Abstract
Nicotinic acetylcholine receptors (nAChRs) have been historically defined as ligand-gated ion channels and function as such in the central and peripheral nervous systems. Recently, however, non-ionic signaling mechanisms via nAChRs have been demonstrated in immune cells. Furthermore, the signaling pathways where nAChRs are expressed can be activated by endogenous ligands other than the canonical agonists acetylcholine and choline. In this review, we discuss the involvement of a subset of nAChRs containing α7, α9, and/or α10 subunits in the modulation of pain and inflammation via the cholinergic anti-inflammatory pathway. Additionally, we review the most recent advances in the development of novel ligands and their potential as therapeutics.
Keywords: Nicotinic acetylcholine receptor subunits α7, α9, and α10; Neuropathic pain; Chronic pain; Inflammatory pain; Chemotherapy-induced neuropathic pain; α-conotoxin RgIA
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) isolated from the electric organ of the Torpedo marmorata ray were first visualized using electron microscopy by Changeux and colleagues in 1973 [1,2]. These studies revealed that the receptors present in the ray electric organ were pentameric structures with a central pore that presumably allowed the flux of ions. Later studies would demonstrate that upon ligand-binding the pentameric structure would, in fact, rotate to open the channel and allow ion flux across the cell membrane generating an electrical current. Nicotinic receptors were later classified as the first member of a superfamily of ligand-gated ion channels that would expand to include γ-aminobutyric acid (GABAA), glycine, 5-hydroxytryptamine type 3, and zinc-activated channels [3,4]. Through molecular biology techniques, it was later discovered that nAChRs are composed of five individual subunits rather than one large polypeptide. In fact, the nAChR subtype found at the neuromuscular junction is composed of four different gene products [5,6]. A total of 16 nicotinic genes have been identified in the human genome and code for α1-α7, α9, α10, β1-β4, δ, ε, and γ subunits. Myriad nAChR subtypes can be formed by different combinations of these subunits. For example, α4 and β2 subunits assemble together and comprise the most abundant nAChR subtype, α4β2 * (the asterisk denotes the potential presence of other subunits), in the mammalian brain. In the peripheral nervous system, α3β4 * nAChRs are most abundant. Subtypes containing more than one gene product are classified as heteromeric while those formed from a single gene product are known as homomeric nAChRs. Human homomeric subtypes have historically only included α7 and α9, but very recently we demonstrated that α10 subunits can assemble as functional homopentamers when heterologously expressed in Xenopus laevis oocytes [7]. Regardless of subunit combination, long-standing scientific precedent has held that nAChRs function as ligand-gated ion channels, but recent work in immune cells has challenged this notion [8].
Immune cells, especially monocytes and lymphocytes, are known to express several nAChR subunits [9–12]. Importantly, distinct subtypes of these cells express α7, α9, and/or α10 subunits [13,14]. The potential nAChR subtypes expressed by immune cells are shown in Fig. 1. However, patch-clamp electrophysiology studies have failed to detect acetylcholine-mediated currents in these cells [15]. Ionic currents mediated by α7 nAChRs have been observed in differentiated macrophages from the human THP-1 monocyte cell line, however [16]. Several ligands of α7, α9, and α10 nAChRs have been shown to modulate the release of inflammatory cytokines from immune cells [17–19]. These ligands include the canonical neurotransmitters acetylcholine and choline, but recently conjugates of choline and phosphocholine with soluble proteins including albumin and C-reactive protein have been shown to act as ligands of α7 and α9α10 nAChRs [20–23]. It is notable that immune cells are not considered ‘excitable’ yet express ion channels that are generally associated with neurons and other excitable cells. Though the exact mechanisms of how signal transduction occurs in immune cells is not fully understood, it is likely that canonical ion-channel functions are not involved. In this work, we review the involvement of nAChRs containing α7, α9, and/or α10 subunits in the modulation of pain and inflammation with a focus on novel ligands and mechanisms.
Fig. 1.
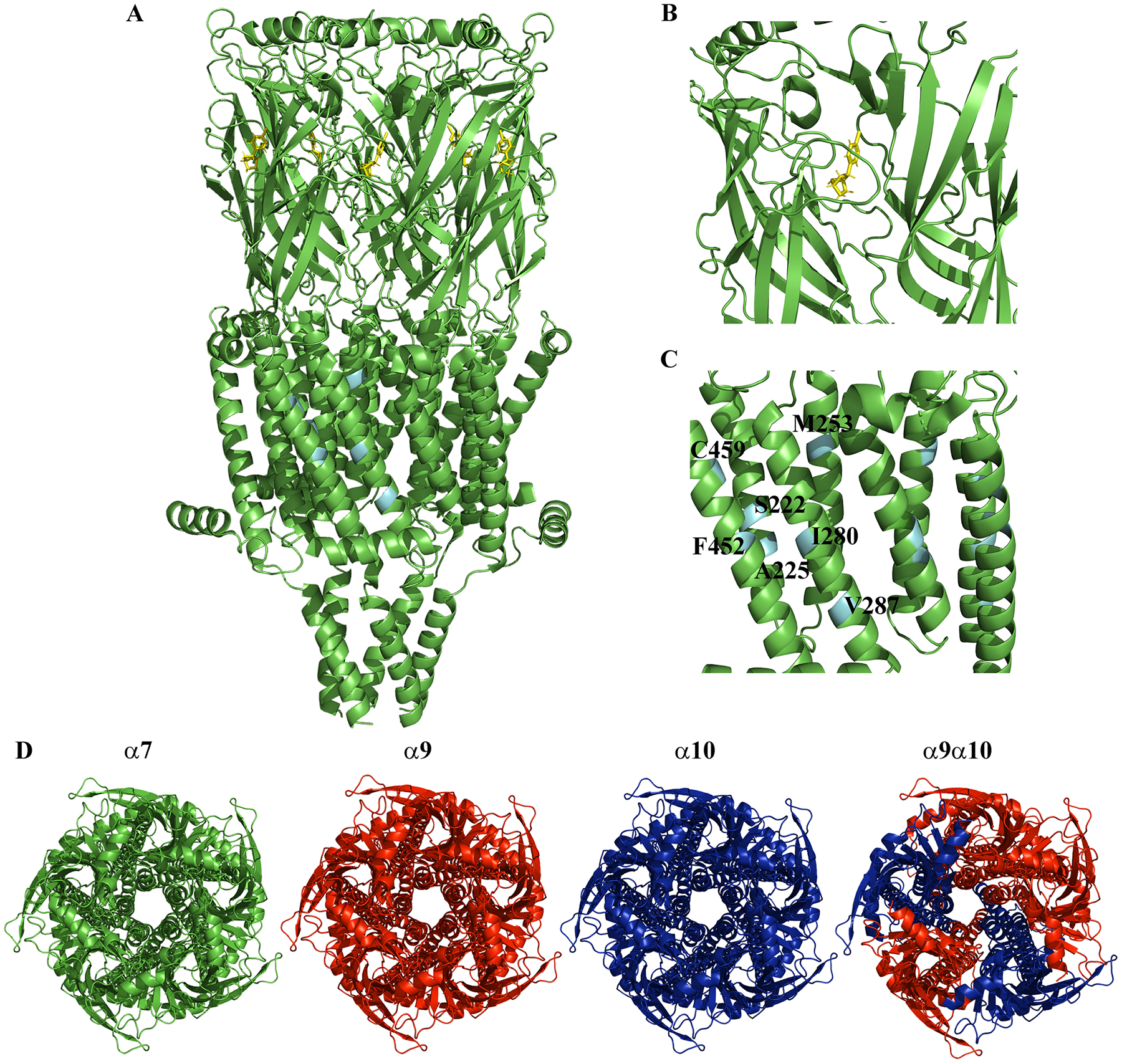
The nicotinic acetylcholine receptors (nAChRs) involved in pain and inflammation. (A) Cartoon rendition of the cryo-EM structure of the human homomeric α7 nAChR (PDB:7KOX) [130]. (B) A 20 Å spherical view of the orthosteric ligand-binding site shown with the frog neurotoxin epibatidine (yellow) from Epipedobates tricolor. (C) A 20 Å spherical view of the allosteric ligand-binding site. The labeled residues are involved in binding different ligands and are cyan colored. (D) Several immune cell types including lymphocytes, monocytes/macrophages, and granulocytes are known to express nAChRs containing α7, α9, and/or α10 nAChR subunits. These subtypes may include homomeric α7 (green), α9 (red) or α10 (blue) nAChRs. Heteromeric α9α10 (red and blue) are also expressed although the stoichiometry and ratio of α9 to α10 subunits has yet to be elucidated. The structures are color coded to depict graphically the different nAChR subtypes and generated using the α7 structure (PDB:7KOO) [130]. The receptors are in the closed position and oriented looking through the channel from extracellular space. All images were generated using PyMOL.
1.1. α7 nAChRs are broadly implicated in modulating the inflammatory responses of immune cells
Seminal studies in the early 2000s identified α7 nAChRs as prominent players in the modulation of inflammatory cytokine release by immune cells. Stimulation of the vagus nerve releases acetylcholine into the blood stream and activates nAChRs expressed by circulating immune cells. In vitro studies using human macrophages showed that stimulation with acetylcholine inhibited the release of the inflammatory cytokines interleukin-1β (IL-1β), IL-6, IL-18 and tumor necrosis factor-α (TNF-α) [24]. Initially the nAChR mediating this response was unknown, but later experiments identified α7 as the principal subtype [25]. The link between the nervous system and the immune system can therefore be attributed to immune cell expressed α7 nAChRs along with the vagus nerve and collectively comprise two critical components of the cholinergic anti-inflammatory pathway (CAP) [26,27]. Mechanistically, stimulation of α7 nAChRs expressed by macrophages activates a number of important biochemical pathways involved in the inflammatory response. One pathway inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and another activates the janus-kinase-2 (JAK)-signal transducer and activator of transcription-3 (STAT3) pathway (JAK2-STAT). Inhibition of NF-κB signaling reduces the expression of and ultimately the release of TNF-α by macrophages and monocytes. A third pathway has been proposed that involves interleukin-1 receptor-associated kinases (IRAK) [28]. Stimulation of monocytes and macrophages with lipopolysaccharide (LPS) induces IRAK expression and TNF-α release through toll-like receptors (TLRs). Increased IRAK expression functions as a negative regulator of TLR functions. Human peripheral blood mononuclear cells (PBMCs) stimulated with nicotine showed up-regulation of IRAK-M expression, and this effect was dependent on α7 nAChR-mediated activation of the JAK2-STAT3 pathway [19]. Macrophages are just one example where a non-ionic mechanism of action mediated by α7 nAChRs has been demonstrated [29]. Non-ionic mechanisms have also been shown in a number of primary monocytes and monocytic cell lines and lymphocytes. In human pathologies, the expression and function of α7 nAChRs by immune cells may attenuate excessive inflammation. Clinical outcomes in patients with sepsis have been shown to closely correlate with levels of α7 nAChR mRNA in PBMCs [30]. Patients with lower levels of α7 nAChR mRNA experienced worse clinical outcomes including increased mortality, whereas those with higher levels showed attenuated signs and symptoms of sepsis. The inflammatory response that occurs in sepsis is reminiscent of the ‘cytokine-storm’ that occurs in COVID-19 disease, and α7 nAChRs have been proposed as pharmacological targets for attenuating the associated inflammatory response [31, 32]. Not surprisingly, broad involvement of α7 nAChRs in a variety of inflammatory conditions has generated immense interest in developing drugs that targets these receptors [33]. A summary of the interaction between α7 nAChRs and the downstream biochemical pathways is presented in Fig. 2.
