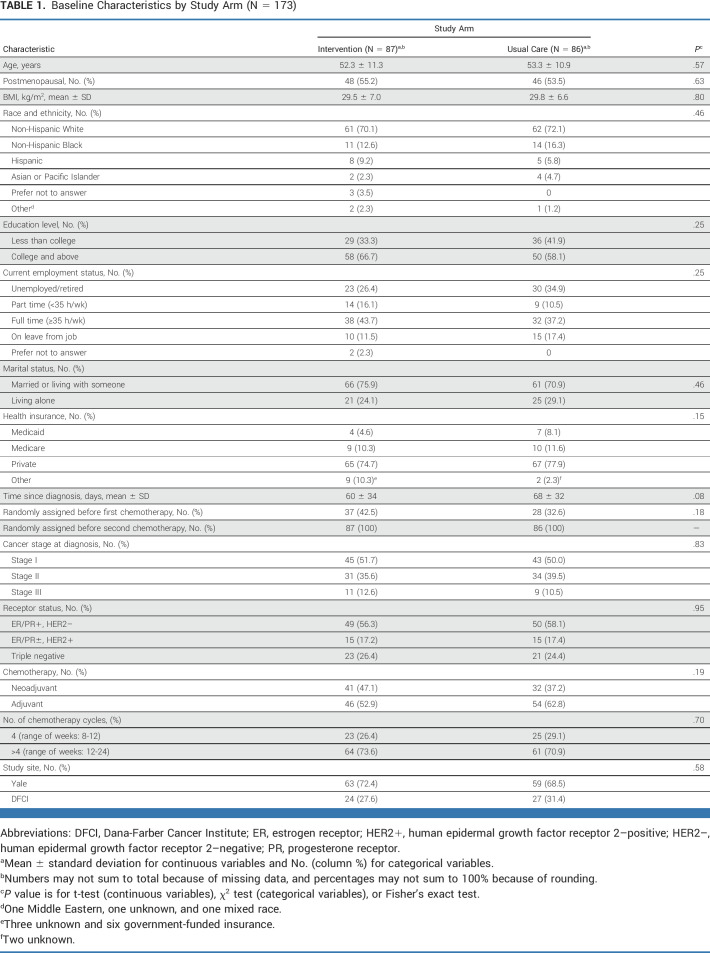
Baseline characteristics were similar between groups (Table 1). Women were age 53 ± 11 years, with a mean BMI of 29.7 ± 6.7 kg/m2, 71% non-Hispanic White, 14% Black, 8% Hispanic, and randomly assigned 64 ± 33 days from diagnosis. Women were diagnosed primarily with stage I breast cancer (51%), with 42% receiving neoadjuvant chemotherapy and 58% receiving adjuvant chemotherapy. The three most common chemotherapy regimens, each accounting for 25% of treatment regimens, were docetaxel and cyclophosphamide (TC) once every 3 weeks for four cycles (TC × 4), dose-dense doxorubicin and cyclophosphamide (ddAC) followed by dose-dense paclitaxel (ddT) once every 2 weeks for 4 weeks each (ddAC × 4 followed by ddT × 4), and dose-dense doxorubicin and cyclophosphamide followed by paclitaxel once weekly for 12 weeks (ddAC followed by weekly T × 12).
TABLE 1.
Baseline Characteristics by Study Arm (N = 173)
Changes in Exercise and Diet
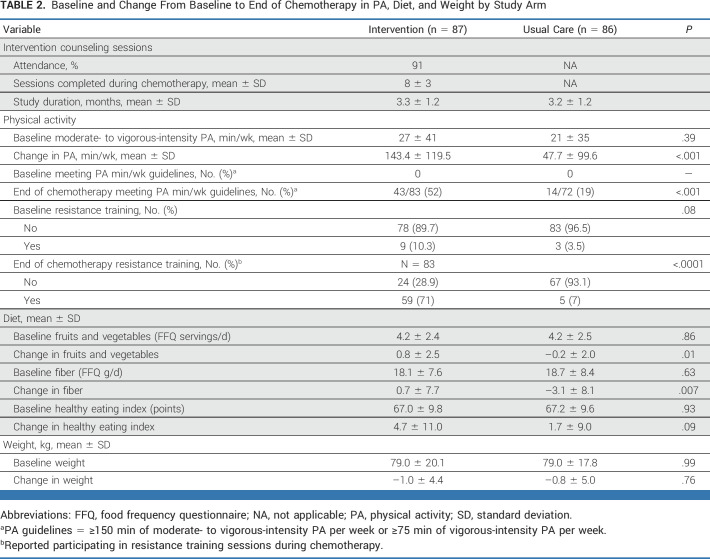
Chemotherapy treatment averaged 3.3 ± 1.2 months. Among women randomly assigned to intervention, a mean of 8 ± 3 study counseling sessions were offered, with 91% attendance.
