Abstract
PURPOSE
Gut microbiota injury in allogeneic hematopoietic cell transplantation (HCT) recipients and patients with AML has been associated with adverse clinical outcomes. Previous studies in these patients have shown improvements in various microbiome indices after fecal microbiota transplantation (FMT). However, whether microbiome improvements translate into improved clinical outcomes remains unclear. We examined this question in a randomized, double-blind, placebo-controlled phase II trial.
METHODS
Two independent cohorts of allogeneic HCT recipients and patients with AML receiving induction chemotherapy were randomly assigned in a 2:1 ratio to receive standardized oral encapsulated FMT versus placebo upon neutrophil recovery. After each course of antibacterial antibiotics, patients received a study treatment. Up to three treatments were administered within 3 months. The primary end point was 4-month all-cause infection rate. Patients were followed for 9 months.
RESULTS
In the HCT cohort (74 patients), 4-month infection density was 0.74 and 0.91 events per 100 patient-days in FMT and placebo arms, respectively (infection rate ratio, 0.83; 95% CI, 0.48 to 1.42; P = .49). In the AML cohort (26 patients), 4-month infection density was 0.93 in the FMT arm and 1.25 in the placebo arm, with an infection rate ratio of 0.74 (95% CI, 0.32 to 1.71; P = .48). Unique donor bacterial sequences comprised 25%-30% of the fecal microbiota after FMT. FMT improved postantibiotic recovery of microbiota diversity, restored several depleted obligate anaerobic commensals, and reduced the abundance of expanded genera Enterococcus, Streptococcus, Veillonella, and Dialister.
CONCLUSION
In allogeneic HCT recipients and patients with AML, third-party FMT was safe and ameliorated intestinal dysbiosis, but did not decrease infections. Novel findings from this trial will inform future development of FMT trials.
INTRODUCTION
Patients with AML and recipients of allogeneic hematopoietic cell transplantation (HCT) experience major disruptions to their intestinal microbiota.1,2 Dysbiosis patterns in these patients are largely similar3 and characterized by microbiota community domination,4-6 diversity loss,7-9 and pathogen outgrowth.10-12 Dysbiosis has been associated with mortality,7,8 acute graft-versus-host disease (aGVHD),13-20 poor immune reconstitution,21-23 and relapse.24,25 Fecal microbiota transplantation (FMT) has been successful in ameliorating dysbiosis26-32 and treating refractory aGVHD.33-38 However, whether FMT-mediated modulation of the microbiota prevents subsequent adverse clinical outcomes remains unclear. Most reported FMT trials in these patients have been small, single-arm, and open-label trials, with a microbiota endpoint as the primary objective.
CONTEXT
Key Objective
Does fecal microbiota transplantation (FMT) decrease infection rates in allogeneic hematopoietic cell transplantation recipients and patients with AML?
Knowledge Generated
In a randomized double-blind placebo-controlled trial, third-party oral FMT was safe but did not decrease infections. FMT ameliorated intestinal dysbiosis by restoring microbiota diversity and commensal bacteria and reducing the abundance of pathobionts.
Relevance (C.F. Craddock)
-
The feasibility and safety of prospective interventional trials of FMT in patients with hematological malignancies undergoing intensive chemotherapy or allo-SCT is demonstrated. Larger randomized trials with clinically relevant endpoints are now indicated in this important patient population.*
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
To evaluate the clinical efficacy of FMT when used as a prophylactic approach, we conducted a randomized, double-blind, placebo-controlled phase II trial using a third-party, oral encapsulated product and with a clinical primary end point. We chose infection as the primary end point because (1) it has a high clinical burden, (2) commensal microbiota enhance antiinfective immunity not only against intestinal pathogens but also distant infections of extraintestinal origin,39 and (3) various types of infection after HCT and induction chemotherapy (eg, bloodstream,5,6,40,41 Clostridioides difficile [CDI],42 and respiratory tract infections43-45) have been associated with intestinal dysbiosis.
METHODS
Trial Oversight
This trial (ClinicalTrials.gov identifier: NCT03678493) was conducted in two parallel independent cohorts of adults treated at the University of Minnesota: allogeneic HCT recipients (HCT cohort) and patients with AML receiving inpatient induction chemotherapy (AML cohort). The Protocol (online only) was approved by our institutional review board and opened to accrual in September 2019. All patients provided written informed consent. Safety monitoring and stopping rules are detailed in the Data Supplement (Supplementary Methods 1, online only).
The trial reached its accrual goal for the HCT cohort in February 2022 after 72 HCT recipients received dose 1. Because of slow accrual in the AML cohort, this cohort was closed to new accrual at the same time. Patients who had already been enrolled but had not yet received dose 1 (two patients) continued on study and were included in the analysis. Data cutoff and unblinding occurred on August 5, 2022, once the last subject in each cohort completed follow-up for the last clinical end point.
Study Design
Patients age 18 years and older were enrolled in this randomized, double-blind, placebo-controlled, phase II trial at the time of admission to the hospital to start conditioning for a T-replete allogeneic HCT or induction chemotherapy for AML. A second set of eligibility criteria were checked when absolute neutrophil count (ANC) reached >1 × 109/L from nadir and antibacterial antibiotics (except those used for Pneumocystis jirovecii prophylaxis) were discontinued for 2 days. If all acute toxicities had resolved to grade 2 or lower at that time and patients were able to swallow capsules and had no evidence of relapse/progression, they received the first study treatment (dose 1). This occurred within 3 days of confirming eligibility, usually on the same day. Consented patients who did not reach the time point for second eligibility screening and those who reached that time point but did not meet the criteria for dose 1 were replaced.
Simple randomization was used to assign patients in a 2:1 ratio to receive third-party FMT or placebo in the form of five oral capsules taken all at once. Details of treatment administration are provided in the Data Supplement (Supplementary Appendix). For patients re-exposed to antibacterial antibiotics after dose 1, up to two more doses of the same type (one dose per exposure) were given until 3 months after consent. The criteria for redosing were similar to those used for dose 1. The protocol did not recommend any changes to the standard of care. We generally use acyclovir for viral, an azole for fungal, and levofloxacin for bacterial prophylaxis for the duration of neutropenia (ANC <1 × 109/L), and cefepime for empiric frontline treatment of neutropenic fever. Patients were followed for 9 months.
The primary end point was all-cause infection rate within 4 months after dose 1. Both microbiologically and clinically documented infections were included. Microbiological documentation included a positive culture or clinically consistent findings from other microbiological assays (eg, polymerase chain reaction). Controversial cases were resolved after consultation with the infectious diseases team. Blood culture isolates considered to be skin-associated bacteria (including viridans group Streptococcus spp. and coagulase-negative Staphylococcus spp.) were classified as bloodstream infection (BSI) if the organism was recovered in ≥2 blood culture sets.46 Clinically documented infection was defined by compelling clinical evidence without a positive microbiological workup (eg, fever and a new lung infiltrate). Secondary end points included specific types of infection (bacterial, viral, or fungal), grade II-IV aGVHD47 (HCT cohort only), and BSI within 7 days after each dose.
Study product manufacturing is detailed in the Data Supplement (Supplementary Appendix). Each FMT capsule contained ≥1 × 1011 bacteria with ≥40% viability and each dose consisted of five capsules. This dose was previously found to be effective in treatment of recurrent CDI.48 Product from four different donors was used and each patient received material manufactured from a single donor.