Fig. 2.
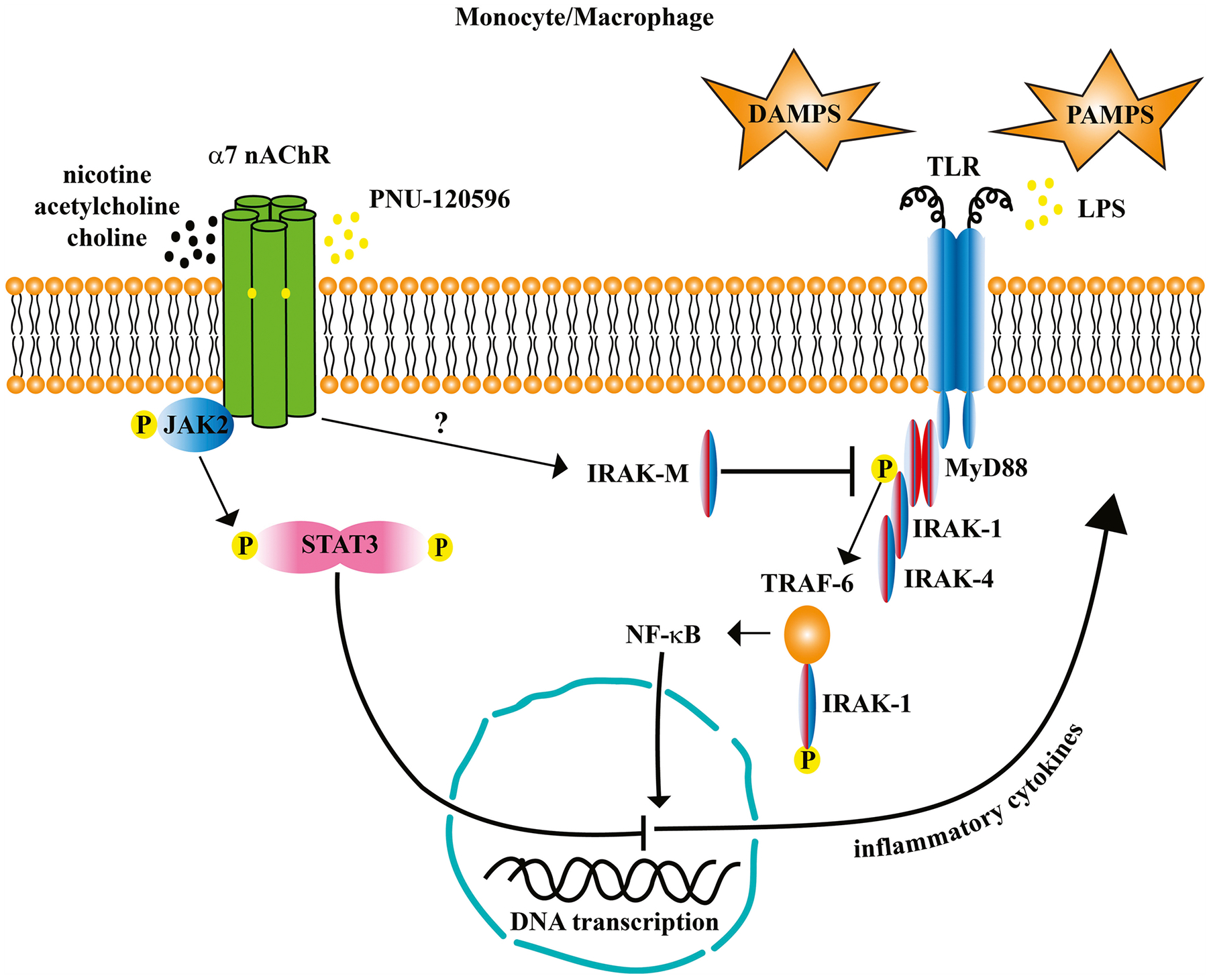
Nicotinic acetylcholine receptor (nAChR) α7 inhibits the production of inflammatory cytokines by suppressing downstream pathways triggered by stimulation of toll-like receptors (TLR) by damage-associated molecular patterns (DAMPS) and pathogen-associated molecular patterns (PAMPS). In this model, stimulation of α7 nAChRs activates the Janus Kinase-2 (JAK2) signal-transducer and activator of transcription-3 (STAT3) signaling pathway. JAK2 phosphorylates STAT3 which dimerizes and translocates to the nucleus where it interferes with nuclear factor kappa-B (NF-κB) binding to DNA and prevents the transcription of genes for inflammatory cytokines. Interleukin-1 receptor associated kinase-M (IRAK-M), an IRAK specific to monocytes and macrophages, inhibits the phosphorylation of IRAK-1 by IRAK-4. The downstream effects are to inhibit the oligomerization of tumor necrosis-factor receptor-associated factor-6 (TRAF-6) and IRAK-1. The TRAF-6/TRAK-1 complex activates NF-κB which translocates to the nucleus and binds to DNA to initiate the production of inflammatory cytokines. How IRAK-M becomes activated by stimulation of α7 nAChRs is currently under investigation but may involve other intermediary molecules such as G-proteins. For brevity, not all intermediaries in the pathways are shown; for a thorough review of NF-κB signaling see Liu et al., [131].
1.2. Novel compounds that target α7 nAChRs are analgesic and antiinflammatory
The development of drugs that target α7 nAChRs is a major focus of research at several academic institutions and in the pharmaceutical industry, and a number of candidate compounds have been developed. Extensive research indicates that activation of α7 nAChRs is antiinflammatory and analgesic. However, recently developed compounds show a more diverse range of mechanisms rather than simple receptor agonism. These mechanisms include partial agonism, silent agonism, and positive allosteric modulation [34,35]. Some ligands show functional properties of both orthosteric agonists and positive allosteric modulators (PAM) and are called ago-PAMs. Agonists of α7 nAChRs have demonstrated efficacy in numerous models of pain and inflammation (Table 1). Two such compounds, PNU-282987 [36] and PHA-543613 [37], have been tested in a wide range of disease models including neuropathic and inflammatory pain, chemotherapeutic-induced neuropathic pain (CIN), acute lung injury, inflammatory bowel disease (IBD), and chronic pain related to posttraumatic stress disorder (PTSD). In the rat chronic constriction injury (CCI) model of neuropathic pain, PNU-282987 was demonstrated to produce acute analgesia in response to noxious mechanical stimuli as measured by an Analgesy-meter [38]. Daily administration of PNU-282987 reduced macrophage infiltrate and attenuated pathophysiological changes of the sciatic nerve that are associated with CCI injury. Certain chemotherapeutics such as oxaliplatin, paclitaxel, and vincristine produce a type of neuropathy in humans that is characterized by decreased pain tolerance and severe cold allodynia. In a model of CIN, cold allodynia (cold plate test) and hyperalgesia (paw pressure and von Frey tests) were reduced by treatment with PNU-282987 [39]. Rats administered the chemotherapeutic oxaliplatin showed lower levels of α7 nAChR protein in the sciatic nerve, DRG, and spinal cord and PNU-282987 prevented this downregulation. PNU-282987 and another α7 nAChR agonist (R)-ICH3 also produced modulatory effects on microglia and astrocyte populations in the oxaliplatin model. Microglia numbers increased in the dorsolateral-periaqueductal grey, thalamus, and somatosensory areas in the brain but not in the dorsal horn of the spinal cord. By contrast, glial-fibrillary acidic protein-positive astrocyte numbers were increased in both the dorsal horn and in pain-related areas of the brain. Stimulation of glial cell and astrocyte populations by α7 nAChR agonists was hypothesized to be neuroprotective by preventing the pathophysiological changes to neurons and nerves induced by oxaliplatin. Both PNU-282987 and PHA-543613 also produced analgesia in the formalin model of inflammatory pain in mice [40,41]. Analgesia was observed in both the acute and tonic phases of pain that are induced by formalin administration. The antinociceptive effects of PNU-282987 were not observed in α7 knockout mice. Both compounds were also effective in the dextran sodium sulfate (DSS) model of colitis in rodents, but there were differences in their clinical profiles. PNU-282987 reduced referred mechanical hyperalgesia (von Frey test) associated with DSS-induced colitis but failed to attenuate the associated pathophysiological changes in colonic histology [42]. By contrast, PHA-543613 showed disease modifying properties including preservation of colon length and prevention of mucosal ulceration [43]. Interestingly, the disease modifying effects of PHA-543613 were only observed in male but not female mice. Although PNU-282987 failed to reduce myeloperoxidase activity, a marker of polymorphonuclear leukocyte recruitment, and levels of keratinocyte-derived chemokine in DSS, it was effective in modifying immune cell activity in the rat model of postoperative ileus (POI) and acute lung injury. Macrophage, but not neutrophil, infiltration into the muscle layer of the intestines was reduced in POI by administration of PNU-282987 [44]. In a model of acute lung injury, neutrophil recruitment, levels of TNF-α, IL-1β, IL-6 levels, and NF- κB activity were all reduced with PNU-282987 [45]. Similarly, PHA-543613 suppressed reactive astrocyte numbers and decreased spinal IL-1β and TNF-α levels in the rat PTSD-related chronic pain model [46]. Lastly, in an inflammation-driven model of systemic skin fibrosis, PHA-543613 prevented or reversed fibrosis in mice [47].
Table 1.