At the end of chemotherapy, women randomly assigned to intervention reported increases in exercise (143.4 ± 119.5 min/wk) compared with UC (47.7 ± 99.6 min/wk; P < .001; Table 2). Seventy percent of women randomly assigned to intervention reported doing strength training during chemotherapy versus 7% of UC (P < .0001).
TABLE 2.
Baseline and Change From Baseline to End of Chemotherapy in PA, Diet, and Weight by Study Arm
Women randomly assigned to intervention increased fruit and vegetable and dietary fiber intake during chemotherapy versus adverse changes in UC (P < .01; Table 2). Both groups experienced an average of 1 kg weight loss during chemotherapy (P = .76).
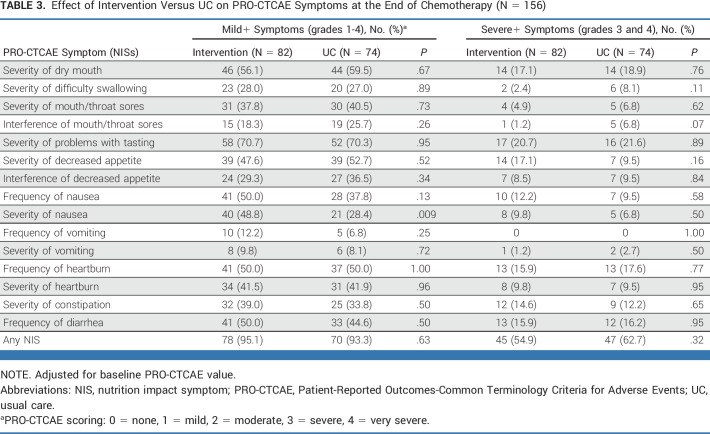
NISs
A majority of women experienced at least one PRO-CTCAE NIS, with 55% and 63% of women randomly assigned to intervention and UC, respectively, experiencing at least one severe NIS (P = .32; Table 3).
TABLE 3.
Effect of Intervention Versus UC on PRO-CTCAE Symptoms at the End of Chemotherapy (N = 156)
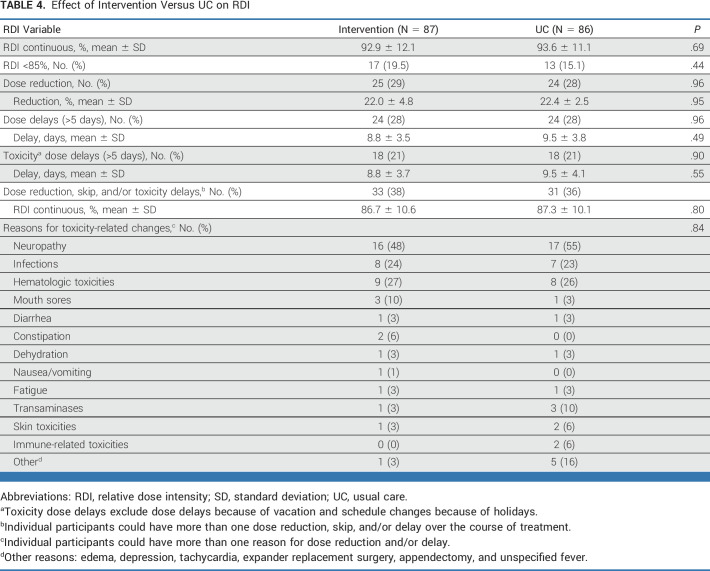
RDI
RDI was 92.9% ± 12.1% and 93.6% ± 11.1% of prescribed chemotherapy for intervention and UC, respectively (P = .69; Table 4). There was no difference in drug-specific RDI by randomization group nor the proportion of patients in the intervention versus UC who achieved ≥85% RDI (81% of intervention v 85% of controls; P = .44). Slightly more than one third of women had a chemotherapy dose reduction and/or dose delay (38% intervention v 36% UC; P = .80). The most common reasons for dose reductions and/or delays were neuropathy, infections, and hematologic toxicities. Tumor stage, treatment regimen, menopausal status at diagnosis, HR status, age (<65 v ≥65 years), BMI (<30 v ≥30 kg/m2), race and ethnicity, living with or without someone, and education level did not modify the effect of the intervention on RDI.