Statistical Analysis
The two cohorts were analyzed independently. The only study procedure that occurred between the two eligibility screening time points was the collection of baseline samples after consent, and only objective criteria (neutrophil recovery and discontinuation of antibacterial antibiotics) were used to determine the second eligibility time point. All patients who met both eligibility criteria were included in safety and efficacy analyses. The expected all-cause infection density in the control arms of both cohorts was 0.9-1.3 events per 100 patient-days in the first 4 months after day 30 of chemotherapy or transplantation, on the basis of our institutional pilot and published data.49 Using Poisson rates to model recurrent event data in each arm, their ratio as measure of treatment efficacy, and a variance-stabilized test statistic, we calculated that a minimum of 72 patients in each cohort would provide 80% power to detect a 50% lower rate of infection in the FMT arm compared with the placebo arm at a two-sided alpha level of 5% (PASS 14, NCSS, LLC, Kaysville, UT). An expected rate of 1.3 events per 100 patient-days was considered for the placebo arm in both cohorts. The intervention was a package defined as up to three treatments within 4 months after dose 1. The objective of the study was to evaluate whether the experimental package as a whole would reduce infections starting with dose 1. With this definition and considering that infection rate over 4 months rather than time to the first infection was the measured end point, we did not censor patients at the time of second or third dose.
Infection data were summarized using infection density. Mean cumulative number of events, estimated by the Nelson-Aalen method,50 was plotted for each arm. The recurrent infection data were compared between the two arms of each cohort using the multiplicative intensity method developed by Wang et al,51 which models the occurrence of recurrent events by a subject-specific nonstationary Poisson process via a latent variable. The treatment arm was considered as the predictor and death as an informative terminal event. This method shares the same spirit as the joint frailty scale-change model but treats death events as informative censoring rather than terminal events.52 Post hoc multivariable regression was performed in the HCT cohort to adjust for conditioning intensity because of its large, by-chance imbalance between the two arms. Package reReg in R 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for both preplanned and post hoc analyses of the primary end point,52 with 95% CIs and P values estimated from 1,000 bootstraps. A two-sided P < .05 was considered statistically significant. The cumulative incidence of grade II-IV aGVHD until 6 months after HCT was calculated in the HCT cohort with death as a competing risk and compared between the two arms using a Gray's test. Post hoc Fine and Gray multivariable proportional-hazard modeling was used to adjust for the GVHD prophylactic regimen, which was not balanced between the two arms. The sample collection schedule and methods for microbiota sequencing and analysis are detailed in the Data Supplement (Supplementary Methods 2).
RESULTS
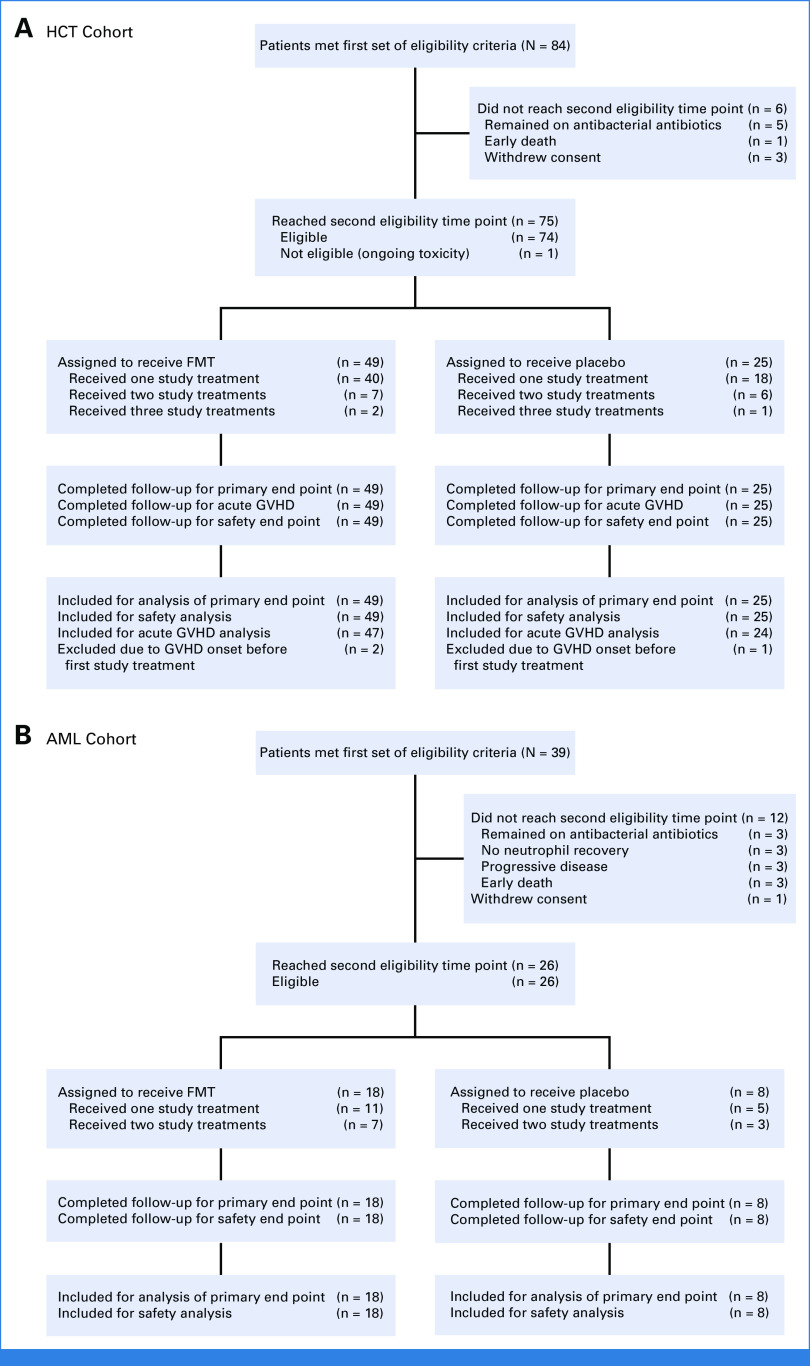
All patients meeting both sets of eligibility criteria were treated. The HCT cohort included 74 patients (FMT, 49; placebo, 25) who received 1 (58 patients), 2 (13 patients), or 3 (3 patients) study treatments. The AML cohort included 26 patients (FMT, 18; placebo, 8) who received 1 (16 patients) or 2 (10 patients) study treatments. There was no loss to follow-up and no missing data (Fig 1).
FIG 1.
CONSORT diagram: (A) HCT cohort and (B) AML cohort. FMT, fecal microbiota transplantation; GVFD, graft-versus-host disease; HCT, hematopoietic cell transplantation.