Novel compounds that target α7 nAChRs, mechanism of action, and effects in models of pain and inflammation.
| Ligand | Mechanism of Action | Disease Model | Pharmacological Effects | Ref. |
|---|---|---|---|---|
| PNU-282987 | Agonist | CCI-induced neuropathic pain | Reduced hyperalgesia; reduced macrophage infiltration of the sciatic nerve; attenuated morphological changes of the sciatic nerve. | [38] |
| CIN | Reduced cold allodynia and hyperalgesia; modulation of glial cell and astrocyte populations in the dorsal horn of the spinal column. | [39] | ||
| Formalin-induced inflammatory pain | Reduced nocifensive behaviors; inhibition of phase I and II pain. | [41] | ||
| DSS induced-colitis | Reduced referred mechanical hyperalgesia. | [42] | ||
| POI | Reduced macrophage infiltration of inflamed intestinal tissue. | [44] | ||
| Acute lung injury | Reduced neutrophil recruitment; reduced TNF-α, IL-1β, IL-6, IL-10 levels; reduced NF- κB activity. | [45] | ||
| PHA-543613 | Agonist | Formalin-induced inflammatory pain | Reduced nocifensive behaviors; inhibition of phase I and II pain. | [40, 41] |
| DSS-colitis | Reduced signs and symptoms of colitis; reduced severity of histological changes in colon morphology. | [43] | ||
| PTSD-related chronic pain | Attenuated mechanical allodynia; suppressed reactive astrocyte numbers; decreased spinal TNF-α and IL-1β levels. | [46] | ||
| Bleomycin-induced systemic sclerosis | Inhibition of profibrotic effects of bleomycin. | [47] | ||
| GTS-21 | Partial agonist | CFA-induced chronic inflammatory pain | Attenuated mechanical hyperalgesia and thermal nociception. | [50] |
| Morphine-exacerbated burn pain | Prevention of mechanical allodynia and thermal hyperalgesia. | [65] | ||
| NS6740 | Silent agonist | Formalin-induced inflammatory pain | Reduced nocifensive behaviors; inhibition of phase I and II pain. | [41, 51] |
| CCI-induced neuropathic pain | Reduced mechanical allodynia. | [51] | ||
| pCF3 diEPP | Partial agonist | CFA-induced chronic inflammatory pain | Reduced mechanical allodynia and paw edema; reduced mechanical allodynia. | [66] |
| GAT-107 | ago-PAM | Formalin-induced inflammatory pain | Reduced nocifensive behaviors; inhibition of phase II pain. | [41, 54] |
| EAE | Reduced signs and symptoms of neuroinflammation; reduced immune cell meningial infiltrate in the spinal cord. | [35] | ||
| CCI-induced neuropathic pain | Attenuation of mechanical allodynia and thermal hyperalgesia. | [54] | ||
| LPS-induced inflammatory pain | Attenuation of mechanical allodynia. | [54] | ||
| PNU-120596 | PAM | Formalin-induced inflammatory pain | Reduced nocifensive behaviors; inhibition of phase II pain. | [40, 41] |
| DSS-induced colitis | Reduced signs and symptoms of colitis; reduced severity of histological changes in colon morphology. | [43] | ||
| TQS | PAM | LPS-induced inflammatory pain | Reduced mechanical and thermal hyperalgesia. | [67] |
| DDD-028 | Unknown | CIN | Reduced mechanical hyperalgesia and mechanical allodynia. | [68] |
CCI, chronic constriction injury; CIN, chemotherapeutic-induced neuropathic pain; DSS, dextran sodium sulfate; CFA, complete Freund’s adjuvant; EAE, experimental autoimmune encephalomyelitis; LPS, lipopolysaccharide; PAM, positive allosteric modulator; POI, post operative ileus; PTSD, posttraumatic stress disorder.
One potential issue with stimulation of α7 nAChRs to treat disease is the fact that this receptor subtype is highly expressed in multiple systems in the body, and over stimulation may result in unwanted effects on systems not related to the disease target of interest. A potential solution would be to use a ligand that lacks full efficacy or to increase endogenous cholinergic tone through allosteric modulation. Partial agonists are ligands that activate the receptor or increase endogenous tone but to a lesser extent than the natural neurotransmitter, and silent agonists are those that lack intrinsic agonist activity and function by working through non-conducting states of the receptor. Examples of such ligand are GTS-21 [48] and NS-6740 [49]. GTS-21, an analog of the plant alkaloid anabasine (Nicotiana glauca), is a partial agonist of α7 nAChRs and has been shown to be analgesic in models of inflammatory pain. In the complete Freund’s adjuvant (CFA) model, GTS-21 attenuated mechanical hyperalgesia (von Frey test) and thermal hyperalgesia (Analgesia Meter and cold plate test) [50]. Likewise, in the formalin model NS-6740 reduced nocifensive behaviors (paw licking) and was analgesic in both the acute and tonic phases of the inflammatory pain response [41]. In a different study, NS-6740 reduced mechanical allodynia (von Frey test) in mice subjected to CCI [51]. Ligands, such as GAT-107, that bind to the orthosteric binding-site as well as an allosteric modulatory site are called ago-PAMs [52,53]. In a mouse model of experimental autoimmune encephalomyelitis (EAE), GAT-107 reduced signs and symptoms of neuroinflammation as well as meningeal infiltration of immune cells in the spinal cord [35]. Mechanical allodynia (von Frey test) was also attenuated in CCI and LPS-induced inflammatory pain by GAT-107 treatment [54]. Interestingly, ligands that have no intrinsic agonist activity, but show only positive allosteric modulation, have also been shown to be analgesic and anti-inflammatory. The PAM PNU-120596 was analgesic in phase II of the formalin model [40,41] and was remarkably effective at reducing the signs and symptoms of DSS-induced colitis in mice including reduced severity of pathophysiological changes in colon histology [43]. These studies suggest that increasing the endogenous cholinergic tone through positive allosteric modulation might be an effective therapeutic strategy for the treatment of pain and inflammation without the risk of inducing desensitization which could result in antagonism of the CAP. However, in some cells over stimulation of α7 nAChRs with PNU-120596 proved to be cytotoxic from elevated levels of intracellular calcium [55]. Nevertheless, these studies provide strong evidence that ligands of α7 nAChRs exert their analgesic and anti-inflammatory properties through modulation of the activities of immune cells and specifically through inhibition of pro-inflammatory cytokine and chemokine release by these cells. A number of other compounds not mentioned here have been developed and assessed for their potential as analgesic and anti-inflammatory drugs as well as treatments for disorders of cognition [56–62]. Lastly, natural products such as formulated curcumin have shown promising results in rodent models of inflammatory pain and CIN [63,64].
1.3. Nicotinic acetylcholine receptors containing α9/α10 subunits are novel targets for pharmacological intervention in pain and inflammatory conditions
CHRNA9 and CHRNA10 are the most recently discovered genes in the nAChR family [15, 69–71]. Initially discovered through a rat cDNA library screen, in situ hybridization studies showed that CHRNA9 expression was localized to a discrete set of tissues outside of the central nervous system including the cochlea, pars tuberalis, and olfactory bulb. In humans, α9 was discovered in epidermal and oral keratinocytes and functionally regulates keratinocyte adhesion [72]. Some years after the discovery of the rat α9 subunit, human CHRNA9 and CHRNA10 were discovered through the screening of libraries obtained from whole embryo and tonsil [15,71]. Importantly, α9 and α10 mRNAs were found in tonsil tissue-derived B-, and T-cells, peripheral blood lymphocytes, and monocytes. Subsequent work refined the expression patterns of α9 and α10 to CD3 +, CD4 +, and CD8 + T-cells as well as CD19 + and CD80 + B-cells [13]. These studies have spurred investigations into the role of α9 and α10 subunits in immune-system function including pathophysiological conditions such as cancer [73,74]. A summary of the interaction between α9/α10 nAChRs and the downstream biochemical pathways is presented in Fig. 3.
Fig. 3.
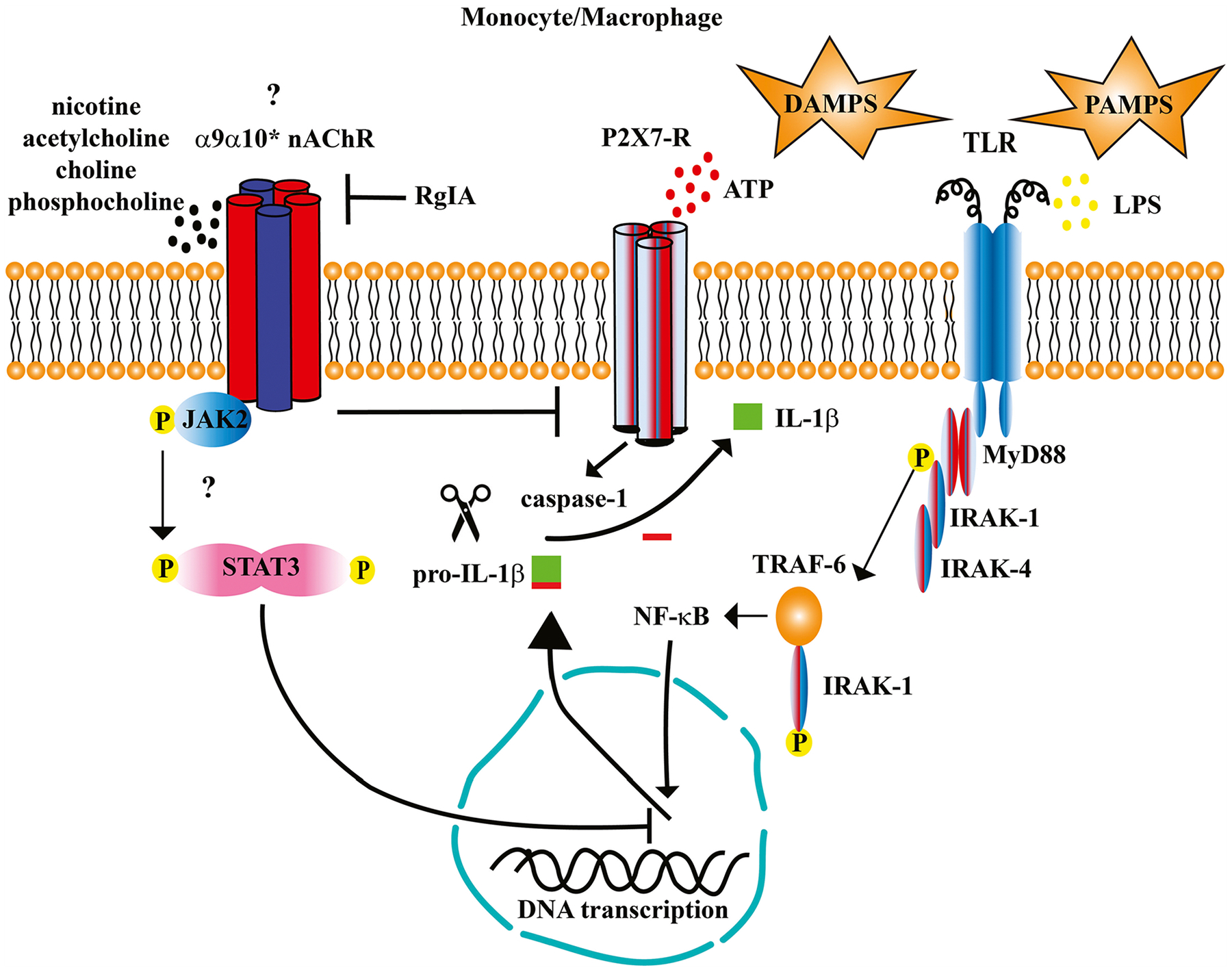
Stimulation of α9α10* (the asterisk indicates that the stoichiometry of the receptor expressed by immune cells is unknown and may also include other subunits) nicotinic acetylcholine receptors (nAChR) inhibits the production of inflammatory cytokines. Danger signals including stimulation of toll-like receptors (TLR) by damage-associated molecular patterns (DAMPS) and pathogen-associated molecular patterns (PAMPS), and stimulation of purinergic P2×7-Rs by ATP increases the production of inflammatory cytokines including interleukin-1β (IL-1β). Stimulation of both TLRs and P2X-Rs is needed for the expression and assembly of the inflammasome complex (not shown) and subsequent cleavage of pro-IL-1β by activated caspase-1. In this model, agonists of monocytic α9α10* nAChRs such as nicotine, choline, and phosphocholine inhibit IL-1β release as demonstrated in human monocytes [20,21]. The mechanisms of how this occurs is currently under investigation, but one potential mechanism involves activation of the Janus Kinase-2 (JAK2) signal-transducer and activator of transcription-3 (STAT3) signaling pathway as demonstrated in breast cancer cells [132]. Stimulation of α9α10* nAChRs has also been shown to inhibit the release of tumor necrosis factor-α (TNF-α) and IL-6, but not the anti-inflammatory cytokine IL-10 in human whole blood cultures [97].