TABLE 4.
Effect of Intervention Versus UC on RDI
pCR
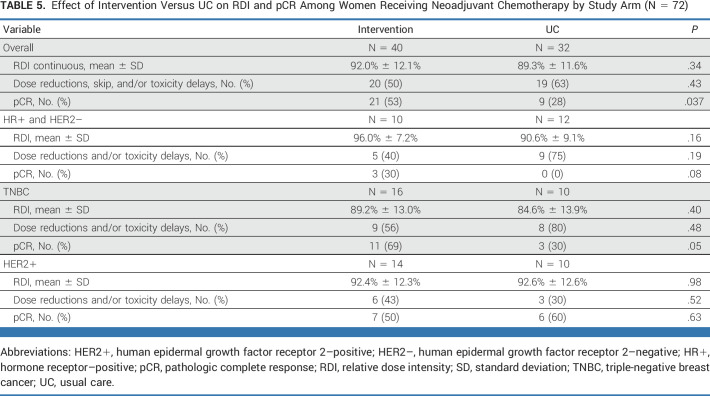
Among the 72 women who received neoadjuvant chemotherapy, women randomly assigned to the intervention arm were more likely to have a pCR (53% v 28% in the UC; P = .037). Findings were limited to women with HR+/HER2– or triple-negative breast cancer subtypes. Breast cancer subtype and RDI were similar for intervention and UC in this subset (Table 5).
TABLE 5.
Effect of Intervention Versus UC on RDI and pCR Among Women Receiving Neoadjuvant Chemotherapy by Study Arm (N = 72)
DISCUSSION
The LEANer trial demonstrated that women with breast cancer initiating chemotherapy were able to make significant improvements in exercise and diet quality during chemotherapy. Women randomly assigned to intervention increased their PA by an average of 143 min/wk. Although 94% of participants undergoing chemotherapy experienced at least one NIS, women randomly assigned to intervention were able to improve their diet quality during chemotherapy compared with UC.
Despite one third of participants experiencing a chemotherapy dose reduction and/or delay, 83% of patients still received >85% RDI and both groups had RDI levels of 93%. Supportive therapies could partially explain high RDI levels. Although trials conducted over 10 years ago had lower RDIs, including one of women randomly assigned to supervised resistance exercise versus Aerobic exercise versus UC, which found RDIs of 90%, 87%, and 84% in the three groups, respectively, our chemotherapy completion rates are similar to more recent trials.31 Sedrak et al31 found 80% of older patients with breast cancer received at least 85% RDI. In recent years, shorter course chemotherapy regimens have been prescribed resulting in higher RDIs. In our trial, 28% of the chemotherapy regimens were four cycles.