HCT Cohort
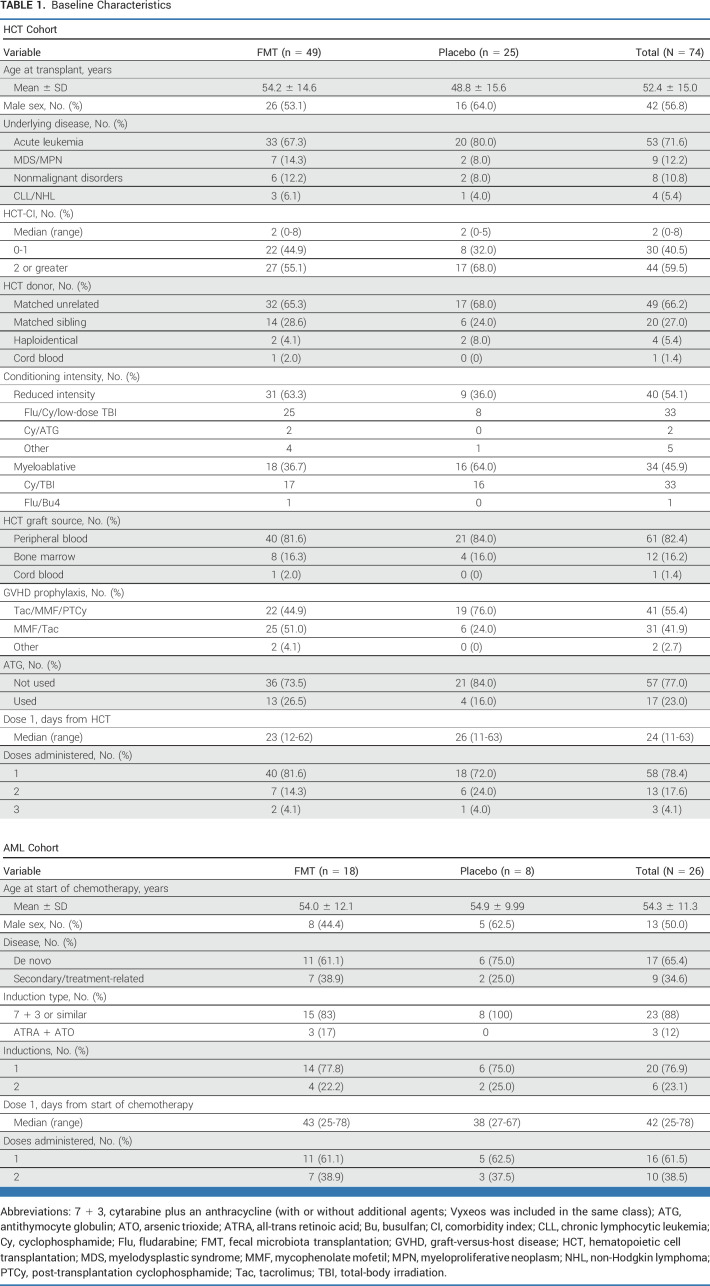
Baseline characteristics were balanced between the two arms (Table 1), except more patients (63.3% v 36.0%) in the FMT arm received reduced-intensity conditioning and fewer (44.9% v 76.0%) received GVHD prophylaxis using a post-transplantation cyclophosphamide (PTCy) backbone. Thirty-three of the 34 patients receiving myeloablative conditioning also received PTCy, indicating multicollinearity between GVHD prophylaxis and conditioning intensity. Given the randomized group assignment, we considered the imbalance in one of these variables to have occurred by chance and the imbalance in the other variable followed due to multicollinearity. All patients were exposed to antibacterial antibiotics before dose 1 (Data Supplement [Supplementary Fig 1]), which was given at a median of 23 (range, 12-62) and 26 (range, 11-63) days after HCT in FMT and placebo arms, respectively. Six patients in the FMT arm and three in the placebo arm died during follow-up. Deaths in the FMT arm were due to aGVHD (three patients), idiopathic pneumonia syndrome (one patient), cryptogenic organizing pneumonia (one patient), and seizure (one patient). All three deaths in the placebo arm were due to relapse.
TABLE 1.
Baseline Characteristics
Grade 3+ adverse events (AEs) are summarized in the Data Supplement (Supplementary Table 1). The most frequent of such events in the FMT arm was aGVHD, occurring as a Common Terminology Criteria for Adverse Events grade 3+ AE in 9 (18.4%) patients versus none in the placebo arm. BSI occurred in 8 (16.3%) FMT recipients versus 3 (12.0%) placebo patients. Details of BSIs are provided in the Data Supplement (Supplementary Table 2). No grade 3+ AE occurred within 24 hours of a dose. There was only 1 grade 3+ GI AE (diarrhea) within 7 days after FMT. BSI within 7 days after FMT occurred in one patient. This was a case of cytomegalovirus (CMV) viremia and the only event that counted toward the stopping rule. The patient was CMV-seropositive and their HCT and FMT donors were both CMV-seronegative, making a relationship with FMT unlikely.
Seventy infections occurred during the infection monitoring window (Data Supplement [Supplementary Figs 2A and 2B], Data Supplement [Supplementary Table 3]). The infection density within 120 days after dose 1 was 0.74 and 0.91 events per 100 patient-days in FMT and placebo arms, respectively. At 120 days after dose 1, the mean cumulative number of events per patient was 0.89 (95% CI, 0.60 to 1.19) and 1.09 (95% CI, 0.55 to 1.64) in the FMT and control arms, respectively, with an infection rate ratio of 0.83 (95% CI, 0.48 to 1.42; P = .49; Fig 2A). After adjusting for conditioning intensity, the infection rate ratio declined to 0.70 (95% CI, 0.38 to 1.30; P = .26; Data Supplement [Supplementary Table 4]). Subgroup analysis for bacterial and viral infections did not indicate a treatment effect (Data Supplement [Supplementary Table 5]). The number of events was not large enough for FMT donor-specific subset analysis.
FIG 2.
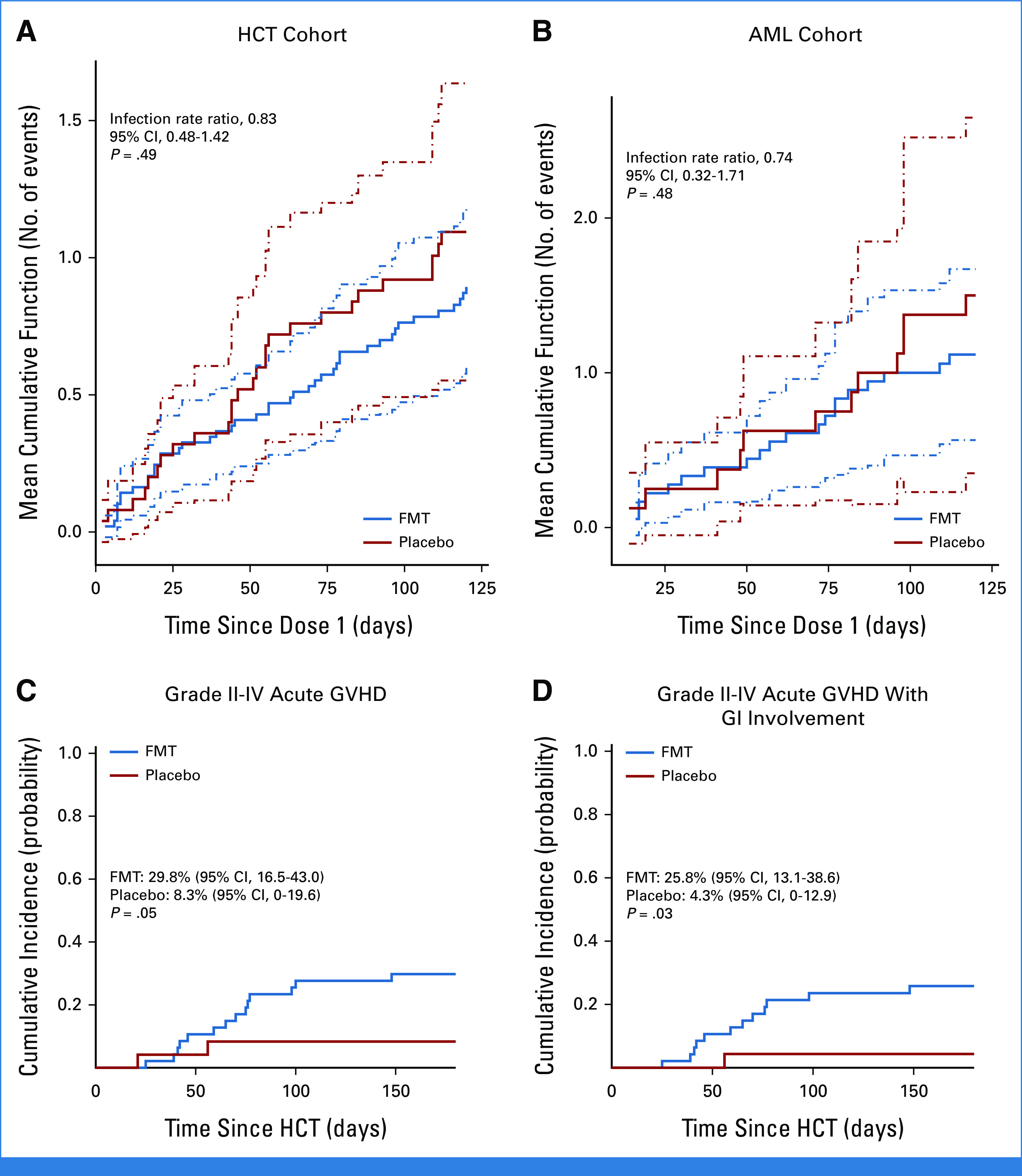
Analysis of the primary and secondary end points. The mean cumulative number of infection events in each arm: (A) HCT cohort and (B) AML cohort. Infection rate ratios are unadjusted and compare FMT to placebo. 95% CIs are shown as dashed lines. Cumulative incidence of (C) grade II-IV acute GVHD overall and (D) grade II-IV acute GVHD with GI involvement in the HCT cohort. FMT, fecal microbiota transplantation; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation.