1.4. α-Conotoxins are analgesic, anti-inflammatory, and reduce signs and symptoms of disease in models of neuropathic pain
The expression of α9 and α10 subunits by immune cells suggests that α9/α10 nAChRs are involved in immune cell functions. Some of the first studies that implicated α9/α10 nAChRs in neuropathic pain came from experiments with α-conotoxins that selectively target α9/α10 nAChRs. One such α-conotoxin, Vc1.1, was shown to accelerate functional recovery of damaged nerves and alleviate pain in the rat CCI model of neuropathic pain [75]. Vc1.1 later entered human clinical trials as ACV-1 but failed to show the same efficacy for reducing human neuropathic pain, consistent with its low affinity for the human α9α10 subtype [76–78]. A second α-conotoxin called [S4Dap]Mr1.1 that is similar in sequence to Vc1.1 has recently been shown to be analgesic in the rat CCI model [79]. α-Conotoxin RgIA has been used in a number of neuropathic and inflammatory pain models and shown to be effective in reducing pain and inflammation. In the rat CCI model, RgIA reduced the signs and symptoms of neuropathy [80]. Specifically, RgIA reduced the histological changes in nerve morphology including decreased axonal compactness and diameter, loss of myelin sheath, and decreased nerve-fiber numbers. The therapeutic effects produced by RgIA in this model may be attributed to modulation in the activity of immune cells since inflammatory infiltrate including lymphocytes and CD86+ macrophages was reduced in the affected nerve and dorsal root ganglion (DRG) [80,81]. These disease-modifying effects were not limited to peripheral nerves as RgIA prevented the activation of microglia and astrocytes in the dorsal horn of the spinal cord. Further evidence that RgIA modulates the activity of immune cells was found in the DSS model of inflammatory bowel diseases (IBD) in mice [82]. In this study, colonic levels of TNF-α were reduced and pathological changes in colon morphology were prevented by administration of RgIA. Therapeutic effects were also observed in models of CIN. Morphological changes in rat DRG induced by oxaliplatin administration were significantly attenuated and the number of glial fibrillary acidic-protein positive astrocytes in the dorsal horn reduced [83]. Analogs of RgIA with increased potency were also analgesic in CIN. RgIA4 prevented oxaliplatin-induced cold allodynia and neuropathic pain in mice [84], and the therapeutic effects were dependent on the presence of CD3+ T-cells [85]. Strikingly, RgIA4 provided sustained protection against oxaliplatin-induced nerve injury in mice long after the last dose of the peptide [86]. Additional analogs of RgIA with increased potency and biostability are being developed as potential therapeutics for treatment of human neuropathic pain conditions [87–90].
Other α-conotoxins and small molecules that target α9α10 nAChRs have also been shown to be therapeutic in various neuropathy models. GeXIVA and is an α-conotoxin that is structurally quite different than Vc1.1, [S4Dap]Mr1.1, and RgIA yet was also analgesic and reduced mechanical hyperalgesia (von Frey test) in rats subjected to CCI [88,91]. Small non-peptidic molecules have been synthesized that are analgesic and anti-inflammatory in CCI-induced neuropathy, formalin-induced inflammatory pain, and CIN. The tetrakis-quaternary ammonium compound ZZ-204G was analgesic across a battery of pain models including CCI, formalin-induced inflammatory pain, and the tail-flick model of thermal nociception [92]. The related compound ZZ1–61c prevented the induction of neuropathy by the chemotherapeutic vincristine [93]. Structurally diverse ligands selective for α9α10 nAChRs that are analgesic provide strong evidence in support of targeting α9/α10 nAChRs for treating neuropathic and inflammatory pain [94,95].
The ligands discussed above are all antagonists of α9α10 nAChRs, but some studies suggest that agonist ligands may have anti-inflammatory and/or analgesic properties. Recently, a number of novel small molecule agonists and antagonists that target α9α10 nAChRs were reported [96]. One of these compounds, the agonist pCF3 diEPP, was effective at inhibiting LPS-induced release of IL-6 from primary mouse macrophages and IL-6, TNF-α, and IL-1β from whole human blood cultures [97]. Additionally, pCF3 diEPP inhibited ATP-induced release of IL-1β from human peripheral blood mononuclear leukocytes. Choline is an agonist of α9α10 nAChRs and has analgesic and anti-inflammatory properties, but most studies attribute the analgesic effects of choline to agonism of α7 nAChRs. It is noteworthy that choline concentrations in the mM range were required for inhibition of TNF-α release from cultured mouse macrophages [98]. In other cell types such as human U937 monocytes, 10 μM choline was sufficient to inhibit IL-1β release [21]. Naturally occurring derivatives of choline have been shown to inhibit the release of cytokines from several immune cell types. Cytidine-5′-diphosphate choline, or CDP-choline, has been shown to be analgesic, but again most of these studies attribute the effects to α7 nAChRs [99–102]. Phosphocholine is an agonist of human monocyte expressed nAChRs containing α7, α9, and α10 subunits [20,21]. In these studies, both choline and phosphocholine inhibited ATP-stimulated release of IL-1β from human and murine monocytes and from the human monocyte U937 cell line. The effects of choline and phosphocholine were significantly inhibited by the potent and selective antagonist RgIA4, suggesting that α9α10 nAChRs are involved in the in modulation of immune cell function by choline and its derivatives. There is a large disparity between the potency of choline for activation of α7 vs α9-containing nAChRs; choline activates α9 and α9α10 nAChRs [71] in the low μM range whereas mM concentrations are required for activation of the α7 subtype. Concentrations in the mM range are probably not achieved in human blood [103], and thus it remains an open question as to which nAChR subtype mediates the therapeutic effects of choline. (Table 2).
Table 2.
Antagonists that target α9-containing nAChRs, mechanism of action, and effects in models of pain and inflammation.
| Ligand | Mechanism of Action | Disease Model | Pharmacological Effects | Ref. |
|---|---|---|---|---|
| Vc1.1 | Antagonist | CCI-induced neuropathic pain | Reduced mechanical allodynia, mechanical hyperalgesia, infiltration of immune cells; disease modifying effects. | [75, 81] |
| Mr1.1 | Antagonist | CCI-induced neuropathic pain | Reduced mechanical hyperalgesia. | [79] |
| RgIA | Antagonist | CCI-induced neuropathic pain CIN | Reduced mechanical allodynia and mechanical hyperalgesia, infiltration of immune cells. Reduced mechanical hyperalgesia, cold allodynia; disease modifying effects | [81] [83, 115] |
| RgIA4 | Antagonist | CIN | Reduced mechanical hyperalgesia and cold allodynia; disease modifying effects. | [84, 85, 116] |
| RgIA-5474 | Antagonist | CIN | Prevention of cold allodynia induction. | [89] |
| RgIA-5628 | Antagonist | CIN | Prevention of cold allodynia induction. | [87] |
| RgIA-5524 | Antagonist | CIN | Prevention of cold allodynia induction. | [90] |
| GeXIVA | Antagonist | CCI-induced neuropathic pain CIN | Reduced mechanical hyperalgesia. Long-lasting inhibition of mechanical and cold allodynia. | [88] [91, 117] |
| ZZ-204 G | Antagonist | CCI-induced neuropathic pain Formalin-induced inflammatory pain | Reduced mechanical hyperalgesia Reduced inflammatory pain. |
[92] [92] |
| ZZ1–61c | Antagonist | CIN | Reduced mechanical allodynia and mechanical hyperalgesia. | [93] |
CCI, chronic constriction injury; CIN, chemotherapeutic-induced neuropathic pain.
1.5. Alternative mechanisms for the therapeutic effects of α-conotoxins that target α9α10 nAChRs
Certain α-conotoxin ligands that target α9α10 nAChRs have been shown to act as agonists of GABAB receptors [104–110]. Structure-activity studies have correlated potency for GABAB receptors with analgesia suggesting that a portion of the therapeutic effects might be accounted for by this mechanism. GABAB receptors are present in DRG, spinal cord and brain. Baclofen is a clinically used GABAB agonist with analgesic activity in animal models. However, its modest therapeutic effects are thought to be mediated through action on central nervous system (CNS) rather than DRG expressed GABAB receptors [111–113]. The size and charge of α-conopeptides would likely significantly limit CNS concentrations of the peptides precluding therapeutic effects at spinal cord or brain GABAB receptors. Separately, it is noteworthy that highly potent α-conopeptide antagonists of α9α10 nAChR, that show analgesic activity, lack effects on GABAB receptors [84,89, 90]. In addition, studies utilizing mice with germline deletions of the α9 nAChR subunit indicate that analgesic activity of the peptides in CIN are dependent on the α9 nAChR subunit [84,85,90]. Furthermore, as reviewed above, disease-modifying aspects of analgesic α-conopeptides are consistent with immune system modulation mediated by α9-containing nAChRs; similar properties have not been noted for baclofen or other GABAB receptor agonists (for further review see [94,114] and references therein).(Table. 3).