We found that chemotherapy dose reductions occurred in 33% of patients, similar to the 27% rate of dose reductions among patients enrolled in an exercise trial published in 2015.21 Similar to that study, we also found neuropathy was the primary reason for dose adjustments. Of note, other participants in our trial could have experienced neuropathy, yet it did not lead to a chemotherapy dose reduction and/or delay. Exercise has been shown to prevent or reduce chemotherapy-induced peripheral neuropathy.32,33
To our knowledge, only one other published study has examined both nutrition and exercise on RDI and it was as a secondary end point. Although Carayol et al found a beneficial effect of a nutrition (nine consultations) and PA (thrice-weekly aerobic and resistance exercise) intervention versus UC on fatigue and quality of life in 143 patients with breast cancer receiving chemotherapy, there was no difference between groups for RDI (97% intervention v 96% UC; P = .39).34 To our knowledge, the LEANer study is the first randomized nutrition and exercise intervention to be administered during chemotherapy with the primary aim of improving RDI. Nutrition counseling from certified oncology dietitians was critical in this study because of the high prevalence of chemotherapy-induced NISs.17 A high-quality diet focused on increased fruits, vegetables, fiber, and protein, and reduced added sugars may reduce inflammation and support immune function, stabilize glucose levels, improve gut microbiome, and maintain muscle mass.11
Among women enrolled in LEANer who received neoadjuvant chemotherapy, pCR rates were 53% in women randomly assigned to intervention versus 28% of women randomly assigned to UC, suggesting the effect of exercise and nutrition on pCR may be via different mechanistic pathways than chemotherapy completion such as immune, inflammatory, and metabolic pathways.30,35-39 In a previous trial of ours, we found exercise altered gene expression in breast tumors, suggesting exercise may have a direct effect on breast cancer.35 Additionally, exercise could enhance blood flow to the tumor and reduce hypoxia in the tumor microenvironment, enhancing chemotherapy delivery to the tumor.36-39 Furthermore, a recent meta-analysis found an association between lower BMI at diagnosis and higher pCR.40 Yet, our findings are hypothesis generating and should be interpreted with caution as pCR was a secondary aim of the study. Future studies of exercise, nutrition, and body composition on pCR and tumor and serum biomarkers are necessary.
Recent ASCO guidelines concluded exercise is safe during chemotherapy, but whether exercise improves chemotherapy tolerance remains unknown.19 To address this evidence gap, the National Cancer Institute launched the Exercise and Nutrition Interventions to Improve Cancer Treatment-Related Outcomes in Cancer Survivors Consortium,41 with four trials in ovarian, rectum, colon, and breast cancer to advance exercise and nutrition intervention research to improve treatment tolerance and patient experience.
Limitations of our study include self-report assessments of PA and diet. We administered the PRO-CTCAE after the last chemotherapy session as a recall of the past 4 weeks rather than the standard PRO-CTCAE recall period of past 7 days; longer recall periods are associated with increasing measurement error. Because of the relatively small number of patients in the neoadjuvant analyses, our findings require validation. The COVID-19 pandemic affected 90 women enrolled after March 12, 2022, resulting in counseling sessions being conducted virtually only and an inability to collect blood and Dual Energy X-ray Absorptiometry scans; thus, assessments of body composition and biomarkers as potential mechanisms mediating the intervention on RDI were not examined. Strengths of our study include detailed EMR review and real-time follow-up with the oncologist to document reasons for chemotherapy dose reductions and delays. Our intervention was delivered virtually with high adherence rates, allowing for future scalability.
In summary, many women newly diagnosed with breast cancer were interested in participating in a trial of exercise and nutrition during chemotherapy, and approximately 8 counseling sessions over an average of 3 months led to improvements in diet quality, PA, and pCR rates, however, not RDI. Clinically relevant effects of nutrition and exercise on RDI may have greater potential in patient populations with lower chemotherapy completion. Given that pCR is an accepted predictor of recurrence and mortality, our findings could provide oncologists with a supportive care intervention that affects the ability to potentially improve survival outcomes.
Tara Sanft
Speakers' Bureau: Hologic/Biotheranostics
Brenda Cartmel
Stock and Other Ownership Interests: Pfizer
Fang-Yong Li
Consulting or Advisory Role: Yiviva
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
Anees B. Chagpar
Leadership: Protean Biodiagnostics
Consulting or Advisory Role: Sanofi/Aventis, Guardant Health, Novartis/Pfizer
Speakers' Bureau: Merck
Andrea Silber
Honoraria: AstraZeneca/Daiichi Sankyo
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2022 ASCO annual meeting, Chicago, IL, June 3-7, 2022.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
All individual participant data collected during the trial, after deidentification, will be shared. In addition, the study Protocol, statistical analysis plan, informed consent form, and analytic code will also be shared. These data will be shared immediately after publication with no end date.