Sixteen grade II-IV aGVHD events (14 with GI involvement) occurred by day 180 after HCT, with a cumulative incidence of 29.8% (95% CI, 16.5 to 43.0) and 8.3% (95% CI, 0 to 19.6) in FMT and placebo arms, respectively (P = .05; Fig 2C). The cumulative incidence of grade II-IV aGVHD with GI involvement was 25.8% (95% CI, 13.1 to 38.6) and 4.3% (95% CI, 0 to 12.9) in FMT and placebo arms, respectively (P = .03; Fig 2D). After adjustment for GVHD prophylaxis, the hazard ratio for FMT versus placebo was 3.1 (95% CI, 0.6 to 14.2; P = .15) for grade II-IV aGVHD and 5.5 (95% CI, 0.7 to 44.0; P = .11) for grade II-IV aGVHD with GI involvement (Data Supplement [Supplementary Table 6]). Further adjustment for conditioning intensity was not possible due to multicollinearity. All 6 cases of stage III-IV GI aGVHD and all three fatal cases of aGVHD occurred in the FMT arm.
AML Cohort
Baseline characteristics were balanced between the two arms (Table 1). All patients were exposed to antibacterial antibiotics before dose 1 (Data Supplement [Supplementary Fig 1]), which was given at a median of 43 (range, 25-78) and 38 (range, 27-67) days after starting chemotherapy in FMT and placebo arms, respectively. One patient (FMT arm) died during follow-up, due to relapse. Grade 3+ AEs are summarized in the Data Supplement (Supplementary Table 1). The most frequent of such events in the FMT arm was BSI, occurring in 10 (55.6%) patients versus 2 (25.0%) patients in the placebo arm. Details of BSIs are provided in the Data Supplement (Supplementary Table 2). No grade 3+ AE occurred within 24 hours and no grade 3+ GI AE within 7 days after FMT. No event counted toward the stopping rule.
Thirty-two infections occurred during the infection monitoring window (Data Supplement [Supplementary Figs 2C and 2D], Data Supplement [Supplementary Table 3]). The infection density within 120 days after dose 1 was 0.93 and 1.25 events per 100 patient-days in the FMT and placebo arm, respectively. At 120 days after dose 1, the mean cumulative number of events per patient was 1.12 (95% CI, 0.57 to 1.67) and 1.50 (95% CI, 0.35 to 2.65) in FMT and control arms, respectively, with an infection rate ratio of 0.74 (95% CI, 0.32 to 1.71; P = .48; Fig 2B). Subgroup analysis for bacterial and viral infections did not indicate a treatment effect (Data Supplement [Supplementary Table 5]). Six patients in the FMT arm and five in the placebo arm proceeded to HCT. Of these, four patients and one patient developed grade II-IV aGVHD, respectively.
Microbiota Effects of FMT
After preprocessing and filtering, 287 stool samples containing 2,126 unique amplicon sequence variants ASVs (5 phyla, 27 families, and 45 genera) were analyzed. Microbiota in HCT and AML cohorts clustered together (Data Supplement [Supplementary Fig 3A]), consistent with similar patterns of injury and recovery as reported previously.3 Thus, we combined these cohorts in future analyses. No clustering per treatment arm was apparent at baseline (P = .27; Fig 3A) or before dose 1 (P = .85; Fig 3B).
FIG 3.
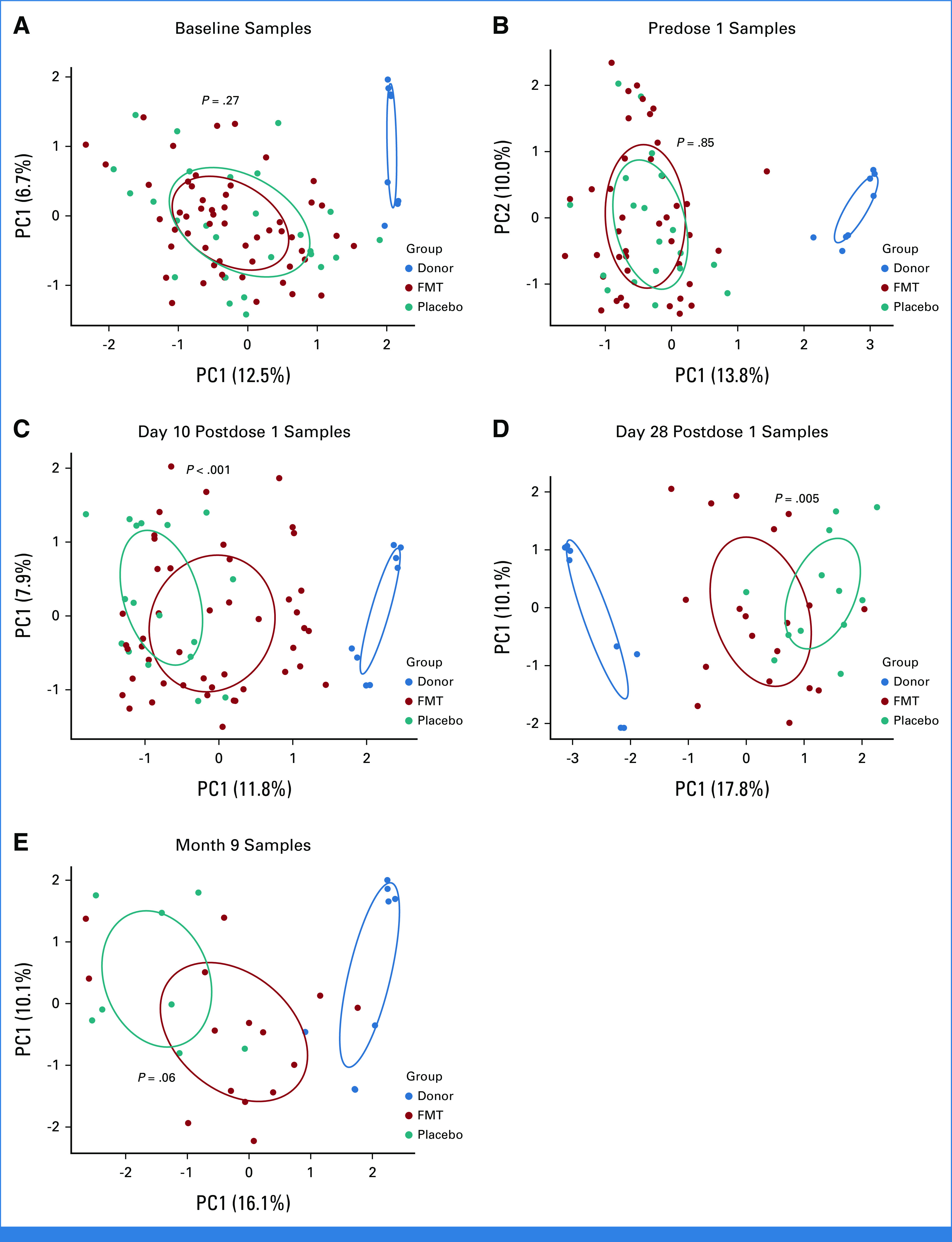
Microbiota clustering per treatment arm at each time point. (A) Baseline, (B) predose 1, (C) day 10 postdose 1, (D) day 28 postdose 1, and (E) month 9. Principal component analysis using Aitchison distances, with numbers in parentheses indicating the proportion of variation explained by the corresponding axis. Each point is a fecal sample and samples with similar microbiome composition are closer together in the plot. 95% confidence ellipses for group centroids are shown. P values are from an adonis test with 999 permutations and compare samples in FMT versus placebo arms. FMT, fecal microbiota transplantation; PC, principal component.