Table 3.
Small molecules that target nAChRs containing α7, α9, or α10 subunits, mechanism of action, and pharmacological effects.
| Ligand | Receptor Target | Mechanism of Action | Pharmacological Effect | Ref. |
|---|---|---|---|---|
| Choline | α7 α9α10 | agonist partial agonist | Decreased TNF-α release from mouse macrophages; inhibition of IL-1β from primary human monocytes. | [21,98] |
| CDP-choline | α7 | agonist | Reduced oxaliplatin-induced neuropathic pain in mice; reversal of CCI-induced mechanical hyperalgesia in mice; decreased mechanical hyperalgesia and paw edema in carrageenan-induced inflammatory pain. | [100–102] |
| Phosphocholine | α9α10 | agonist | Inhibition of IL-1β from human U937 monocytes, monocytic THP-1, THP-1 derived M1-like macrophages, primary monocytes, and PBMCs. | [21,22] |
| pCF3 diEPP | α9α10 | partial agonist | Inhibition of TNF-α, IL-1β, and Il-6 release from whole human blood cultures; reduced IL-1β release from PBMCs, monocytic THP-1, THP-1 derived M1-like macrophages, and primary monocytes. | [97] |
CDP-choline; cytidine-5′-diphosphate choline; PBMCs, peripheral blood mononuclear cells.
2. Conclusions
A growing body of evidence establishes nAChRs containing α7, α9, and/or α10 subunits as promising molecular targets for pharmacotherapy of disease that involves pain and inflammation. It appears increasingly likely that inhibition of pain and inflammatory states by nAChRs occurs through modulation of immune cell function. However, at the present time the exact composition and stoichiometry of the nAChRs involved in modulating immune cell function isn’t precisely known. Long-standing convention holds that α7 subunits form homopentamers and that α9 and α10 subunits are expressed together as heteropentamers in human. Recently, however, it was demonstrated that α7 subunits can combine with β2 subunits to form α7β2 heteromers and are expressed in certain areas of mammalian basal forebrain [118–120]. Immune cells are also known to express β2 subunits, and it is unknown whether they combine with α7 subunits in these cells. The stoichiometry of immune cell expressed receptors containing α9 and α10 subunits is perhaps more complicated. Not only can α9α10 nAChRs vary with respect to the ratio of α9 to α10 subunits [78,121], which alters the pharmacology of the receptor, but the individual subunits themselves can also be expressed as homopentamers [7,69,71]. Nevertheless, studies using ligands selective for α7 and α9α10 nAChRs strongly suggest that a number of immune cell types express these two subtypes [122]. Prospective pharmacotherapeutics may benefit from being subtype selective to avoid off-target effects such as reinforcing behaviors that, for example, have hindered the development of an analgesic drug that targets α4β2* nAChRs [123]. As mentioned above, agonists and PAMs of α7 nAChRs may produce unwanted side effects from elevated intracellular calcium levels which can be cytotoxic. Furthermore, α7 nAChRs are highly expressed in numerous areas of the CNS and PNS. Human adrenal chromaffin cells, for example, have enriched expression of α7 nAChRs and are involved in the stimulus-secretion coupling response [124]. Ligands that activate α7 nAChRs have been shown to be excitatory and increase catecholamine release from human chromaffin cells [125,126]. Such actions could increase plasma catecholamine levels and potentially trigger cardiovascular side effects. Therefore, research investigating potential side effects that may occur from systemic administration of α7 nAChR ligands is essential. Pharmacotherapeutics targeting α9α10 nAChRs might produce fewer side effects because of their restricted expression patterns in comparison to α7 nAChRs. α9α10 nAChR are expressed by immune cells, skin, anterior pituitary, and a limited number of other tissues but not in the CNS [127], and therefore agonist ligands would likely be devoid of reinforcing behaviors. Interestingly, mRNA for the α9 subunit has been found in human [128] and α10 in rat [129] DRG though responses attributable to α9α10 nAChRs have thus far not been identified. Additional research is needed to further understand the physiological as well as the pathological roles of α7 and α9α10 nAChRs in pain and inflammation.
Funding
This work was funded by the National Institute of Health [R35 GM136430].
Abbreviations:
- CCI
chronic constriction injury
- CIN
chemotherapy-induced neuropathic pain
- EAE
experimental autoimmune encephalomyelitis
- PTSD
posttraumatic stress disorder
- PAM
positive allosteric modulator
- LPS
lipopolysaccharide
Footnotes
CRediT authorship contribution statement
The authors contributed equally to manuscript conceptualization, writing the original draft, and writing, reviewing and, editing the revised manuscript.
Declaration of Interest Statement
The University of Utah has filed patent applications on conopeptides including those described in the present manuscript and on which J.M. M. is listed as an inventor.
The multifaceted activities of nervous and non-nervous neuronal nicotinic acetylcholine receptors in physiology and pathology. Eds: Dr Cecilia Gotti, Prof Francesco Clementi, Prof Michele ZOli.
Data availability
No data was used for the research described in the article.
References
- [1].Cartaud J, Benedetti EL, Cohen JB, Meunier JC, Changeux JP, Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata, FEBS Lett. 33 (1) (1973) 109–113. [DOI] [PubMed] [Google Scholar]
- [2].Changeux JP, Discovery of the first neurotransmitter receptor: the acetylcholine nicotinic receptor, Biomolecules 10 (4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Davies PA, Wang W, Hales TG, Kirkness EF, A novel class of ligand-gated ion channel is activated by Zn2+, J. Biol. Chem 278 (2) (2003) 712–717. [DOI] [PubMed] [Google Scholar]
- [4].Thompson AJ, Lester HA, Lummis SC, The structural basis of function in Cysloop receptors, Q Rev. Biophys 43 (4) (2010) 449–499. [DOI] [PubMed] [Google Scholar]
- [5].Raftery MA, Hunkapiller MW, Strader CD, Hood LE, Acetylcholine receptor: complex of homologous subunits, Science 208 (4451) (1980) 1454–1456. [DOI] [PubMed] [Google Scholar]
- [6].Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B, Molecular distinction between fetal and adult forms of muscle acetylcholine receptor, Nature 321 (6068) (1986) 406–411. [DOI] [PubMed] [Google Scholar]
- [7].Hone AJ, McIntosh JM, Alkaloid ligands enable function of homomeric human alpha10 nicotinic acetylcholine receptors, Front Pharm. 13 (2022), 981760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Richter K, Grau V Signaling of nicotinic acetylcholine receptors in mononuclear phagocytes. Pharmacological Research. 2023; in press. [DOI] [PubMed] [Google Scholar]
- [9].Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K, Physiological functions of the cholinergic system in immune cells, J. Pharm. Sci 134 (1) (2017) 1–21. [DOI] [PubMed] [Google Scholar]
- [10].Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H, Expression and function of genes encoding cholinergic components in murine immune cells, Life Sci. 80 (24–25) (2007) 2314–2319. [DOI] [PubMed] [Google Scholar]
- [11].Kawashima K, Fujii T, Extraneuronal cholinergic system in lymphocytes, Pharm. Ther 86 (1) (2000) 29–48. [DOI] [PubMed] [Google Scholar]
- [12].Hao J, Simard AR, Turner GH, Wu J, Whiteaker P, Lukas RJ, Shi FD, Attenuation of CNS inflammatory responses by nicotine involves alpha7 and nonalpha7 nicotinic receptors, Exp. Neurol 227 (1) (2011) 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peng H, Ferris RL, Matthews T, Hiel H, Lopez-Albaitero A, Lustig LR, Characterization of the human nicotinic acetylcholine receptor subunit alpha (alpha) 9 (CHRNA9) and alpha (alpha) 10 (CHRNA10) in lymphocytes, Life Sci. 76 (3) (2004) 263–280. [DOI] [PubMed] [Google Scholar]
- [14].Nakata Y, Miura K, Yamasaki N, Ogata S, Miura S, Hosomi N, Kaminuma O, Expression and function of nicotinic acetylcholine receptors in induced regulatory T cells, Int J. Mol. Sci 23 (2022) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA, Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10), Genomics 73 (3) (2001) 272–283. [DOI] [PubMed] [Google Scholar]
- [16].Siniavin AE, Streltsova MA, Kudryavtsev DS, Shelukhina IV, Utkin YN, Tsetlin VI, Activation of alpha7 nicotinic acetylcholine receptor upregulates HLADR and macrophage receptors: potential role in adaptive immunity and in preventing immunosuppression, Biomolecules 10 (4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chernyavsky AI, Arredondo J, Skok M, Grando SA, Auto/paracrine control of inflammatory cytokines by acetylcholine in macrophage-like U937 cells through nicotinic receptors, Int Immunopharmacol. 10 (3) (2010) 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mikulski Z, Hartmann P, Jositsch G, Zaslona Z, Lips KS, Pfeil U, Kurzen H, Lohmeyer J, Clauss WG, Grau V, Fronius M, Kummer W, Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration, Respir. Res 11 (2010) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maldifassi MC, Atienza G, Arnalich F, Lopez-Collazo E, Cedillo JL, Martin-Sanchez C, Bordas A, Renart J, Montiel C, A new IRAK-M-mediated mechanism implicated in the anti-inflammatory effect of nicotine via alpha7 nicotinic receptors in human macrophages, PLoS One 9 (9) (2014), e108397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Richter K, Mathes V, Fronius M, Althaus M, Hecker A, Krasteva-Christ G, Padberg W, Hone AJ, McIntosh JM, Zakrzewicz A, Grau V, Phosphocholine - an agonist of metabotropic but not of ionotropic functions of alpha9-containing nicotinic acetylcholine receptors, Sci. Rep 6 (2016) 28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hecker A, Kullmar M, Wilker S, Richter K, Zakrzewicz A, Atanasova S, Mathes V, Timm T, Lerner S, Klein J, Kaufmann A, Bauer S, Padberg W, Kummer W, Janciauskiene S, Fronius M, Schweda EK, Lochnit G, Grau V, Phosphocholine-modified macromolecules and canonical nicotinic agonists inhibit ATP-induced IL-1beta release, J. Immunol 195 (5) (2015) 2325–2334. [DOI] [PubMed] [Google Scholar]