AUTHOR CONTRIBUTIONS
Conception and design: Tara Sanft, Maura Harrigan, Brenda Cartmel, Fang-Yong Li, Dawn L. Hershman, Karen Basen-Engquist, Beth A. Jones, Jennifer A. Ligibel, Melinda L. Irwin
Financial support: Melinda L. Irwin
Administrative support: Michelle Zupa
Provision of study materials or patients: Maura Harrigan, Courtney McGowan, Michelle Zupa, Anees B. Chagpar, Jennifer A. Ligibel, Melinda L. Irwin
Collection and assembly of data: Tara Sanft, Maura Harrigan, Courtney McGowan, Brenda Cartmel, Michelle Zupa, Fang-Yong Li, Thai Hien Nguyen, Dawn L. Hershman, Anees B. Chagpar, Anna Tanasijevic, Jennifer A. Ligibel, Melinda L. Irwin
Data analysis and interpretation: Tara Sanft, Maura Harrigan, Courtney McGowan, Brenda Cartmel, Fang-Yong Li, Leah M. Ferrucci, Leah Puklin, Anlan Cao, Marian L. Neuhouser, Dawn L. Hershman, Beth A. Jones, Tish Knobf, Andrea Silber, Melinda L. Irwin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Trial of Exercise and Nutrition on Chemotherapy Completion and Pathologic Complete Response in Women With Breast Cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tara Sanft
Speakers' Bureau: Hologic/Biotheranostics
Brenda Cartmel
Stock and Other Ownership Interests: Pfizer
Fang-Yong Li
Consulting or Advisory Role: Yiviva
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
Anees B. Chagpar
Leadership: Protean Biodiagnostics
Consulting or Advisory Role: Sanofi/Aventis, Guardant Health, Novartis/Pfizer
Speakers' Bureau: Merck
Andrea Silber
Honoraria: AstraZeneca/Daiichi Sankyo
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7e30, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Longo DL, Duffey PL, DeVita VT, et al. : The calculation of actual or received dose intensity: A comparison of published methods. J Clin Oncol 9:2042-2051, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Bonadonna G, Valagussa P, Moliterni A, et al. : Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up. N Engl J Med 332:901-906, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Weycker D, Barron R, Edelsberg J, et al. : Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat 133:301-310, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Neilson HK, Farris MS, Stone CR, et al. : Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: A systematic review and meta-analysis. Menopause 24:322-344, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Schmid D, Leitzmann MF: Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol 25:1293-1311, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski R, Aragaki A, Anderson G, et al. : Dietary modification and breast cancer mortality: Long-term follow-up of the Women's Health Initiative randomized trial. J Clin Oncol 38:1419-1428, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard-Barbash R, Friedenrecih C, Courneya K, et al. : Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J Natl Cancer Inst 104:815-840, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruinsma T, Dyer A, Rogers C, et al. : Effects of diet and exercise-induced weight loss on biomarkers of inflammation in breast cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 30:1048-1062, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell KL, Winters-Stone KM, Wiskemann J, et al. : Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51:2375-2390, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock CL, Thomson CA, Sullivan KR, et al. : American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 72:230-262, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Hastert TA, Beresford SA, Patterson RE, et al. : Adherence to WCRF/AICR cancer prevention recommendations and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 22:1498-1508, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arem H, Mama SK, Duan X, et al. : Prevalence of healthy behaviors among cancer survivors in the United States: How far have we come? Cancer Epidemiol Biomarkers Prev 29:1179-1187, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin ML, Crumley D, McTiernan A, et al. : Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity and Lifestyle (HEAL) Study. Cancer 97:1746-1757, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. : Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 4:798-804, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Peterson B, Winer E: Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 19:2381-2389, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Prado CM, Laviano A, Gillis C, et al. : Examining guidelines and new evidence in oncology nutrition: A position paper on gaps and opportunities in multimodal approaches to improve patient care. Support Care Cancer 30:3073-3083, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salas S, Cottet V, Dossus L, et al. : Nutritional factors during and after cancer: Impacts on survival and quality of life. Nutrients 14:2958, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ligibel JA, Bohlke K, May AM, et al. : Exercise, diet and weight management during cancer treatment: ASCO Guideline. J Clin Oncol 49:2491-2507, 2022 [DOI] [PubMed] [Google Scholar]