Even at baseline, patient microbiota clustered away from donor microbiota, indicating injured communities (Data Supplement [Supplementary Fig 3B]). Patient microbiota at baseline was enriched in frequently pathogenic (eg, Enterococcus, Staphylococcus), mucolytic (Ruminococcus gnavus/torques groups),53 and typically oral (eg, Rothia,54 Dialister55) genera and depleted in several obligate anaerobic bacteria (eg, Faecalibacterium) known to produce butyrate.56 Expansion of mucolytic bacteria in patients with acute leukemia and HCT recipients has been reported.18,40,57-59 Further expansion of genera such as Enterococcus and Staphylococcus and depletion of the largely butyrogenic family Lachnospiraceae and genera Blautia, Roseburia, and Faecalibacterium occurred between baseline and before dose 1 (Data Supplement [Supplementary Fig 4]).
Postintervention samples clustered according to the treatment arm (Figs 3C-3E), with post-FMT microbiota moving toward donor microbiota, indicating an FMT effect. Post-FMT samples were enriched in obligate anaerobic commensal families Coriobacteriaceae (eg, genus Collinsella) and Rikenellaceae (eg, genus Alistipes), whereas postplacebo samples were enriched in Streptococcus, Enterococcus, Veillonella, and Dialister (Data Supplement [Supplementary Fig 5]). FMT was highly effective in decreasing Enterococcus and Dialister, which had expanded greatly before dose 1 and restoring Collinsella, which had drastically declined before dose 1 and failed to have any spontaneous recovery after placebo. Both arms showed Blautia recovery toward donor levels (Fig 4). Microbiota alpha diversity was similar at baseline and predose 1 between the two arms. After treatment, while diversity in postplacebo samples showed slight recovery over time, it did not reach baseline values. By contrast, diversity in post-FMT samples significantly increased and even exceeded baseline (Fig 5A).
FIG 4.
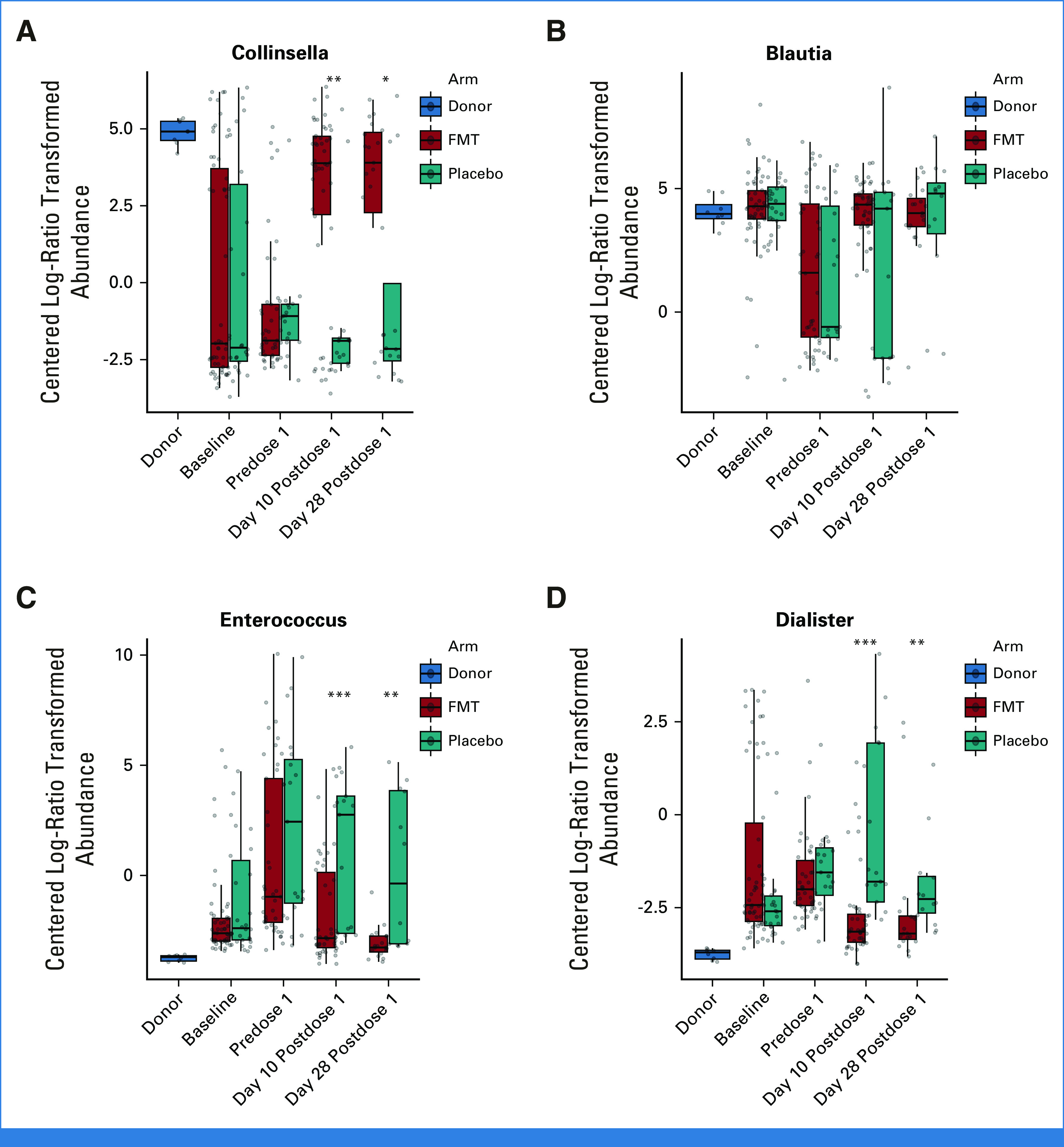
Effect of FMT on the abundance of select genera: (A) Collinsella, (B) Blautia, (C) Enterococcus, and (D) Dialister. Centered log-ratio abundances are shown. The horizontal line within each box shows the median. *P < .05, **P < .01, ***P < .001. P values are from a Wilcoxon test and compare the two arms at each time point. FMT, fecal microbiota transplantation.
FIG 5.