- [22].Zakrzewicz A, Richter K, Agne A, Wilker S, Siebers K, Fink B, Krasteva-Christ G, Althaus M, Padberg W, Hone AJ, McIntosh JM, Grau V, Canonical and novel non-canonical cholinergic agonists inhibit ATP-induced release of monocytic interleukin-1beta via different combinations of nicotinic acetylcholine receptor subunits alpha7, alpha9 and alpha10, Front Cell Neurosci. 11 (2017) 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Richter K, Sagawe S, Hecker A, Kullmar M, Askevold I, Damm J, Heldmann S, Pohlmann M, Ruhrmann S, Sander M, Schluter KD, Wilker S, Konig IR, Kummer W, Padberg W, Hone AJ, McIntosh JM, Zakrzewicz AT, Koch C, Grau V, C-reactive protein stimulates nicotinic acetylcholine receptors to control ATP-mediated monocytic inflammasome activation, Front Immunol. 9 (2018) 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin, Nature 405 (6785) (2000) 458–462. [DOI] [PubMed] [Google Scholar]
- [25].Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ, Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation, Nature 421 (6921) (2003) 384–388. [DOI] [PubMed] [Google Scholar]
- [26].Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ, The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation, Mol. Med 9 (5–8) (2003) 125–134. [PMC free article] [PubMed] [Google Scholar]
- [27].Alen NV, The cholinergic anti-inflammatory pathway in humans: state-of-the-art review and future directions, Neurosci. Biobehav Rev 136 (2022), 104622. [DOI] [PubMed] [Google Scholar]
- [28].Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr., Medzhitov R, Flavell RA, IRAK-M is a negative regulator of Toll-like receptor signaling, Cell 110 (2) (2002) 191–202. [DOI] [PubMed] [Google Scholar]
- [29].Baez-Pagan CA, Delgado-Velez M, Lasalde-Dominicci JA, Activation of the macrophage alpha7 nicotinic acetylcholine receptor and control of inflammation, J. Neuroimmune Pharm 10 (3) (2015) 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cedillo JL, Arnalich F, Martin-Sanchez C, Quesada A, Rios JJ, Maldifassi MC, Atienza G, Renart J, Fernandez-Capitan C, Garcia-Rio F, Lopez-Collazo E, Montiel C, Usefulness of alpha7 nicotinic receptor messenger RNA levels in peripheral blood mononuclear cells as a marker for cholinergic antiinflammatory pathway activity in septic patients: results of a pilot study, J. Infect. Dis 211 (1) (2015) 146–155. [DOI] [PubMed] [Google Scholar]
- [31].Changeux JP, Amoura Z, Rey FA, Miyara M, A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications, C. R. Biol 343 (1) (2020) 33–39. [DOI] [PubMed] [Google Scholar]
- [32].Gauthier AG, Lin M, Wu J, Kennedy TP, Daley LA, Ashby CR Jr., Mantell LL, From nicotine to the cholinergic anti-inflammatory reflex - Can nicotine alleviate the dysregulated inflammation in COVID-19? J. Immunotoxicol 18 (1) (2021) 23–29. [DOI] [PubMed] [Google Scholar]
- [33].Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI, New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: a focus on alpha7 nAChRs, Curr. Neuropharmacol 16 (4) (2018) 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bouzat C, Lasala M, Nielsen BE, Corradi J, Esandi MDC, Molecular function of alpha7 nicotinic receptors as drug targets, J. Physiol 596 (10) (2018) 1847–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mizrachi T, Marsha O, Brusin K, Ben-David Y, Thakur GA, Vaknin-Dembinsky A, Treinin M, Brenner T, Suppression of neuroinflammation by an allosteric agonist and positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor GAT107, J. Neuroinflamm 18 (1) (2021) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, Higdon NR, Hoffmann WE, Hurst RS, Myers JK, Rogers BN, Wall TM, Wolfe ML, Wong E, Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors, J. Med Chem 48 (4) (2005) 905–908. [DOI] [PubMed] [Google Scholar]
- [37].Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, Olson KL, Jacobsen EJ, Wolfe ML, Groppi VE, Hanchar AJ, Thornburgh BA, Cortes-Burgos LA, Wong EH, Staton BA, Raub TJ, Higdon NR, Wall TM, Hurst RS, Walters RR, Hoffmann WE, Hajos M, Franklin S, Carey G, Gold LH, Cook KK, Sands SB, Zhao SX, Soglia JR, Kalgutkar AS, Arneric SP, Rogers BN, Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure–activity relationship, J. Med Chem 49 (14) (2006) 4425–4436. [DOI] [PubMed] [Google Scholar]
- [38].Pacini A, Di Cesare Mannelli L, Bonaccini L, Ronzoni S, Bartolini A, Ghelardini C, Protective effect of alpha7 nAChR: behavioural and morphological features on neuropathy, Pain 150 (3) (2010) 542–549. [DOI] [PubMed] [Google Scholar]
- [39].Di Cesare Mannelli L, Pacini A, Matera C, Zanardelli M, Mello T, De Amici M, Dallanoce C, Ghelardini C, Involvement of alpha7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation, Neuropharmacology 79 (2014) 37–48. [DOI] [PubMed] [Google Scholar]
- [40].Umana IC, Daniele CA, Miller BA, Abburi C, Gallagher K, Brown MA, Mason P, McGehee DS, Nicotinic modulation of descending pain control circuitry, Pain 158 (10) (2017) 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Donvito G, Bagdas D, Toma W, Rahimpour E, Jackson A, Meade JA, AlSharari S, Kulkarni AR, Carroll F Ivy, A.H. Lichtman, R.L. Papke, G. A. Thakur, Damaj M. Imad, The interaction between alpha 7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-alpha represents a new antinociceptive signaling pathway in mice, Exp. Neurol 295 (2017) 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Costa R, Motta EM, Manjavachi MN, Cola M, Calixto JB, Activation of the alpha-7 nicotinic acetylcholine receptor (alpha7 nAchR) reverses referred mechanical hyperalgesia induced by colonic inflammation in mice, Neuropharmacology 63 (5) (2012) 798–805. [DOI] [PubMed] [Google Scholar]
- [43].AlSharari SD, Bagdas D, Akbarali HI, Lichtman PA, Raborn ES, Cabral GA, Carroll FI, McGee EA, Damaj MI, Sex differences and drug dose influence the role of the alpha7 nicotinic acetylcholine receptor in the mouse dextran sodium sulfate-induced colitis model, Nicotine Tob. Res 19 (4) (2017) 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kimura H, Imura YK, Tomiyasu H, Mihara T, Kaji N, Ohno K, Unno T, Tanahashi Y, Jan TR, Tsubone H, Ozaki H, Hori M, Neural anti-inflammatory action mediated by two types of acetylcholine receptors in the small intestine, Sci. Rep 9 (1) (2019) 5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pinheiro NM, Santana FP, Almeida RR, Guerreiro M, Martins MA, Caperuto LC, Camara NO, Wensing LA, Prado VF, Tiberio IF, Prado MA, Prado CM, Acute lung injury is reduced by the alpha7nAChR agonist PNU-282987 through changes in the macrophage profile, FASEB J. 31 (1) (2017) 320–332. [DOI] [PubMed] [Google Scholar]
- [46].Sun R, Zhang W, Bo J, Zhang Z, Lei Y, Huo W, Liu Y, Ma Z, Gu X, Spinal activation of alpha7-nicotinic acetylcholine receptor attenuates posttraumatic stress disorder-related chronic pain via suppression of glial activation, Neuroscience 344 (2017) 243–254. [DOI] [PubMed] [Google Scholar]
- [47].Stegemann A, Flis D, Ziolkowski W, Distler JHW, Steinbrink K, Bohm M, The alpha7 nicotinic acetylcholine receptor: a promising target for the treatment of fibrotic skin disorders, J. Invest Dermatol 140 (12) (2020) 2371–2379. [DOI] [PubMed] [Google Scholar]
- [48].Woodruff-Pak DS, Li YT, Kem WR, A nicotinic agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain, Brain Res 645 (1–2) (1994) 309–317. [DOI] [PubMed] [Google Scholar]
- [49].Briggs CA, Gronlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, Olsen GM, Peters D, Bunnelle WH, Gopalakrishnan M, Role of channel activation in cognitive enhancement mediated by alpha7 nicotinic acetylcholine receptors, Br. J. Pharm 158 (6) (2009) 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang X, Xu F, Wang L, Li J, Zhang J, Huang L, The role of dorsal root ganglia alpha-7 nicotinic acetylcholine receptor in complete Freund’s adjuvant-induced chronic inflammatory pain, Inflammopharmacology 29 (5) (2021) 1487–1501. [DOI] [PubMed] [Google Scholar]
- [51].Papke RL, Bagdas D, Kulkarni AR, Gould T, AlSharari SD, Thakur GA, Damaj MI, The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor, Neuropharmacology 91 (2015) 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Thakur GA, Kulkarni AR, Deschamps JR, Papke RL, Expeditious synthesis, enantiomeric resolution, and enantiomer functional characterization of (4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (4BP-TQS): an allosteric agonist-positive allosteric modulator of alpha7 nicotinic acetylcholine receptors, J. Med Chem 56 (21) (2013) 8943–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gill JK, Dhankher P, Sheppard TD, Sher E, Millar NS, A series of alpha7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties, Mol. Pharm 81 (5) (2012) 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bagdas D, Wilkerson JL, Kulkarni A, Toma W, AlSharari S, Gul Z, Lichtman AH, Papke RL, Thakur GA, Damaj MI, The alpha7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain, Br. J. Pharm 173 (16) (2016) 2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guerra-Alvarez M, Moreno-Ortega AJ, Navarro E, Fernandez-Morales JC, Egea J, Lopez MG, Cano-Abad MF, Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca(2+) release from the endoplasmic reticulum, J. Neurochem 133 (3) (2015) 309–319. [DOI] [PubMed] [Google Scholar]