- 20.Courneya KS, Segal RJ, Mackey JR, et al. : Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 25:4396-4404, 2007 [DOI] [PubMed] [Google Scholar]
- 21.van Waart H, Stuiver MM, van Harten WH, et al. : Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J Clin Oncol 33:1918-1927, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Bland K, Zadravec K, Landry T, et al. : Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol 136:79-85, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Caan B, Meyerhardt J, Brown J, et al. : Recruitment strategies and design considerations in a trial of resistance training to prevent dose-limiting toxicities in colon cancer patients undergoing chemotherapy. Contemp Clin Trials 101:106242, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Minckwitz G, Untch M, Blohmer J, et al. : Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinstic breast cancer subtypes. J Clin Oncol 30:1796-1804, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Sanft T, Harrigan M, Cartmel B, et al. : Effect of healthy diet and exercise on chemotherapy completion rate in women with breast cancer: The Lifestyle, Exercise and Nutrition Early after Diagnosis (LEANer) study: Study protocol for a randomized clinical trial. Contemp Clin Trials 109:106508, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kriska AM, Knowler WC, LaPorte RE, et al. : Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 13:401-411, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Patterson RE, Kristal AR, Tinker LF, et al. : Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 9:178-187, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Kang D, Kim S, Kim H, et al. : Surveillance of symptom burden using the patient-reported outcome version of the common terminology criteria for adverse events in patients with various types of cancer during chemoradiation therapy: Real-world study. JMIR Public Health Surveill 9:e44105, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson C, Harrigan M, George SM, et al. : Changes in diet quality in a randomized weight loss trial in breast cancer survivors: The Lifestyle, Exercise and Nutrition (LEAN) Study. NPJ Breast Cancer 2:16026, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrigan M, Cartmel B, Loftfield E, et al. : Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol 34:669-676, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedrak MS, Sun C, Ji J, et al. : Low-intensity adjuvant chemotherapy for breast cancer in older women: Results from the Prospective Multicenter HOPE Trial. J Clin Oncol 41:316-326, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bland KA, Kirkham AA, Bovard J, et al. : Effect of exercise on taxane chemotherapy-induced peripheral neuropathy in women with breast cancer: A randomized controlled trial. Clin Breast Cancer 19:411-422, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Kleckner I, Kamen C, Gewandter J, et al. : Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: A multi-center, randomized controlled trial. Support Care Cancer 26:1019-1028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carayol M, Ninot G, Senesse P, et al. : Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: The “APAD1” randomized controlled trial. BMC Cancer 19:737, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligibel JA, Dillon D, Giobbie-Hurder A, et al. : Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: Results from the Pre-Operative Health and Body (PreHAB) study. Clin Cancer Res 25:5398-5406, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Morielli AR, Heer E, et al. : Effects of exercise on cancer treatment efficacy: A systematic review of preclinical and clinical studies. Cancer Res 81:4889-4895, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hojman P, Gehl J, Christensen JF, et al. : Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab 27:10-21, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Betof AS, Lascola CD, Weitzel D, et al. : Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst 107:djv040, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zylstra J, Whyte GP, Beckmann K, et al. : Exercise prehabilitation during neoadjuvant chemotherapy may enhance tumour regression in oesophageal cancer: Results from a prospective non-randomised trial. Br J Sports Med 56:402-409, 2022 [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Zhang S, Yee D, et al. : Impact of body mass index on pathological complete response following neoadjuvant chemotherapy in operable breast cancer: A meta-analysis. Breast Cancer 28:618-629, 2021 [DOI] [PubMed] [Google Scholar]
- 41.National Cancer Instititute: Exercise and nutrition interventions to improve cancer treatment-related outcomes (ENICTO) in cancer survivors, 2023. https://cancercontrol.cancer.gov/brp/hbrb/enicto-consortium
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All individual participant data collected during the trial, after deidentification, will be shared. In addition, the study Protocol, statistical analysis plan, informed consent form, and analytic code will also be shared. These data will be shared immediately after publication with no end date.