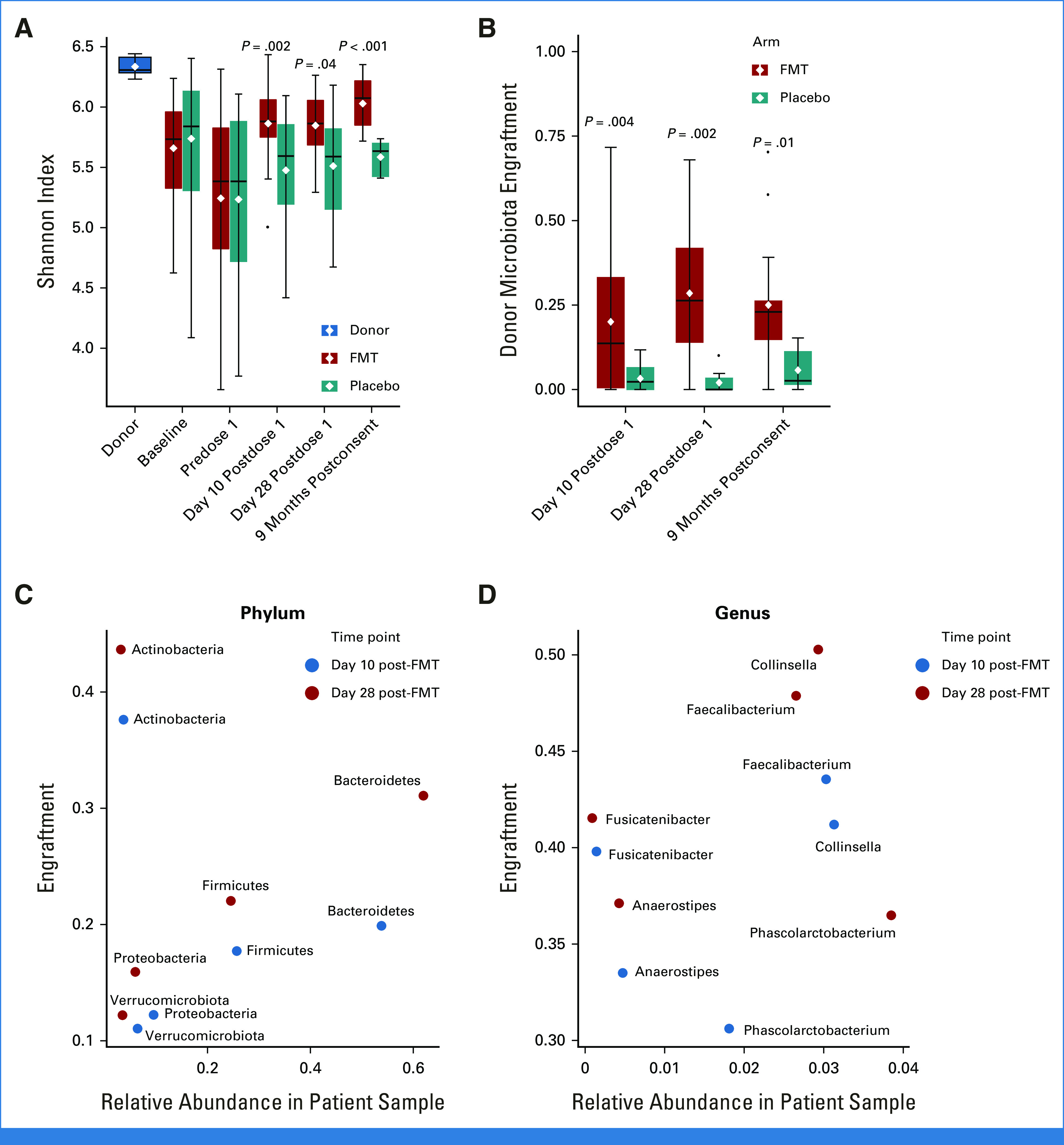
Alpha diversity and donor microbiota engraftment. (A) Alpha diversity, as measured by Shannon's index. (B) Donor microbiota engraftment in day-10 postdose, day-28 postdose, and 9-month postconsent samples as determined by SourceTracker. SourceTracker estimates the attribution of microbiota in a post-treatment sample to donor microbiota. In choosing the donor source for each post-FMT sample, only the specific donor whose product was administered to the patient was used as donor. By contrast, because placebo capsules did not contain any donor material, the calculated engraftment rates from all four presumptive donors were averaged to derive donor attribution of postplacebo microbiota. There were 77 post-FMT and 37 postplacebo samples in this analysis. The distribution of samples across time points was 60 samples at 10 days post-treatment, 31 samples at 28 days post-treatment, and 23 samples at 9 months. As expected, donor attribution of postplacebo microbiota was minimal. The horizontal line within each box indicates the median and the white diamond indicates the mean. P values comparing FMT and placebo arms are from a Wilcoxon test. The relationship between (C) phylum- and (D) genus-level engraftment rates and relative abundances at days 10 and 28 post-FMT. The x-axis shows the mean relative abundance of the taxon of interest in post-FMT samples, and the y-axis shows the attribution of that taxon to donor microbiota. FMT, fecal microbiota transplantation.
Sustained donor microbiota engraftment of approximately 25% was achieved (Fig 5B; Data Supplement [Supplementary Analysis 1]). Taxa with the largest donor attribution were Actinobacteria (genus Collinsella), Faecalibacterium, Fusicatenibacter, and Anaerostipes (Figs 5C and 5D). Faecalibacterium and Anaerostipes are butyrate producers60 depleted in aGVHD.61 Exploratory analyses did not offer a microbiome explanation for the higher incidence of aGVHD in the FMT arm (Data Supplement [Supplementary Analysis 2]).
DISCUSSION
To our knowledge, we conducted the first randomized, double-blind, placebo-controlled trial of third-party FMT with a clinical primary end point in allogeneic HCT recipients and patients with AML. Because some degree of spontaneous microbiota recovery is expected to occur after normalization of diet and discontinuation of antibiotics, randomization uniquely positioned us to evaluate the additional effects of FMT on the trajectory of microbiota recovery. Several novel observations were made. First, FMT was highly effective in decreasing the fecal abundance of Enterococcus and oral bacteria such as Dialister. Enterococcus expansion has been associated with aGVHD,62 BSI,5,6 and mortality.4 Similarly, ectopic colonization of oral bacteria in the gut has been associated with colitis and inflammatory bowel disease.63 Second, FMT was essential for the recovery of Collinsella. Collinsella depletion has been associated with neutropenic fever after HCT64 and microscopic colitis.65 This genus elicits systemic immunoglobulin responses in inflammatory bowel disease66 and has been associated with response to FMT in patients with ulcerative colitis.67 At least one strain of Collinsella is known to produce butyrate.68 Finally, Blautia showed equivalent recovery in both arms, arguing against an essential FMT impact. Blautia depletion has been associated with an increased risk of fatal aGVHD.69 FMT improved microbiota diversity, consistent with a previous randomized trial.30
Although these favorable effects on the microbiota support the potential efficacy of FMT in preventing adverse clinical outcomes associated with microbiota injury such as aGVHD, infections, and mortality, we could not demonstrate improved outcomes in the FMT arm. We chose all-cause infections, rather than a specific infection type/site, as the primary end point to test whether FMT could modulate systemic immunity not only against infections of intestinal origin but also distant-site infections originating from extraintestinal pathogens. As an example, the gut microbiota has been linked to susceptibility to respiratory tract infections.43-45 Including infections unrelated to the gut microbiota in the same composite end point might have diluted a potential protective FMT effect. For safety reasons, we did not administer the study product before hematopoietic engraftment. With the pre-engraftment period being the highest-risk interval for infections,70 the incidence of a large fraction of infections could not be influenced by FMT, and this might have also diluted an overall beneficial effect. FMT did not result in BSI, similar to a recent report.71 Strict donor and product evaluation was key in this safety and should continue in future studies.
The unexpected higher incidence of aGVHD in the FMT arm is best explained by the imbalance in the GVHD prophylactic regimen between the two arms despite randomization. Grade II-IV aGVHD rates in the control arm where GVHD prophylaxis was predominantly PTCy-based were markedly lower than our historical rates without PTCy (8.3% v approximately 40%). The high frequency of PTCy use in the placebo arm likely decreased aGVHD incidence. In addition, the trial was not powered for aGVHD as a secondary end point and the number of events was small.
The optimal schedule and route of administration of FMT is unknown. Most studies have administered FMT on a single day or on two consecutive days. FMT was administered as oral capsules over 2 days at a median of 27 days after HCT in a previous trial, with improvement in microbiota indices.29 Besides oral encapsulated form,29,35,38 FMT has been administered by enema,26,28,30 via nasogastric/nasoduodenal tube,26,27,31,33,36 or by upper32 or lower endoscopy.34 The source of FMT has been third-party26,27,31,32,34,36 or autologous,28,30 with no compelling comparative data. We preferred a third-party product because many patients have already been exposed to antibiotics by the time their own stool could be banked for FMT. Patient subsets more likely to benefit from FMT are also unknown. In one study, 25 allogeneic HCT recipients were randomly assigned after neutrophil engraftment to receive autologous FMT versus no intervention.30 A unique aspect of this study was that only patients with a low fecal abundance of Bacteroidetes were treated. The most frequent time frame for FMT in HCT recipients has been after neutrophil engraftment, consistent with the observed association between microbiota diversity at the time of engraftment and transplant-related mortality.7 Some studies administered FMT before HCT to eradicate pre-existing MDROs.32
The minimum required engraftment for clinical efficacy is also unknown.33,35 Our engraftment rate is in the same range as in previous FMT trials in patients with AML and HCT patients.29,34,36 The current product (single dose, same dose range) yielded engraftment rates of 50%-60% in patients with CDI.72 The reasons for a lower engraftment rate in this trial are unclear but likely include more severe mucoepithelial damage and a less-receptive environment for new microbiota. With short-amplicon sequencing, donor engraftment results may underestimate true engraftment rates. This is because while novel donor strains often replace related strains of the same species in the patient, completely novel donor species are less likely to be competitive against the patient's indigenous microbiota.73 Because taxonomy deeper than the genus level is not reliable in short-amplicon sequencing, unique donor strains (and even species) cannot be identified with certainty. Shotgun sequencing can partially overcome this barrier.