- [56].Loram LC, Taylor FR, Strand KA, Maier SF, Speake JD, Jordan KG, James JW, Wene SP, Pritchard RC, Green H, Van Dyke K, Mazarov A, Letchworth SR, Watkins LR, Systemic administration of an alpha-7 nicotinic acetylcholine agonist reverses neuropathic pain in male Sprague Dawley rats, J. Pain 13 (12) (2012) 1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Potasiewicz A, Kos T, Ravazzini F, Puia G, Arias HR, Popik P, Nikiforuk A, Pro-cognitive activity in rats of 3-furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor, Br. J. Pharm 172 (21) (2015) 5123–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kucinski A, Syposs C, Wersinger S, Bencherif M, Stachowiak MK, Stachowiak EK, alpha7 neuronal nicotinic receptor agonist (TC-7020) reverses increased striatal dopamine release during acoustic PPI testing in a transgenic mouse model of schizophrenia, Schizophr. Res 136 (1–3) (2012) 82–87. [DOI] [PubMed] [Google Scholar]
- [59].Wood C, Kohli S, Malcolm E, Allison C, Shoaib M, Subtype-selective nicotinic acetylcholine receptor agonists can improve cognitive flexibility in an attentional set shifting task, Neuropharmacology 105 (2016) 106–113. [DOI] [PubMed] [Google Scholar]
- [60].Pichat P, Bergis OE, Terranova JP, Urani A, Duarte C, Santucci V, Gueudet C, Voltz C, Steinberg R, Stemmelin J, Oury-Donat F, Avenet P, Griebel G, Scatton B, SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia, Neuropsychopharmacology 32 (1) (2007) 17–34. [DOI] [PubMed] [Google Scholar]
- [61].Pieschl RL, Miller R, Jones KM, Post-Munson DJ, Chen P, Newberry K, Benitex Y, Molski T, Morgan D, McDonald IM, Macor JE, Olson RE, Asaka Y, Digavalli S, Easton A, Herrington J, Westphal RS, Lodge NJ, Zaczek R, Bristow LJ, Li YW, Effects of BMS-902483, an alpha7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents, Eur. J. Pharm 807 (2017) 1–11. [DOI] [PubMed] [Google Scholar]
- [62].van Maanen MA, Papke RL, Koopman FA, Koepke J, Bevaart L, Clark R, Lamppu D, Elbaum D, LaRosa GJ, Tak PP, Vervoordeldonk MJ, Two novel alpha7 nicotinic acetylcholine receptor ligands: in vitro properties and their efficacy in collagen-induced arthritis in mice, PLoS One 10 (1) (2015), e0116227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].El Nebrisi EG, Bagdas D, Toma W, Al Samri H, Brodzik A, Alkhlaif Y, Yang KS, Howarth FC, Damaj IM, Oz M, Curcumin acts as a positive allosteric modulator of alpha(7)-nicotinic acetylcholine receptors and reverses nociception in mouse models of inflammatory pain, J. Pharm. Exp. Ther 365 (1) (2018) 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Caillaud M, Thompson D, Toma W, White A, Mann J, Roberts JL, Bigbee JW, Gewirtz DA, Damaj MI, Formulated curcumin prevents paclitaxel-induced peripheral neuropathy through reduction in neuroinflammation by modulation of alpha7 nicotinic acetylcholine receptors, Pharmaceutics 14 (2022) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ren Y, Zhou Y, You Z, Deng H, Kem WR, Mao J, Zhang W, Martyn JAJ, The nonopioid cholinergic agonist GTS-21 mitigates morphine-induced aggravation of burn injury pain together with inhibition of spinal microglia activation in young rats, Br. J. Anaesth 129 (6) (2022) 959–969. [DOI] [PubMed] [Google Scholar]
- [66].Quadri M, Bagdas D, Toma W, Stokes C, Horenstein NA, Damaj MI, Papke RL, The antinociceptive and anti-inflammatory properties of the alpha7 nAChR Weak Partial Agonist p-CF(3) N,N-diethyl-N′-phenylpiperazine, J. Pharm. Exp. Ther 367 (2) (2018) 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Abbas M, Alzarea S, Papke RL, Rahman S, Effects of alpha7 nicotinic acetylcholine receptor positive allosteric modulator on BDNF, NKCC1 and KCC2 expression in the hippocampus following lipopolysaccharide-induced allodynia and hyperalgesia in a mouse model of inflammatory pain, CNS Neurol. Disord. Drug Targets 20 (4) (2021) 366–377. [DOI] [PubMed] [Google Scholar]
- [68].Micheli L, Rajagopalan R, Lucarini E, Toti A, Parisio C, Carrino D, Pacini A, Ghelardini C, Rajagopalan P, Di Cesare Mannelli L, Pain relieving and neuroprotective effects of non-opioid compound, DDD-028, in the rat model of paclitaxel-induced neuropathy, Neurotherapeutics 18 (3) (2021) 2008–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S, Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells, Cell 79 (4) (1994) 705–715. [DOI] [PubMed] [Google Scholar]
- [70].Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J, alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells, Proc. Natl. Acad. Sci. USA 98 (6) (2001) 3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F, A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit, Mol. Pharm 61 (1) (2002) 150–159. [DOI] [PubMed] [Google Scholar]
- [72].Nguyen VT, Ndoye A, Grando SA, Novel human alpha9 acetylcholine receptor regulating keratinocyte adhesion is targeted by Pemphigus vulgaris autoimmunity, Am. J. Pathol 157 (4) (2000) 1377–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pucci S, Zoli M, Clementi F, Gotti C, alpha9-containing nicotinic receptors in cancer, Front Cell Neurosci. 15 (2021), 805123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Russo P, Del Bufalo A, Milic M, Salinaro G, Fini M, Cesario A, Cholinergic receptors as target for cancer therapy in a systems medicine perspective, Curr. Mol. Med 14 (9) (2014) 1126–1138. [DOI] [PubMed] [Google Scholar]
- [75].Satkunanathan N, Livett B, Gayler K, Sandall D, Down J, Khalil Z, Alpha-conotoxin Vc1.1 alleviates neuropathic pain and accelerates functional recovery of injured neurones, Brain Res 1059 (2) (2005) 149–158. [DOI] [PubMed] [Google Scholar]
- [76].AdisInsight. A.C.V.1. 2016;〈http://adisinsight.springer.com/drugs/800020787〉 Accessed: April 17th 2017(accessed 17 April 2017).
- [77].Yu R, Kompella SN, Adams DJ, Craik DJ, Kaas Q, Determination of the alpha-conotoxin Vc1.1 binding site on the alpha9alpha10 nicotinic acetylcholine receptor, J. Med Chem 56 (9) (2013) 3557–3567. [DOI] [PubMed] [Google Scholar]
- [78].Indurthi DC, Pera E, Kim HL, Chu C, McLeod MD, McIntosh JM, Absalom NL, Chebib M, Presence of multiple binding sites on alpha9alpha10 nAChR receptors alludes to stoichiometric-dependent action of the alpha-conotoxin, Vc1.1, Biochem Pharm. 89 (1) (2014) 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liang J, Tae HS, Zhao Z, Li X, Zhang J, Chen S, Jiang T, Adams DJ, Yu R, Mechanism of action and structure-activity relationship of alpha-conotoxin Mr1.1 at the Human alpha9alpha10 Nicotinic Acetylcholine Receptor, J. Med Chem (2022). [DOI] [PubMed] [Google Scholar]
- [80].Di Cesare Mannelli L, Cinci L, Micheli L, Zanardelli M, Pacini A, McIntosh JM, Ghelardini C, alpha-conotoxin RgIA protects against the development of nerve injury-induced chronic pain and prevents both neuronal and glial derangement, Pain 155 (10) (2014) 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM, Molecular mechanism for analgesia involving specific antagonism of alpha9alpha10 nicotinic acetylcholine receptors, Proc. Natl. Acad. Sci. USA 103 (47) (2006) 17880–17884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].AlSharari SD, Toma W, Mahmood HM, Michael McIntosh J, Imad, Damaj M, The alpha9alpha10 nicotinic acetylcholine receptors antagonist alpha-conotoxin RgIA reverses colitis signs in murine dextran sodium sulfate model, Eur. J. Pharm 883 (2020), 173320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pacini A, Micheli L, Maresca M, Branca JJ, McIntosh JM, Ghelardini C, Di Cesare Mannelli L, The alpha9alpha10 nicotinic receptor antagonist alpha-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment, Exp. Neurol 282 (2016) 37–48. [DOI] [PubMed] [Google Scholar]
- [84].Romero HK, Christensen SB, Di Cesare Mannelli L, Gajewiak J, Ramachandra R, Elmslie KS, Vetter DE, Ghelardini C, Iadonato SP, Mercado JL, Olivera BM, McIntosh JM, Inhibition of alpha9alpha10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain, Proc. Natl. Acad. Sci. USA 114 (10) (2017) 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Huynh PN, Christensen SB, McIntosh JM, RgIA4 prevention of acute oxaliplatin-induced cold allodynia requires alpha9-containing nicotinic acetylcholine receptors and CD3(+) T-Cells, Cells 11 (22) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Christensen SB, Hone AJ, Roux I, Kniazeff J, Pin JP, Upert G, Servent D, Glowatzki E, McIntosh JM, RgIA4 potently blocks mouse alpha9alpha10 nAChRs and provides long lasting protection against oxaliplatin-induced cold allodynia, Front Cell Neurosci. 11 (2017) 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zheng N, Christensen SB, Blakely A, Dowell C, Purushottam L, McIntosh JM, Chou DH, Development of conformationally constrained alpha-RgIA analogues as stable peptide antagonists of human alpha9alpha10 nicotinic acetylcholine receptors, J. Med Chem 63 (15) (2020) 8380–8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Luo S, Zhangsun D, Harvey PJ, Kaas Q, Wu Y, Zhu X, Hu Y, Li X, Tsetlin VI, Christensen S, Romero HK, McIntyre M, Dowell C, Baxter JC, Elmslie KS, Craik DJ, Cloning McIntosh JM, synthesis, and characterization of alphaO-conotoxin GeXIVA, a potent alpha9alpha10 nicotinic acetylcholine receptor antagonist, Proc. Natl. Acad. Sci. USA 112 (30) (2015) E4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gajewiak J, Christensen SB, Dowell C, Hararah F, Fisher F, Huynh PN, Olivera BM, McIntosh JM, Selective penicillamine substitution enables development of a potent analgesic peptide that acts through a non-opioid-based mechanism, J. Med Chem 64 (13) (2021) 9271–9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zheng N, Christensen SB, Dowell C, Purushottam L, Skalicky JJ, McIntosh JM, Chou DH, Discovery of methylene thioacetal-incorporated alpha-RgIA analogues as potent and stable antagonists of the human alpha9alpha10 nicotinic acetylcholine receptor for the treatment of neuropathic pain, J. Med Chem 64 (13) (2021) 9513–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Li X, Hu Y, Wu Y, Huang Y, Yu S, Ding Q, Zhangsun D, Luo S, Anti-hypersensitive effect of intramuscular administration of alphaO-conotoxin GeXIVA[1,2] and GeXIVA[1,4] in rats of neuropathic pain, Prog. Neuropsychopharmacol. Biol. Psychiatry 66 (2016) 112–119. [DOI] [PubMed] [Google Scholar]
- [92].Holtman JR, Dwoskin LP, Dowell C, Wala EP, Zhang Z, Crooks PA, McIntosh JM, The novel small molecule alpha9alpha10 nicotinic acetylcholine receptor antagonist ZZ-204G is analgesic, Eur. J. Pharm 670 (2–3) (2011) 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wala EP, Crooks PA, McIntosh JM, Holtman JR Jr., Novel small molecule alpha9alpha10 nicotinic receptor antagonist prevents and reverses chemotherapy-evoked neuropathic pain in rats, Anesth. Analg 115 (3) (2012) 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hone AJ, Servent D, McIntosh JM, alpha9-containing nicotinic acetylcholine receptors and the modulation of pain, Br. J. Pharm 175 (11) (2018) 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li X, Tae HS, Chu Y, Jiang T, Adams DJ, Yu R, Medicinal chemistry, pharmacology, and therapeutic potential of alpha-conotoxins antagonizing the alpha9alpha10 nicotinic acetylcholine receptor, Pharm. Ther 222 (2021), 107792. [DOI] [PubMed] [Google Scholar]
- [96].Papke RL, Andleeb H, Stokes C, Quadri M, Horenstein NA, Selective agonists and antagonists of alpha9 Versus alpha7 nicotinic acetylcholine receptors, ACS Chem. Neurosci 13 (5) (2022) 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Richter K, Papke RL, Stokes C, Roy DC, Espinosa ES, Wolf PMK, Hecker A, Liese J, Singh VK, Padberg W, Schluter KD, Rohde M, McIntosh JM, Morley BJ, Horenstein NA, Grau V, Simard AR, Comparison of the antiinflammatory properties of two nicotinic acetylcholine receptor ligands, phosphocholine and pCF3-diEPP, Front Cell Neurosci. 16 (2022), 779081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P, Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain, Br. J. Anaesth 105 (2) (2010) 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hamurtekin E, Gurun MS, The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system, Brain Res 1117 (1) (2006) 92–100. [DOI] [PubMed] [Google Scholar]
- [100].Gurun MS, Parker R, Eisenach JC, Vincler M, The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor, Anesth. Analg 108 (5) (2009) 1680–1687. [DOI] [PubMed] [Google Scholar]
- [101].Bagdas D, Sonat FA, Hamurtekin E, Sonal S, Gurun MS, The antihyperalgesic effect of cytidine-5′-diphosphate-choline in neuropathic and inflammatory pain models, Behav. Pharm 22 (5–6) (2011) 589–598. [DOI] [PubMed] [Google Scholar]
- [102].Kanat O, Bagdas D, Ozboluk HY, Gurun MS, Preclinical evidence for the antihyperalgesic activity of CDP-choline in oxaliplatin-induced neuropathic pain, J. BUON 18 (4) (2013) 1012–1018. [PubMed] [Google Scholar]
- [103].Garcia E, Shalaurova I, Matyus SP, Wolak-Dinsmore J, Oskardmay DN, Connelly MA, Quantification of choline in serum and plasma using a clinical nuclear magnetic resonance analyzer, Clin. Chim. Acta 524 (2022) 106–112. [DOI] [PubMed] [Google Scholar]
- [104].Adams DJ, Berecki G, Mechanisms of conotoxin inhibition of N-type (Ca(v)2.2) calcium channels, Biochim Biophys. Acta 1828 (7) (2013) 1619–1628. [DOI] [PubMed] [Google Scholar]
- [105].Adams DJ, Callaghan B, Berecki G, Analgesic conotoxins: block and G protein-coupled receptor modulation of N-type (Ca(V) 2.2) calcium channels, Br. J. Pharm 166 (2) (2012) 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yousuf A, Wu X, Bony AR, Sadeghi M, Huang YH, Craik DJ, Adams DJ, aO-Conotoxin GeXIVA isomers modulate N-type calcium (Ca(V) 2.2) channels and inwardly-rectifying potassium (GIRK) channels via GABA(B) receptor activation, J. Neurochem 160 (2) (2022) 154–171. [DOI] [PubMed] [Google Scholar]
- [107].Huynh TG, Cuny H, Slesinger PA, Adams DJ, Novel mechanism of voltage-gated N-type (Cav2.2) calcium channel inhibition revealed through alpha-conotoxin Vc1.1 activation of the GABA(B) receptor, Mol. Pharm 87 (2) (2015) 240–250. [DOI] [PubMed] [Google Scholar]
- [108].Cai F, Xu N, Liu Z, Ding R, Yu S, Dong M, Wang S, Shen J, Tae HS, Adams DJ, Zhang X, Dai Q, Targeting of N-Type calcium channels via GABA(B)-receptor activation by alpha-conotoxin Vc1.1 variants displaying improved analgesic activity, J. Med Chem 61 (22) (2018) 10198–10205. [DOI] [PubMed] [Google Scholar]
- [109].Bony AR, McArthur JR, Komori A, Wong AR, Hung A, Adams DJ, Analgesic alpha-Conotoxin Binding Site on the Human GABA(B) Receptor, Mol. Pharm 102 (4) (2022) 196–208. [DOI] [PubMed] [Google Scholar]
- [110].Wright AB, Norimatsu Y, McIntosh JM, Elmslie KS, Limited efficacy of alpha-conopeptides, Vc1.1 and RgIA, to inhibit sensory neuron Ca(V) current, eNeuro 2 (1) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bowery NG, GABAB receptor: a site of therapeutic benefit, Curr. Opin. Pharm 6 (1) (2006) 37–43. [DOI] [PubMed] [Google Scholar]
- [112].Goudet C, Magnaghi V, Landry M, Nagy F, Wt Gereau R, Pin JP, Metabotropic receptors for glutamate and GABA in pain, Brain Res Rev 60 (1) (2009) 43–56. [DOI] [PubMed] [Google Scholar]
- [113].Gangadharan V, Agarwal N, Brugger S, Tegeder I, Bettler B, Kuner R, Kurejova M, Conditional gene deletion reveals functional redundancy of GABAB receptors in peripheral nociceptors in vivo, Mol. Pain 5 (2009) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sadeghi M, McArthur JR, Finol-Urdaneta RK, Adams DJ, Analgesic conopeptides targeting G protein-coupled receptors reduce excitability of sensory neurons, Neuropharmacology 127 (2017) 116–123. [DOI] [PubMed] [Google Scholar]
- [115].Dyachenko IA, Palikova YA, Palikov VA, Korolkova YV, Kazakov VA, Egorova NS, Garifulina AI, Utkin YN, Tsetlin VI, Kryukova EV, alpha-Conotoxin RgIA and oligoarginine R8 in the mice model alleviate long-term oxaliplatin induced neuropathy, Biochimie 194 (2022) 127–136. [DOI] [PubMed] [Google Scholar]
- [116].Huynh PN, Giuvelis D, Christensen S, Tucker KL, McIntosh JM, RgIA4 accelerates recovery from paclitaxel-induced neuropathic pain in rats, Mar. Drugs 18 (2019) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang H, Li X, Zhangsun D, Yu G, Su R, Luo S, The alpha9alpha10 nicotinic acetylcholine receptor antagonist alphaO-conotoxin GeXIVA[1,2] alleviates and reverses chemotherapy-induced neuropathic pain, Mar. Drugs 17 (5) (2019) 265 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J, A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides, J. Neurosci 29 (4) (2009) 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Moretti M, Zoli M, George AA, Lukas RJ, Pistillo F, Maskos U, Whiteaker P, Gotti C, The novel alpha7beta2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: biochemical and pharmacological characterization, Mol. Pharm 86 (3) (2014) 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wu J, Liu Q, Tang P, Mikkelsen JD, Shen J, Whiteaker P, Yakel JL, Heteromeric alpha7beta2 nicotinic acetylcholine receptors in the brain, Trends Pharm. Sci 37 (7) (2016) 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB, Stoichiometry of the alpha9alpha10 nicotinic cholinergic receptor, J. Neurosci 25 (47) (2005) 10905–10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Grau V, Richter K, Hone AJ, McIntosh JM, Conopeptides [V11L;V16D]ArIB and RgIA4: powerful tools for the identification of novel nicotinic acetylcholine receptors in monocytes, Front Pharm. 9 (2018) 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Meyer MD, Neuronal nicotinic acetylcholine receptors as a target for the treatment of neuropathic pain, Drug Dev. Res 67 (4) (2006) 355–359. [Google Scholar]
- [124].Perez-Alvarez A, Hernandez-Vivanco A, Alonso YGS, Tabernero A, McIntosh JM, Albillos A, Pharmacological characterization of native alpha7 nicotinic ACh receptors and their contribution to depolarization-elicited exocytosis in human chromaffin cells, Br. J. Pharm 165 (4) (2012) 908–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hone AJ, Michael McIntosh J, Rueda-Ruzafa L, Passas J, de Castro-Guerin C, Blazquez J, Gonzalez-Enguita C, Albillos A, Therapeutic concentrations of varenicline in the presence of nicotine increase action potential firing in human adrenal chromaffin cells, J. Neurochem 140 (1) (2017) 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Jimenez-Pompa A, Sanz-Lazaro S, Hone AJ, Rueda-Ruzafa L, Medina-Polo J, Gonzalez-Enguita C, Blazquez J, de Los Rios C, Michael McIntosh J, Albillos A, Therapeutic concentrations of varenicline increases exocytotic release of catecholamines from human and rat adrenal chromaffin cells in the presence of nicotine, Neuropharmacology 195 (2021), 108632. [DOI] [PubMed] [Google Scholar]
- [127].Elgoyhen AB, The alpha9alpha10 acetylcholine receptor: a non-neuronal nicotinic receptor. Pharmacological Research. 2023;in press. [DOI] [PubMed] [Google Scholar]
- [128].Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim JY, Kim TH, Zhang MQ, Dussor G, Price TJ, Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research, Pain 159 (7) (2018) 1325–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hone AJ, Meyer EL, McIntyre M, McIntosh JM, Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the alpha6beta4* subtype, FASEB J. 26 (2) (2012) 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Noviello CM, Gharpure A, Mukhtasimova N, Cabuco R, Baxter L, Borek D, Sine SM, Hibbs RE, Structure and gating mechanism of the alpha7 nicotinic acetylcholine receptor, Cell 184 (8) (2021) 2121–2134, e2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Liu T, Zhang L, Joo D, Sun SC, NF-kappaB signaling in inflammation, Signal Transduct. Target Ther 2 (2017) 17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Guha P, Bandyopadhyaya G, Polumuri SK, Chumsri S, Gade P, Kalvakolanu DV, Ahmed H, Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, alpha9 nicotinic acetylcholine receptor and STAT3, Breast Cancer Res Treat. 145 (1) (2014) 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