In conclusion, this randomized, double-blind placebo-controlled trial confirmed safety of FMT and its efficacy on the microbiota in patients with AML and patients with allogeneic HCT. The findings from this trial should inform the design of future definitive trials. A randomized trial in allogeneic HCT recipients with acute GVHD as the primary end point and with stratification for GVHD prophylaxis and conditioning intensity is warranted. Our observed small effect size for infection argues against using infection as the primary end point in future trials unless several hundred patients can be enrolled. If such a large trial is infeasible, we recommend limiting the inclusion criteria to patients with known or likely more severe microbiota injury (eg, recent CDI or pathogen colonization, exposure to antianaerobic antibiotics) in whom the impact of FMT may be greater. In the AML cohort, we enrolled only patients receiving inpatient chemotherapy. The increasing use of novel primarily outpatient antileukemia regimens reduced the pool of eligible inpatients, thereby slowing accrual. FMT trials targeting such patients who also have significant antibiotic exposure and likely develop dysbiosis will be of interest. Finally, a larger total microbiota dose delivered over several days might improve engraftment and potentially treatment efficacy.
ACKNOWLEDGMENT
DNA sequencing of the fecal samples was performed by the University of Minnesota Genomics Center. Sequence data were analyzed using the resources of the Minnesota Supercomputing Institute.
Shernan G. Holtan
Consulting or Advisory Role: Incyte, Bristol Myers Squibb, CSL Behring (Inst)
Research Funding: Incyte, VITRAC Therapeutics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/471226
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics
Research Funding: Incyte
Alexander Khoruts
Patents, Royalties, Other Intellectual Property: I have patents on preparation of fecal microbiota for transplantation
No other potential conflicts of interest were reported.
See accompanying article, p. 5320
DISCLAIMER
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
SUPPORT
Supported in part by the National Institutes of Health's National Center for Advancing Translational Sciences grants KL2TR002492 and UL1TR002494 and the National Cancer Institute's grant P30CA07759. Additional support was provided by the Chainbreaker grant (University of Minnesota). The manufacturing of the FMT product and the corresponding placebo was supported by Achieving Cures Together, a nonprofit organization.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Armin Rashidi, Qing Cao, Xianghua Luo, Daniel J. Weisdorf, Alexander Khoruts, Christopher Staley
Financial support: Alexander Khoruts
Administrative support: Andrea Hoeschen, Alexander Khoruts
Provision of study materials or patients: Shernan G. Holtan, Daniel J. Weisdorf, Alexander Khoruts
Collection and assembly of data: Armin Rashidi, Maryam Ebadi, Tauseef Ur Rehman, Heba Elhusseini, David Kazadi, Hossam Halaweish, Mohammad H. Khan, Andrea Hoeschen, Qing Cao, Amanda J. Kabage, Sharon Lopez, Shernan G. Holtan, Christopher Staley
Data analysis and interpretation: Armin Rashidi, Qing Cao, Xianghua Luo, Shernan G. Holtan, Daniel J. Weisdorf, Alexander Khoruts, Christopher Staley
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Double-Blind Phase II Trial of Fecal Microbiota Transplantation Versus Placebo in Allogeneic Hematopoietic Cell Transplantation and AML
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shernan G. Holtan
Consulting or Advisory Role: Incyte, Bristol Myers Squibb, CSL Behring (Inst)
Research Funding: Incyte, VITRAC Therapeutics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/471226
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics
Research Funding: Incyte
Alexander Khoruts
Patents, Royalties, Other Intellectual Property: I have patents on preparation of fecal microbiota for transplantation
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shono Y, van den Brink MRM: Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer 18:283-295, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rashidi A, Weisdorf DJ: Microbiota-based approaches to mitigate infectious complications of intensive chemotherapy in patients with acute leukemia. Transl Res 220:167-181, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rashidi A, Kaiser T, Graiziger C, et al. : Gut dysbiosis during antileukemia chemotherapy versus allogeneic hematopoietic cell transplantation. Cancer 126:1434-1447, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Messina JA, Tan CY, Ren Y, et al. : Enterococcus intestinal domination is associated with increased mortality in the acute leukemia chemotherapy population. Clin Infect Dis 10.1093/cid/ciab1043 [Epub ahead of print on December 20, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ubeda C, Taur Y, Jenq RR, et al. : Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332-4341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taur Y, Xavier JB, Lipuma L, et al. : Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 55:905-914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peled JU, Gomes ALC, Devlin SM, et al. : Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med 382:822-834, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taur Y, Jenq RR, Perales M-A, et al. : The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124:1174-1182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber D, Oefner PJ, Hiergeist A, et al. : Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 126:1723-1728, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Rashidi A, Zhu Z, Kaiser T, et al. : Vancomycin-resistance gene cluster, vanC, in the gut microbiome of acute leukemia patients undergoing intensive chemotherapy. PLoS One 14:e0223890, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Vliet MJ, Tissing WJE, Dun CAJ, et al. : Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis 49:262-270, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kaysen A, Heintz-Buschart A, Muller EEL, et al. : Integrated meta-omic analyses of the gastrointestinal tract microbiome in patients undergoing allogeneic hematopoietic stem cell transplantation. Transl Res 186:79-94.e1, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Swimm A, Giver CR, DeFilipp Z, et al. : Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood 132:2506-2519, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meedt E, Hiergeist A, Gessner A, et al. : Prolonged suppression of butyrate-producing bacteria is associated with acute gastrointestinal graft-vs-host disease and transplantation-related mortality after allogeneic stem cell transplantation. Clin Infect Dis 74:614-621, 2022 [DOI] [PubMed] [Google Scholar]
- 15.Ilett EE, Jørgensen M, Noguera-Julian M, et al. : Associations of the gut microbiome and clinical factors with acute GVHD in allogeneic HSCT recipients. Blood Adv 4:5797-5809, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golob JL, Pergam SA Srinivasan S, et al. : Stool microbiota at neutrophil recovery is predictive for severe acute graft vs host disease after hematopoietic cell transplantation. Clin Infect Dis 65:1984-1991, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legoff J, Resche-Rigon M, Bouquet J, et al. : The eukaryotic gut virome in hematopoietic stem cell transplantation: New clues in enteric graft-versus-host disease. Nat Med 23:1080-1085, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Shono Y, Docampo MD, Peled JU, et al. : Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 8:339ra71, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenq RR, Ubeda C, Taur Y, et al. : Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 209:903-911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgos da Silva M, Ponce DM, Dai A, et al. : Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease. Blood 140:2385-2397, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staffas A, Burgos da Silva M, Slingerland AE, et al. : Nutritional support from the intestinal microbiota improves hematopoietic reconstitution after bone marrow transplantation in mice. Cell Host Microbe 23:447-457.e4, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miltiadous O, Waters NR, Andrlová H, et al. : Early intestinal microbial features are associated with CD4 T-cell recovery after allogeneic hematopoietic transplant. Blood 139:2758-2769, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrlová H, Miltiadous O, Kousa AI, et al. : MAIT and Vδ2 unconventional T cells are supported by a diverse intestinal microbiome and correlate with favorable patient outcome after allogeneic HCT. Sci Transl Med 14:eabj2829, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peled JU, Devlin SM, Staffas A, et al. : Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol 35:1650-1659, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicente-Dueñas C, Janssen S, Oldenburg M, et al. : An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 136:2003-2017, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battipaglia G, Malard F, Rubio MT, et al. : Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 104:1682-1688, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilinski J, Grzesiowski P, Sorensen N, et al. : Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: Results of a prospective, single-center study. Clin Infect Dis 65:364-370, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Malard F, Vekhoff A, Lapusan S, et al. : Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat Commun 12:3084, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFilipp Z, Peled JU, Li S, et al. : Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv 2:745-753, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taur Y, Coyte K, Schluter J, et al. : Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med 10:eaap9489, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghani R, Mullish BH, McDonald JAK, et al. : Disease prevention not decolonization: A model for fecal microbiota transplantation in patients colonized with multidrug-resistant organisms. Clin Infect Dis 72:1444-1447, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merli P, Putignani L, Ruggeri A, et al. : Decolonization of multi-drug resistant bacteria by fecal microbiota transplantation in five pediatric patients before allogeneic hematopoietic stem cell transplantation: Gut microbiota profiling, infectious and clinical outcomes. Haematologica 105:2686-2690, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakihana K, Fujioka Y, Suda W, et al. : Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 128:2083-2088, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spindelboeck W, Halwachs B, Bayer N, et al. : Antibiotic use and ileocolonic immune cells in patients receiving fecal microbiota transplantation for refractory intestinal GvHD: A prospective cohort study. Ther Adv Hematol 12:204062072110583, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaito S, Toya T, Yoshifuji K, et al. : Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv 2:3097-3101, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Lier YF, Davids M, Haverkate NJE, et al. : Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med 12:eaaz8926, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Li X, Zhou Y, et al. : Safety and efficacy of fecal microbiota transplantation for grade IV steroid refractory GI-GvHD patients: Interim results from FMT2017002 trial. Front Immunol 12:678476, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goeser F, Sifft B, Stein-Thoeringer C, et al. : Fecal microbiota transfer for refractory intestinal graft-versus-host disease - experience from two German tertiary centers. Eur J Haematol 107:229-245, 2021 [DOI] [PubMed] [Google Scholar]
- 39.Belkaid Y, Hand TW: Role of the microbiota in immunity and inflammation. Cell 157:121-141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rashidi A, Kaiser T, Graiziger C, et al. : Specific gut microbiota changes heralding bloodstream infection and neutropenic fever during intensive chemotherapy. Leukemia 34:312-316, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Stoma I, Littmann ER, Peled JU, et al. : Compositional flux within the intestinal microbiota and risk for bloodstream infection with gram-negative bacteria. Clin Infect Dis 73:e4627-e4635, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YJ, Arguello ES, Jenq RR, et al. : Protective factors in the intestinal microbiome against Clostridium difficile infection in recipients of allogeneic hematopoietic stem cell transplantation. J Infect Dis 215:1117-1123, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haak BW, Littmann ER, Chaubard J-L, et al. : Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood 131:2978-2986, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris B, Morjaria SM, Littmann ER, et al. : Gut microbiota predict pulmonary infiltrates after allogeneic hematopoietic cell transplantation. Am J Respir Crit Care Med 194:450-463, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Zou Y, Zhang Y, et al. : Characteristics in gut microbiome is associated with chemotherapy-induced pneumonia in pediatric acute lymphoblastic leukemia. BMC Cancer 21:1190, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Healthcare Safety Network (NHSN) : Patient Safety Component Manual, 2022. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf [Google Scholar]
- 47.Rowlings PA, Przepiorka D, Klein JP, et al. : IBMTR Severity Index for grading acute graft-versus-host disease: Retrospective comparison with Glucksberg grade. Br J Haematol 97:855-864, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Staley C, Hamilton MJ, Vaughn BP, et al. : Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota; pragmatic cohort study. Am J Gastroenterol 112:940-947, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bejanyan N, Brunstein CG, Cao Q, et al. : Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv 2:909-922, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson W: Confidence limits for recurrence data—Applied to cost or number of product repairs. Technometrics 37:147-157, 1995 [Google Scholar]
- 51.Wang M-C, Qin J, Chiang C-T: Analyzing recurrent event data with informative censoring. J Am Stat Assoc 96:1057-1065, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiou SH, Xu G, Yan J, et al. : Regression modeling for recurrent events possibly with an informative terminal event using R package reReg. J Stat Softw 105:1‐34, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sicard J-F, Le Bihan G, Vogeleer P, et al. : Interactions of intestinal bacteria with components of the intestinal mucus. Front Cell Infect Microbiol 7:387, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilbert SA, Mark Welch JL, Borisy GG: Spatial ecology of the human tongue dorsum microbiome. Cell Rep 30:4003-4015.e3, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Downes J, Munson M, Wade WG: Dialister invisus sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol 53:1937-1940, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Siles M, Duncan SH, Garcia-Gil LJ, et al. : Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J 11:841-852, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rashidi A, Ebadi M, Rehman TU, et al. : Altered microbiota-host metabolic cross talk preceding neutropenic fever in patients with acute leukemia. Blood Adv 5:3937-3950, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajagopala SV, Singh H, Yu Y, et al. : Persistent gut microbial dysbiosis in children with acute lymphoblastic leukemia (ALL) during chemotherapy. Microb Ecol 79:1034-1043, 2020 [DOI] [PubMed] [Google Scholar]
- 59.Schwabkey ZI, Wiesnoski DH, Chang C-C, et al. : Diet-derived metabolites and mucus link the gut microbiome to fever after cytotoxic cancer treatment. Sci Transl Med 14:eabo3445, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louis P, Flint HJ: Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1-8, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Payen M, Nicolis I, Robin M, et al. : Functional and phylogenetic alterations in gut microbiome are linked to graft-versus-host disease severity. Blood Adv 4:1824–1832, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein-Thoeringer CK, Nichols KB, Lazrak A, et al. : Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 366:1143-1149, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Read E, Curtis MA, Neves JF: The role of oral bacteria in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 18:731-742, 2021 [DOI] [PubMed] [Google Scholar]
- 64.Murthy HS, Gharaibeh RZ, Al-Mansour Z, et al. : Baseline gut microbiota composition is associated with major infections early after hematopoietic cell transplantation. Biol Blood Marrow Transplant 26:2001-2010, 2020 [DOI] [PubMed] [Google Scholar]
- 65.Sun S, Blakley IC, Fodor AA, et al. : Microbial associations with microscopic colitis. Clin Transl Gastroenterol 13:e00528, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vujkovic-Cvijin I, Welles HC, Ha CWY, et al. : The systemic anti-microbiota IgG repertoire can identify gut bacteria that translocate across gut barrier surfaces. Sci Transl Med 14:eabl3927, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W-H, Jin Z-Y, Yang Z-H, et al. : Fecal microbiota transplantation ameliorates active ulcerative colitis by downregulating pro-inflammatory cytokines in mucosa and serum. Front Microbiol 13:818111, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin P, Zou Y, Dai Y, et al. : Characterization a novel butyric acid-producing bacterium Collinsella aerofaciens subsp. shenzhenensis subsp. nov. Microorganisms 7:78, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenq RR, Taur Y, Devlin SM, et al. : Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 21:1373-1383, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ustun C, Young J-AH, Papanicolaou GA, et al. : Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl 54:1254-1265, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eshel A, Sharon I, Nagler A, et al. : Origins of bloodstream infections following fecal microbiota transplantation: A strain-level analysis. Blood Adv 6:568-573, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Staley C, Kaiser T, Vaughn BP, et al. : Durable long-term bacterial engraftment following encapsulated fecal microbiota transplantation to treat Clostridium difficile infection. MBio 10:e01586-19, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li SS, Zhu A, Benes V, et al. : Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 352:586-589, 2016 [DOI] [PubMed] [Google Scholar]